Introduction
Osteosarcoma is the most common primary tumor of
bone tissue; it is most common in young people and exhibits a high
degree of malignancy (1,2). The treatment of osteosarcoma is
neoadjuvant chemotherapy and surgery (3). The five-year survival rate remains at
60–70%, and there has been no significant increase during the past
10 years (4). The primary reason for
this is that the mechanism of pathogenesis is not clear. The
formation and development of the tumor is a multifactorial,
multi-stage and a gradual process; early diagnosis and timely
intervention are essential for the prognosis of patients (5). In previous years, the associations
between cell proliferation and cell signaling have become an area
of interest (6,7).
Previous studies have identified that nuclear
receptor co-activator 5 (NCOA5) is a nuclear receptor co-regulator
that is widely expressed (8,9). NCOA5 is also a co-regulator for the
estrogen receptor and orphan nuclear hormone receptor co-activator
BD73 (10). The transcription of
NCOA5 is mediated by estrogen receptors to achieve homeostatic
regulation of human B cells. NCOA5 gene defects may lead to cancer
and diabetes (11,12).
To investigate the association between the
expression of NCOA5 and the prognosis of postoperative patients
with osteosarcoma, immunohistochemistry (IHC) and reverse
transcription-polymerase chain reaction (RT-PCR) were employed in
the present studyt to measure the levels of NCOA5 protein in
patients with human osteosarcoma, using normal bone tissues as the
controls. The association between these groups and the mechanism of
the formation of osteosarcoma were explored to create novel targets
for early diagnosis of this disease.
Patients and methods
Patient samples
Samples of human osteosarcoma were collected between
February 2012 and June 2017 from 145 patients who received surgical
resection at The First People's Hospital of Yancheng City
(Yancheng, China), and had been diagnosed by pathological
confirmation. Detailed clinical data were available for all the
patients, including sex, age, differentiation, invasion depth,
lymph node metastasis and Union for International Cancer Control
stage tumor node metastasis (TNM) stage (13), and none had received preoperative
chemotherapy or radiotherapy. Human osteosarcoma patients included
75 males and 70 females, (age range between 15–63 years; average
age, 40.9±11.6 years). Normal bone tissue specimens were collected
by surgical resection from 100 individuals to serve as a control
group. These included 55 males and 45 females, (age range 16–68
years; average age, 34.4±10.3 years). No statistically significant
differences were detected in age or sex between the two groups. The
protocol used in the present study was in accordance with the
Medical Ethics and Human Clinical Trial Committee of The First
People's Hospital of Yancheng City, (Identification No. HMU Ethics
20120003). Written informed consent was obtained by all
participants in the present study.
Immunohistochemical staining
The EnVision and DAB chromogenic reagent kit
(Agilent Technologies, Inc., Santa Clara, CA, USA) was used for
immunostaining to detect the distribution of NCOA5 (Antibody
Diagnostic; Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Immunohistochemical procedures were performed in
strict accordance with the manufacturer's protocol (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The EnVision and
3,3′-diaminobenzidine chromogenic reagent kits (Antibody
Diagnostic; Applied Biosystems) were used for immunohistochemical
staining. All slide staining was performed under the same
conditions: The tissue was sliced to 4-µm thickness, dehydrated
with 21% sucrose, de-waxed and subjected to antigen retrieval in
0.01 mol/l citric acid pH 6.0 at 95°C for 10 min. Normal goat serum
(10%; Toyobo Life Science, Osaka, Japan) was placed on the slide
and incubated for 10 min at room temperature. Slides were then
incubated with the corresponding specific primary antibodies (mouse
anti-osteonection/NCOA5; cat. no. ab70831; Abcam, Cambridge, UK;
1:1,000) for 1.5 h at room temperature. Tissue sections were then
washed with PBS 3 times for 3 min. Subsequently, tissue sections
were incubated with secondary antibody horseradish
peroxidase-conjugated mouse anti-osteonection/NCOA5 IgG (1:1,000;
cat. no. ab125508; Abcam) for 30 min at room temperature. Proteins
were detected using 3,3′-Diaminobenzidine (5 min at room
temperature), while the nucleus was stained with 10% hematoxylin at
37°C for 7 min. Tissue samples were then dehydrated in 75, 85, 95
and 100% gradient ethanol series, cleared by 1% xylene and sealed
with rubber cement. Each batch of staining contained a positive
control sourced from patients with confirmed osteosarcoma [with the
known positive section reagent (cat. no. ab92431; Abcam; 1:1,000)]
and a negative control, in which the corresponding specific
antibody was replaced by PBS.
Samples with nuclei with yellow or tan reactant
particles were considered positive. A total of four independent
experiments were conducted, and four fields of view were assessed
by optical microscopy at ×200 magnification. Analysis of the
staining scores was semi-quantitative: Final staining scores were
calculated by multiplying the positive cell percentage score and
the staining intensity score. The positive cell staining percentage
score criteria were as follows: 0–15%, 0; >15–30%, 1;
>30–45%, 2; and >45%, 3. These staining scores were then
classified as follows: >2, negative (−); 2–4, weakly positive
(+); 4–6, positive (++); and ≥6, strongly positive (+++). For the
convenience of statistical analysis, samples within the (−) group
were defined as the negative expression group (−), and samples
within the (+), (++) and (+++) groups were defined as the positive
expression group (+).
Detection of NCOA5 mRNA expression by
RT-PCR
Total RNA was isolated from tissues using
TRIzol® reagent (NanoDrop Technologies; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol and was
quantified using a NanoDrop spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). A total of 2 µg RNA
was reverse transcribed to complementary DNA using the QuantiNova
Reverse Transcription kit (50) according to the manufacturer's
protocol (cat. no. 205411; Qiagen GmbH, Hilden, Germany). Samples
were then amplified by semi-quantitative PCR with β-actin as a
reference. The sequences of the primers (Sangon Biotech., Co.,
Ltd., Shanghai, China) are listed in Table I. Thermocycler conditions were as
follows: Pre-denaturation at 94°C for 4 min, followed by 30 cycles
of 94°C for 10 sec, 55°C for 30 sec and 72°C for 60 sec.
 | Table I.Primer sequences for reverse
transcription-polymerase chain reaction analysis. |
Table I.
Primer sequences for reverse
transcription-polymerase chain reaction analysis.
| Primers | Primer sequences
(5′-3′) | Product size
(bp) |
|---|
| NCOA5 |
|
|
|
Forward |
CTGCTGGCAGACAACAGGTA | 344 |
|
Reverse |
CTGTTTGCTGCTGTGGAAAA | 344 |
| B-actin |
|
|
|
Forward |
TGACGTGGACATCCGCAAAG | 231 |
|
Reverse |
CTGGAAGGTGGACAGCGAGG | 231 |
Amplification of NCOA5 by PCR was examined using 1%
agarose gel electrophoresis. The absorbance value of the sample
gene and the reference (β-actin) were read, and the results were
expressed as the ratio (sample value/reference value). If the ratio
of osteosarcoma to reference absorbance values was positive, NCOA5
mRNA expression was considered to be positive; if it was negative,
NCOA5 mRNA expression of the sample was categorized as
negative.
Statistical methods
SPSS 13.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. The χ2 test
was used to compare the distribution of NCOA5 between osteosarcoma
and normal bone tissues. The Kaplan-Meier survival analysis with
log-rank test was performed to analyze the association between the
expression levels of the proteins in cancer tissue or other
clinicopathological characteristics and the survival rate of
patients. Associations between the expression of NCOA5 and overall
survival of postoperative patients with osteosarcoma were assessed
using Cox regression analysis. P<0.05 was considered to indicate
a statistically significantly difference.
Results
Cell nucleus staining distribution of
NCOA5 in human osteosarcoma and normal bone tissues
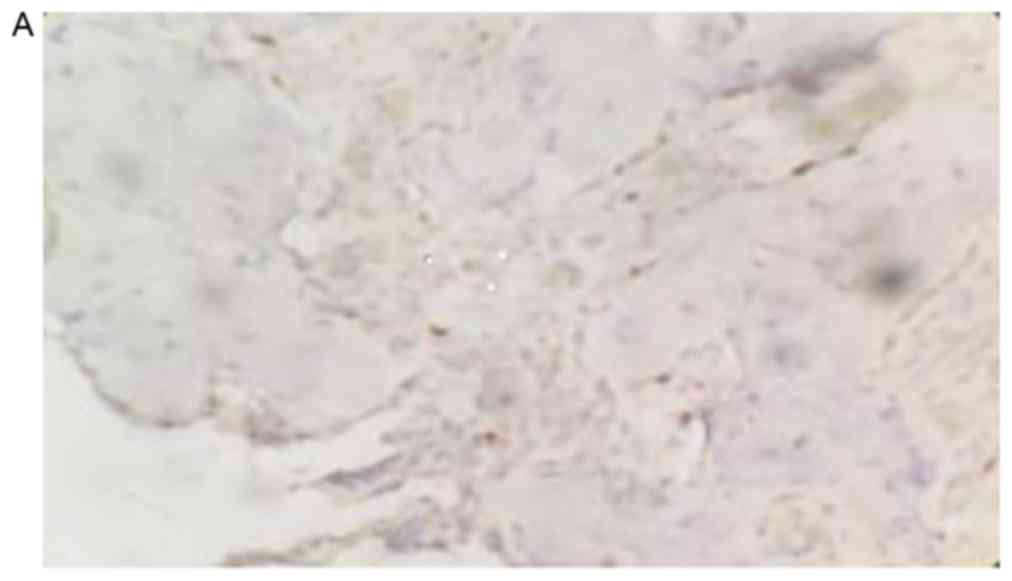
The positive rate of NCOA5 staining in human
osteosarcoma tissues was 26.21% (38/145), which was significantly
lower compared with the positive rate of NCOA5 in normal bone
tissues, which was 82.00% (82/100; P<0.05; Fig. 1).
NCOA5 mRNA expression in human
osteosarcoma and normal bone tissues
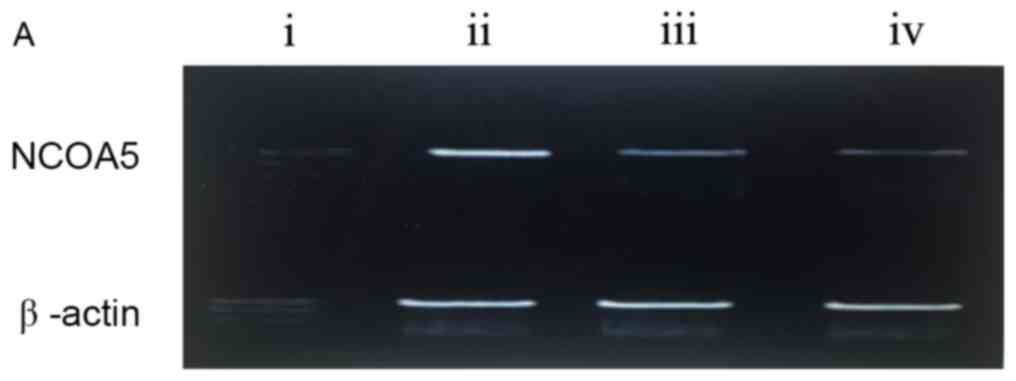
The results from RT-PCR indicate that mRNA NCOA5 was
expressed in osteosarcoma and normal bone tissues (P<0.05;
Table I). The positive rate of NCOA5
mRNA expression in osteosarcoma was 17.24% (25/145), which was
significantly lower compared with that in normal bone tissue
(84.00%, 84/100; χ2=33.166; P<0.001; Fig. 2). Compared with the normal bone
tissue, the rate of NCOA5 expression was significantly lower in
pulmonary metastatic osteosarcoma tissues.
Association between the expression of
NCOA5 mRNA and the clinicopathological characteristics of
osteosarcoma
The expression levels of NCOA5 mRNA and protein in
osteosarcoma were consistent and were lower than those in the
normal controls. NCOA5 exhibited a low level of expression in
osteosarcoma, and was not associated with sex, age or tumor size.
However, it was associated with recurrence and metastasis
(P<0.01; Table II).
 | Table II.Association between NCOA5 mRNA and
protein expression with clinicopathological features in
osteosarcoma. |
Table II.
Association between NCOA5 mRNA and
protein expression with clinicopathological features in
osteosarcoma.
| Characteristics | n | NCOA5 mRNA positive
rate, n (%) | χ2 | P-value | NCOA5 protein
positive rate, n (%) | χ2 | P-value |
|---|
| Sex |
|
| 0.190 | 0.663 |
| 0.008 | 0.927 |
| Male | 75 | 15 (20.0) |
|
| 12 (16.0) |
|
|
|
Female | 70 | 11 (15.7) |
|
| 11 (15.7) |
|
|
| Age (years) |
|
| 0.121 | 0.728 |
| 0.005 | 0.945 |
|
<40 | 73 | 13 (17.8) |
|
| 12 (16.4) |
|
|
| ≥40 | 72 | 10 (13.9) |
|
| 13 (18.1) |
|
|
| Tumor diameter
(cm) |
|
| 0.351 | 0.553 |
| 0.072 | 0.789 |
| ≥10 | 63 | 11 (17.5) |
|
| 10 (15.9) |
|
|
|
<10 | 82 | 13 (15.9) |
|
| 15 (18.3) |
|
|
| Pulmonary
metastasis |
|
| 7.601 | 0.006 |
| 7.601 | 0.006 |
| Yes | 65 | 26 (40.0) |
|
| 26 (40.0) |
|
|
| No | 80 | 5 (6.3) |
|
| 5 (6.3) |
|
|
Association between the expression of
NCOA5 and overall survival of postoperative patients with
osteosarcoma
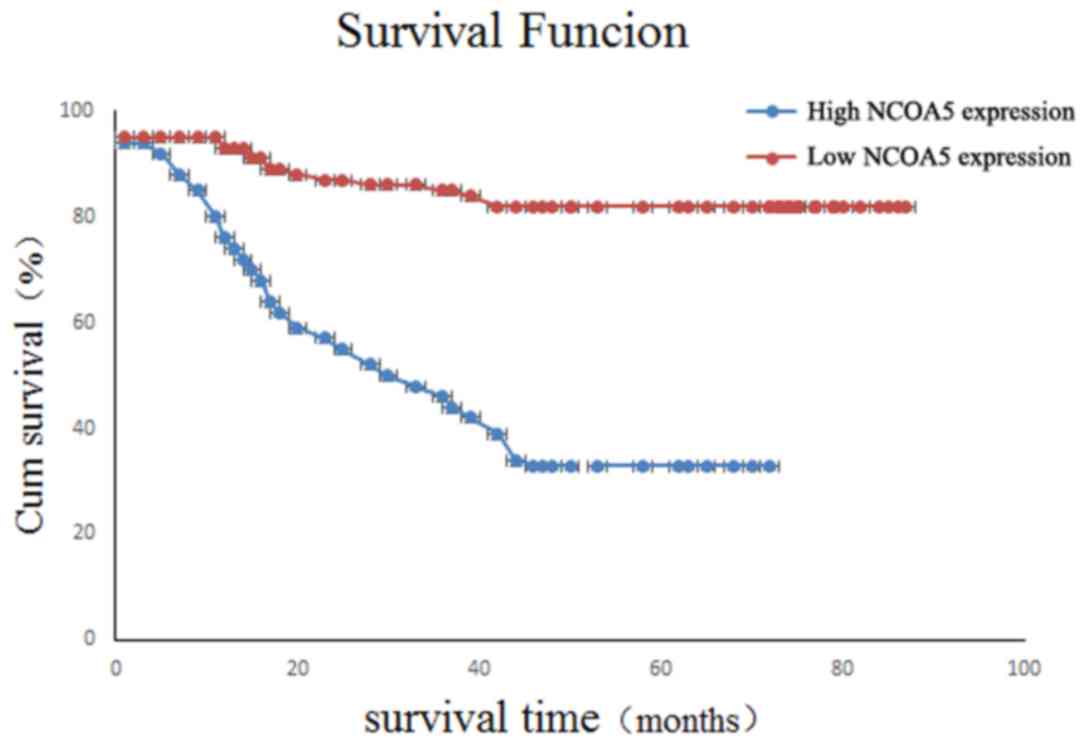
The overall survival time of NCOA5-positive patients
was (60.92±3.45) months after a median follow-up of 61.5 months
(61.5±77.3 months). The overall survival of NCOA5-negative patients
was (25.68±5.65) months. Kaplan-Meier survival analysis
demonstrated that there was a significant difference between these
data (χ2=0.990; P=0.031; Fig.
3).
In addition, adjuvant therapy, sex, age, gross
morphology, tumor invasion depth and lymph node metastasis were not
identified as independent factors affecting the overall survival of
postoperative patients (Table
III).
 | Table III.Analysis of survival factors in
patients with osteosarcoma. |
Table III.
Analysis of survival factors in
patients with osteosarcoma.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Clinicopathological
features | B | SE | P-value | 95%CI | B | SE | P-value | 95%CI |
|---|
| Age | 0.658 | 0.604 | 0.269 |
| 0.612 | 0.598 | 0.325 |
|
| Sex | 0.060 | 0.602 | 0.940 |
| 0.068 | 0.615 | 0.915 |
|
| Tumor size | 1.756 | 0.765 | 0.042 | 1.02–3.08 | 1.211 | 0.544 | 0.031 | 1.06–3.13 |
| Tumor location | 0.052 | 0.512 | 0.819 |
| 0.063 | 0.610 | 0.875 |
|
| Pathological
type | 0.598 | 0.512 | 0.294 |
| 0.626 | 0.651 | 0.312 |
|
| Lymph node
metastasis | 1.796 | 0.621 | <0.001 | 1.42–4.26 | 1.826 | 0.631 | <0.001 | 1.55–4.52 |
| Low NCOA5
expression | 3.003 | 1.071 | 0.003 | 1.41–6.08 | 3.012 | 1.076 | 0.003 | 1.48–6.23 |
Discussion
Previous studies have indicated that the occurrence
and development of tumors is a complicated process (14); disorders in the regulation of cell
growth and proliferation may affect the occurrence and development
of tumors (15). Concurrently, the
abnormal expression of tumor-associated genes, and the abnormal
activation of cell signal transduction and cell proliferation cycle
are also involved in numerous aspects of tumor development
(16,17). Cell growth and proliferation in the
human body are affected and controlled by a number of factors
(18,19). In particular, cell signaling proteins,
growth factors and their receptors, apoptotic proteins and
transcription factors, and the changes to these factors are closely
associated with the occurrence and development of tumors (20,21).
Previous studies have also revealed that the NCOA5
protein may be associated with interleukin (IL)-6, tumor necrosis
factor (TNF)-α and nuclear factor (NF)-κB (22–24).
Prevention of the overexpression of IL-6 may limit the occurrence
and development of certain types of cancer. TNF-α was significantly
increased in NCOA5 gene-deficient animal tumors. However, this gene
defect is not reversible in vivo, and the specific mechanism
requires additional study (25).
Previous studies have also identified that the
decrease in NCOA5 expression in esophageal squamous cell carcinoma
(ESCC) tissue is associated with the differentiation status of the
tumor and the TNM stage, while is not associated with age, sex,
weight loss, tumor location or lymph node metastasis (26,27). In
addition, the expression of NCOA5 in normal tissues was higher
compared with that in tumor tissues. As the level of tumor
differentiation and TNM stage are important indicators of the
malignant degree of tumors, the results of the present study
suggest that the decreased expression of NCOA5 may be involved in
the promotion of tumor progression (28–30).
One limitation of the current study is the
relatively small sample size. Nevertheless, to the best of our
knowledge, the present study is among the largest studies
addressing NCOA5 protein expression in osteosarcoma. The results of
the present study indicated that the expression of NCOA5 in
osteosarcoma was significantly lower compared with that in normal
bone tissue. In addition, the expression levels of NCOA5 protein in
benign bone tumor tissues were significantly higher than in
osteosarcoma tissues, which may indicate an association between the
occurrence and development of tumors and low NCOA5 expression.
The present study demonstrated that the expression
of NCOA5 protein in human specimens is closely associated with the
occurrence of osteosarcoma. The expression of NCOA5 in osteosarcoma
tissues was significantly lower compared with that in normal bone
tissue. This indicates that NCOA5 may be a tumor-suppressor gene in
humans.
The results of the present study suggested that the
expression of NCOA5 is consistent in different types of
osteosarcoma tissues, such as those with or without pulmonary
metastasis. The expression of NCOA5 in osteosarcoma was
significantly lower compared to that in normal bone tissue. The low
expression of NCOA5 may be a cause of osteosarcoma, and therefore,
it may be important to detect the expression of NCOA5 in
osteosarcoma for the diagnosis of this disease.
In the present study, it was also identified that
the expression of NCOA5 protein in patients with osteosarcoma was
associated with survival prognosis, and the clinical features of
the tumor were significantly associated with the survival rate,
differentiation and staging.
In conclusion, the results of the present study
indicate the potential role of NCOA5 in the progression of
osteosarcoma, highlighting its low expression as an independent
prognostic factor. Furthermore, additional studies examining NCOA5
may also assist in developing novel therapeutic strategies for
osteosarcoma.
Acknowledgements
The present study was supported by Jiangsu
Pharmaceutical Association (grant no. 201542) and the Science and
Technology commission of Yancheng City (grant no. YK2015002).
References
|
1
|
Huang J, Liu K, Song D, Ding M, Wang J,
Jin Q and Ni J: Krüppel-like factor 4 promotes high-mobility group
box 1-induced chemotherapy resistance in osteosarcoma cells. Cancer
Sci. 107:242–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li YS, Deng ZH, Zeng C and Lei GH: JNK
pathway in osteosarcoma: Pathogenesis and therapeutics. J Recept
Signal Transduct Res. 36:465–470. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lamplot JD, Denduluri S, Qin J, Li R, Liu
X, Zhang H, Chen X, Wang N, Pratt A, Shui W, et al: The current and
future therapies for human osteosarcoma. Curr Cancer Ther Rev.
9:55–77. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chakravarthi PS, Kattimani VS, Prasad LK
and Satish PR: Juxtacortical osteosarcoma of the mandible:
Challenges in diagnosis and management. Natl J Maxillofac Surg.
6:127–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Commandeur AE, Styer AK and Teixeira JM:
Epidemiological and genetic clues for molecular mechanisms involved
in uterine leiomyoma development and growth. Hum Reprod Update.
21:593–615. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou W, Zhu Y, Chen S, Xu R and Wang K:
Fibroblast growth factor receptor 1 promotes MG63 cell
proliferation and is associated with increased expression of
cyclin-dependent kinase 1 in osteosarcoma. Mol Med Rep. 13:713–719.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang J, Cheng D, Zhou S, Zhu B, Hu T and
Yang Q: Overexpression of X-box binding protein 1 (XBP1 correlates
to poor prognosis and up-regulation of PI3K/mTOR in human
osteosarcoma. Int J Mol Sci. 16:28635–28646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu CY and Feng GS: NCOA5, a molecular
link between type 2 diabetes and liver cancer. Hepatobiliary Surg
Nutr. 3:106–108. 2014.PubMed/NCBI
|
|
9
|
Tetel MJ, Auger AP and Charlier TD: Who's
in charge? Nuclear receptor coactivator and corepressor function in
brain and behavior. Front Neuroendocrinol. 30:328–342. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao S, Li A, Liu F, Chen F, Williams M,
Zhang C, Kelley Z, Wu CL, Luo R and Xiao H: NCOA5
Haploinsufficiency results in glucose intolerance and subsequent
hepatocellular carcinoma. Cancer Cell. 24:725–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lahusen T, Henke RT, Kagan BL, Wellstein A
and Riegel AT: The role and regulation of the nuclear receptor
co-activator AIB1 in breast cancer. Breast Cancer Res Treat.
116:225–237. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chauhan C, Zraly CB, Parilla M, Diaz MO
and Dingwall AK: Histone recognition and nuclear receptor
co-activator functions of Drosophila cara mitad, a homolog of the
N-terminal portion of mammalian MLL2 and MLL3. Development.
139:1997–2008. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen VW, Ruiz BA, Hsieh MC, Wu XC, Ries LA
and Lewis DR: Analysis of stage and clinical/prognostic factors for
lung cancer from SEER registries: AJCC staging and collaborative
stage data collection system. Cancer. 120 Suppl 23:S3781–S3792.
2014. View Article : Google Scholar
|
|
14
|
Chinenov Y, Gupte R and Rogatsky I:
Nuclear receptors in inflammation control: Repression by GR and
beyond. Mol Cell Endocrinol. 380:55–64. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pavlin MR, Brunzelle JS and Fernandez EJ:
Agonist ligands mediate the transcriptional response of nuclear
receptor heterodimers through distinct stoichiometric assemblies
with coactivators. J Biol Chem. 289:24771–24778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gyamfi MA and Wan YJ: Pathogenesis of
alcoholic liver disease: The role of nuclear receptors. Exp Biol
Med (Maywood). 235:547–560. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brand TM, Iida M, Li C and Wheeler DL: The
nuclear epidermal growth factor receptor signaling network and its
role in cancer. Discov Med. 12:419–432. 2011.PubMed/NCBI
|
|
18
|
Poos K, Smida J, Maugg D, Eckstein G,
Baumhoer D, Nathrath M and Korsching E: Genomic heterogeneity of
osteosarcoma-shift from single candidates to functional modules.
PLoS One. 10:e01230822015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Basu-Roy U, Basilico C and Mansukhani A:
Perspectives on cancer stem cells in osteosarcoma. Cancer Lett.
338:158–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao R, Ni D, Tian Y, Ni B and Wang A:
Aberrant ADAM10 expression correlates with osteosarcoma
progression. Eur J Med Res. 19:92014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Talmadge JE and Gabrilovich DI: History of
myeloid-derived suppressor cells. Nat Rev Cancer. 13:739–752. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dhar D, Seki E and Karin M: NCOA5, IL-6,
Type-2 diabetes and HCC: The deadly quartet. Cell Metab. 19:6–7.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cumsille P, Coronel A, Conca C, Quiñinao C
and Escudero C: Proposal of a hybrid approach for tumor progression
and tumor-induced angiogenesis. Theor Biol Med Model. 12:132015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arvelo F, Sojo F and Cotte C: Tumour
progression and metastasis. Ecancermedicalscience. 10:6172016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gillespie MA, Gold ES, Ramsey SA, Podolsky
I, Aderem A and Ranish JA: An LXR-NCOA5 gene regulatory complex
directs inflammatory crosstalk-dependent repression of macrophage
cholesterol efflux. EMBO J. 34:1244–1258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee E, Pandey NB and Popel AS: Crosstalk
between cancer cells and blood endothelial and lymphatic
endothelial cells in tumour and organ microenvironment. Expert Rev
Mol Med. 17:e32015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Marcu LG and Harriss-Phillips WM: In
Silico modelling of treatment-induced tumour cell kill:
Developments and advances. Comput Math Methods Med.
2012:9602562012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang M, Yuan J, Peng C and Li Y: Collagen
as a double-edged sword in tumor progression. Tumour Biol.
35:2871–2882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin G, Sun XJ, Han QB, Wang Z, Xu YP, Gu
JL, Wu W, Zhang GU, Hu JL, Sun WY and Mao WM: Epidermal growth
factor receptor protein overexpression and gene amplification are
associated with aggressive biological behaviors of esophageal
squamous cell carcinoma. Oncol Lett. 10:901–906. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shiomi H, Eguchi Y, Tani T, Kodama M and
Hattori T: Cellular distribution and clinical value of
urokinase-type plasminogen activator, its receptor, and plasminogen
activator inhibitor-2 in esophageal squamous cell carcinoma. Am J
Pathol. 156:567–575. 2000. View Article : Google Scholar : PubMed/NCBI
|