Introduction
Human Liver Cancer (HLC) is a malignant tumor of the
digestive system, typically with a poor prognosis, and its
mortality rate ranks in third place worldwide of all
cancer-associated mortalities (1).
Male morbidity is higher compared with female morbidity (1). A report from 2006 revealed that 662,000
patients succumb to HLC annually (2).
The morbidity of HLC varies across regions; Eastern and Southeast
Asia, a number of Western Pacific islands, and Saharan and Southern
Africa have high morbidity rates compared with other countries. The
morbidity rate of HLC in Asia is ~70%, and the corresponding
morbidity rates of Eastern and Southern Europe, the Caribbean,
Central America and Western Asia are close behind; the morbidity
rates of other countries are lower in comparison (3,4).
Tumors arise from aberrations in the cell cycle;
mutations in a number of cell cycle-associated genes may result in
altered expression and activities of other cell cycle-associated
genes, leading to tumor development (4). Abnormalities associated with the
G1/S checkpoint serve an important role in tumor
initiation, as DNA replication and repair and take place during the
G1/S phase. Cyclin D1, cellular tumor antigen p53 (p53),
cyclin-dependent kinase inhibitor 1 (p21) and signal transducer and
activator of transcription 3 serve important roles in the
regulation of the G1/S phase of the cell cycle (5,6).
Apoptosis is part of the normal life cycle of cells,
and is a way to regulate the stability of cell populations
(7). Apoptosis maintains the turnover
of normal cells and eliminates abnormal cells in vivo;
however, abnormal apoptosis is associated with the occurrence of a
number of diseases (8). Tumor
necrosis factor receptor superfamily member 6 (Fas) and Fas ligand
(FasL) are transmembrane proteins that are present on the surface
of cells (9). When Fas on the surface
of one cell binds to FasL on the membrane of another cell, the cell
that expresses Fas undergoes apoptosis. The Fas/FasL signaling
pathway is the primary method of mediating liver cell apoptosis
(10,11). Therefore, Fas/FasL signaling pathway
abnormalities and their association with the occurrence and
progression of HLC require further investigation.
Over the last 20 years, a number of studies have
investigated the potential anticancer effects of Chinese herbs and
extracts, including harmaline, which is an active ingredient
extracted from the fleabane seeds of renascent herbs. Harmaline
exhibits numerous clinical effects, including protection from
radiation, reduction of inflammation, analgesia, antipruritic
effects, immunosuppression, relief from psoriasis and antitumor
effects (12). The antitumor effects
of harmaline, first studied in the 1970s (12), are of particular interest for the
current study and other studies. A previous study revealed that
harmaline exhibits a number of antitumor effects and has a low risk
of producing toxic side effects (13). Harmaline has been demonstrated to have
antitumor effects in gastric carcinoma in vivo and in
vitro (14). Therefore, in the
present study, the anticancer and apoptosis-promoting effects of
harmaline were investigated in human liver carcinoma cells.
Materials and methods
Cell culture
Human liver carcinoma (HepG2) cells were obtained
from the Animal Centre of Guangxi University of Chinese Traditional
Medicine (Nanning, Guangxi, China) and cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 10% fetal bovine
serum (FBS; both Gibco, Thermo Fisher Scientific, Inc., Waltham,
MA, USA), 100 U/ml penicillin and 100 µg/ml streptomycin (Thermo
Fisher Scientific Inc.) in a humidified incubator with 5%
CO2 at 37°C.
Cell viability assay
HepG2 cells (1×103) were seeded in a
96-well plate and treated with 0–10 µM of harmaline (Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) for 12, 24 and 48 h in a humidified
incubator with 5% CO2 at 37°C. Following incubation, 20
µl of MTT (0.5 mg/ml) was added to the wells, and the plates were
subsequently incubated at 37°C for 4 h. Following incubation, 150
µl dimethyl sulfoxide was added to each well prior to incubation
for a further 20 min at 37°C. The absorbance of the plates at 490
nm was subsequently recorded using a PowerWave HT microplate
spectrophotometer (BioTek Instruments, Inc., Winooski, VT, USA).
The structural formula of harmaline is illustrated in Fig. 1.
Flow cytometry
HepG2 cells (1×106) were seeded in a
6-well plate and treated with 0–10 µM harmaline for 48 h in a
humidified incubator with 5% CO2 at 37°C. HepG2 cells
were washed in PBS, re-suspended in binding buffer from a Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide kit (BD
Biosciences, San Jose, CA, USA) and stained using 5 µl Annexin
V-FITC and 5 µl propidium iodide (BD Biosciences) for 15 min in the
dark at room temperature, according to the manufacturer's protocol.
The percentage of apoptotic cells was measured using a flow
cytometer (COULTER® EPICS® ALTRA™
Flow Cytometer; Beckman Coulter, Inc., Brea, CA, USA) and CellQuest
software 3.0 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Caspase-8/3 activity
HepG2 cells (1×103) were seeded into
96-well plates and treated with 0–10 µM of harmaline for 48 h in a
humidified incubator with 5% CO2 at 37°C. HepG2 cells
(1×106) were seeded onto a 6-well plate and treated with
0–10 µM harmaline for 48 h in a humidified incubator with 5%
CO2 at 37°C. HepG2 cells were lyzed in
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China). Total protein was quantified using a
BCA assay kit (Beyotime Institute of Biotechnology), and 5 µg/lane
of total protein was incubated with the chromogenic substrates
Ac-IETD-pNA (caspase-8; catalog no., C1152; Beyotime Institute of
Biotechnology) and Ac-DEVD-pNA (caspase-3; catalog no., C1116;
Beyotime Institute of Biotechnology) at 37°C for 1 h in the dark.
The absorbance was subsequently recorded using a PowerWave HT
microplate spectrophotometer at 405 nm.
Western blot analysis
HepG2 cells (1×106) were seeded into a
6-well plate and treated with 0–10 µM harmaline for 48 h in a
humidified incubator with 5% CO2 at 37°C. HepG2 cells
were lyzed in radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology, Haimen, China). Total protein was
quantified using a BCA assay kit (Beyotime Institute of
Biotechnology), and 50 µg/lane of total protein was loaded and run
on a 12% gel using SDS-PAGE. Separated proteins were transferred to
a nitrocellulose membrane (Thermo Fisher Scientific, Inc.). The
membrane was blocked with 5% non-fat milk in Tris-buffered
saline-0.1% Tween (TBST) for 1 h at 37°C and incubated with
anti-p53 (cat. no. sc-55476; dilution, 1:300), anti-p21 (cat. no.
sc-271532; dilution, 1:300) anti-Fas (cat. no. sc-8009; dilution,
1:300), anti-FasL (cat. no. sc-33716; dilution, 1:300),
anti-caspase-8 (cat. no., sc-7890; dilution, 1:300) (all Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-GAPDH (cat. no.,
AG019; dilution, 1:2,000, Beyotime Institute of Biotechnology)
antibodies overnight at 4°C. The membrane was washed with TBST and
incubated with a goat anti-rabbit horseradish peroxidase-conjugated
secondary antibody (cat. no., A0239; dilution, 1:5,000; Beyotime
Institute of Biotechnology) for 1 h at 37°C. Protein bands detected
using an BeyoECL Plus (Beyotime Institute of Biotechnology) and
analyzed using Bio-Rad Laboratories Quantity One software 3.0
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). This experiment
was repeated in triplicate.
Statistical analysis
All results are presented as the mean ± SD using
SPSS 19.0 (IMB Corp., Armonk, NY, USA). Data were analyzed using
one-way repeated measures analysis of variance followed by Duncan's
multiple comparison tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Harmaline decreases HepG2 cell
viability
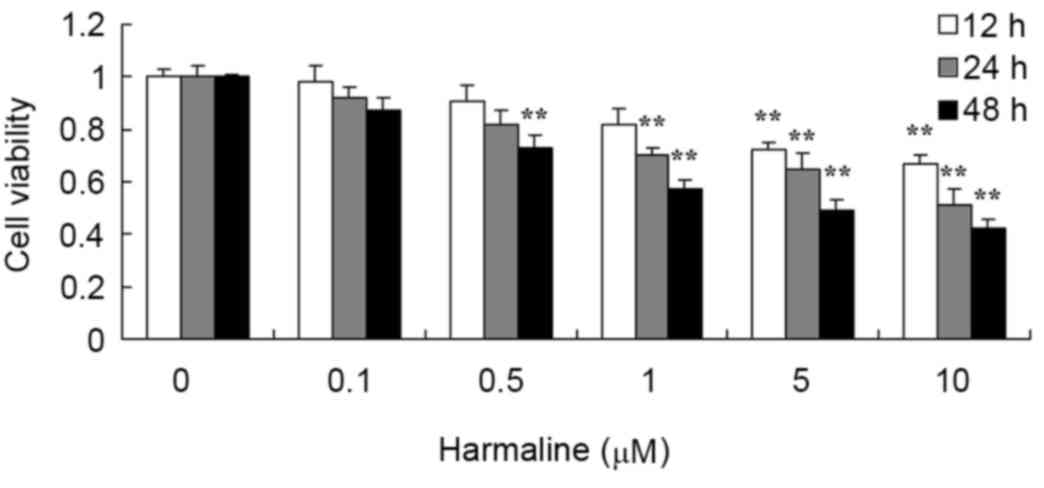
Following treatment with 0–10 µM harmaline for 12,
24 or 48 h, HepG2 cell viability was analyzed using an MTT assay.
Harmaline decreased the viability of HepG2 cells in a time- and
dose-dependent manner (Fig. 2).
Following treatment with 5 and 10 µM harmaline, the viability of
HepG2 cells was significantly decreased at 12, 24 and 48 h compared
with the untreated negative control group (P<0.01).
Additionally, 1 µM harmaline significantly decreased the viability
of HepG2 cells at 24 and 48 h (both P<0.01), and 0.5 µM
harmaline significantly decreased the viability at 48 h
(P<0.01).
Harmaline increases HepG2 cell
apoptosis
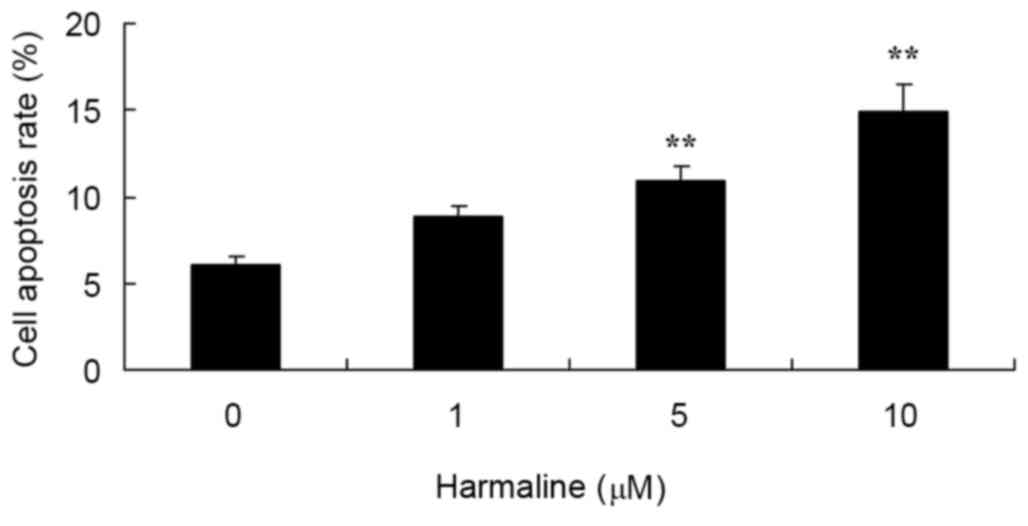
Following treatment with 0–10 µM of harmaline for 48
h, HepG2 cell apoptosis was analyzed by flow cytometry. Doses of 5
and 10 µM harmaline significantly increased the cell apoptosis rate
of HepG2 cells compared with the untreated negative control group
(Fig. 3; P<0.01).
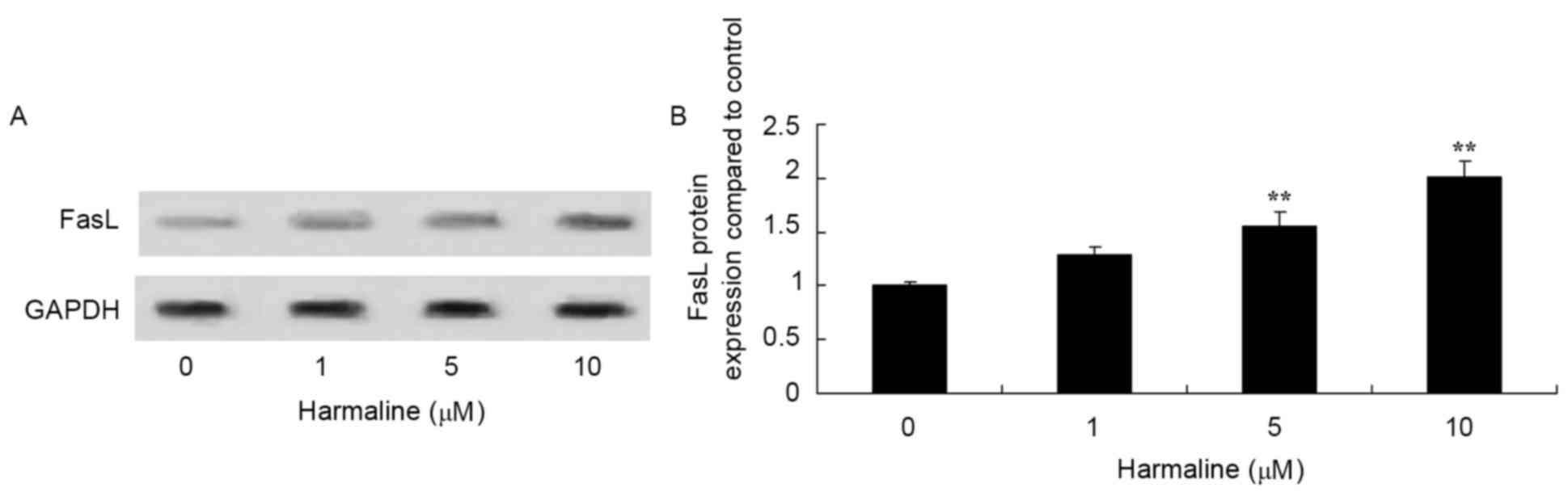
Harmaline increases HepG2 cell p53,
p21, Fas and FasL expression
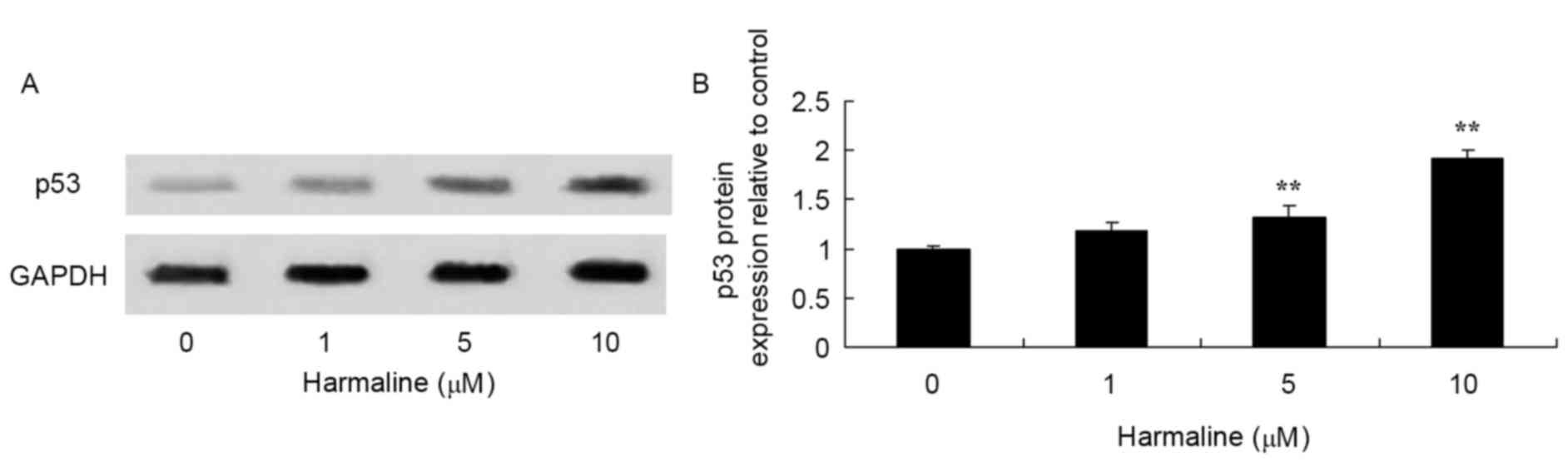
Western blot analysis was performed to assess the
effect of harmaline on p53, p21, Fas, FasL and caspase-8 protein
expression in HepG2 cells. Compared with the negative control,
treatment with 5 and 10 µM harmaline for 48 h significantly
increased p53, p21, Fas and FasL protein expression in HepG2 cells
compared with the untreated negative control group (Figs. 4–7;
P<0.01). These results indicate that the p53/p21 and Fas/FasL
signaling pathways serve a role in the anticancer effect of
harmaline.
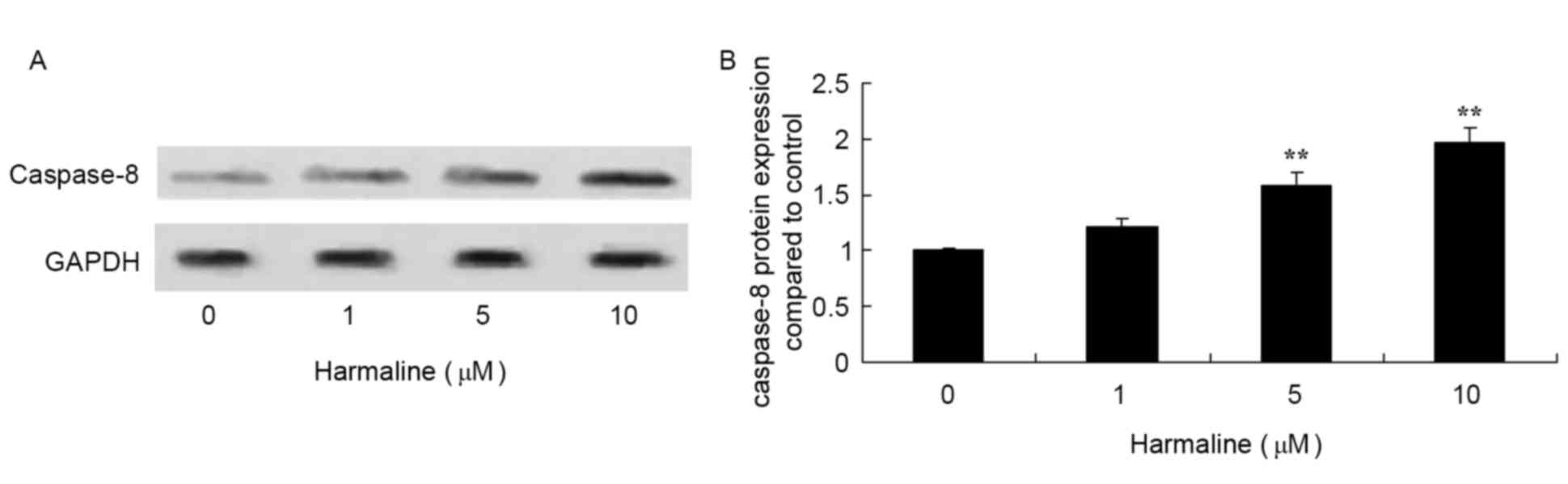
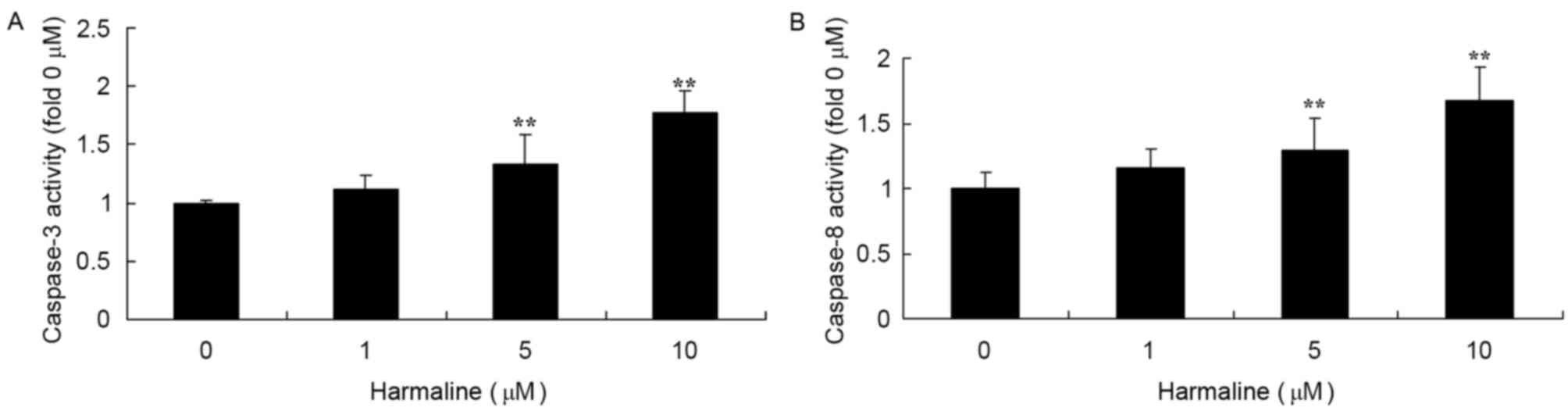
Harmaline increases caspase-8
expression and caspase-3/−8 activity in HepG2 cells
In order to explore the underlying molecular
mechanisms of harmaline-induced cell apoptosis, the expression and
activity of caspase-3/−8 was measured in HepG2 cells following
harmaline treatment using a western blot analysis and an ELISA,
respectively. Doses of 5 and 10 µM harmaline significantly
increased caspase-8 protein expression (Fig. 8; P<0.01) and caspase-3/−8 activity
(Fig. 9; P<0.01) compared with the
untreated negative control group.
Discussion
The morbidity rate of HLC is rising, therefore the
development of treatments that inhibit the occurrence and
development of HLC are required (15). The development of HLC is a complex and
relatively slow, and there is no definitive pathological definition
of HLC in the clinic (16).
Therefore, investigating the changes in gene expression that are
associated with HLC may aid in the diagnosis and inhibition of HLC
occurrence and development (17). The
results from the present study demonstrate that harmaline
significantly decreases the viability and significantly increases
the apoptosis rate of HepG2 cells.
The gene that encodes p53 is a tumor suppressor gene
located on human chromosome 17p. The p53 protein is a 53 kDa
protein that serves a role in several core cellular processes,
including transcription, DNA repair, the cell cycle, genome
stability, chromosomal separation, apoptosis and vascularization.
Under normal conditions, p53 is expressed at a low level (18). Mutations in the gene encoding p53 have
been identified in various types of human cancer, including HLC
(18). Activation of the gene
encoding p53 is associated with changes in various cell activities
and surroundings, including the following: Increased sensitivity to
DNA damage induced by UV-light, gamma rays, X-rays and
topoisomerase; cell stress (anoxia and decreased cell adhesion)
(18–20). Following DNA damage and prior to DNA
replication, the development of neoplasia can be prevented through
DNA repair, apoptosis and/or G1/S arrest, which the p53
signaling pathway can regulate (18,19). The
results from the present study demonstrated that harmaline
significantly increases p53 protein expression in HepG2 cells,
indicating that harmaline affects the p53 signaling pathway.
Following DNA damage and p53 activation,
transcription of p21 is induced, which arrests cells in
G1 (21). In addition, p21
may act synergistically with proliferating cell nuclear antigen to
inhibit DNA synthesis (22). However,
p21 can stabilize the interaction between cyclin-dependent kinase
(CDK)-4/6 and cyclin D, promoting the formation of cyclin D/CDK
complexes (23). p21 is the
transcriptional target of p53 and serves an essential role in
mediating the effects DNA damage, including adriamycin- and gamma
radiation-induced DNA damage, in addition to cell growth inhibition
(24). Overexpression of p21 leads to
the arrest of cells in G1/G2 or S phase
(24). However, cells lacking p21
cannot mediate the effects of p53 upregulation following DNA damage
(25). In addition, the consistent
expression of p21 and p53 is important in G2 following
DNA injury. The results from the present study demonstrate that
harmaline significantly increases p21 protein expression in HepG2
cells, indicating that harmaline affects the p21 signaling
pathway.
Membrane-bound FasL and soluble FasL may be
cross-linked with Fas to form a trimer, leading to the subsequent
interaction of the Fas molecule DD (DD) with other DDs in the
trimer. This subsequently leads to the activation of caspase-8
zymogens by the DD of Fas-associated protein with death domain
(FADD), which activates caspase-8 and results in the apoptosis of
cells that express Fas (10).
Fas/FasL-mediated apoptosis serves an important physiological role
in cancer (10). The results from the
present study demonstrated that harmaline significantly increases
Fas, FasL and caspase-8 protein expression and activity in HepG2
cells.
A previous study identified Fas expression in HLC
and liver para-carcinoma; however, compared with liver
para-carcinoma, the level of cirrhosis in HLC was significantly
reduced (7). Fas expression in the
non-cancerous hepatic tissues of patients with HLC is upregulated
compared with that in HLC cells (8).
Compared with patients with Fas-negative diseases, the number of
intrahepatic lesions in patients with HLC is significantly
decreased (26). Survival times of
patients with increased levels of Fas in cancer tissues or sera is
improved compared to those without (26). Fas/FasL-mediated apoptosis in HLC
cells is inhibited through downregulated or short Fas expression.
In this circumstance, tumor cell apoptosis is reduced, and
therefore tumor cells proliferate, acquire survival and metastasize
(27).
The results of the present study demonstrated that
harmaline significantly increases caspase-8/3 activity in HepG2
cells. Wang et al (26)
demonstrated that harmaline induces G2/M cell cycle
arrest and apoptosis through upregulation of Fas/FasL in SGC-7901
gastric cancer cells. In addition, the data from the current study
demonstrated that harmaline significantly inhibits the viability
and increases the apoptosis of HepG2 cells, which was associated
with increased expression of p53/p21, Fas/FasL and caspase-8, and
increased caspases-8/3 activity. These results indicate that
harmaline affects the p53/p21 and Fas/FasL signaling pathways, and
thus may be a potential drug for treating liver cancer.
References
|
1
|
Lee SB, Park YI, Dong MS and Gong YD:
Identification of 2,3,6-trisubstituted quinoxaline derivatives as a
Wnt2/β-catenin pathway inhibitor in non-small-cell lung cancer cell
lines. Bioorg Med Chem Lett. 20:5900–5904. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jarnagin WR, Schwartz LH, Gultekin DH,
Gönen M, Haviland D, Shia J, D'Angelica M, Fong Y, Dematteo R, Tse
A, et al: Regional chemotherapy for unresectable primary liver
cancer: Results of a phase II clinical trial and assessment of
DCE-MRI as a biomarker of survival. Ann Oncol. 20:1589–1595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heo J, Reid T, Ruo L, Breitbach CJ, Rose
S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, et al: Randomized
dose-finding clinical trial of oncolytic immunotherapeutic vaccinia
JX-594 in liver cancer. Nat Med. 19:329–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qu K, Xu X, Liu C, Wu Q, Wei J, Meng F,
Zhou L, Wang Z, Lei L and Liu P: Negative regulation of
transcription factor FoxM1 by p53 enhances oxaliplatin-induced
senescence in hepatocellular carcinoma. Cancer Lett. 331:105–114.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, Ye Y, Chui JH, Zhu GY, Li YW, Fong
DW and Yu ZL: Oridonin induces G2/M cell cycle arrest and apoptosis
through MAPK and p53 signaling pathways in HepG2 cells. Oncol Rep.
24:647–651. 2010.PubMed/NCBI
|
|
6
|
Chao CH, Chen CM, Cheng PL, Shih JW, Tsou
AP and Lee YH: DDX3, a DEAD box RNA helicase with tumor
growth-suppressive property and transcriptional regulation activity
of the p21waf1/cip1 promoter, is a candidate tumor suppressor.
Cancer Res. 66:6579–6588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li MS, Ma QL, Chen Q, Liu XH, Li PF, Du GG
and Li G: Alpha-fetoprotein triggers hepatoma cells escaping from
immune surveillance through altering the expression of Fas/FasL and
tumor necrosis factor related apoptosis-inducing ligand and its
receptor of lymphocytes and liver cancer cells. World J
Gastroenterol. 11:2564–2569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Guo CL, Yang XH, Cheng W, Xu Y, Li JB, Sun
YX, Bi YM, Zhang L and Wang QC: Expression of Fas/FasL in CD8+ T
and CD3+ Foxp3+ Treg cells-relationship with apoptosis of
circulating CD8+ T cells in hepatocellular carcinoma patients.
Asian Pac J Cancer Prev. 15:2613–2618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang WT, Hsieh BS, Cheng HL, Lee KT and
Chang KL: Progesterone augments epirubicin-induced apoptosis in
HA22T/VGH cells by increasing oxidative stress and upregulating
Fas/FasL. J Surg Res. 188:432–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jung YJ, Kim YJ, Kim LH, Lee SO, Park BL,
Shin HD and Lee HS: Putative association of Fas and FasL gene
polymorphisms with clinical outcomes of hepatitis B virus
infection. Intervirology. 50:369–376. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nakamura M, Nagano H, Sakon M, Yamamoto T,
Ota H, Wada H, Damdinsuren B, Noda T, Marubashi S, Miyamoto A, et
al: Role of the Fas/FasL pathway in combination therapy with
interferon-alpha and fluorouracil against hepatocellular carcinoma
in vitro. J Hepatol. 46:77–88. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nasehi M, Meskarian M, Khakpai F and
Zarrindast MR: Harmaline-induced amnesia: Possible role of the
amygdala dopaminergic system. Neuroscience. 312:1–9. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Amin B, Malekzadeh M, Heidari MR and
Hosseinzadeh H: Effect of Crocus sativus extracts and its active
constituent safranal on the harmaline-induced tremor in mice. Iran
J Basic Med Sci. 18:449–458. 2015.PubMed/NCBI
|
|
14
|
Sasaki K, Bower JM and Llinás R: Multiple
purkinje cell recording in rodent cerebellar cortex. Eur J
Neurosci. 1:572–586. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Drozdov I, Bornschein J, Wex T, Valeyev
NV, Tsoka S and Malfertheiner P: Functional and topological
properties in hepatocellular carcinoma transcriptome. PLoS One.
7:e355102012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
George J and Patel T: Noncoding RNA as
therapeutic targets for hepatocellular carcinoma. Semin Liver Dis.
35:63–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Peng YF, Ni HM, Li Y, Shi YH, Ding
WX and Fan J: Basal autophagy and feedback activation of akt are
associated with resistance to metformin-induced inhibition of
hepatic tumor cell growth. PLoS One. 10:e01309532015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ashur-Fabian O, Har-Zahav A, Shaish A,
Wiener Amram H, Margalit O, Weizer-Stern O, Dominissini D, Harats
D, Amariglio N and Rechavi G: apoB and apobec1, two genes key to
lipid metabolism, are transcriptionally regulated by p53. Cell
Cycle. 9:3761–3770. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu Q, Teng GJ, Zhang Y, Niu HZ, Zhu GY, An
YL, Yu H, Li GZ, Qiu DH and Wu CG: Enhancement of p53 gene transfer
efficiency in hepatic tumor mediated by transferrin receptor
through trans-arterial delivery. Cancer Biol Ther. 7:218–224. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Formigari A, Gregianin E and Irato P: The
effect of zinc and the role of p53 in copper-induced cellular
stress responses. J Appl Toxicol. 33:527–536. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang MF, Zhang ZY, Fu J, Yang YF and Yun
JP: Correlation between expression of p53, p21/WAF1, and MDM2
proteins and their prognostic significance in primary
hepatocellular carcinoma. J Transl Med. 7:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Farah IO, Begum RA and Ishaque AB:
Differential protection and transactivation of P53, P21, Bcl2,
PCNA, cyclin G, and MDM2 genes in rat liver and the HepG2 cell line
upon exposure to pifithrin. Biomed Sci Instrum. 43:116–121.
2007.PubMed/NCBI
|
|
23
|
Sheahan S, Bellamy CO, Dunbar DR, Harrison
DJ and Prost S: Deficiency of G1 regulators P53, P21Cip1 and/or pRb
decreases hepatocyte sensitivity to TGFbeta cell cycle arrest. BMC
Cancer. 7:2152007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu T, Qin W, Hou L and Huang Y:
MicroRNA-17 promotes normal ovarian cancer cells to cancer stem
cells development via suppression of the LKB1-p53-p21/WAF1 pathway.
Tumour Biol. 36:1881–1893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ou X, Lu Y, Liao L, Li D, Liu L, Liu H and
Xu H: Nitidine chloride induces apoptosis in human hepatocellular
carcinoma cells through a pathway involving p53, p21, Bax and
Bcl-2. Oncol Rep. 33:1264–1274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Wang C, Jiang C, Zeng H and He X:
Novel mechanism of harmaline on inducing G2/M cell cycle arrest and
apoptosis by up-regulating Fas/FasL in SGC-7901 cells. Sci Rep.
5:186132015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vekemans K, Braet F and Wisse E:
DiO-labeled CC531s colon carcinoma cells traverse the hepatic
sinusoidal endothelium via the Fas/FasL pathway. J Gastrointest
Surg. 8:371–372. 2004. View Article : Google Scholar : PubMed/NCBI
|