Introduction
Cervical cancer (CC) is the leading gynecological
malignancy worldwide, the global incidence increased from 378,000
cases per year in 1980 to 454,000 cases per year in 2010, with a
0.6% annual rate of increase (1).
High-risk-human papillomavirus (HR-HPV) persistent infection is a
main factor on cervical intraepithelial neoplasia (CIN) and CC
(2,3),
but not all women infected with HPV develop CC. It is indicated
that other factors may involved along with HPV in inducing cervical
carcinogenesis. HPV DNA often attack host cell DNA in the process
of carcinogenesis and occur mostly in the gene fragile site.
Interesting, low folate status could increase the probability of
occurring gene fragile site further promoting the integration
between HPV DNA and host cell DNA (4). Folate is a water-soluble B-vitamin and
an important cofactor in one-carbon metabolism in which folate
participates in DNA synthesis and regulating methylation reactions,
adequate folate status is an important determinant of normal DNA
methylation status (5,6). Deficient folate levels have been shown
in murine prostate cancer models to result in CpG island
hypermethylation and misincorporation of uracil into DNA strands,
leading to genetic and epigenetic instability that is
characteristic of carcinogenesis (7).
Epidemiological studies have demonstrated an inverse relationship
between folate status and the risks of some malignancies including
cancer of colon, esophagus, stomach, pancreas, lung, cervix, ovary,
breast and leukemia (8,9), and folate deficiency contributes to CC
risk at several sites (10), but
there were less reports about the effects of folate on the
progression from the normal cervix (NC), CIN to cancer. Additional,
Red cell folate is not affected by folic acid intake and is more
representative of the actual folic acid levels in the body, and
though our previous researches have suggested that serum folate
levels were inversely associated with CC risk (11), it is still remains unclear that the
modification of red blood cell (RBC) folate in the progression of
cervical carcinogenesis.
Converging evidence from epidemiological and
molecular studies have suggested that the alteration of DNA
methylation is one of most consistent epigenetic changes in human
cancers (12–14), aberrant promoter hypermethylation is
an important mechanism for function loss of certain tumor
suppressor genes in tumors (15–17).
Fragile histidine triad (FHIT) gene is a tumor suppressor gene
which could regulate cell apoptosis and proliferation (18) and is rich in benign squamous
epithelial cells. Some study showed that FHIT gene expression
decreased or was absent in tumorigenesis, especially epithelial
tumour (19). Aberrant FHIT gene
expression caused by promoter region hypermethylation has been
found in some cancers, our previous researches also have suggested
that FHIT gene hypermethylation and protein low expression could
increase the risk of CC and precancerous lesions (20). LOH-dependent FHIT decreased expression
have been linked with high proliferation and low apoptotic index in
tumor cells, it has also been proven that co-hypermethylation of
FHIT gene and p16 (a tumor suppressor gene) gene in early stage of
cancer is poor prognostic factor and can confer cisplatin
resistance in cancer cells (21).
However, the effects of folate on FHIT gene methylation and
expression in CC is unclear.
Folate deficiency and FHIT gene hypermethylation
were the risk factors of cervical lesions, considering folate and
FHIT inactivation closely related to DNA methylation, we undertook
a study intended to evaluate the associations between RBC folate
levels and FHIT gene methylation status, mRNA and protein
expression levels in population with multistage cervical lesions.
Meanwhile, we observed the role of folate on cell proliferation and
apoptosis of CC cells CaSki (HPV16 positive) and C33A (HPV
negative) and evaluated whether the effects were developed through
mediating DNA methylation status and expression of FHIT gene. A
deeper understanding of the role of folate and FHIT gene in human
cervical tissues and cancer cells can provide insight into early
mechanisms of cervical carcinogenesis.
Materials and methods
Patients and samples
The participants were diagnosed by pathology,
including 80 women with NC, 110 patients with CIN1 (n=55) and
CIN2/3 (n=55) and 64 patients with cervical squamous cell carcinoma
(SCC). All the study participants were collected from Shanxi Tumor
Hospital and Second Hospital of Shanxi Medical University (Taiyuan,
China), and lived in the Shanxi province for more than five years.
Participants were excluded with nutritional megaloblastic anemia,
hemolytic disease, leukemia, liver disease, other malignant tumors
and taking B vitamins within three months. The information of
demographic characteristics, lifestyle, personal hygiene behavior
and reproductive factors known to be associated with cervical
lesions was collected using structured questionnaire. Prior to
surgery or other treatments, 5 ml anticoagulant blood and non
anticoagulant blood were obtained from all participants after an
overnight fasting and stored at −80°C until analysis. Cervical
tissues were obtained from all participants who underwent surgery
or biopsy under colposcope, and was stored in −80°C refrigerator
immediately. Informed consent will be signed by eligible
participants who agree to participate in the study. The study was
approved by Shanxi Medical University Science Research Ethics
Committee.
Cells culture and folate
interventions
CC cell lines C33A (HPV negative) and CaSki (HPV16
positive) were cultured in MEM-EBSS and RPMI-1640 culture medium
(Chinese Academy of Medical Sciences) at 37°C in a 5%
CO2 atmosphere, respectively. MEM-EBSS and RPMI-1640
culture medium: 10% fetal bovine serum and 100 units/ml of
penicillin and streptomycin were dissolved in ready-to-use
MEM-EBSS/RPMI-1640 culture solution. CaSki and C33A cells in the
logarithmic phase of growth were cultured for 24 h and then
transfered to folate culture medium at concentrations of 1.0 µg/ml
(as control group), 10, 100, 250, 500 and 1,000 µg/ml for 48 h.
Folate culture medium: first, prepare 2 mg/ml folate reserve
solution (dissolve 2 g folate into 100 ml MEM-EBSS/RPMI-1640
culture solution, and adjust PH to 7.2 with 7.4% NaOH), and then
add appropriate amount of folate reserve solution and 10% fetal
bovine serum and 100 units/ml of penicillin and streptomycin to
ready-to-use MEM-EBSS/RPMI-1640 culture solution, so that final
concentration were 1.0, 10, 100, 250, 500 and 1,000 µg/ml.
Testing HPV16 infection by PCR
Cervical tissue DNA was extracted by proteinase K
digestion and a modified phenol-chloroform protocol. HPV16 DNA was
determined by PCR as described before (22). The HPV16E2 primers were as follows:
P1, AAG GGC GTA ACC GAA ATC GGT; P2, CAT ATA CCT CAC GTC GCA G, and
the HPV16 E6 primers were as follows: P1, CTT GGG CAC CGA AGA AAC
AC; P2, TTG GTC ACG TTG CCA TTC AC. Either E2 or E6 positive can be
determined as HPV16 positive.
Detecting RBC folate levels by
microbiological method
Serum and whole blood samples were treated with 1%
ascorbic acid and folate concentration were determined by
microbiological method as described before (11). Each sample was analyzed in duplicate.
Serum folate concentrations were obtained automatically by standard
curve. RBC folate concentration = [whole blood folate
concentration-serum folate concentration ×
(1-hematocrit)]/hematocrit.
Detecting cells proliferation ability
by cell counting and apoptosis by flow cytometry
The C33A and CaSki cells were cultured with
different folate concentrations (1.0, 10, 250, 500 and 1,000 µg/ml)
for 48 h. The cell proliferation ability were measured by cell
counting using microscopy (4×10) after trypan blue staining, and
apoptosis were tested by flow cytometry with Annexin V-FITC/PI
apoptosis detection kit (KGA108; Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China). Proliferation inhibition rate = (control group
cell counts-interventional group cell counts)/control group cell
counts.
Detecting FHIT gene methylation status
by MSP
Cervical tissues and cells DNA was extracted by
phenol-chloroform extraction. 20 µl DNA solution was used for
detecting FHIT gene methylation status by methylation-specific
polymerase chain reaction (MSP) as described before (23). MSP primers for FHIT (Gene ID:2272)
were as follows; FHIT (M) P1, 5′-TTGGGGCGCGGGTTTGGGTTTTTACGC-3′;
P2, 5′-CGTAAACGACGCCGACCCCACTA-3′; FHIT (U) P1,
5′-TTGGGGTGTGGGTTTGGGTTTTTATG-3′; P2,
5′-CATAAACAACACCAACCCCACTA-3′. The PCR product was separated on a
2% agarose gel electrophoresis at 74 bp. FHIT gene methylated and
unmethylated bands were obtained with Vilber Lourmat (VILBER
CV-A50C; Marne La Valée, France).
Measuring FHIT mRNA expression levels
in cervical tissues and cells by qPCR
Total RNA extracted from tissue and cells samples
was isolated with Trizol reagent (Takara Bio, Inc., Otsu, Japan)
following the manufacturer's instruction. qPCR was carried out
using an Mx3005p™ Real-Time PCR Detection System (Stratagene;
Agilent Technologies, Inc., Santa Clara, CA, USA) and the Prime
Script™ RT-PCR kit (Takara Bio, Inc.) according to the
manufacturer's instructions to confirm the expression levels of
mRNA. In brief, the reverse transcription reaction with a BioRad
PTC-200 PCR Detection System (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) was carried out in a 10 µl volume with 1 µg of total RNA,
at 37°C for 15 min, 85°C for 5 sec, and then the cDNA were stored
at 4°C until use. A total of 2 µl of the RT product was used in
each PCR. The PCR cycling began with template denature at 95°C for
30 sec, followed by 40 cycles of 95°C for 5 sec, annealing at 60°C
for 30 sec and extension at 95°C for 15 sec, and followed by 60°C
for 30 sec and 95°C for 15 sec. The FHIT primers were as follows:
upstream 5′-GCAGCTCTGCGGGTCTACTTTC-3′; and downstream
5′-TCTTCAAACTGGTTGGCAATAGCTC-3′. The β-actin (GAPDH) primers were
as follows: upstream 5′-TGGCACCCAGCACAATGAA-3′; and downstream
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. Relative quantification of FHIT
mRNA was performed using 2−∆∆Cq, The higher
2−∆∆Cq was, the higher the levels of mRNA were.
Measuring the expression levels of
FHIT protein in cervical tissues and cells by western blotting
Cervical tissues and cells were lysed in WIP (Tissue
and Cell lysis solution; Bioss Inc., Beijing, China) and PMSF
(Amresco). Protein was quantitated with BCA protein assay (Wuhan
Boster Biological Technology, Ltd., Wuhan, China). The FHIT protein
expression levels were detected as described before (24) using rabbit monoclonal anti-FHIT
(1:800; Abcam, Cambridge, UK). Densitometric analysis was performed
by Quantity One software (Bio-Rad Laboratories, Inc.). Relative
expression quantity of FHIT protein was represented by OD ratio of
the target band to β-actin band.
Statistical analysis
All the in vitro experiments were
independently repeated three times. Data analyses were performed
with SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA) statistical
software. Differences between groups were assessed by ANOVA,
Kruskal-Wallis H test, Bonferroni test, Chi-square test and trend
of Chi-square test. Correlation was analyzed by spearman rank
correlation. Multinomial logistic regression model was used to
estimate odds ratio (OR) and adjusted OR (aOR) after adjusting
potential covariates and OR 95% confidence intervals (95% CI).
Statistical significance was set at α=0.05.
Results
Demographic characteristics and
relevant factors of cervical lesions
Demographic characteristics and relevant factors
analysis showed that low education degree, seldom vaginal cleaning,
higher number of pregnancy and parity, gynecological history,
peasant occupation and induced abortion history were risk factors
for cervical lesions (Table I).
While, there were no significant differences on the distribution of
age, birth place, race and marital status in each group
(P>0.05).
 | Table I.Related factors analysis of cervical
lesions. |
Table I.
Related factors analysis of cervical
lesions.
| Variables | NC | CIN1 | CIN2/3 | SCC |
χ2 | P-value |
|---|
| HPV16
infection |
|
|
|
| 36.86 | <0.001 |
|
Yes | 14 (17.5) | 23 (41.8) | 31 (56.3) | 41 (64.1) |
|
|
| No | 66 (82.5) | 32 (58.2) | 24 (43.6) | 23 (35.9) |
|
|
| Occupation |
|
|
|
| 17.12 | 0.001 |
|
Peasant | 48 (60.0) | 27 (49.1) | 30 (54.5) | 53 (82.8) |
|
|
|
Other | 32 (40.0) | 28 (50.9) | 25 (45.5) | 11 (17.2) |
|
|
| Education
degree |
|
|
|
| 24.14 | <0.001 |
| Under
middle school | 17 (21.3) | 19 (34.5) | 21 (38.2) | 39 (60.9) |
|
|
| Middle
school above | 63 (78.7) | 36 (65.5) | 34 (61.8) | 25 (39.1) |
|
|
| Frequency of
vaginal cleaning |
|
|
|
| 31.79 | <0.001 |
| ≥3
times/week | 46 (57.5) | 18 (32.7) | 26 (47.3) | 16 (25.0) |
|
|
| 3
times/week-1 time/month | 32 (40.0) | 31 (56.4) | 16 (29.1) | 33 (51.6) |
|
|
| ≤1
time/month | 2 (2.5) | 6 (10.9) | 13 (23.6) | 15 (23.4) |
|
|
| Gynecological
history |
|
|
|
| 21.19 | <0.001 |
|
Yes | 6 (7.5) | 16 (29.1) | 19 (34.5) | 24 (37.5) |
|
|
| No | 74 (92.5) | 39 (70.9) | 36 (65.5) | 40 (62.5) |
|
|
| Induced abortion
history |
|
|
|
| 15.81 | 0.001 |
|
Yes | 29 (36.3) | 32 (58.2) | 33 (60.0) | 43 (67.2) |
|
|
| No | 51 (63.7) | 23 (41.8) | 22 (40.0) | 21 (32.8) |
|
|
| Number of
parity |
|
|
|
| 12.57 | 0.05 |
| ≤2 | 35 (43.8) | 17 (30.9) | 27 (49.1) | 16 (25.0) |
|
|
|
3–4 | 20 (25.0) | 18 (32.7) | 16 (29.1) | 18 (28.1) |
|
|
|
>4 | 25 (31.2) | 20 (36.4) | 12 (21.8) | 30 (46.9) |
|
|
| Number of
pregnancy |
|
|
|
| 15.94 | 0.01 |
| ≤2 | 38 (47.5) | 27 (49.1) | 18 (32.7) | 20 (31.3) |
|
|
|
3–4 | 30 (37.5) | 17 (30.9) | 15 (27.3) | 21 (32.8) |
|
|
|
>4 | 12 (15.0) | 11 (20.0) | 22 (40.0) | 23 (35.9) |
|
|
RBC folate levels
The levels of RBC folate were significantly
different in NC, CIN and SCC groups (H=43.68, P<0.001), and
showed decreasing trend
(χ2trend=21.91, P<0.001).
After multiple comparisons by adopting the Bonferroni test, we
found levels of RBC folate in CIN2/3 and SCC groups were
significantly lower than in CIN1 or NC groups, in CIN2/3 group was
significantly lower than in CIN1 group. Further, we defined the 50%
point value of RBC folate levels in NC group (275.42) as cut-off
point to carry on qualitative analysis, the results showed that the
OR and aOR in SCC group was higher than in CIN groups (Table II).
 | Table II.RBC folate levels (ng/ml) in
different groups. |
Table II.
RBC folate levels (ng/ml) in
different groups.
| Group | n | RBC folate
(M±Q)a | Low levels
(≤275.42) | OR (95% CI) | aORb (95% CI) |
|---|
| NC | 80 |
275.42±54.64c | 40 (50.0) | 1.00 | 1.00 |
| CIN1 | 55 |
259.36±43.67c | 34 (61.8) | 1.62
(0.81–3.26) | 2.00
(0.92–4.35) |
| CIN2/3 | 55 |
249.23±34.59d | 47 (85.5) | 5.88
(2.47–14.00) | 7.96
(2.97–21.35) |
| SCC | 64 |
226.04±54.69d | 52 (81.3) | 4.33
(2.02–9.32) | 8.53
(3.12–23.29) |
|
|
| H=43.68,
P<0.001 |
χ2trend=21.91,
P<0.001 |
FHIT gene methylation status and
expression levels
FHIT gene methylation rate in CIN1 (16.4%), CIN2/3
(25.5%) and SCC (40.6%) groups was significantly higher than in NC
(5.0%) group. Along with the severity of cervical lesions, FHIT
gene methylation rate and OR and aOR gradually increased
(χ2trend=28.34, P<0.001)
(Table III).
 | Table III.FHIT gene methylation status in
different groups. |
Table III.
FHIT gene methylation status in
different groups.
| Group | N | FHIT methylation n
(%) |
χ2 | P-value | OR (95% CI) | ORa (95% CI) |
|---|
| NC | 80 | 4 (5.0) |
|
| 1.00 | 1.00 |
| CIN1 | 55 | 9 (16.4) | 4.35 | 0.04 | 3.72
(1.08–12.76) | 3.32
(0.91–12.17) |
| CIN2/3 | 55 | 14 (25.5) | 9.74 | 0.002 | 6.49
(2.01–20.99) | 5.43
(1.53–19.32) |
| SCC | 64 | 26 (40.6) | 20.03 | <0.001 | 13.00
(4.23–39.94) | 11.57
(3.23–41.43) |
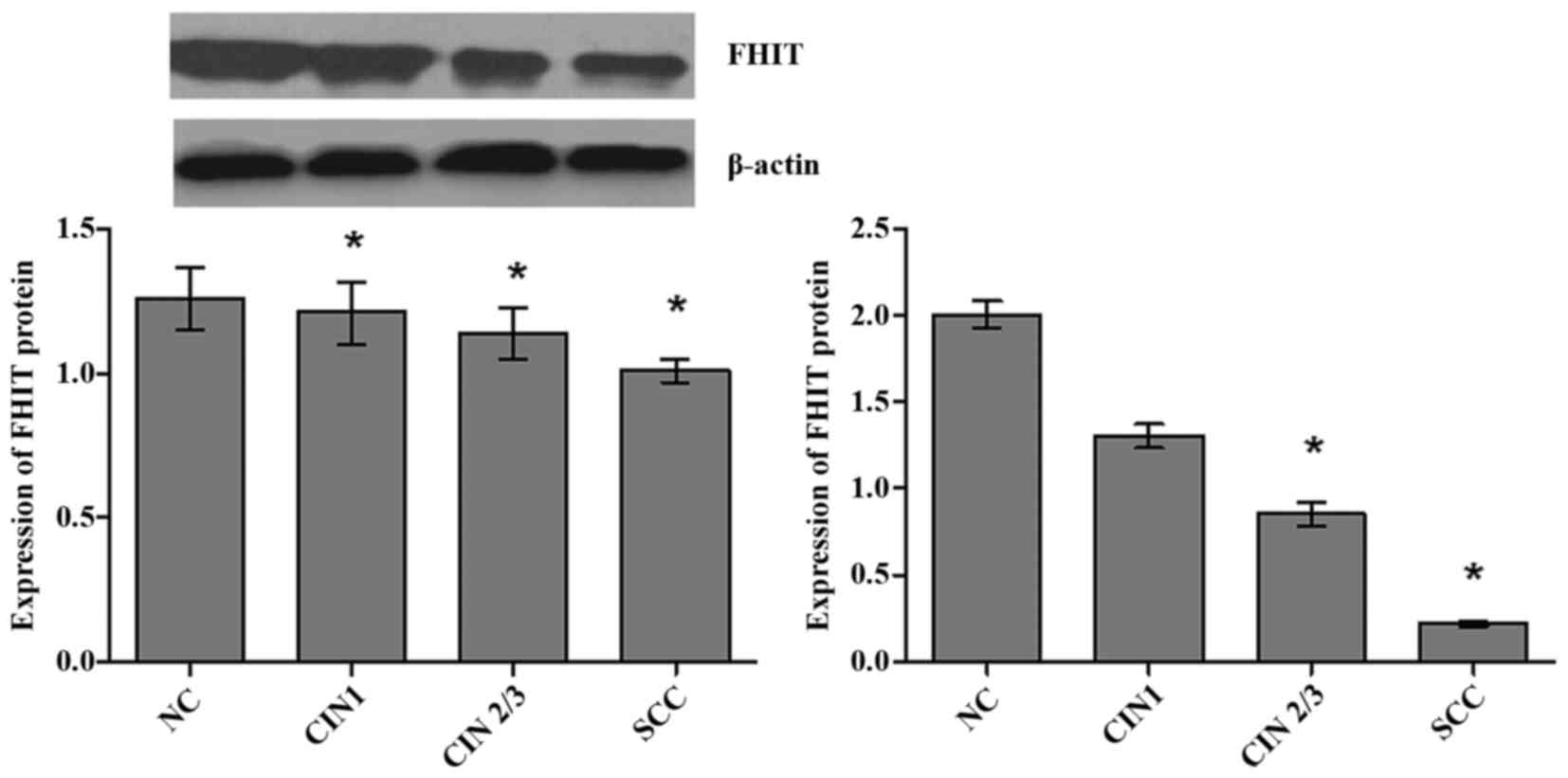
The average expression levels of FHIT mRNA were
significantly different in NC, CIN1, CIN2/3 and SCC groups
(H=60.17, P<0.001), and showed an decreasing trend with
the severity of cervical lesions (Fig.
1). The expression levels of FHIT mRNA in groups of SCC
(P<0.001) and CIN2/3 (P=0.003) were significantly lower than NC
group after using Bonferroni test. The positive rate of low FHIT
mRNA was increased gradually with the severity of the cervix
lesions (χ2trend=40.58, P<0.001)
with 50% point value of mRNA expression levels of FHIT in NC group
as a cut-off point.
The average expression levels of FHIT protein were
significantly different in cervical lesions groups (H=52.23,
P<0.001), and showed an decreasing trend with growing severity
of cervical lesions (Fig. 1). The
expression levels of FHIT protein were significantly lower in
groups of SCC (P<0.001), CIN2/3 (P<0.001) and CIN1 (P=0.001)
than NC group after using Bonferroni test. Furthermore, 50% point
value of expression levels of FHIT protein in NC group (1.26) was
defined as cut-off point, our results showed that positive rate of
FHIT protein low expression increased gradually with the severity
of cervix lesions (χ2trend=56.22,
P<0.001).
FHIT gene methylation status and
expression levels analysis by folate status and cervical lesions
status
Spearman rank correlation showed that RBC folate
levels were positively correlated with the FHIT mRNA
(r=0.17, P=0.007) and protein (r=0.21, P=0.004)
expression levels. According to median concentration of folate
(275.42 ng/ml) in NC group, subjects were divided into low folate
concentration group and high folate concentration group. The median
expression levels of mRNA (2.00) and protein (1.26) in NC group as
the cut-off point of FHIT mRNA and protein low level. FHIT gene
methylation status, mRNA and protein expression levels were
analyzed by RBC folate levels and the severity of cervical
abnormality (Table IV). FHIT gene
methylation rate and positive rate of low mRNA and protein
expression was consistently higher in low folate concentration
group than in high folate concentration group for all lesions
groups. Table IV also showed that
patients with lower folate status were more likely to become the
status of FHIT gene methylation (CIN1: aOR=5.6, 90% CI, 1.0–31.7;
CIN2/3: aOR=47.5, 90% CI, 7.6–297.5; SCC: aOR=52.3, 90% CI,
10.2–268.3), mRNA low expression (CIN1: aOR=2.6, 90% CI, 0.9–7.1;
CIN2/3: aOR=17.5, 90% CI, 3.6–84.6; SCC: aOR=24.8, 90% CI,
2.8–47.6) and protein low expression (CIN1: aOR=5.6, 90% CI,
1.8–17.7; CIN2/3: aOR=24.8, 90% CI, 7.6–76.8; SCC: aOR=44.7, 90%
CI, 7.2–280.1).
 | Table IV.Methylation status and expression
levels of FHIT by folate and cervical lesions status. |
Table IV.
Methylation status and expression
levels of FHIT by folate and cervical lesions status.
|
| Variables | NC n (%) | CIN1 n (%) | CIN2/3 n (%) | SCC n (%) |
|---|
| RBC-F | FHIT
methylation |
|
|
|
|
| ≥275.42 | − | 38 (47.5) | 17 (30.9) | 4 (7.3) | 8 (12.5) |
| ≥275.42 | + | 2 (2.5) | 4 (7.3) | 4 (7.3) | 4 (6.3) |
|
| aOR (90% CI) | 1.0 | 1.7 (0.8–3.6) | 9.2 (3.0–28.5) | 3.7 (1.5–9.2) |
| <275.42 | − | 38 (47.5) | 29 (52.7) | 20 (36.4) | 23 (35.9) |
|
| aOR (90% CI) | 1.00 | 4.5 (0.7–26.8) | 19.0
(2.6–138.4) | 9.5 (1.5–61.1) |
| <275.42 | + | 2 (2.5) | 5 (9.1) | 27 (49.1) | 32 (50.0) |
|
| OR (90% CI) | 1.0 | 5.6 (1.0–31.7) | 47.5
(7.6–297.5) | 52.3
(10.2–268.3) |
| RBC-F | FHIT mRNA |
|
|
|
|
| ≥275.42 | ≥2.00 | 17 (21.3) | 9 (16.4) | 2 (3.6) | 0 (0.0) |
| ≥275.42 | <2.00 | 23 (28.7) | 12 (21.8) | 6 (10.9) | 12 (18.8) |
|
| aOR (90% CI) | 1.0 | 0.9 (0.3–2.7) | 4.4 (0.9–22.5) | 3.5 (1.6–28.6) |
| <275.42 | ≥2.00 | 23 (28.7) | 11 (20.0) | 12 (21.8) | 1 (1.6) |
|
| aOR (90% CI) | 1.0 | 1.0 (0.3–2.9) | 2.2 (0.4–12.4) | 4.3 (1.8–10.5) |
| <275.42 | <2.00 | 17 (21.3) | 23 (41.8) | 35 (63.6) | 51 (79.7) |
|
| aOR (90% CI) | 1.0 | 2.6 (0.9–7.1) | 17.5
(3.6–84.6) | 24.8
(2.8–47.6) |
| RBC-F | FHIT protein |
|
|
|
|
| ≥275.42 | ≥1.26 | 21 (26.3) | 5 (9.1) | 0 (0.0) | 0 (0.0) |
| ≥275.42 | <1.26 | 19 (23.7) | 16 (29.1) | 8 (14.5) | 12 (18.8) |
|
| aOR (90% CI) | 1.0 | 1.9 (06–6.5) | 3.4 (1.1–11.3) | 14.6
(3.1–68.6) |
| <275.42 | ≥1.26 | 22 (27.5) | 10 (18.2) | 5 (9.1) | 2 (3.1) |
|
| aOR (90% CI) | 1.0 | 3.5 (1.1–11.5) | 7.9 (2.9–21.2) | 10.2
(4.1–25.1) |
| <275.42 | <1.26 | 18 (22.5) | 24 (43.6) | 42 (76.4) | 50 (78.1) |
|
| aOR (90% CI) | 1.0 | 5.6 (1.8–17.7) | 24.8
(7.6–76.8) | 44.7
(7.2–280.1) |
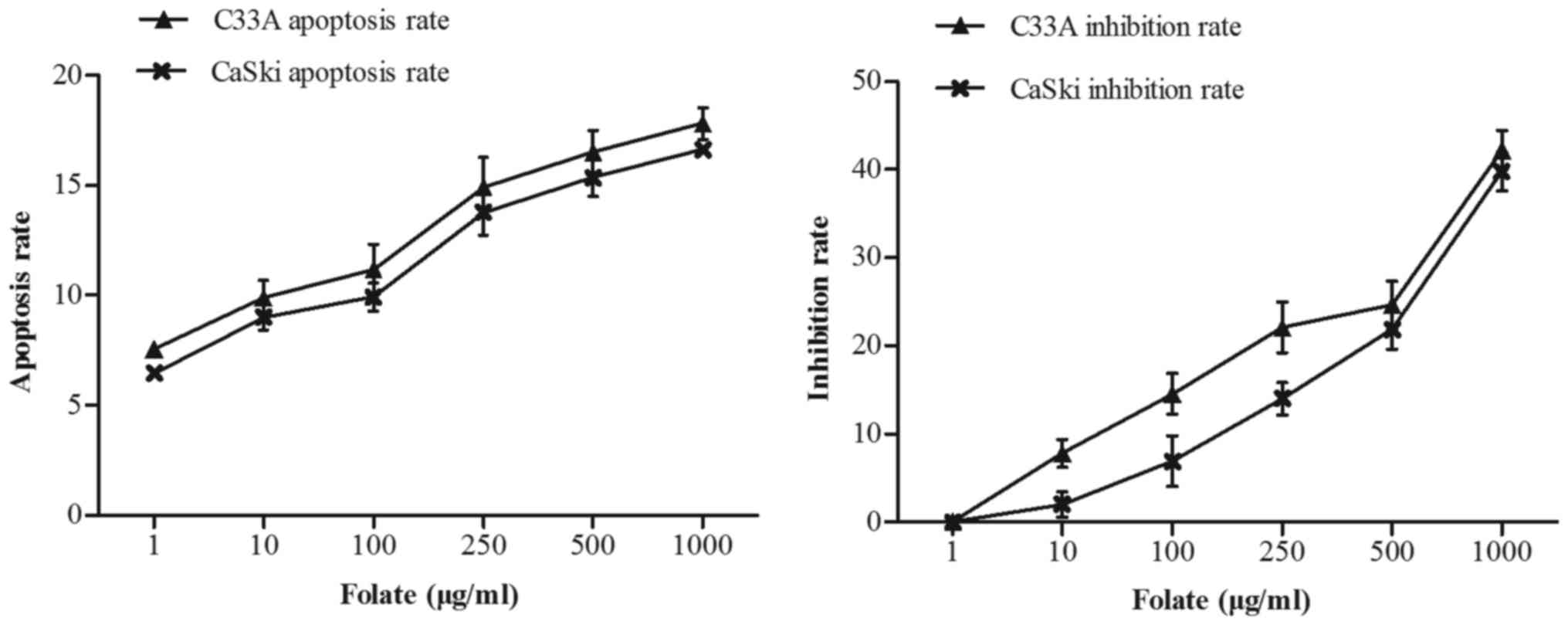
Effect of folate on cells
proliferation and apoptosis
C33A and CaSki cells were treated with increasing
concentrations of folate (1–1,000 µg/ml) for 48 h. We found that
the number of live cells was decreased in two cell lines with
increased concentration of folate. The proliferation inhibition
rate (C33A: r=0.98, P<0.001; CaSki: r=0.98, P<0.001) and
apoptosis rate (C33A: r=0.97, P<0.001; CaSki: r=0.99,
P<0.001) both gradually increased (Fig. 2) along with the rising concentrations
of folate. Cell proliferation ability was inhibited by folate in
dose-dependent manners, showing the higher the folate dosage, the
higher the degree of inhibition. The inhibition rate and apoptosis
rate of C33A cell was higher than CaSki cell, but there was no
statistically significant difference (P>0.05).
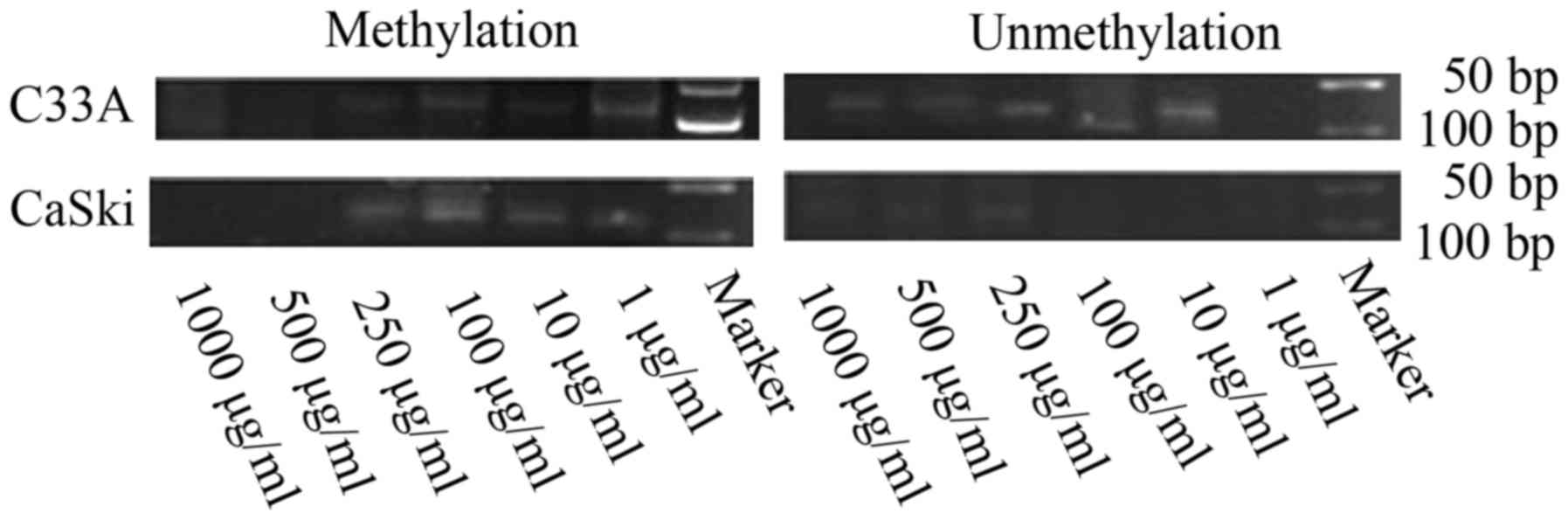
FHIT gene methylation status and
expression levels in C33A and CaSki cells treated with different
concentrations of folate
Along with rising concentrations of folate, the
degree of FHIT gene methylation in CC cells gradually weaken
(Fig. 3). Both C33A and CaSki cell
lines showed methylation positive at the concentration of 1 and 10
µg/ml, partly methylation positive at the concentration of 100 and
250 µg/ml, methylation negative at the concentration of 500 and
1,000 µg/ml.
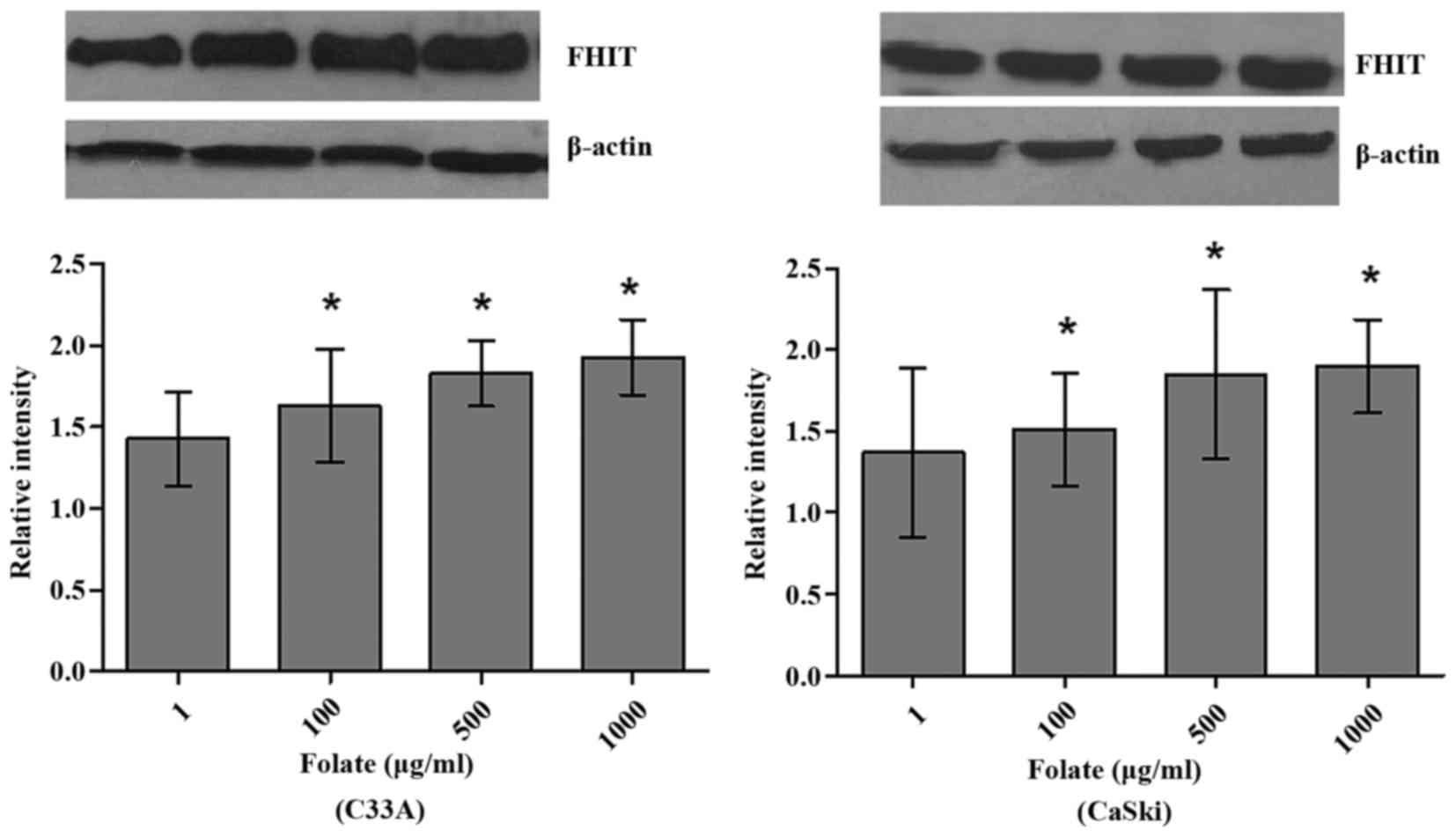
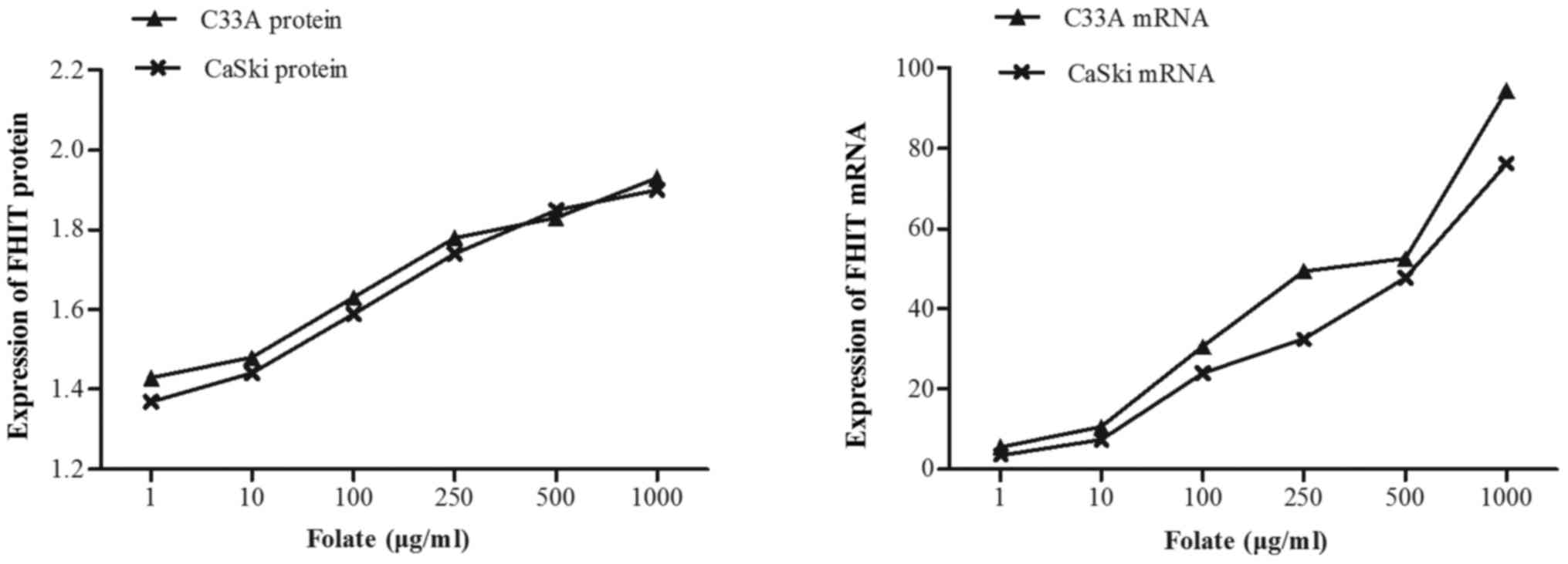
Expression levels of FHIT mRNA and protein in C33A
(HPV-negative) and CaSki (HPV16-positive) cells were significantly
different at different folate concentrations (Table V). Both expression levels of FHIT
protein (Fig. 4) and mRNA increased
in C33A and CaSki cell lines along with the rising concentrations
of folate, showing positive correlation between folate and protein
expression (C33A: r=0.969, P<0.001; CaSki: r=0.979, P<0.001)
and positive correlation between folate and mRNA expression (C33A:
r=0.917, P<0.001; CaSki: r=0.930, P<0.001) (Fig. 5). Further multiple comparisons showed
that except at concentration of 10 µg/ml, FHIT mRNA and protein
expression were all significantly different between 1.0 µg/ml
(control) and other groups. At the same folate levels, both the
expression of FHIT mRNA and protein were not different in C33A cell
and in CaSki cell (P>0.05).
 | Table V.Effects of folate on the expression
of FHIT gene in two cervical cancer cells. |
Table V.
Effects of folate on the expression
of FHIT gene in two cervical cancer cells.
|
| C33A | CasKi |
|---|
|
|
|
|
|---|
| Folate (µg/ml) | FHIT protein | FHIT mRNA | FHIT protein | FHIT mRNA |
|---|
| 1 | 1.44±0.24 | 4.58±1.87 | 1.38±0.26 | 3.39±1.37 |
| 10 | 1.47±0.31 | 11.58±1.70 |
1.43±0.21a | 8.68±2.45 |
| 100 |
1.62±0.26a | 29.56±1.94 |
1.59±0.18a | 24.57±3.70 |
| 250 |
1.78±0.23a | 49.17±5.12 |
1.73±0.21a | 35.09±6.62 |
| 500 |
1.79±0.14a | 52.94±8.24 |
1.85±0.26a |
48.17±10.09a |
| 1,000 |
1.92±0.18a |
97.59±13.18a |
1.91±0.15a |
73.14±5.12a |
| F | 68.77 | 24.74 | 103.05 | 20.97 |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
Discussion
CC is a complex disease caused by multiple factors,
persistent HPV infection is reported to be a key factor determining
risk of developing intraepithelial cervical lesions and CC. HPV,
particularly types 16, is transmitted sexually and when in contact
with the transformation zone of the cervix is known to contribute
to invasive cervical carcinoma. But recurrent or persistent
infection with the oncogenic HPV is necessary but not sufficient
for the development of CC (25,26), there
may be other carcinogenic factors or some factors that work
together with HPV for carcinogenesis. Our study was designed to
comprehensively evaluate the effects of folate on the progression
from the NC, low-grade cervical intraepithelial neoplasia,
high-grade cervical intraepithelial neoplasia to CC at the angle of
groups, meanwhile, the effect of folate on cervical carcinogenesis
was demonstrated by folate intervention at the angle of vitro.
There have not been largely conducted epidemiologic
studies designed to examine where in the cervical carcinogenesis
continuum nutrients such as folate and vitamin B 12 may influence
the natural history of the disease (27). Folate participates in the synthesis of
DNA as the precursor of purine and pyrimidine and is directly
involved in DNA methylation process, through provision of methyl
groups by the 5-methyltetrahydrofolate, these biological functions
suggest changes of folate levels have the biological basis of
cancer risk. Our results based on the population and in
vitro studies showed that levels of RBC folate were decreased
with the severity of cervical lesions, proliferation inhibition
rate and apoptosis rate gradually increased in CC cells (HPV16
positive or negative) with the increasing folate concentrations.
Shyr and colleagues (28) reported
folic acid (0–10 µmol/l) concentration-dependently decreased DNA
synthesis and proliferation in cultured human umbilical venous
endothelial cells (HUVEC). Other research have shown that folic
acid can promote dose dependent cell proliferation in folate
receptor α (FRα)-positive HeLa cells, but not in FRα-negative
HEK293 cells (29). The study of
Huang indicated that hepatoma HepG2 cells cultivated in
folate-deficient medium have a low folate concentration, decreased
growth and viability, and increased apoptotic propensity (30). Hearnden (31) recently demonstrated that head and neck
SCC (HNSCC) cells cultured in methyl donor deplete conditions
showed significantly reduced cell proliferation, impaired cell
migration, and a dose-dependent increase in apoptosis when compared
to cells cultured in complete medium. Maybe the mechanism of the
effects of folate on proliferation and apoptosis of CC cells is
complex and needs to be further studied.
FHIT belongs to histidine triad gene family, which
encodes Hydrolase of Ap3A and is located on chromosome 3p14.2 and
encompasses the common fragile site FRA3B. FHIT is now considered
as a tumor suppressor gene and the loss or aberrant transcripts of
FHIT gene is associated with carcinogenesis. FHIT inhibits the
serine/threonine kinase Akt, a key effector in PI3K pathway,
promoting survival and cell growth in response to extracellular
signals. The tumor suppressor genes function of this gene is
reflected by regulation of programmed cell death and suppression of
tumor metastasis. FHIT protein also plays a role in the modulation
of response to DNA damage, for example, preventing the replication
of stress-induced DNA damage (21,32). DNA
methylation is a common well-balanced regulatory process and
important epigenetic determinant in gene expression (an inverse
relationship). Promoter hypermethylation of tumor suppressor genes
leads to silencing or diminishing expression of tumor suppressor
gene in carcinoma and is recognized as the hallmark of human cancer
(33–35). Multiple studies have found the
reduction of FHIT expression in precancerous lesions, indicating
its potential suppressing role in carcinogenesis (36). Our results showed that mtehylation
rate of FHIT gene increased, however, expression of FHIT protein
and mRNA steadily decreased with the severity of cervical lesions,
which suggested that FHIT gene function loss significantly
contributed to CC as well as its precancerous neoplastic lesions.
Czarnecka et al (21).
demonstrated that FHIT promoter hypermethylation status, low
protein in patients with non-small cell lung cancer, but high mRNA
levels. Widschwendter et al (37) also reported an increase in the
frequency of promoter methylation in tumor suppressor genes DAPK
and CDH1 from low-grade cervical neoplasia to CC in their small
study of Austrian women.
In mammals, the vitamin folate is a key source of
the one carbon group used to methylate DNA, DNA methylation is an
epigenetic modification critical to normal genome regulation and
development (11). So the biological
function of folate and FHIT suggest that influence of folate to
cervical carcinogenesis may be associated with the activity and
function of FHIT gene, poor folate status may contribute to CC risk
through effects on one-carbon metabolism and DNA methylation
(38) there is enormous interest in
assessing the potential for changes in folate intake to modulate
DNA methylation as a mechanistic link to cancer. Research in rat
liver showed a significant 20% decrease in genomic DNA methylation
associated with a severe degree of dietary folate deficiency of 4
weeks' duration (39). Currently,
correlation between folate and FHIT gene epigenetic characteristics
in the progression of cervical carcinogenesis still remains
unclear, the effect of folate on FHIT gene methylation and
expression is largely unknown. In our study, our results revealed
that FHIT gene methylation rate and positive rate of low mRNA and
protein expression was consistently higher in low folate
concentration group than in high folate concentration group. In
vitro studies found that the degree of FHIT gene methylation in
HPV16 positive and negative CC cells gradually weaken with the
increased supplementation of folate, however, expression levels of
FHIT protein and mRNA were strengthened. The study of Liu et
al (40) have investigated the
effects of maternal folic acid (FA) supplementation on genes
expressions relating to cell apoptosis (p53, Bcl-2 and Bax) in
intrauterine growth retarded (IUGR) and normal body weight (NBW)
piglets, and showed that the expression of p53 and Bax was higher,
but expression of Bcl-2 was significantly lower in jejunum of IUGR
piglets compared with NBW piglets.
The results of this cross-sectional study support a
role for folate in modulating the risk of CC. There was complicated
relationship between folate and FHIT gene hypermethylation. Folate
is likely to be only one of many factors influencing promoter
hypermethylation of tumor suppressor genes. In-depth studies, such
as prospective cohort study, will provide the etiological evidence
of folate and tumor suppressor genes in CC.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (nos. 81273157, 30872166 and 81473060)
and Special Public Welfare Industry Research of National Health and
Family Planning Commission (no. 20140 2010).
References
|
1
|
Forouzanfar MH, Foreman KJ, Delossantos
AM, Lozano R, Lopez AD, Murray CJ and Naghavi M: Breast and
cervical cancer in 187 countries between 1980 and 2010: A
systematic analysis. Lancet. 378:1461–1484. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Depuydt CE, Thys S, Beert J, Jonckheere J,
Salembier G and Boqers JJ: Linear viral load increase of a single
HPV-type in women with multiple HPV infections predicts progression
to cervical cancer. Int J Cancer. 139:2021–2032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang JT, Ding L, Jiang SW, Hao J, Zhao WM,
Zhou Q, Yang ZK and Zhang L: Folate deficiency and aberrant
expression of DNA methyltransferase 1 were associated with cervical
cancerization. Curr Pharm Des. 20:1639–1646. PubMed/NCBI
|
|
4
|
Blount BC, Mack MM, Wehr CM, MacGregor JT,
Hiatt RA, Wang G, Wickramasinghe SN, Everson RB and Ames BN: Folate
deficiency causes uracil misincorporation into human DNA and
chromosome breakage: Implications for cancer and neuronal damage.
Proc Natl Acad Sci U S A. 94:pp. 3290–3295. 1997; View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duthie SJ: Folic acid deficiency and
cancer: Mechanisms of DNA instability. Br Med Bull. 55:578–592.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flatley JE, Sarqent A, Kitchener HC,
Russell JM and Powers HJ: Tumour suppressor gene methylation and
cervical cell folate concentration are determinants of high-risk
human papillomavirus persistence: A nested case control study. BMC
Cancer. 14:8032014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi LC, Limburg H, Miao Q, Woolcott
C, Bedard LL, Massey TE and Aronson KJ: Folate intake, alcohol
consumption and the methyl enetetrahydrofolate reductase (MTHFR)
C677T gene polymorphism: influence on prostate cancer risk and
interactions. Front Oncol. 2:1002012.PubMed/NCBI
|
|
8
|
Choi SW and Mason JB: Folate status:
Effects on pathways of colorectal carcinogenesis. J Nutr. 132(suppl
8): 2413S–2418S. 2002.PubMed/NCBI
|
|
9
|
Kim YI: Folate and carcinogenesis:
Evidence, mechanisms and implications. J Nutr Biochem. 10:66–88.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Flatley JE, McNeir K, Balasubramani L,
Tidy J, Stuart EL, Young TA and Powers HJ: Folate status and
aberrant DNA methylation are associated with HPV infection and
cervical pathogenesis. Cancer Epidemiol Biomarkers Prev.
18:2782–2789. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun XS, Ding L, Chen F, Wu T and Wang J:
Effects of folate deficiency with HPVl6 infection on cervix
cancerization. Zhonghua Liu Xing Bing Xue Za Zhi. 35:437–41.
2014.PubMed/NCBI
|
|
12
|
Kanai Y and Hirohashi S: Alterations of
DNA methylation associated with abnormalities of DNA
methyltransferases in human cancers during transition from a
precancerous to a malignant state. Carcinogenesis. 28:2434–42.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones PA: DNA methylation errors and
cancer. Cancer Res. 56:2463–2467. 1996.PubMed/NCBI
|
|
14
|
Zhang A, Månér S, Betz R, Angström T,
Stendahl U, Bergman F, Zetterberg A and Wallin KL: Genetic
alterations in cervical carcinomas: Frequent low-level
amplifications of oncogenes are associated with human
papillomavirus infection. Int J Cancer. 101:427–433. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kulis M and Esteller M: DNA methylation
and cancer. Adv Genet. 70:27–56. 2010.PubMed/NCBI
|
|
16
|
Abouzeid HE, Kassem AM, Abdel Wahab AH,
El-mezayen HA, Sharad HA and Abdel Rahman S: Promoter
hypermethylation of RASSF1A, MGMT and HIC-1 genes in benign and
malignant colorectal tumors. Tumour Biol. 32:845–852. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su Y, Wang X, Li J, Xu J and Xu L: The
clinicopathological significance and drug target potential of FHIT
in breast cancer, a meta-analysis and literature review. Drug Des
Devel Ther. 9:5439–5445. 2015.PubMed/NCBI
|
|
18
|
Al-Temaimi RA, Jacob S, Al-Ali W, Thomas
DA and Al-Mulla F: Reduced FHIT expression is associated with
mismatch repair deficient and high CpG island methylator phenotype
colorectal cancer. J Histochem Cytochem. 61:627–638. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herzog CR, Crist KA, Sabourin CL, Kelloff
GJ, Boone CW, Stoner GD and You M: Chromosome 3p tumor suppressor
gene alterations in cervical carcinomas. Mol Careinog. 30:159–168.
2001. View
Article : Google Scholar
|
|
20
|
Liu XZ, Nan J, Li J, Li Y, Ding L and Wang
JT: Interaction between fragile histidine triad methylation,
protein expression and human papillomavirus 16 infection in
cervical carcinogenesis. Zhonghua Liu Xing Bing Xue Za Zhi.
37:858–862. 2016.(In Chinese). PubMed/NCBI
|
|
21
|
Czarnecka KH, Miqdalska-Sek M, Domanska D,
Pastuszak-Lewandoska D, Dutkowska A, Kordiak J, Nawrot E,
Kiszlkiewicz J, Antczak A and Brzeziańska-Lasota E: FHIT promoter
methylation status, low protein and high mRNA levels in patients
with non-small cell lung cancer. Int J Oncol. 49:1175–1184.
2016.PubMed/NCBI
|
|
22
|
Wang JT, Gao ES, Cheng YY, Yan JW and Ding
L: Analysis on synergistic action between estrogen, progesterone
and human papillomaviruses in cervical cancer. Zhonghua Liu Xing
Bing Xue Za Zhi. 26:370–373. 2005.(In Chinese). PubMed/NCBI
|
|
23
|
Bai LX, Wang JT, Ding L, Jiang SW, Kang
HJ, Gao CF, Chen X, Chen C and Zhou Q: Folate deficiency and FHIT
hypermethlation and HPV 16 infection promote cervical
cancerization. Asian Pac J Cancer Prev. 15:9313–9317. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen X, Wang J, Bai L, Ding L, Bai L, Xu J
and Sun XS: Interaction between folate deficiency and aberrant
expression related to fragile histidine triad gene in the
Progression of cervical cancerization. Zhonghua Liu Xing Bing Xue
Za Zhi. 36:387–392. 2015.(In Chinese). PubMed/NCBI
|
|
25
|
Guzmán-Olea E, Bermúdez-Morales VH,
Peralta-Zaragoza O, Torres-Poveda K and Madrid-Marine V: Molecular
mechanism and potential targets for blocking hpv-induced lesion
development. J Oncol. 2012:2783122012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kyrqiou M, Mitra A and Moscicki AB: Does
the vaginal microbiota plays a role in the development of cervical
cancer? Trans Res. 179:168–182. 2017. View Article : Google Scholar
|
|
27
|
Piyathilake CJ, Henao OL, Macaluso M,
Cornwell PE, Melleth S, Heimburqer and Partridqe EE: Folate is
associated with the natural history of high-riskt human
papillomaviruses. Cancer Res. 64:1–8793. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin SY, Lee WR, Su YF, Hsu SP, Lin HC, Ho
PY, Hou TC, Chou YP, Kuo CT and Lee WS: Folic acid inhibits
endothelial cell proliferation through activating the cSrc/ERK
2/NF-jB/p53 pathway mediated by folic acid receptor. Angiogenesis.
15:671–683. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hansen MF, Greibe E, Skovbjerq S, Rohde S,
Kristensen AC, Jensen TR, Stentoft C, Kjær KH, Kronborq CS and
Martensen PM: Folic acid mediates activation of the pro-oncogene
STAT3 via the Folate Receptor alpha. Cell Signal. 27:1356–1368.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang RF, Ho YH, Lin HL, Wei JS and Liu
TZ: Folate deficiency induces a cell cycle-specific apoptosis in
HepG2 cells. J Nutr. 129:25–31. 1999.PubMed/NCBI
|
|
31
|
Hearnden V, Powers HJ, Elmoqassabi A, Lowe
R and Murdoch C: Methyl-donor depletion of head and neck cancer
cells in vitro establishes a less aggressivetumour cell phenotype.
Eur J Nutr. March 1–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sozzi G, Pastorino U, Moiraghi L,
Tagliabue E, Pezzella F, Ghirelli C, Tornielli S, Sard L, Huebner
K, Pierotti MA, et al: Loss of FHIT function in lung cancer and
preinvasive bronchial lesions. Cancer Res. 58:5032–5037.
1998.PubMed/NCBI
|
|
33
|
Kwan KY and Wong CS: DNA methylation of
tumor suppressor protein-coding and non-coding genes in multiple
myeloma. Epigenomics. 7:985–1001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
lliopoulos D, Guler G, Han SY, Johnston D,
Druck T, McCorkell KA, Palazzo J, McCue PA, Baffa R and Huebner K:
Fragile genes as biomarker: Epigenetic control of WWOX and FHIT in
lung, breast and bladder cancer. Oncogene. 24:1625–1633. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dhillon VS, Shahid M and Husain SA: CpG
methylation of the FHIT, FANCF, cyclin-D2, BRCA2 and RUNX3 genes in
Granulosa cell tumors (GCFs) of ovarian origin. Mol Cancer.
3:332004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Geng X, Pu W, Tan Y, Lu Z, Wang A, Tan L,
Chen S, Guo S, Wang J and Chen X: Quantitative assessment of the
diagnostic role of FHIT promoter methylation in non-small cell lung
cancer. Oncotarget. 8:6845–6856. 2017.PubMed/NCBI
|
|
37
|
Widschwendter A, Gattringer C, Ivarsson L,
Fiegl H, Schneitter A, Ramoni A, Müller HM, Wiedemair A, Jerabek S,
Müller-Holzner E, et al: Analysis of aberrant DNA methylation and
human papillomavirus DNA in cervicovaginal specimens to detect
invasive cervical cancer and its precursors. Clin Cancer Res.
10:3396–3400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y, Guo C, Hu H, Zheng L, Ma J, Jiang
L, Zhao E and Li H: Folate intake, serum folate levels and
esophageal cancer risk: An overall and dose-response meta-analysis.
Oncotarget. 8:10458–10469. 2017.PubMed/NCBI
|
|
39
|
Kim YI: Folate and DNA methylation: A
mechanistic link between folate deficiency and colorectal cancer?
Cancer Epidemiol Biomarkers Prev. 13:511–519. 2004.PubMed/NCBI
|
|
40
|
Liu J, Chen D, Mao X and Yu B: Effects of
maternal folic acid supplementation on morphology and
apoptosis-related gene expression in jejunum of newborn
intrauterine growth retarded piglets. Arch Anim Nutr. 65:376–385.
2011. View Article : Google Scholar : PubMed/NCBI
|