Introduction
Prostate cancer (PCa) is the most common malignancy
of the male genitourinary system and primarily occurs in aging men
(1). PCa is the second leading cause
of cancer-associated mortality in developed countries (2). Conventional treatments for PCa include
surgery, chemotherapy, hormonal therapy, radiation therapy,
radiofrequency ablation, high-intensity focused ultrasound and
cryosurgery (3–5). Currently, androgen-deprivation therapy
(ADT) is widely used for PCa treatment; however, the majority of
PCa patients relapse within 1 to 3 years and develop
androgen-independent disease, which is unresponsive to ADT
(6–8).
At present, there is no effective therapy for recurrent PCa
(9). Therefore, a novel therapeutic
method for PCa is required. Recently, the US Food and Drugs
Administration approved novel compounds for the treatment of PCa,
including abiraterone acetate (10),
enzalutamide (11), sipuleucel-T
(12), and Alpharadin (radium-233)
(13). One study proposed that
certain non-antitumor chemicals may have an antitumor effect
(14). Sulfur, which is widely used
for detoxifying the body and the treatment of scabies in
traditional Chinese medicine (15,16), has
been shown to suppress the growth of PCa in vivo (17). Itraconzole, a common triazole
antifungal drug in widespread clinical use, has shown evidence of
clinical anticancer effects, including against PCa (18).
Oxibendazole
(methyl-5-n-propoxy-2-benzimidazole-carbamate; OBZ), was first
synthesized in 1973 (19). OBZ is a
benzimidazole drug that is used to treat infection by roundworms,
strongyles, pinworms, threadworms and lungworm infestation in
horses and other animals (20–22). The
benzimidazole derivatives exhibit, among others, anti-ulcerative
(23), anti-inflammatory (24), antibacterial (25), and anti-carcinogenic (26) bioactivities. These drugs are widely
available for veterinary use and a number of them, such as
thiabendazole, albendazole and mebendazole, have been used in human
medicine for several years (27). A
previous study found that benzimidazoles could have potential
cytostatic effects, through the inhibition of microtubule formation
and glucose uptake (28). Notably,
albendazole, mebendazole and flubendazole, which are all
benzimidazole drugs, have been observed to inhibit tumor growth
(29).
The ability of OBZ to suppress growth in PCa was
assessed in the present study using the PCa 22Rv1 and PC-3 cell
lines. Previous studies have shown that growth of recurrent PCa
cells (also termed androgen-independent PCa cells) depends on the
androgen receptor (AR) or the AR signaling pathway (ASP), although
it is independent of androgens themselves (30,31). The
22Rv1 cell line is an androgen-independent PCa epithelial cell line
that is representative of clinical recurrent PCa (32). This cell line expresses AR and
prostate-specific antigen (PSA) (33,34), which
has been widely used as a clinically diagnostic biomarker and a key
prognostic factor for PCa (35).
However, the androgen-independent PC-3 cells do not express AR or
PSA (36). The two markers are
frequently used to evaluate the anti-PCa effects of diverse
chemicals (37–39).
In the present study, 22Rv1 cells were studied in
vitro and in vivo, and PC-3 cells were studied in
vitro. The aim of the study was to investigate the inhibitory
effect of oxibendazole on prostate cancer cells.
Materials and methods
Drugs and animals
OBZ was purchased from Selleck Chemicals, Inc.
(Houston, TX, USA) and was prepared as homogeneous suspensions in
corn oil and administered to the nude mice by gavage. A total of 20
specific pathogen-free (SPF) male BALB/c nude mice aged between 6
and 8 weeks (weight range, 18–25 g) were purchased from Shanghai
SLAC Laboratory Animal Co., Ltd. (Shanghai, China), maintained
under SPF conditions with a 12 h light-dark cycle and provided with
food and water ad libitum. Mice in the experiment were
randomly divided into two groups, the control and the OBZ-treated
groups, with 10 mice in each group. Ethical approval for the
present study was obtained from the Animal Ethics Committee of
Shanghai Institute of Planned Parenthood Research (Shanghai,
China).
Cell culture and transfection
Human PCa 22Rv1 and PC-3 cell lines were purchased
from Shanghai Institute of Cell Life Science Resource Center
(Shanghai, China). The cells were maintained in RPMI-1640 medium
(Hyclone; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin, at 37°C with an
atmosphere of 5% CO2. Transfection with 100 nM
microRNA-204 (miR-204) inhibitor (cat. no. miR20000265; RiboBio
Co., Ltd., Guangzhou, China) was performed with
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) in 22Rv1 cells according to the manufacturer's
instructions. An GFP-expressing miR-204-expressing recombinant
lentivirus and GFP-expressing control were purchased from Kangchen
Bio-tech (Shanghai, China). The delivery of miR-204 to the 22Rv1
and PC-3 cells was performed by 1×108 TU/ml viral
infection according to the manufacturer's instructions.
Cell proliferation assays
Cells were seeded in 96-well plates at a cell
density of 1×104 cells per well in 100 µl RPMI-1640
medium containing 10% fetal bovine serum and 1%
penicillin-streptomycin, and incubated at 37°C with an atmosphere
of 5% CO2 overnight. 22Rv1 and PC-3 cells were treated
with 0.12, 0.25, 0.50, 1.00, 2.00 and 3.00 µM OBZ for 48 h, or with
0.25 and 1.00 µM OBZ for 96 h. In order to assess the role of
miR-204 in mediating the effect of OBZ, 22Rv1 and PC-3 cells were
transfected with the miR-204 or miR-204 inhibitor, followed by
treatment with 1 µM OBZ or dimethyl sulfoxide (DMSO; as a control)
for 48 h. Cells were trypsinized and live cell numbers were counted
in four areas under an inverted microscope (magnification, ×40)
using a hemocytometer and the trypan blue exclusion assay.
Flow cytometry
Apoptosis was determined using a double-staining
Annexin V-Fluorescein Isothiocyanate (FITC) Apoptosis Detection kit
(BD Biosciences Inc., Franklin Lakes, NJ, USA). The 22Rv1 and PC-3
cells were treated with DMSO control or 0.25 µM OBZ. After 48 h,
the cells were collected, washed in phosphate-buffered saline (PBS)
and suspended in binding buffer. The cells were then stained using
annexin V and propidium iodide (PI) (5 µl). Following incubation
for 15 min at room temperature in the dark, the cells were diluted
and analyzed using a flow cytometer (Beckman Coulter, Inc.,
Atlanta, GA, USA). When green fluorescence (FITC) was plotted
against red fluorescence (PI), the cell populations could be
detected in a dot-plot that indicated the following conditions:
Viable cells (FITC−/PI−), early apoptotic
cells (FITC+/PI−) and late apoptotic cells
(FITC+/PI+). The data were reported as the
percentage of early apoptotic cells
(FITC+/PI−) and late apoptotic cells
(FITC+/PI+).
Xenograft tumor development in nude
mice
At the exponential growth stage, 22Rv1 cells were
harvested, washed and suspended in PBS. A trypan blue exclusion
assay was performed to ensure cell viability (>99%) prior to
inoculation. The cells were counted and 2×106 cells
suspended in 0.1 ml PBS were subcutaneously injected into the right
flank of each mouse. At 10 days after tumor cell inoculation, each
mouse in the OBZ-treated group was provided with 25 mg/kg
homogeneous suspension of OBZ by intragastric gavage. The treatment
was administered once a day for 14 days; mice in the control group
was provided with the same amount of corn oil. Tumor size was
measured in two dimensions every other day. Tumor volume (measured
in cm3) was calculated using the following formula:
Tumor volume=axb2×0.5 (a, length; b, width).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. First-strand cDNA synthesis was
performed on the total RNAs using Quant Reverse Transcriptase
(Toyobo Co., Ltd., Osaka, Japan) according to the manufacturer's
instructions with a PCR machine (cat. no. 580BR6819; RiboBio Co.,
Ltd, Guangzhou, China). Gene amplifications were performed with a
Eco Real time PCR System (Model Ec-100-1001; Illumina, Inc., San
Diego, CA, USA) using SYBR-Green (Thermo Fisher Scientific, Inc.).
Gene expression in cells and tumors was measured using qPCR. The
cycling conditions of GAPDH, AR, ARN1 and CD44 were as followed:
95°C for 3 min, 95°C for 20 sec and 58°C for 20 sec, for 40 cycles.
The cycling conditions of U6, miR-204 and miR-34a were as follows:
95°C for 1 min, 95°C for 15 sec and 60°C for 15 sec, for 40 cycles.
Bulge-loop™ miRNA qRT-PCR Primer Sets (one RT primer and a pair of
qPCR primers for each set) specific for miR-34a were designed by
RiboBio. Primers for β-actin, AR and 5′-3′ exoribonuclease 1 (XRN1)
were purchased from Sangon Biotech, Inc. (Shanghai, China). The
sequences of the primers are included in Table I. The relative expression of genes was
calculated using the 2−ΔΔCq method (40). The mean Cq was calculated from
triplicate PCRs.
 | Table I.Primer sequences for target
genes. |
Table I.
Primer sequences for target
genes.
| Gene name | Primer
sequence |
|---|
| GAPDH | Forward:
5′-CCTGTACGCCAACACAGTGC-3′ |
|
| Reverse:
5′-ATACTCCTGCTTGCTGATCC-3′ |
|
| Forward:
5′-TTCCCTCCCTATCTAACCCTC-3′ |
| AR | Reverse:
5′-TCTAAACTTCCCGTGGCATAA-3′ |
|
| Forward:
5′-GGAAACAACAGGAATGGGAAGC-3′ |
| XRN1 | Reverse:
5′-ACCAGCACATTAGGCACTCAC-3′ |
|
| Forward:
5′-AGCAACCAAGAGGCAAGAAA-3′ |
| CD44 | Reverse:
5′-GTGTGGTTGAAATGGTGCTG-3′ |
|
| Reverse
transcription: 5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| U6 | Forward:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
| Reverse:
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| miR-204 | Reverse
transcription:
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACAGGCATA-3′ |
|
| Forward:
5′-GGTTCCCTTTGTCATCC-3′ |
|
| Reverse:
5′-TGCGTGTCGTGGAGTC-3′ |
Western blotting
Total protein was extracted from cells and tumors
using radioimmunoprecipitation assay buffer [50 mM Tris (pH 8), 150
mM NaCl, 1% Triton X-100, 5% sodium deoxycholate and 0.1% SDS]
supplemented with inhibitors of proteinase and phosphatase. The
proteins (20 µg) were separated by 12% SDS-PAGE and then
transferred onto a polyvinylidene fluoride membrane. The membrane
was blocked with 5% skimmed milk for 1 h at room temperature,
washed with Tris-buffered saline with Tween-20 (TBST) buffer three
times, and incubated overnight at 4°C with anti-glyceraldehyde
3-phosphate (GAPDH; cat. no. AG019; 1:1,000 dilution), anti-tumor
protein 53 (p53; cat. no. AP062; 1:1,000 dilution), anti-p21 (cat.
no. AP021; 1:1,000 dilution) and anti-PSA (cat. no. ab53774;
1:1,000 dilution). Next, the membrane was washed 3×15 min with TBST
and incubated with the horseradish peroxidase-conjugated secondary
antibodies (anti-mouse, cat. no. A0216; 1:3,000 dilution;
anti-rabbit; cat. no. A0208; 1:3,000 dilution) at room temperature.
All primary antibodies were purchased from Beyotime Institute of
Biotechnology (Haimen, China) except anti-PSA (Abcam, Cambridge,
UK). The antibody-reactive bands were visualized using enhanced
chemiluminescence detection reagents and a gel imaging system
(Tanon Science & Technology, Co., Ltd., Shanghai, China).
Statistical analysis
Data were analyzed using SPSS software, version 11.5
(SPSS, Inc., Chicago, IL, USA). Differences in the continuous
variable ‘tumor volume’ were compared using a one-way analysis of
variance followed by a Student-Newman-Keuls test. Densitometry
analysis of western blots was performed using Image J software
(version 1.37, National Institute of Health, Bethesda, MD, USA).
Data are presented as the mean ± standard error of the mean.
P<0.05 was considered to indicate a statistically significant
difference.
Results
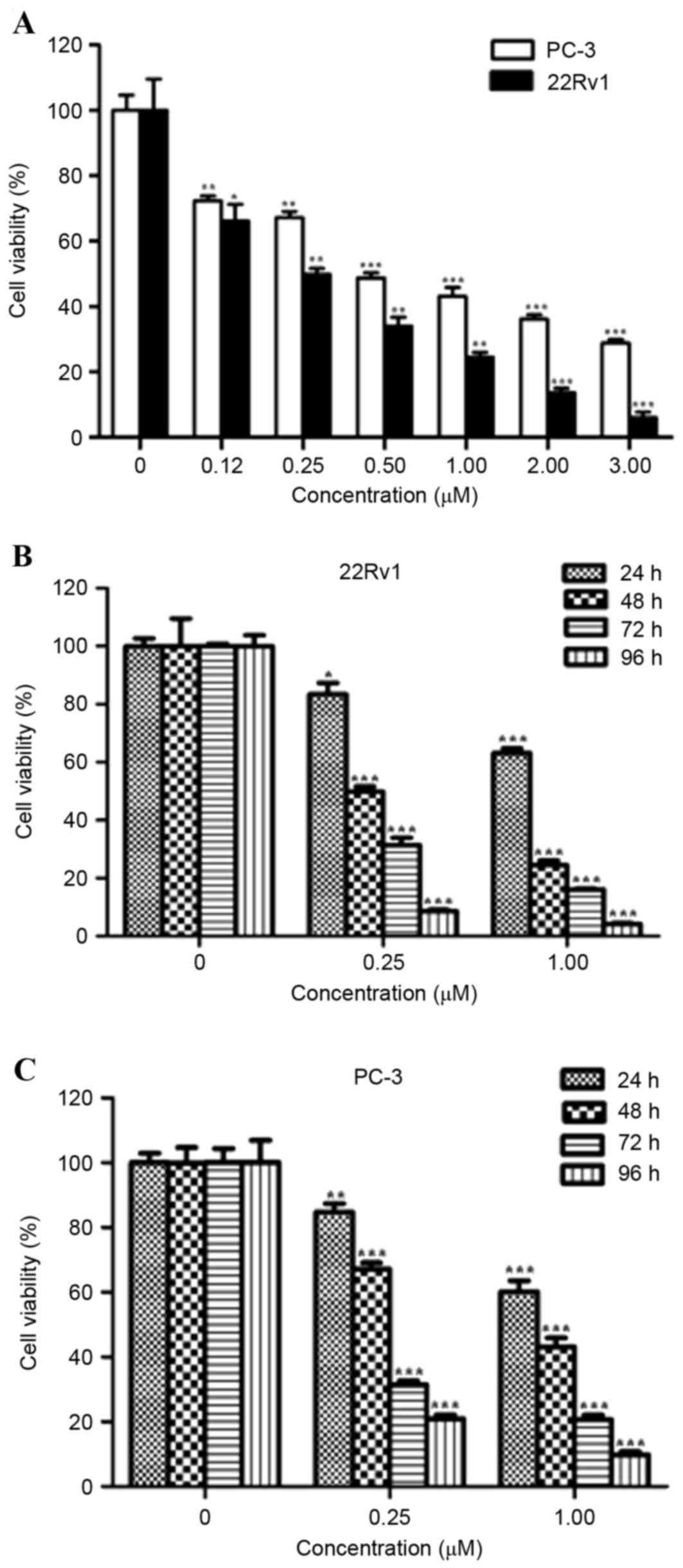
OBZ inhibits growth of 22Rv1 and PC-3
cells
The ability of OBZ to inhibit the growth of 22Rv1
and PC-3 cells was determined by counting cell number. OBZ markedly
inhibited the cell viability of 22Rv1 and PC-3 cells in a
dose-dependent manner (Fig. 1A). As
little as 0.12 µM of OBZ was observed to significantly inhibit the
growth of the 22Rv1 and PC-3 cells, respectively (both P<0.05).
The 22Rv1 cells were more sensitive to OBZ treatment, with a
half-maximal inhibitory concentration (IC50) value of
0.25 µM, compared with 0.64 µM in PC-3 cells. OBZ inhibited the
cell viability of 22Rv1 and PC-3 cells in a time-dependent manner
(Fig. 1B and C). These results
demonstrated that OBZ inhibited the growth of PCa cells in
vitro with varied efficiency.
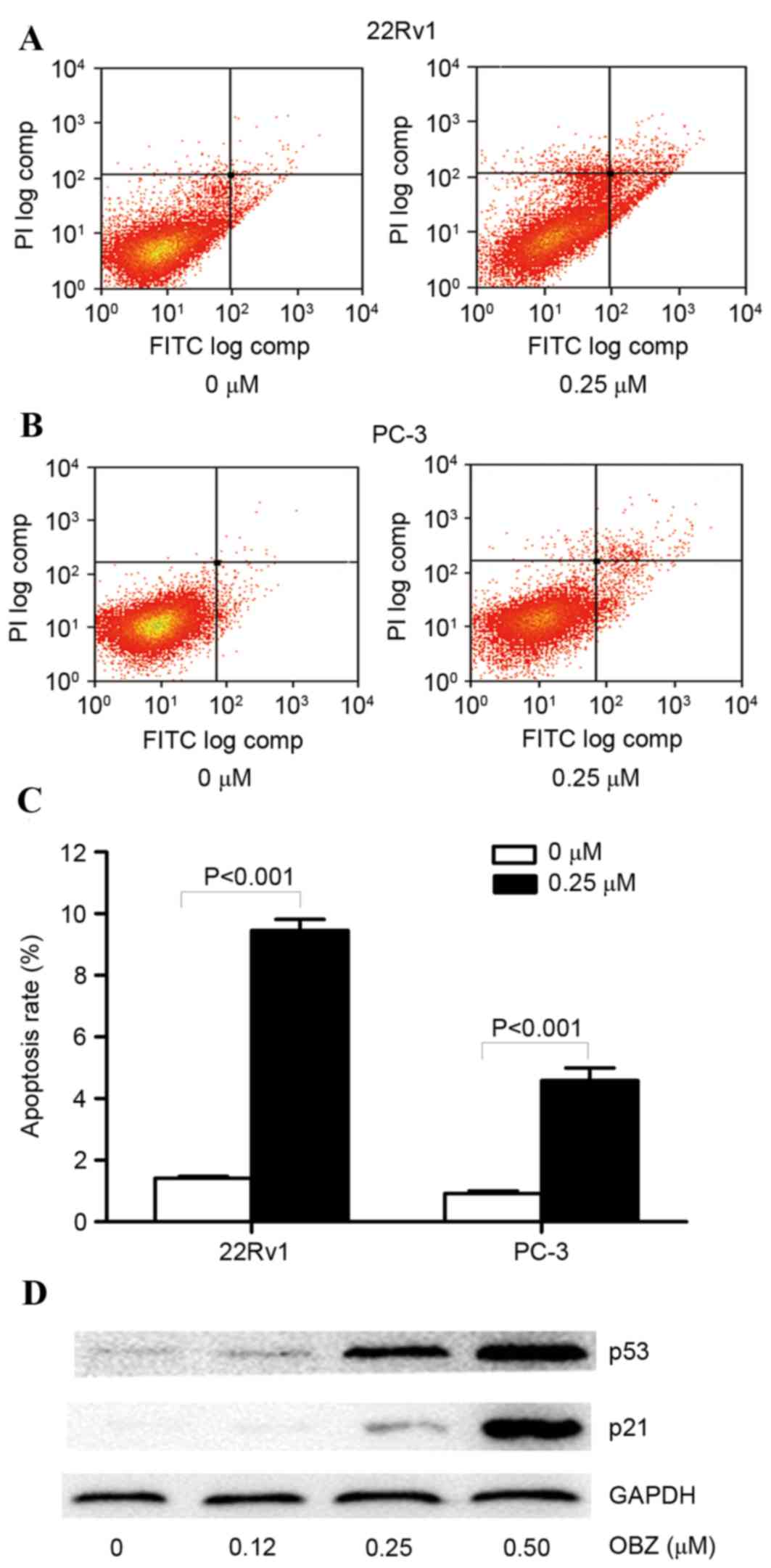
OBZ causes apoptosis of 22Rv1 and PC-3
cells
The apoptosis-inducing capability of OBZ in 22Rv1
and PC-3 cells was evaluated by Annexin V-FITC and PI double
staining. Provided that the IC50 value was 0.25 µM in
22Rv1 cells, 0.25 µM OBZ was used to treat 22Rv1 and PC-3 cells for
48 h. A notable increase in the number of apoptotic cells was
observed in the OBZ-treated group compared with DMSO-treated cells
(the negative control) (Fig. 2A and
B). The apoptotic rate of 22Rv1 cells was 1.41% in DMSO-treated
cells and 9.45% in OBZ-treated cells. The apoptotic rate in PC-3
cells was 0.92 and 4.58% in DMSO- and OBZ-treated cells,
respectively (Fig. 2C). These results
indicated that treatment with OBZ resulted in an increased
apoptotic rate in PCa cells, and the apoptosis-inducing capability
of OBZ was more marked in 22Rv1 compared with PC-3 cells.
To investigate the mechanism of the inhibitory
effect of OBZ in PCa cells, the effect of OBZ on the expression of
p53 and p21 (41,42), which are known to be the key
regulators of apoptosis, was measured via western blot analysis in
the OBZ-treated cells. An OBZ dose of ≥0.25 µM markedly increased
p53 and p21 expression in 22Rv1 cells (Fig. 2D). These results suggested that
upregulation of p53 and p21 served an important role in the
OBZ-induced apoptosis of 22Rv1 cells.
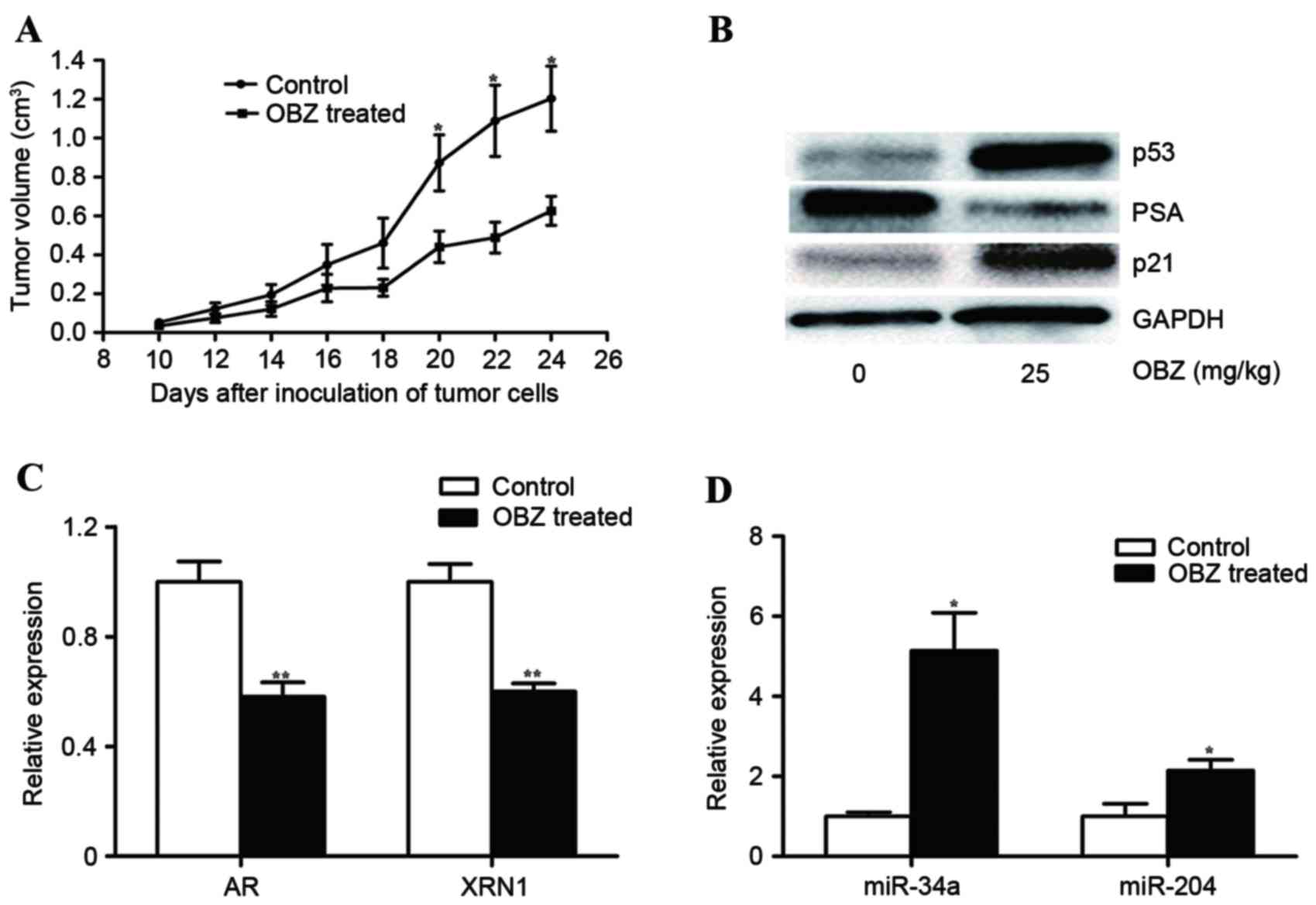
OBZ inhibits 22Rv1 tumor growth in
nude mice
The antitumor effect of OBZ was next evaluated in
vivo. First, 22Rv1 cells were injected into the right flank of
nude mice. Approximately 10 days after injection of the cells, the
tumor sizes were measurable. On day 10, the mice were treated with
OBZ (25 mg/kg) by intragastric gavage. The treatment was
administered once a day for 14 days. The control group of mice was
treated in the same way, but OBX was substituted with corn oil. OBZ
significantly repressed tumor growth in a time-dependent manner,
with a significant difference identified at 20 days after cancer
cell inoculation (P<0.05; Fig.
3A). The mean tumor volume of the OBZ-treated group was 0.63
cm3, whereas in the control group it was 1.20
cm3; OBZ inhibited growth of the tumor by ~47.96%.
Additionally, the mean body weight of the tumor-bearing mice was
24.06±1.28 and 23.10±3.39 g in the OBZ-treated and control groups,
respectively. However, this difference was not statistically
significant, demonstrating that OBZ did not exert a significant
general toxic effect in vivo, consistent with the results of
a previous study (43).
The mechanism for the tumor-suppressive effect of
OBZ in vivo was also studied. First, the expression of p53
and p21 in the tumor tissues of OBZ-treated mice and their negative
control was measured using western blot analysis. The density of
the two proteins increased by 821.34 and 75.18%, respectively, in
the OBZ-treated tumor compared with the control samples (Fig. 3B). Therefore, the in vivo
results are consistent with those observed in vitro.
Given that PSA is a clinically diagnostic biomarker
and key prognostic factor for PCa (35), anti-PCa chemicals have also been
assessed for their effect on reducing the level of PSA either in
PCa cells or in the blood (44). The
level of PSA in OBZ-treated 22Rv1 tumors was measured via western
blotting in the present study. The concentration of PSA decreased
by 78.16% more in OBZ-treated tumors compared with the control,
indicating that OBZ repressed PSA expression.
The AR serves a critical role in the progression and
recurrence of PCa (45), and is also
the transcriptional activator of PSA expression (46). Therefore, downregulation of PSA by OBZ
indicates that OBZ affects the AR or ASP. Recently, a study
reported the existence of the AR-miR-204-XRN1 axis in PCa cells,
which has a dual regulatory function in mediating the growth of
different PCa cells (47). In this
axis, androgen/AR raises the level of XRN1, a target of miR-204, by
inhibiting expression of miR-204. Accordingly, whether OBZ affects
the AR-miR-204-XRN1 axis in 22Rv1 tumors was investigated. Levels
of AR, miR-204 and XRN1 in the mouse tumors were measured by qPCR.
Expression of AR and XRN1 was significantly lower in OBZ-treated
tumors than in the control (both P<0.01; Fig. 3C). By contrast, OBZ raised the
expression of miR-204 ~115% (Fig.
3D). Together, these results indicated that OBZ interfered with
the AR-miR-204-XRN1 axis in PCa tumors.
miR-34a has been reported to be a tumor suppressive
miRNA (48). miR-34a represses the
growth of PCa cells by targeting the AR (49,50). As
such, the level of miR-34a in OBZ-treated 22Rv1 tumors was measured
in the present study. OBZ treatment raised the expression of
miR-34a by ~5-fold (Fig. 3D). These
results, therefore, were consistent with the hypothesis that OBZ
suppresses AR expression by raising the level of miR-34a in 22Rv1
cells.
Manipulated expression of miR-204
affects the sensitivity of 22Rv1 cells to OBZ
To evaluate the role of a disturbed AR-miR-204-XRN1
axis in the inhibition of growth of 22Rv1 tumors using OBZ, the
effect of OBZ on miR-204 expression was studied in cultured 22Rv1
cells and PC-3 cells. miR-204 has been widely shown to be a tumor
suppressive gene in multiple types of cancer, including
AR-expressing PCa cells (47,51–53).
However, miR-204 has been reported to be an oncomiR in the PC-3
cell line (47), which is AR-negative
and represents a neuroendocrine-like PCa (NEPC) cell (54). The present results, based on RT-qPCR
data, showed that 1.00 µM OBZ significantly raised the expression
of miR-34a and miR-204 in 22Rv1 cells by 197.07 and 185.11%,
respectively (both P<0.05; Fig.
4A). Additionally, 1.00 µM OBZ significantly raised the
expression of miR-34a and miR-204 in PC-3 cells by 44.77 and
87.46%, respectively (both P<0.05; Fig. 4B).
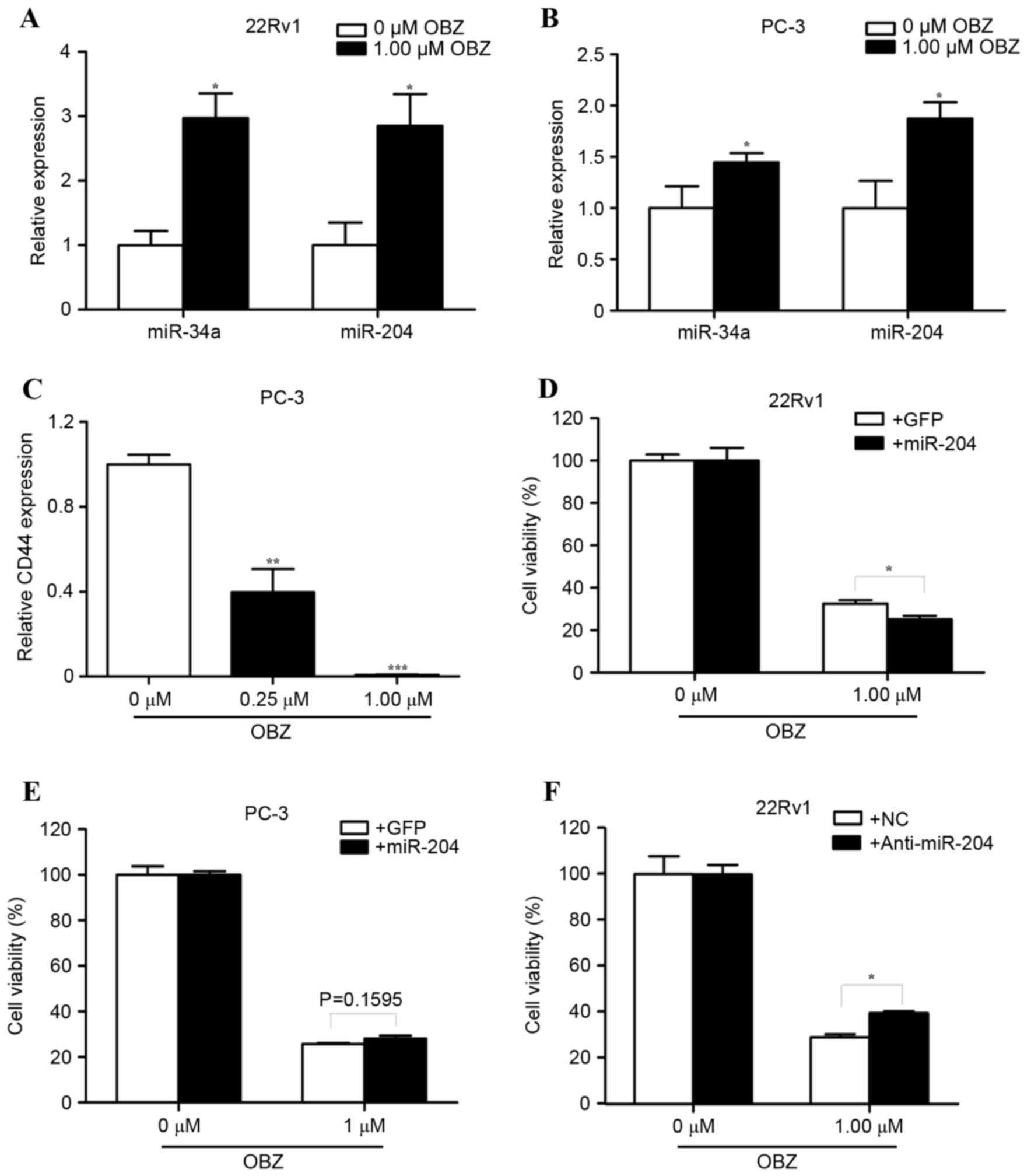 | Figure 4.Altered expression of miR-204 affects
the sensitivity of 22Rv1 cells to OBZ treatment. Following
treatment with 1.00 µM OBZ for 48 h, the total RNA was prepared
from (A) 22Rv1 and (B) PC-3 cells. The relative expression level of
miR-34a and miR-204 was determined with RT-qPCR. (C) Following
treatment with 0.25 or 1.00 µM OBZ for 48 h, the relative level of
CD44 was determined with RT-qPCR in PC-3 cells. (D) 22Rv1 and (E)
PC-3 cells were stably transfected with an miR-204 or control (GFP)
virus. At 24 h after transfection, 1.00 µM OBZ was added for a
further 48 h. The cell viability was determined by a trypan blue
exclusion assay. (F) 22Rv1 cells were transiently transfected with
an miR-204 inhibitor or NC nucleotide. At 8 h after transfection,
1.00 µM OBZ was added for a further 48 h. The cell viability was
determined by a trypan blue exclusion assay. All values are the
mean ± standard error of the mean from three independent
experiments. *P<0.05, **P<0.01, ***P<0.001. miR-204,
microRNA 204; OBZ, oxibendazole; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; CD44, cluster
of differentiation 44; GFP, green fluorescent protein; NC, negative
control. |
Cluster of differentiation 44 (CD44) is a PCa
stem-cell marker that is expressed in NEPC cells, including PC-3
cells, but not in AR-expressing PCa cells (54–56). A
previous report demonstrated that CD44 is the target gene of
miR-34a (57). Considering that OBZ
raised the expression of miR-34a in PC-3 cells in the present
study, it was speculated that OBZ may suppress the expression of
CD44. Notably, 0.25 µM OBZ significantly reduced the expression of
CD44 compared with the control (by 60.22%; P<0.01). CD44
expression was almost completely repressed in PC-3 cells that were
treated with 1.00 µM OBZ (Fig. 4C).
These results supported the hypothesis that OBZ represses CD44
expression by raising the level of miR-34a in PC-3 cells.
If the upregulation of miR-204 is essential for the
growth inhibition mediated by OBZ, then ectopic expression of
miR-204 should enhance its inhibitory efficiency. To test the
hypothesis, 22Rv1 cells and PC-3 cells were stably infected with a
recombinant lentivirus expressing miR-204 and green fluorescent
protein (GFP). As a control, the cells were stably infected with a
recombinant lentivirus expressing only GFP. Overexpression of
miR-204 significantly raised the inhibitory effect of OBZ (by
22.77%; P<0.05; Fig. 4D),
suggesting that miR-204 is important to the tumor-suppressive
effect of OBZ in 22Rv1 cells. However, the ectopic expression of
miR-204 did not significantly change the effect of OBZ in PC-3
cells (P=0.1595; Fig. 4E). To exclude
further any potential artificial effect exerted by the
overexpression of miR-204 in 22Rv1 cells, the study also assessed
whether knockdown of miR-204 could change effect of OBZ. 22Rv1
cells were transiently transfected with a miR-204 inhibitor or a
non-targeting control prior to treatment with OBZ. OBZ exerted its
inhibitory effect with a lower (by 36.11%) efficiency in cells that
were transfected with the miR-204 inhibitor than in those
transfected with the control (Fig.
4F). Taken together, these results indicated that upregulation
of miR-204 is key for the tumor-suppressive effect of OBZ in 22Rv1
cells.
Discussion
The present study demonstrated that OBZ markedly
inhibited the growth of androgen-independent PCa cells in cultured
cells and xenograft models (Figs. 1
and 3), at least partially by
inducing the apoptosis of the PCa cells (Fig. 2).
AR serves a key role in PCa progression (45), and is the target of drugs used in PCa
therapy. AR-regulated genes have been extensively studied in
primary and recurrent PCa, but the key genes under the control of
AR have remained elusive. Activation of the AR in the PCa
AR/miR-204/XRN1/miR-34a positive feedback loop (47) upregulates XRN1 expression by
repressing miR-204 expression, whereas XRN1 selectively degrades
miR-34a, eventually resulting in the raised expression of AR, since
AR is a target gene of miR-34a.
The regulation of the AR/miR-204/XRN1/miR-34a
positive feedback loop is severely disturbed in OBZ-treated 22Rv1
tumors, given that OBZ upregulated the expression of miR-204 and
miR-34a, but inhibited the expression of AR and XRN1 in the present
study (Fig. 3B and C). miR-204 has
been reported to act as a tumor suppressor, and is markedly
downregulated in various types of solid malignant tumors (51–53) and
AR-positive PCa tumors (47). The
tumor suppressor activity of miR-204 is mediated not only by its
capability to reduce apoptosis and inhibit the
epithelial-to-mesenchymal transition (EMT), but also by its power
to increase the efficiency of chemotherapy of cancer (58–60). For
example, overexpression of miR-204 increased the responsiveness of
gastric cancer cells to 5-fluorouracil and oxaliplatin treatment in
gastric cancer (52). Similarly,
ectopic expression of miR-204 markedly raised the sensitivity of
22Rv1 cells to OBZ in the present study (Fig. 4D). Consistent with these findings,
knockdown of miR-204 promoted OBZ resistance (Fig. 4F). These results, together with the
observation that OBZ treatment upregulated miR-204, indicates that
miR-204 serves a key role in mediating the anticancer effect of
OBZ.
Besides interfering with the AR/miR-204/XRN1/miR-34a
regulatory loop, OBZ also upregulated the expression of p53 and
p21. The upstream event induced by OBZ in 22Rv1 cells is currently
unknown. It has been noted that the effect of OBZ on the expression
of miR-34a is markedly stronger in 22Rv1 cells, which express
wild-type p53, than in the p53-null PC3 cells (Fig. 4A and B) (61). The discrepancy is consistent with the
hypothesis that OBZ raises miR-34a expression by upregulating p53
in 22Rv1 cells. However, a further study approach is warranted.
ADT has been used clinically to treat PCa for >70
years. However, ADT can only induce apoptosis in AR-positive cells
(or prostatic adenocarcinoma cells) in primary cancer, but does not
have a marked effect on AR-negative PCa cells, such as those of
NEPC (62). Although NEPC cells only
represent a small population (~1%) of PCa cells, they are
distributed randomly within prostatic adenocarcinomas and secrete a
variety of growth factors that can promote the proliferation of
adjacent prostatic adenocarcinoma cells via a paracrine mechanism
in an androgen-ablated environment (63,64).
Therefore, it has been proposed that NEPC cells should be targeted
by treatment in order to prevent the recurrence of PCa (65). PC-3 cells have previously been
characterized as small cell neuroendocrine carcinoma cells, a
subtype of NEPC cells (54). Notably,
OBZ repressed the growth of PC-3 cells in vitro, an
observation that requires further study in vivo. CD44 is a
marker of NEPC cells and is highly expressed in PC-3 cells
(54–56). Recent research found that CD44 is
important to the tumorigenicity of PC-3 cells (66). CD44 expression is markedly repressed
in PC-3 cells treated with 1.00 µM OBZ (Fig. 4C), indicating that CD44 serves an
indispensable role in the anticancer effect of OBZ.
It should be noted that the capability of OBZ to
cause apoptosis and inhibit growth is markedly higher in 22Rv1
cells than PC-3 cells. This is likely to be caused by the two
following mechanisms, one associated with p53 and the other with
miR-204. Apoptosis can be induced by different stimuli, including
chemotherapy, which allows p53 to regulate cellular fate by
activating the transcription of several pro-apoptotic BCL2 family
members (67). As aforementioned,
upregulation of p53 is likely to be the pivotal step mediating the
anticancer effect of OBZ in 22Rv1 cells. However, the p53 gene is
not present in PC-3 cells (61). As
reported previously, miR-204 is a tumor-suppressive gene in 22Rv1
cells, but acts as an oncomiR in PC-3 cells (47). Accordingly, the OBZ-dependent
upregulation of miR-204 should, in theory, partially neutralize the
growth-inhibitory effect of OBZ in PC-3 cells. However, the method
by which OBZ raises expression of miR-204 in PC-3 cells is
currently unknown.
Previous studies have shown that OBZ is safe for use
in ruminants, in laboratory animals and in humans at concentrations
up to 30 mg/kg (43,68), evidence that supports the further
study of OBZ as a novel anti-PCa drug.
The present study demonstrated that OBZ markedly
inhibited the growth of androgen-independent tumors by the
upregulation of miR-204 in vitro and in vivo. These
findings support the potential application of OBZ alone or in
combination with other drugs, such as enzalutamide, in the clinical
treatment of PCa, particularly recurrent PCa.
Acknowledgements
This study was supported by grants from the National
Science Foundation of China (nos. 81270760 and 81571495), the
National Basic Research Program of China (no. 2014CB943104) and the
Shanghai Municipal Committee of Science and Technology (no.
15431902800).
Glossary
Abbreviations
Abbreviations:
|
ADT
|
androgen deprivation therapy
|
|
AR
|
androgen receptor
|
|
ASP
|
androgen signaling pathway
|
|
NEPC
|
neuroendocrine-like prostate
cancer
|
|
OBZ
|
oxibendazole
|
|
PCa
|
prostate cancer
|
|
PSA
|
prostate-specific antigen
|
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peyromaure M, Valéri A, Rebillard X,
Beuzeboc P, Richaud P, Soulié M and Salomon L: CCAFU:
Characteristics of prostate cancer in men less than 50-year-old.
Prog Urol. 19:803–809. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crouzet S, Rouviere O, Martin X and Gelet
A: High-intensity focused ultrasound as focal therapy of prostate
cancer. Curr Opin Urol. 24:225–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao X and Chua KJ: Regulating the
cryo-freezing region of biological tissue with a controlled thermal
device. Med Eng Phys. 36:325–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smaletz O, Scher HI, Small EJ, Verbel DA,
McMillan A, Regan K, Kelly WK and Kattan MW: Nomogram for overall
survival of patients with progressive metastatic prostate cancer
after castration. J Clin Oncol. 20:3972–3982. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Asmane I, Céraline J, Duclos B, Rob L,
Litique V, Barthélémy P, Bergerat JP, Dufour P and Kurtz JE: New
strategies for medical management of castration-resistant prostate
cancer. Oncology. 80:1–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saad F and Hotte SJ: Guidelines for the
management of castrate-resistant prostate cancer. Can Urol Assoc J.
4:380–384. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petrylak DP: Current state of
castration-resistant prostate cancer. Am J Manag Care. 19 18
Suppl:S358–S365. 2013.PubMed/NCBI
|
|
10
|
de Bono JS, Logothetis CJ, Molina A,
Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F,
et al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanimoto T, Hori A and Kami M:
Sipuleucel-T immunotherapy for castration-resistant prostate
cancer. N Engl J Med. 363:1966; author reply 1967–8. 2010.
|
|
13
|
Joung JY, Ha YS and Kim IY: Radium Ra 223
dichloride in castration-resistant prostate cancer. Drugs Today
(Barc). 49:483–490. 2013. View Article : Google Scholar
|
|
14
|
Pantziarka P, Bouche G, Meheus L, Sukhatme
V and Sukhatme VP: Repurposing drugs in your medicine cabinet:
Untapped opportunities for cancer therapy? Future Oncol.
11:181–184. 2015. View Article : Google Scholar
|
|
15
|
Pruksachatkunakorn C, Damrongsak M and
Sinthupuan S: Sulfur for scabies outbreaks in orphanages. Pediatr
Dermatol. 19:448–453. 2002. View Article : Google Scholar
|
|
16
|
Kenawi MZ, Morsy TA, Abdalla KF and el
Hady HM: Treatment of human scabies by sulfur and permethrin. J
Egypt Soc Parasitol. 23:691–696. 1993.
|
|
17
|
Duan F, Li Y, Chen L, Zhou X, Chen J, Chen
H and Li R: Sulfur inhibits the growth of androgen-independent
prostate cancer. Oncol Lett. 9:437–441. 2015. View Article : Google Scholar
|
|
18
|
Pantziarka P, Sukhatme V, Bouche G, Meheus
L and Sukhatme VP: Repurposing drugs in oncology
(ReDO)-itraconazole as an anti-cancer agent. Ecancermedicalscience.
9:5212015. View Article : Google Scholar
|
|
19
|
Theodorides VJ, Chang J, DiCUOLLO CJ,
Grass GM, Parish RC and Scott GC: Oxibendazole, a new broad
spectrum anthelmintic effective against gastrointestinal nematodes
of domestic animals. Br Vet J. 129:xcontdvii–scvi. 1973. View Article : Google Scholar
|
|
20
|
Kates KC, Colglazier ML and Enzie FD:
Oxibendazole: Critical anthelmintic trials in equids. Vet Rec.
97:442–444. 1975.
|
|
21
|
Theodorides VJ, Nawalinski T, Freeman JF
and Murphy JR: Efficacy of oxibendazole against gastrointestinal
nematodes of cattle. Am J Vet Res. 37:1207–1209. 1976.
|
|
22
|
Overgaauw PA and Boersema JH: Anthelmintic
efficacy of oxibendazole against some important nematodes in dogs
and cats. Vet Q. 20:69–72. 1998. View Article : Google Scholar
|
|
23
|
Rao PS, Ray UK, Gupta PB, Rao DV, Islam A,
Rajput P and Mukkanti K: Identification, isolation and
characterization of new impurity in rabeprazole sodium. J Pharm
Biomed Anal. 52:620–624. 2010. View Article : Google Scholar
|
|
24
|
Gaba M, Singh S and Mohan C:
Benzimidazole: An emerging scaffold for analgesic and
anti-inflammatory agents. Eur J Med Chem. 76:494–505. 2014.
View Article : Google Scholar
|
|
25
|
He Y, Yang J, Wu B, Risen L and Swayze EE:
Synthesis and biological evaluations of novel benzimidazoles as
potential antibacterial agents. Bioorg Med Chem Lett. 14:1217–1220.
2004. View Article : Google Scholar
|
|
26
|
Li Y, Tan C, Gao C, Zhang C, Luan X, Chen
X, Liu H, Chen Y and Jiang Y: Discovery of benzimidazole
derivatives as novel multi-target EGFR VEGFR-2 and PDGFR kinase
inhibitors. Bioorg Med Chem. 19:4529–4535. 2011. View Article : Google Scholar
|
|
27
|
Velik J, Baliharová V, Fink-Gremmels J,
Bull S, Lamka J and Skálová L: Benzimidazole drugs and modulation
of biotransformation enzymes. Res Vet Sci. 76:95–108. 2004.
View Article : Google Scholar
|
|
28
|
Králová V, Hanušová V, Staňková P,
Knoppová K, Čáňová K and Skálová L: Antiproliferative effect of
benzimidazole anthelmintics albendazole, ricobendazole, and
flubendazole in intestinal cancer cell lines. Anticancer Drugs.
24:911–919. 2013. View Article : Google Scholar
|
|
29
|
Hanusova V, Skalova L, Kralova V and
Matouskova P: Potential anti-cancer drugs commonly used for other
indications. Curr Cancer Drug Targets. 15:35–52. 2015. View Article : Google Scholar
|
|
30
|
Sridhar SS, Freedland SJ, Gleave ME,
Higano C, Mulders P, Parker C, Sartor O and Saad F:
Castration-resistant prostate cancer: From new pathophysiology to
new treatment. Eur Urol. 65:289–299. 2014. View Article : Google Scholar
|
|
31
|
Debes JD and Tindall DJ: Mechanisms of
androgen-refractory prostate cancer. N Engl J Med. 351:1488–1490.
2004. View Article : Google Scholar
|
|
32
|
Nagabhushan M, Miller CM, Pretlow TP,
Giaconia JM, Edgehouse NL, Schwartz S, Kung HJ, de Vere WR,
Gumerlock PH, Resnick MI, et al: CWR22: The first human prostate
cancer xenograft with strongly androgen-dependent and relapsed
strains both in vivo and in soft agar. Cancer Res. 56:3042–2046.
1996.PubMed/NCBI
|
|
33
|
Shen MM and Abate-Shen C: Molecular
genetics of prostate cancer: New prospects for old challenges.
Genes Dev. 24:1967–2000. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sramkoski RM, Pretlow TG II, Giaconia JM,
Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D and
Jacobberger JW: A new human prostate carcinoma cell line, 22Rv1. In
Vitro Cell Dev Biol Anim. 35:403–409. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sturgeon CM, Hoffman BR, Chan DW, Ch'Ng
SL, Hammond E, Hayes DF, Liotta LA, Petricoin EF, Schmitt M, Semmes
OJ, et al: National academy of clinical biochemistry laboratory
medicine practice guidelines for use of tumor markers in clinical
practice: Quality requirements. Clin Chem. 54:e1–e10. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sardana G, Jung K, Stephan C and Diamandis
EP: Proteomic analysis of conditioned media from the PC3, LNCaP,
and 22Rv1 prostate cancer cell lines: Discovery and validation of
candidate prostate cancer biomarkers. J Proteome Res. 7:3329–3338.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li G, Petiwala SM, Nonn L and Johnson JJ:
Inhibition of CHOP accentuates the apoptotic effect of α-mangostin
from the mangosteen fruit (Garcinia mangostana) in 22Rv1 prostate
cancer cells. Biochem Biophys Res Commun. 453:75–80. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kasimsetty SG, Bialonska D, Reddy MK,
Thornton C, Willett KL and Ferreira D: Effects of pomegranate
chemical constituents/intestinal microbial metabolites on CYP1B1 in
22Rv1 prostate cancer cells. J Agric Food Chem. 57:10636–10644.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang F, Jiang X, Song L, Wang H, Mei Z, Xu
Z and Xing N: Quercetin inhibits angiogenesis through
thrombospondin-1 upregulation to antagonize human prostate cancer
PC-3 cell growth in vitro and in vivo. Oncol Rep. 35:1602–1610.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McDonald ER III, Wu GS, Waldman T and
El-Deiry WS: Repair defect in p21 WAF1/CIP1-/-human cancer cells.
Cancer Res. 56:2250–2255. 1996.PubMed/NCBI
|
|
42
|
Smith ML, Chen IT, Zhan Q, Bae I, Chen CY,
Gilmer TM, Kastan MB, O'Connor PM and Fornace AJ Jr: Interaction of
the p53-regulated protein Gadd45 with proliferating cell nuclear
antigen. Science. 266:1376–1380. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Theodorides VJ, DiCuollo CJ, Nawalinski T,
Miller CR, Murphy JR, Freeman JF, Killeen JC Jr and Rapp WR:
Toxicologic and teratologic studies of oxibendazole in ruminants
and laboratory animals. Am J Vet Res. 38:809–814. 1977.PubMed/NCBI
|
|
44
|
Stenman UH: Prostate-specific antigen,
clinical use and staging: An overview. Br J Urol. 79 Suppl
1:S53–S60. 1997. View Article : Google Scholar
|
|
45
|
Heinlein CA and Chang C: Androgen receptor
in prostate cancer. Endocr Rev. 25:276–308. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim J and Coetzee GA: Prostate specific
antigen gene regulation by androgen receptor. J Cell Biochem.
93:233–241. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ding M, Lin B, Li T, Liu Y, Li Y, Zhou X,
Miao M, Gu J, Pan H, Yang F, et al: A dual yet opposite
growth-regulating function of miR-204 and its target XRN1 in
prostate adenocarcinoma cells and neuroendocrine-like prostate
cancer cells. Oncotarget. 6:7686–7700. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hermeking H: The miR-34 family in cancer
and apoptosis. Cell Death Differ. 17:193–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Östling P, Leivonen SK, Aakula A, Kohonen
P, Mäkelä R, Hagman Z, Edsjö A, Kangaspeska S, Edgren H, Nicorici
D, et al: Systematic analysis of microRNAs targeting the androgen
receptor in prostate cancer cells. Cancer Res. 71:1956–1567. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shi XB, Xue L, Ma AH, Tepper CG,
Gandour-Edwards R, Kung HJ and DeVere WR: Tumor suppressive miR-124
targets androgen receptor and inhibits proliferation of prostate
cancer cells. Oncogene. 32:4130–4138. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Mikhaylova O, Stratton Y, Hall D, Kellner
E, Ehmer B, Drew AF, Gallo CA, Plas DR, Biesiada J, Meller J and
Czyzyk-Krzeska MF: VHL-regulated MiR-204 suppresses tumor growth
through inhibition of LC3B-mediated autophagy in renal clear cell
carcinoma. Cancer Cell. 21:532–546. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sacconi A, Biagioni F, Canu V, Mori F, Di
Benedetto A, Lorenzon L, Ercolani C, Di Agostino S, Cambria AM,
Germoni S, et al: miR-204 targets Bcl-2 expression and enhances
responsiveness of gastric cancer. Cell Death Dis. 3:e4232012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Ying Z, Li Y, Wu J, Zhu X, Yang Y, Tian H,
Li W, Hu B, Cheng SY and Li M: Loss of miR-204 expression enhances
glioma migration and stem cell-like phenotype. Cancer Res.
73:990–999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Tai S, Sun Y, Squires JM, Zhang H, Oh WK,
Liang CZ and Huang J: PC3 is a cell line characteristic of
prostatic small cell carcinoma. Prostate. 71:1668–1679. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Palapattu GS, Wu C, Silvers CR, Martin HB,
Williams K, Salamone L, Bushnell T, Huang LS, Yang Q and Huang J:
Selective expression of CD44, a putative prostate cancer stem cell
marker, in neuroendocrine tumor cells of human prostate cancer.
Prostate. 69:787–798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Simon RA, di Sant'Agnese PA, Huang LS, Xu
H, Yao JL, Yang Q, Liang S, Liu J, Yu R, Cheng L, et al: CD44
expression is a feature of prostatic small cell carcinoma and
distinguishes it from its mimickers. Hum Pathol. 40:252–258. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu C, Kelnar K, Liu B, Chen X,
Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, et
al: The microRNA miR-34a inhibits prostate cancer stem cells and
metastasis by directly repressing CD44. Nat Med. 17:211–215. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yin Y, Zhang B, Wang W, Fei B, Quan C,
Zhang J, Song M, Bian Z, Wang Q, Ni S, et al: miR-204-5p inhibits
proliferation and invasion and enhances chemotherapeutic
sensitivity of colorectal cancer cells by downregulating RAB22A.
Clin Cancer Res. 20:6187–6199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ryan J, Tivnan A, Fay J, Bryan K, Meehan
M, Creevey L, Lynch J, Bray IM, O'Meara A, Tracey L, et al:
MicroRNA-204 increases sensitivity of neuroblastoma cells to
cisplatin and is associated with a favourable clinical outcome. Br
J Cancer. 107:967–976. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu J and Li Y: Trichostatin A and
Tamoxifen inhibit breast cancer cell growth by miR-204 and ERα
reducing AKT/mTOR pathway. Biochem Biophys Res Commun. 467:242–247.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
van Bokhoven A, Varella-Garcia M, Korch C,
Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ and Lucia
MS: Molecular characterization of human prostate carcinoma cell
lines. Prostate. 57:205–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wen S, Niu Y, Lee SO and Chang C: Androgen
receptor (AR) positive vs negative roles in prostate cancer cell
deaths including apoptosis, anoikis, entosis, necrosis and
autophagic cell death. Cancer Treat Rev. 40:31–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vashchenko N and Abrahamsson PA:
Neuroendocrine differentiation in prostate cancer: Implications for
new treatment modalities. Eur Urol. 47:147–155. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jin RJ, Wang Y, Masumori N, Ishii K,
Tsukamoto T, Shappell SB, Hayward SW, Kasper S and Matusik RJ:
NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in
castrated mice. Cancer Res. 64:5489–5495. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Beltran H, Rickman DS, Park K, Chae SS,
Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, et
al: Molecular characterization of neuroendocrine prostate cancer
and identification of new drug targets. Cancer Discov. 1:487–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li W, Cohen A, Sun Y, Squires J, Braas D,
Graeber TG, Du L, Li G, Li Z, Xu X, et al: The role of CD44 in
glucose metabolism in prostatic small cell neuroendocrine
carcinoma. Mol Cancer Res. 14:344–353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chi SW: Structural insights into the
transcription-independent apoptotic pathway of p53. BMB Rep.
47:167–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang YX, Zhou JX, Xue ZQ, Wu YX, Chen JY,
Wu HZ, Ji MH, Shen YP, Cao GQ, Wu ZX, et al: Clinical observations
on the treatment of hookworm, Ascaris and Trichuris infection with
oxibendazole. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za
Zhi. 8:100–103. 1990.(In Chinese). PubMed/NCBI
|