Introduction
Prostate cancer is the second most common type of
cancer in men, and the leading cause of cancer-associated mortality
in men worldwide (1). Although
surgery is the most effective treatment for patients with early
stage prostate cancer, the tumor is unresectable for patients with
advanced prostate cancer, and systemic chemotherapy is considered
as the alternative option (2).
However, acquired chemoresistance due to the repeated use of
chemotherapeutic drugs is a common, critical problem, which limits
the clinical application of chemotherapy (3,4). There is
an urgent requirement to develop novel strategies that may inhibit
or delay the occurrence of chemoresistance.
Doxorubicin, which belongs to the family of
antitumor antibiotics, is an effective type of chemotherapeutic
drug. This antibiotic is able to embed into the double helical
structure of cell DNA, inhibiting the synthesis of RNA and DNA,
thereby inducing the apoptosis of cancer cells (5,6). Although
doxorubicin is widely used in the treatment of prostate cancer,
there is a high rate of chemotherapeutic failure due to the
acquisition of doxorubicin resistance (7,8). To delay
the occurrence of doxorubicin resistance, combination treatments
are routinely used to offset the aforementioned drug-resistance
mechanisms, and resensitize tumor cells to doxorubicin. It has been
reported that curcumin improves the efficacy of doxorubicin
treatment in breast cancer (9).
Another study reported that the addition of microRNA-122
oligonucleotides was able to reverse doxorubicin resistance in
hepatocellular carcinoma cells (10).
The aforementioned studies provide evidence to demonstrate that
combination treatment with other drugs are effective to inhibit or
delay the occurrence of doxorubicin resistance.
Quercetin, which is widely distributed in
plant-based foods, is a flavonoid. The reported pharmacological
effects of quercetin include antioxidant, anti-inflammatory, and
anti-proliferative activity (11,12).
Modern drug researchers have demonstrated that quercetin acts as a
potential anti-tumor agent in multiple types of cancer by
regulating the apoptosis pathway (13,14).
However, the anti-tumor effect of single quercetin treatment is
limited. Therefore, the aim of the present study was to assess
whether the combination of quercetin with doxorubicin reversed the
resistance of prostate cancer cells to doxorubicin-based therapy
in vitro.
Materials and methods
Cell culture
The present study was approved by the Ethics
Committee of Tongde Hospital of Zhejiang (Hangzhou, China). The
human prostate cancer cell line PC3 was obtained from the American
Type Culture Collection (ATCC; Manassas, VA, USA).
Doxorubicin-resistant PC3 cells (PC3/R) were established by
continuous exposure of normal PC3 cells to increasing
concentrations of doxorubicin (Sigma Aldrich; Merck KGaA,
Darmstadt, Germany). Briefly, PC3 cells were incubated with
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 0.05 µg/ml
doxorubicin for 2 months. Subsequently, the dose of doxorubicin was
increased every 3 weeks by 0.01 µg/ml up to a final concentration
of 0.15 µg/ml. Prior to the experiments, the PC3/R cells were
cultured in Dulbecco's modified Eagle's medium supplemented with
10% fetal bovine serum for 2 weeks at 37°C in a humidified 5%
CO2 incubator.
Drug sensitivity assay
The sensitivity of PC3 and PC3/R cells to
doxorubicin, and the effects of quercetin on doxorubicin-induced
cell death of PC3/R cells were measured by
3-(4,5-dimethylthiazol-z-yl)-2,5-diphenyl tetrazolium bromide (MTT;
Sigma Aldrich; Merck KGaA) assay. Cells were seeded into 96-well
plates at a density of 5×103 per well and cultured
overnight. Cells were treated with various concentrations (0, 0.1,
0.2, 0.5, 1.0, 1.5, 2, 4, 6, 8, 10 or 15 g/ml) of doxorubicin in
the presence or absence of quercetin (10 µm; Sigma Aldrich; Merck
KGaA) for 48 h at 37°C. Subsequently, 20 µl MTT (5 mg/ml) was added
and cells were incubated at 37°C for an additional 4 h. Following
this, 150 µl DMSO was added to dissolve the formazan crystals. The
absorbance in each well was measured at 570 nm using a microplate
reader. The half maximal inhibitory concentration (IC50)
of doxorubicin in PC3 and PC3/R cells was calculated according to
the viability curves.
Western blot analysis
A total of 5×106 cells were harvested for
total protein extraction and lysed with radioimmunoprecipitation
assay buffer (Cell Signaling Technology, Inc., Danvers, MA, USA)
for 30 min at 4°C. The cell lysate (50 µg) samples were separated
by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis
and transferred to PVDF membranes. Following transfer, membranes
were incubated in 5% skimmed milk for 1 h at room temperature.
Subsequently, the membranes were incubated with primary antibodies
overnight at 4°C. Antibodys used were as follows: Anti-PI3K (cat.
no. 4249; 1:1,000 dilution), anti-phosphorylated (p-) PI3K (cat.
no. 13857; 1:1,000 dilution), anti-AKT (cat. no. 4685; 1:1,000
dilution), anti-p-AKT (cat. no. 4060; 1:1,000 dilution), anti-c-met
(cat. no. 8198; 1:1,000 dilution), anti-caspase-9 (cat. no. 9502;
1:1,000 dilution), anti-cleaved caspase-9 (cat. no. 9505; 1:1,000
dilution), anti-caspase-3 (cat. no. 9665; 1:1,000 dilution),
anti-cleaved caspase-3 (cat. no. 9664; 1:1,000 dilution) and
anti-β-actin (cat. no. 4970; 1:1,000 dilution), all purchased from
Cell Signaling Technology, Inc. The following day, the membranes
were washed three times using Tris-buffered saline with 1% Tween-20
and incubated for 2 h at 37°C with horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat no.
7074; 1:2,000 dilution; Cell Signaling Technology, Inc.). Protein
bands were visualized using an enhanced chemiluminescence detection
kit (Pierce; Thermo Fisher Scientific, Inc.).
Assessment of apoptosis
Cells were collected and washed with ice-cold PBS.
Apoptosis was determined in PC3 and PC3/R cells using a dual
staining method with Annexin V-fluorescein isothiocyanate
(Sigma-Aldrich; Merck KgaA) and propidium iodide for 20 min in the
dark at room temperature. The percentage of apoptotic cells in
total 104 cells was quantified by flow cytometry
(FACSCanto; BD Biosciences, Franklin Lanes, NJ, CA, USA) and
analyzed by using FlowJo software (version 10; Tree Star, Inc.,
Ashland, OR, USA).
Detection of mitochondrial membrane
potential (MMP, ∆Ψm) and reactive oxygen species (ROS)
A total of 1×106 cells were harvested and
washed with ice-cold PBS. For MMP detection, cells were stained
with 5,5′, 6,6′-Tetrachloro-1,1′,3,3′-tetraethyl imidacarbo cyanine
iodide (Molecular Probes; Thermo Fisher Scientific, Inc.) for 15
min in the dark at room temperature. For measurement of ROS, cells
were stained with dihydroethidium (Molecular Probes; Thermo Fisher
Scientific, Inc.) for 15 min in the dark at room temperature.
Detection of MMP and ROS were analyzed by flow cytometry
(FACSCanto; BD Biosciences) and FlowJo 10 software.
Recombinant plasmid construction and
transfection
Total RNAs from PC3 cells were extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Total RNA was reverse
transcribed into cDNA using the Prime Script RT reagent kit (Takara
Biotechnology Co., Ltd. Dailan, China) following the manufacturer's
protocol. Following synthesis of PC3 cDNA, the open reading frame
of the c-met gene was amplified by polymerase chain reaction (PCR)
using Takara LA Taq® (Takara Biotechnology Co.,
Ltd.) using the following primers: Forward, c-met HindIII,
5′-ACGAAGCTTATGAAGGCCCCCGCTGTGCTT-3′, and reverse, c-met
XhoI, ACGCTCGAGTGATGTCTCCCAGAAGGAGGCTGGT. The reaction
conditions were as follows: 94°C for 1 min, 30 cycles of 98°C for
10 sec, 68°C for 5 min and 72°C for 10 min. PCR products and the
pcDNA3.1 plasmid (Invitrogen; Thermo Fisher Scientific, Inc.) were
digested with HindIII/XhoI (Takara Biotechnology Co., Ltd.)
for 2 h at 37°C. To ligate the c-met fragment into the pcDNA3.1
plasmid, the digestion products were incubated with T4 DNA ligase
(Takara Biotechnology Co., Ltd.) overnight at 16°C and the
recombinant plasmid was named the c-met vector. For transfection,
the 5×105 PC3/R cells were plated and cultured to reach
80% confluency. C-met vector (2 µg/ml) or empty vector (used for
control) was transiently transfected into the cells with
Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the the manufacturer's protocol.
Statistical analysis
Statistical analyses were conducted on all the
experiments, which were repeated in triplicate and data were
expressed as the mean ± standard deviation. For comparison
analysis, two-tailed unpaired Student's t-test was used to evaluate
the statistical differences between two groups. One-way analysis of
variance and Bonferroni's post-hoc test were used to determine the
differences between three or more groups. Statistical analysis was
performed using SPSS software (version 15.0; SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Resistance of PC3/R cells to
doxorubicin
To investigate the resistance of PC3 prostate cancer
cells to doxorubicin, a PC3/R cell line was established by
continuous exposure of routine PC3 cells to doxorubicin. The
results presented in Fig. 1A
demonstrated that IC50 of doxorubicin to PC3/R was
11.25-fold higher than the parental PC3 cells. This indicated that
the established PC3/R cell line demonstrated significant drug
resistance to doxorubicin. Due to data published from previous
studies, which provided evidence that the PI3K/AKT pathway
regulates the chemotherapeutic resistance of cells (15,16), the
activation of PI3K and AKT in PC3/R cells compared with parental
PC3 cells, in response to doxorubicin, was investigated. Notably,
activation of the PI3K/AKT pathway was significantly increased in
PC3/R cells compared with parental PC3 cells, in the presence or
absence of equal doses of doxorubicin (Fig. 1B). The results from the present study
suggested that the hyper-activation of the PI3K/AKT pathway may be
responsible for the drug resistance to doxorubicin in PC3/R
cells.
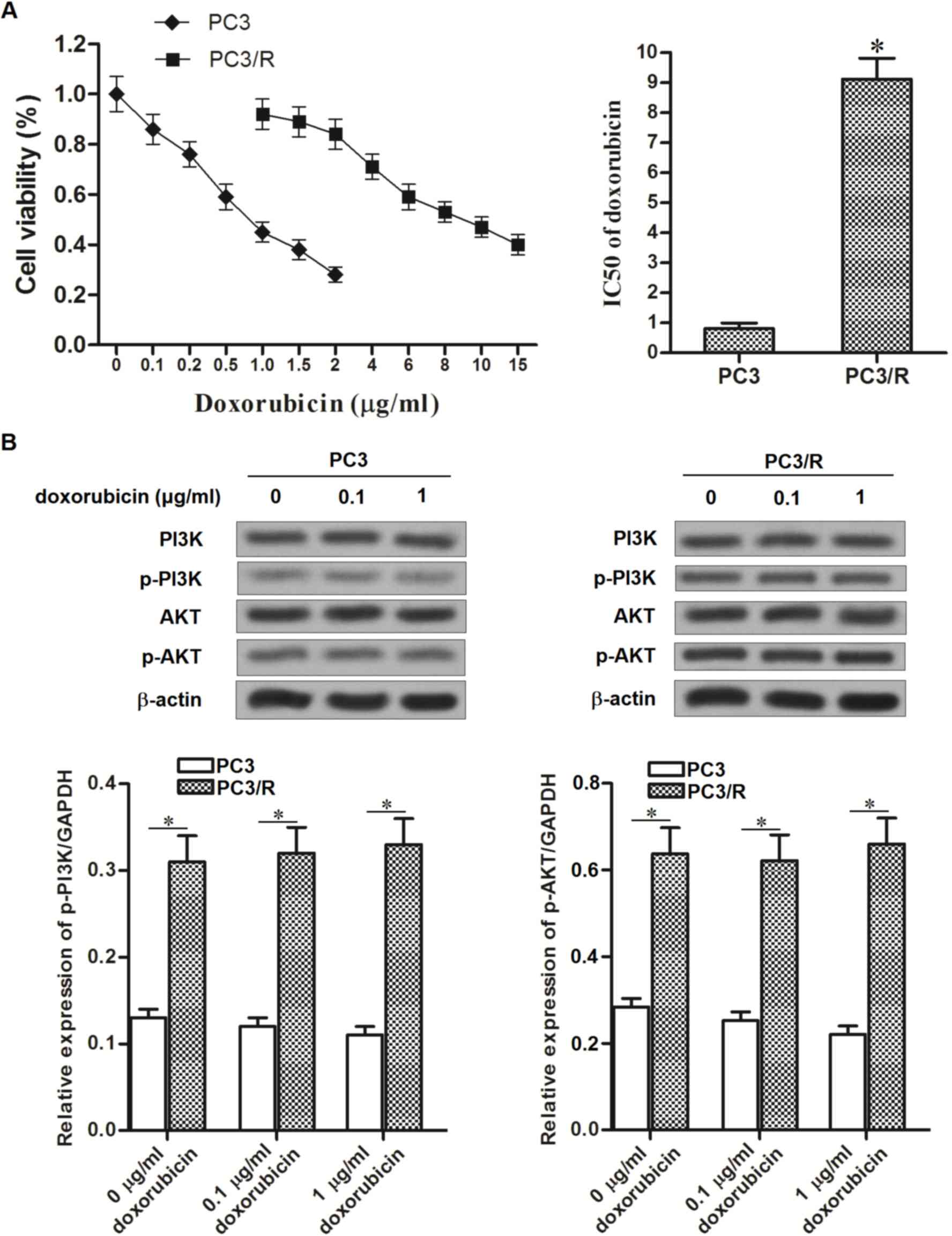 | Figure 1.Comparison of doxorubicin sensitivity
between PC3/R and PC3 cell lines. (A) Following treatment with
doxorubicin at the indicated concentrations for 48 h, the cell
viability of PC3/R and PC3 were detected by MTT assay. The
IC50 of PC3/R and PC3 to doxorubicin was calculated
according to the viability curves. (B) Following 48 h of
doxorubicin treatment, the activation of PI3K/AKT pathway was
evaluated by western blot analysis in PC3/R and PC3 cells.
*P<0.05 vs. PC3 cell line. PC3/R, prostate cancer 3/resistant;
PC3, prostate cancer 3; MTT,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
IC50, half maximal inhibitory concentration. P13K,
phosphoinositide 3-kinase; AKT, protein kinase B; P-,
phosphorylated. |
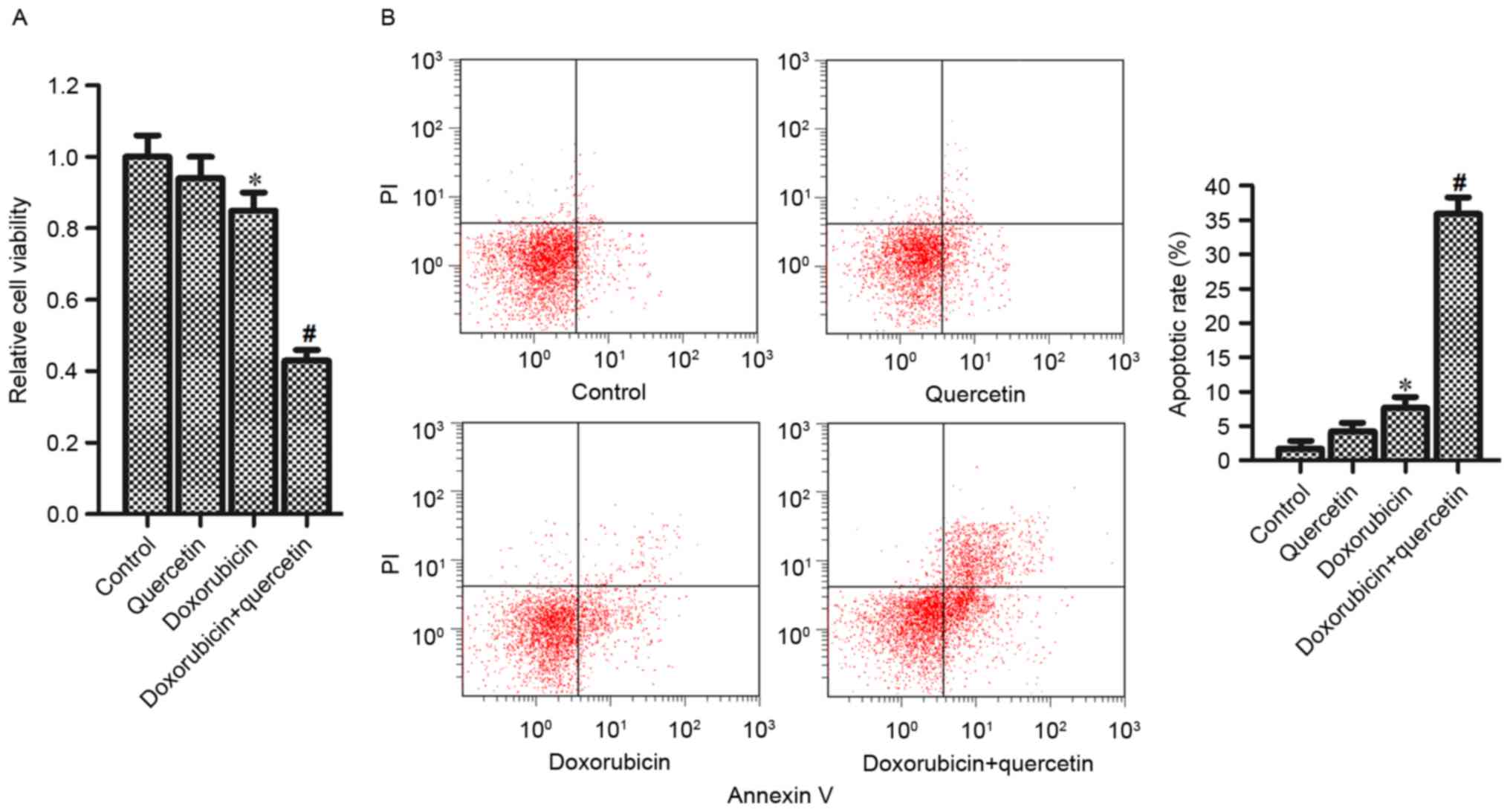
Quercetin increased the sensitivity of
PC3/R cells to doxorubicin
To investigate the effect of quercetin on
doxorubicin resistance, PC3/R cells were co-treated with
doxorubicin and quercetin. Analysis of cell viability revealed that
although single quercetin treatment alone had no effect on the
viability of PC3/R cells, in combination with doxorubicin
treatment, quercetin significantly enhanced the effects of
doxorubicin and reduced PC3/R cell viability compared with cells
treated with doxorubicin alone (Fig.
2A). In addition, the results of flow cytometry indicated that
the combination of quercetin and doxorubicin induced significantly
increased apoptosis in PC3/R cells compared with cells treated with
doxorubicin alone (Fig. 2B). Taken
together, these results demonstrated that quercetin in combination
with doxorubicin was able to reverse drug resistance in doxorubicin
resistant prostate cancer cells.
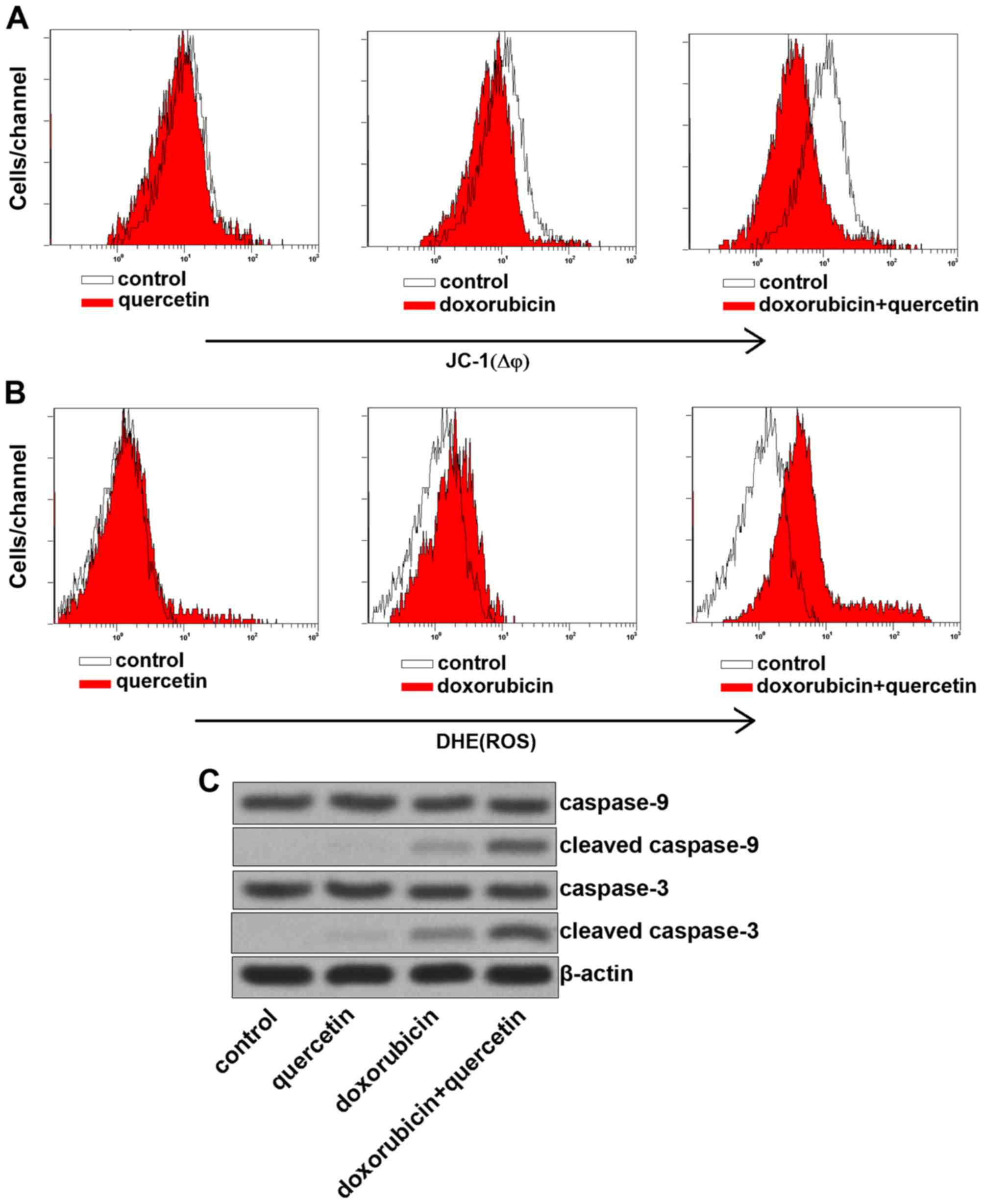
Combination treatment with quercetin
and doxorubicin induced apoptosis via the mitochondrial
pathway
To investigate the mechanism by which quercetin
promoted doxorubicin-induced apoptosis, MMP was measured in PC3/R
cells, co-treated with quercetin and doxorubicin. The results
presented in Fig. 3A demonstrated
that combination treatment with quercetin and doxorubicin induced a
significant decrease of MMP in PC3/R cells compared with cells
treated with doxorubicin alone. Furthermore, ROS, which are
considered to be key apoptotic inducers (17) were released from the mitochondria into
the cytoplasm, due to MMP collapse induced by co-treatment with
quercetin and doxorubicin (Fig. 3B).
The expression of caspase-3 and −9 in response to quercetin and
doxorubicin treatment were further investigated, as these are known
to be activated downstream of the mitochondrial pathway (Fig. 3C). The effects of doxorubicin
treatment were enhanced by combination treatment with quercetin to
induce expression of cleaved caspase-3 and −9. The results from the
present study therefore demonstrated that quercetin promoted
doxorubicin-induced apoptosis via the mitochondrial pathway.
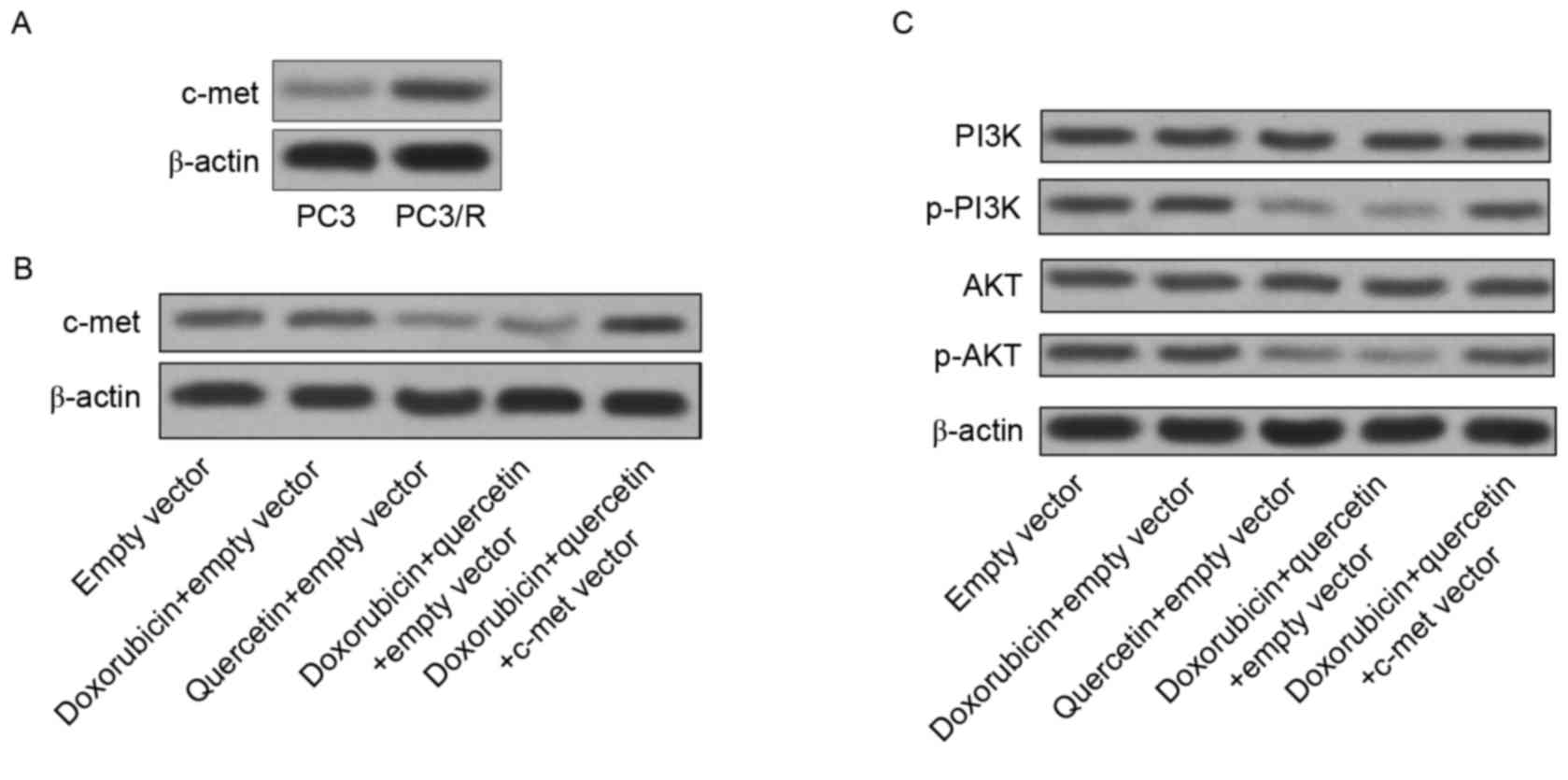
C-met is the target of quercetin in
PC3/R cells
The results from the present study have indicated
thus far that PC3/R cells demonstrate hyper-activation of PI3K/AKT
compared with normal PC3 cells. To elucidate how quercetin
facilitates doxorubicin-induced cell death in PC3/R cells, the
target of quercetin in the PI3K/AKT pathway in PC3/R cells was
explored. The expression level of c-met, which is upstream of PI3K
signaling (18), was significantly
increased in PC3/R cells compared with in PC3 cells (Fig. 4A). Thus, hyper-activation of the
c-met/PI3K/AKT pathway may be responsible for doxorubicin
resistance in PC3/R cells. However, treatment with quercetin
without doxorubicin significantly downregulated c-met expression
compared to the control group (Fig.
4B). In addition, the activation of the PI3K/AKT pathway was
also inhibited by quercetin treatment. Furthermore, the induced
expression of c-met abolished the inhibition of the PI3K/AKT
pathway induced by quercetin (Fig.
4C). Taken together, the results revealed that quercetin
targeted c-met to inhibit the PI3K/AKT pathway in
doxorubicin-resistant prostate cancer cells.
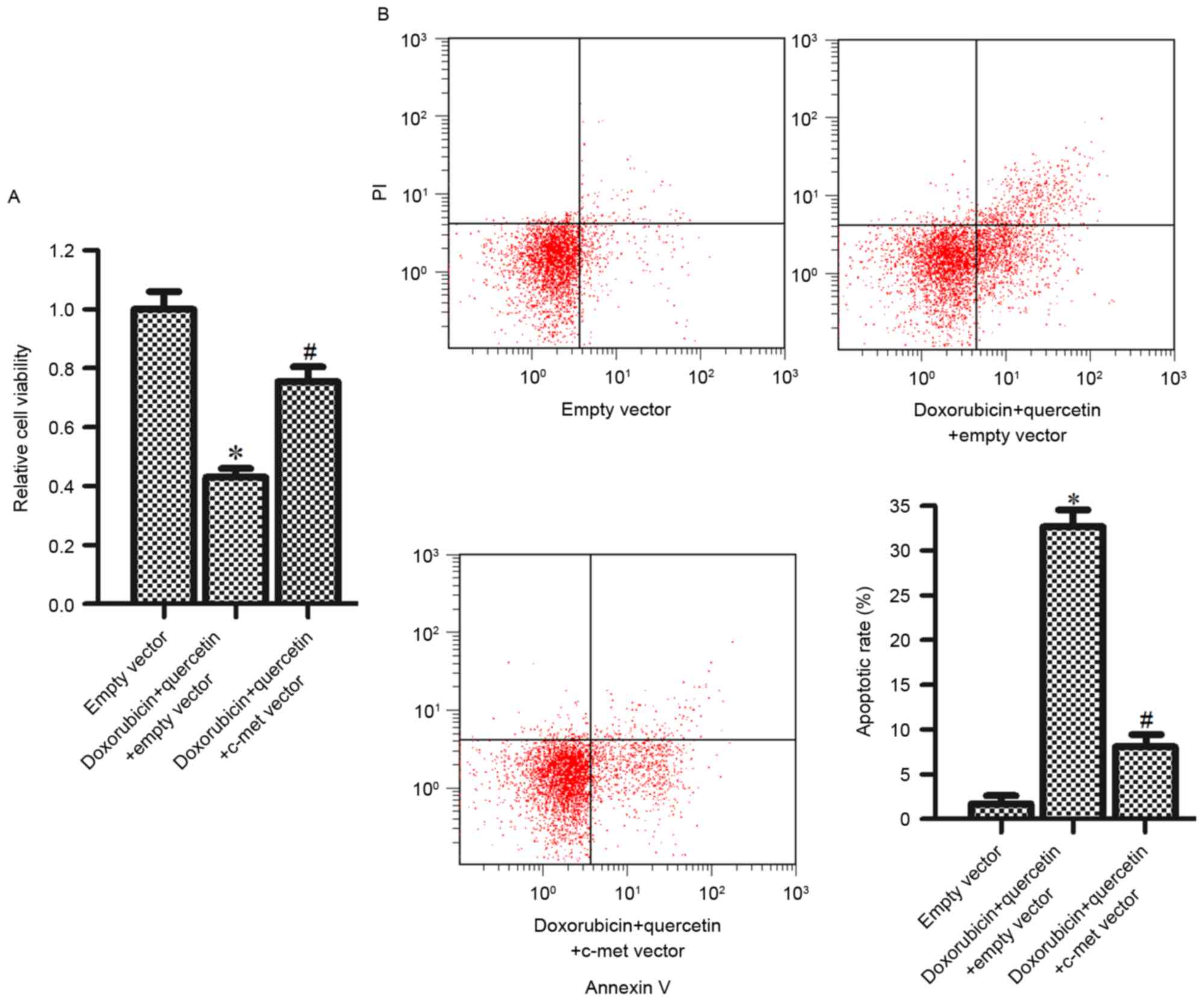
Quercetin increased the sensitivity of
PC3/R to doxorubicin via downregulating c-met expression
To study the function of c-met and the synergistic
effects of quercetin and doxorubicin, c-met expression was induced
in PC3/R cells to rescue cell death induced by this combined
treatment. It was observed that introduction of the c-met vector
significantly inhibited the cytotoxicity, induced in cells by the
combination treatment with quercetin and doxorubicin (Fig. 5A). Furthermore, quercetin and
doxorubicin induced-apoptosis was also inhibited by the c-met
vector (Fig. 5B). The results from
the present study suggested that the synergistic effects of
quercetin and doxorubicin were dependent on the downregulation of
c-met.
Discussion
C-met is the receptor for hepatocyte growth factor
(HGF). The extracellular sema domain of c-met mediates binding to
HGF, which activates receptor auto-phosphorylation (19). The activation of c-met has been
reported to stimulate multiple downstream genes to promote
tumorigenesis in various types of cancer (20). Overexpression of c-met stimulates
proliferation, migration and invasion in various types of cancer
including prostate cancer (21,22).
Furthermore, activation of the c-met pathway was revealed to be an
important mechanism for acquired resistance to chemotherapy.
Previous studies have reported that c-met receptor activation
protects cancer cells against DNA-damaging agents. For example,
activation of the c-met pathway triggered the expression of focal
adhesion kinase and downregulated apoptosis-inducing factor
expression to develop cisplatin resistance in lung cancer (23). In human multiple myeloma, c-met
knockdown resulted in decreased drug-resistance and increased
chemosensitivity to doxorubicin (24). Therefore, the inhibition of c-met and
its downstream signaling targets has been considered as a potential
strategy to enhance the therapeutic efficacy for the treatment of
cancer (25,26).
Within the c-met pathway, the auto-phosphorylation
of c-met leads to phosphorylation of PI3K and the subsequent
generation of phosphatidylinositol-3,4,5-trisphosphate (PIP3).
Then, cellular PIP3 triggers AKT activation (27,28). It
has been reported that PI3K/AKT signaling stimulates various
biological processes in cancer, and functions as a key regulator in
cell proliferation, survival, migration and apoptosis (29,30).
Hyper-activation of the PI3K/AKT pathway is observed in several
types of cancer, such as colorectal (31), ovarian (32) and prostate cancer (33). Previous studies have revealed a direct
link between highly activated PI3K/AKT and poor prognosis and tumor
recurrence in prostate cancer (33).
In addition, as the PI3K/AKT pathway regulates the apoptosis of
cancer cells, it accelerates chemoresistance in cancer cells when
under the influence of chemotherapeutic drugs (34). Therefore, intervention of this pathway
is considered to be a potential strategy to delay or reverse the
occurrence of drug resistance in cancer.
Quercetin is a natural flavonoid compound. Previous
studies have demonstrated that quercetin is involved in inducing
the apoptosis pathway in cancers (35). Therefore, combination treatment with
quercetin and several anti-tumor agents, including docetaxel, tumor
necrosis factor-related apoptosis-inducing ligand, and
2-methoxyestradiol have proved to be effective synergistic
treatments for prostate cancer (36–38). The
fact that doxorubicin treatment in prostate cancer frequently leads
to the acquirement of doxorubicin resistance is a major problem. To
study the potential function of quercetin in doxorubicin resistance
in prostate cancer, a doxorubicin-resistant PC3 cell line PC3/R was
established. Notably, it was observed that the addition of
quercetin was able to reverse doxorubicin resistance, by increasing
the sensitivity of PC3/R cells to doxorubicin-induced apoptosis
in vitro.
The activated c-met/PI3K/AKT pathway protects cancer
cells from apoptotic signals and promotes cancer survival (39). In the present study, it was revealed
that c-met was further upregulated in response to the acquisition
of doxorubicin-resistance in prostate cancer cells. Therefore, it
is suggested that c-met is associated with drug-resistance in
prostate cancer. Furthermore, it was demonstrated that quercetin
treatment significantly inhibited c-met expression in PC3/R cells.
Subsequently, the inhibition of the PI3K/AKT pathway, which is
downstream of c-met, was also observed. Furthermore, as the
combination treatment with quercetin inhibited the PI3K/AKT
pathway, doxorubicin-induced dysfunction of mitochondria, release
of ROS, cleavage of caspase-3/9 and apoptosis in PC3/R cells
occurred as a consequence. Additionally, quercetin-induced
doxorubicin cytotoxicity in PC3/R cells was impaired in response to
the overexpression of c-met, induced by its expression plasmid.
Taken together, these results provide evidence that combination
treatment with quercetin is able to reverse doxorubicin-resistance
in prostate cancer cells by targeting the c-met/PI3K/AKT pathway.
In conclusion, the present study may provide a novel treatment
protocol for inhibiting or delaying drug resistance to
doxorubicin-based therapy.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kopczyńska E: Role of microRNAs in the
resistance of prostate cancer to docetaxel and paclitaxel. Contemp
Oncol (Pozn). 19:423–427. 2015.PubMed/NCBI
|
|
3
|
Zhang W, Meng Y, Liu N, Wen XF and Yang T:
Insights into chemoresistance of prostate cancer. Int J Biol Sci.
11:1160–1170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karnak D and Xu L: Chemosensitization of
prostate cancer by modulating Bcl-2 family proteins. Curr Drug
Targets. 11:699–707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
He H, Tian W, Chen H and Deng Y:
MicroRNA-101 sensitizes hepatocellular carcinoma cells to
doxorubicin-induced apoptosis via targeting Mcl-1. Mol Med Rep.
13:1923–1929. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rivankar S: An overview of doxorubicin
formulations in cancer therapy. J Cancer Res Ther. 10:853–858.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
De Luca P, Vazquez ES, Moiola CP, Zalazar
F, Cotignola J, Gueron G, Gardner K and De Siervi A: BRCA1 loss
induces GADD153-mediated doxorubicin resistance in prostate cancer.
Mol Cancer Res. 9:1078–1090. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carey JP, Knowell AE, Chinaranagari S and
Chaudhary J: Id4 promotes senescence and sensitivity to
doxorubicin-induced apoptosis in DU145 prostate cancer cells.
Anticancer Res. 33:4271–7278. 2013.PubMed/NCBI
|
|
9
|
Lv L, Qiu K, Yu X, Chen C, Qin F, Shi Y,
Ou J, Zhang T, Zhu H, Wu J, et al: Amphiphilic Copolymeric micelles
for doxorubicin and curcumin co-delivery to reverse multidrug
resistance in breast cancer. J Biomed Nanotechnol. 12:973–985.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pan C, Wang X, Shi K, Zheng Y, Li J, Chen
Y, Jin L and Pan Z: MiR-122 reverses the doxorubicin-resistance in
hepatocellular carcinoma cells through regulating the tumor
metabolism. PLoS One. 11:e01520902016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bischoff SC: Quercetin: Potentials in the
prevention and therapy of disease. Curr Opin Clin Nutr Metab Care.
11:733–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Erlund I: Review of the flavonoids
quercetin, hespertin and naringenin. dietary sources,
bioactivities, bioavailability and epidemiology. Nutr Res.
24:851–874. 2004. View Article : Google Scholar
|
|
13
|
Ramos S: Effects of dietary flavonoids on
apoptotic pathways related to cancer chemoprevention. J Nutr
Biochem. 18:427–442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Tang C, Li L, Li R and Fan Y:
Quercetin sensitizes glioblastoma to t-AUCB by dual inhibition of
Hsp27 and COX-2 in vitro and in vivo. J Exp Clin Cancer Res.
35:612016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
West KA, Castillo SS and Dennis PA:
Activation of the PI3K/Akt pathway and chemotherapeutic resistance.
Drug Resist Updat. 5:234–248. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kang Q and Yan S: Piperlongumine reverses
doxorubicin resistance through the PI3K/Akt signaling pathway in
K562/A02 human leukemia cells. Exp Ther Med. 9:1345–1350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zorov DB, Juhaszova M and Sollott SJ:
Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS
release. Physiol Rev. 94:909–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung KA, Choi BH and Kwak MK: The
c-MET/PI3K signaling is associated with cancer resistance to
doxorubicin and photodynamic therapy by elevating BCRP/ABCG2
expression. Mol Pharmacol. 87:465–476. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferracini R, Longati P, Naldini L, Vigna E
and Comoglio PM: Identification of the major autophosphorylation
site of the Met⁄hepatocyte growth factor receptor tyrosine kinase.
J Biol Chem. 266:19558–19564. 1991.PubMed/NCBI
|
|
20
|
Knudsen BS and Vande Woude G: Showering
c-Met dependent cancers with drugs. Curr Opin Genet Dev. 18:87–96.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han Y, Luo Y, Zhao J, Li M and Jiang Y:
Overexpression of c-Met increases the tumor invasion of human
prostate LNCaP cancer cells in vitro and in vivo. Oncol Lett.
8:1618–1624. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yasuda K, Nagakawa O, Akashi T, Fujiuchi
Y, Koizumi K, Komiya A, Saiki I and Fuse H: Serum active hepatocyte
growth factor (AHGF) in benign prostatic disease and prostate
cancer. Prostate. 69:346–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen JT, Huang CY, Chiang YY, Chen WH,
Chiou SH, Chen CY and Chow KC: HGF increases cisplatin resistance
via down-regulation of AIF in lung cancer cells. Am J Respir Cell
Mol Biol. 38:559–565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Que W and Chen J: Knockdown of c-Met
inhibits cell proliferation and invasion and increases
chemosensitivity to doxorubicin in human multiple myeloma U266
cells in vitro. Mol Med Rep. 4:343–349. 2011.PubMed/NCBI
|
|
25
|
Toschi L and Jänne PA: Single-agent and
combination therapeutic strategies to inhibit hepatocyte growth
factor⁄MET signaling in cancer. Clin Cancer Res. 14:5941–5946.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hung TH, Li YH, Tseng CP, Lan YW, Hsu SC,
Chen YH, Huang TT, Lai HC, Chen CM, Choo KB and Chong KY: Knockdown
of c-MET induced apoptosis in ABCB1-overexpressed
multidrug-resistance cancer cell lines. Cancer Gene Ther.
22:262–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yao Y, Dou C, Lu Z, Zheng X and Liu Q:
MACC1 suppresses cell apoptosis in hepatocellular carcinoma by
targeting the HGF/c-MET/AKT pathway. Cell Physiol Biochem.
35:983–996. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trovato M, Torre ML, Ragonese M, Simone A,
Scarfì R, Barresi V, Giuffrè G, Benvenga S, Angileri FF, Tuccari G,
et al: HGF/c-met system targeting PI3K/AKT and
STAT3/phosphorylated-STAT3 pathways in pituitary adenomas: an
immunohistochemical characterization in view of targeted therapies.
Endocrine. 44:735–743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Engelman JA: Targeting PI3K signalling in
cancer: Opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang BH and Liu LZ: PI3K/PTEN signaling
in angiogenesis and tumorigenesis. Adv Cancer Res. 102:19–65. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Danielsen SA, Eide PW, Nesbakken A, Guren
T, Leithe E and Lothe RA: Portrait of the PI3K/AKT pathway in
colorectal cancer. Biochim Biophys Acta. 1855:104–121.
2015.PubMed/NCBI
|
|
32
|
Li H, Zeng J and Shen K: PI3K/AKT/mTOR
signaling pathway as a therapeutic target for ovarian cancer. Arch
Gynecol Obstet. 290:1067–1078. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toren P and Zoubeidi A: Targeting the
PI3K/Akt pathway in prostate cancer: Challenges and opportunities
(review). Int J Oncol. 45:1793–1801. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang KD and Ling MT: Targeting
drug-resistant prostate cancer with dual PI3K/mTOR inhibition. Curr
Med Chem. 21:3048–3056. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang F, Song L, Wang H, Wang J, Xu Z and
Xing N: Quercetin in prostate cancer: Chemotherapeutic and
chemopreventive effects, mechanisms and clinical application
potential (Review). Oncol Rep. 33:2659–2668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang P, Henning SM, Magyar CE, Elshimali
Y, Heber D and Vadgama JV: Green tea and quercetin sensitize PC-3
xenograft prostate tumors to docetaxel chemotherapy. J Exp Clin
Cancer Res. 35:732016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jung YH, Heo J, Lee YJ, Kwon TK and Kim
YH: Quercetin enhances TRAIL-induced apoptosis in prostate cancer
cells via increased protein stability of death receptor 5. Life
Sci. 86:351–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang F, Song L, Wang H, Wang J, Xu Z and
Xing N: Combination of quercetin and 2-Methoxyestradiol enhances
inhibition of human prostate cancer LNCaP and PC-3 cells xenograft
tumor growth. PLoS One. 10:e01282772015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiao D, Wang J, Lu W, Tang X, Chen J, Mou
H and Chen QY: Curcumin inhibited HGF-induced EMT and angiogenesis
through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways
in lung cancer. Mol Ther Oncolytics. 3:160182016. View Article : Google Scholar : PubMed/NCBI
|