Introduction
Osteosarcoma is a primary malignant bone tumor and
originates from osteoblast-like tumor cells or osteoid tumor cells
(1). Osteosarcoma often occurs in
individuals at 10–25 years of age. The morbidity fastigium is at
~18 years of age (2). Currently,
therapeutic measures include radical limb salvage operations,
pre-operative neoadjuvant chemotherapy and post-operative
multi-medicine combined chemotherapy (3). For patients whose tumors are restricted
to protopathy, the 5-year long-term survival rate is up to 50–70%
(4). Often, once bone tumors have
been diagnosed, lung metastatic foci have already formed and
patients are unresponsive to chemo-radiotherapy and operations
(5). Furthermore, in patients with
neoplasm recurrence in later periods, the 5-year long-term survival
rate is only 15–20% (5). Current
therapeutic methods include multi-medicine combined chemotherapy,
surgical resection and radiotherapy with several courses (4). However, if clinical metastasis is
revealed in the first diagnosis or patients are unresponsive to
chemo-radiotherapy, particularly for patients with osseous
metastasis, prognosis is often negative, and the long-term survival
rate is decreased to 15–25% (3).
The p53 gene is located on chromosome 17p13.1 and is
a negative regulatory factor of the cell growth cycle. Furthermore,
it has been demonstrated to be associated with various important
biological functions, including the regulation of the cell cycle,
DNA repair, cell differentiation and apoptosis (6). The p53 gene is a cancer suppressor gene
with a higher mutation rate in malignant tumors (7). Previous research has demonstrated that
functional inactivation of the p53 gene serves a crucial function
in the occurrence and developmental process of common types of
tumor, including lung cancer and breast cancer (8).
In the process of apoptosis, the mitochondrion is
the center of apoptosis regulation, with cytochrome c
serving a critical function upon its release from the
mitochondrion. Once cytochrome c is released into the
cytoplasm, it may activate caspases and trigger a cascade reaction,
thus resulting in apoptosis. The B cell lymphoma-2 (Bcl-2) protein
family regulates the release of cytochrome c. The apoptosis
induction factor ensures the order of the process of apoptosis. The
endoplasmic reticulum may improve the sensitivity of the
mitochondrion to pro-apoptosis factors through stress, recruitment
and activation, so as to allow ease of cytochrome c release
from the intermembrane space of the mitochondrion.
In a previous study in 1966, camptothecin was
extracted and separated from the plant Camptotheca acuminata
(Nyssaceae) in China and was revealed to act on DNA topoisomerase I
with good specificity and demonstrates antitumor activity (9). However, due to serious side effects, it
is not applied in clinical practice. By studying the
pharmacological action mechanism and structure-function
relationship of camptothecin, researchers have developed a series
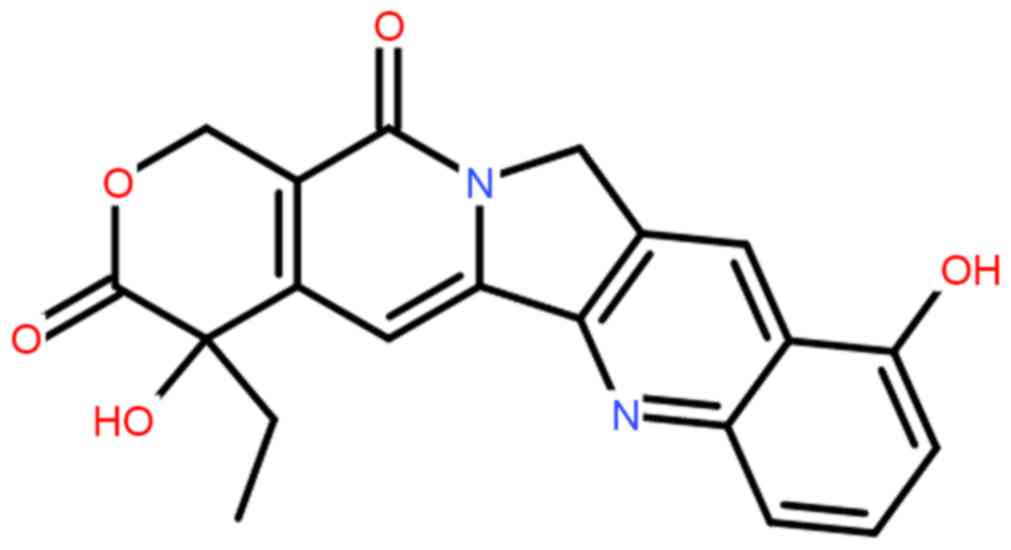
of derivatives of camptothecin. 10-hydroxycamptothecin (HCPT;
Fig. 1) is a derivative of
camptothecin that has medical uses. HCPT may act on DNA
topoisomerase I selectively and form stable medical topoisomerase
I-DNA complex compounds to disturb the duplication of DNA (10). Such a unique mechanism makes it
difficult for HCPT to form cross tolerance with other
antineoplastic drugs. Thus, it may form part of a combination
treatment with a number of medicines for clinical uses (11). The primary negative effects include
myelosuppression and a gastrointestinal reaction. These effects are
due to dose-limiting toxicity (12).
Currently, HCPT is primarily used in the form of a sodium salt
injection. The present study evaluated the anticancer effects of
HCPT in inducing the apoptosis of human osteosarcoma cells, and its
apoptosis-inducing molecular mechanisms were investigated.
Materials and methods
Cell culture and hypoxia
treatment
Human osteosarcoma MG-63 cells were purchased from
the Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai,
China) and grown in Dulbecco's modified Eagle medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (Biowest LLC, Kansas City, MO, USA), penicillin
(100 U/ml) and streptomycin (100 µg/ml) at 37°C in a humidified
atmosphere of 5% CO2.
Cell viability and cytotoxicity
MG-63 cells were cultured in 96-well plates
(~1×104 cells/well) and treated with HCPT (0, 20, 40 and
80 nM) for 24 and 48 h at 37°C. MTT solution (5 mg/ml) in PBS was
added to the cells and incubated for 2 h at 37°C. Dimethylsulfoxide
was added to the cells prior to incubation for 20 min at 37°C. Cell
proliferation was measured using an ELISA reader (Beckman Coulter,
Inc., Brea, CA, USA) at a wavelength of 540 nm. Lactate
dehydrogenase (LDH; 10 µl) was added to the cells using an LDH
cytotoxicity assay kit (C0016, Beyotime Institute of Biotechnology,
Haimen, China) according to the manufacturer's protocol.
Cytotoxicity was measured using the ELISA reader (Beckman Coulter,
Inc.) at a wavelength of 500 nm.
Cell apoptosis analysis
MG-63 cells were cultured in 6-well plates
(~2×106 cells/well) and treated with HCPT (0, 20, 40 and
80 nM) for 48 h. Cells were then suspended in 500 µl binding buffer
(BD Pharmingen; BD Biosciences, San Jose, CA, USA) following
centrifugation at 2,000 × g for 5 min at 4°C, and were then
supplemented with 5 µl Annexin V (BD Pharmingen; BD Biosciences)
and 5 µl propidium iodide (BD Pharmingen; BD Biosciences) for 15
min in the dark at room temperature. Cell apoptosis was analyzed
using a FC 500 MPL flow cytometer (Beckman Coulter, Inc.) and
analyzed using FlowJo software (version 7.6.1; FlowJo LLC, Ashland,
OR, USA).
Caspase-9 and caspase-3 activity
MG-63 cells were cultured in 96-well plates
(~1×104 cells/well) and treated with HCPT (0, 20, 40 and
80 nM) for 48 h at 37°C. Acetyl
(Ac)-Leu-Glu-His-Asp-p-nitroanilide (pNA) substrate for
caspase-9 and Ac-Asp-Glu-Val-Asp-pNA substrate for caspase-3 were
added to the cells and cultured for 2 h at 37°C (all were purchased
from Beyotime Institute of Biotechnology). Caspase-9 and caspase-3
activity was measured using an ELISA reader (Beckman Coulter, Inc.)
at a wavelength of 405 nm.
Western blot analysis
For the analysis of apoptosis, MG-63 cells were
cultured in 6-well plates (~2×106 cells/well) and
treated with HCPT (0, 20, 40 and 80 nM) for 48 h at 37°C. MG-63
cells were lysed in radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology) containing a phenylmethylsulfonyl
fluoride protease inhibitor mixture (PMSF, Beyotime Institute of
Biotechnology) at 4°C for 15 min. Protein concentration was
determined using the Bradford reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Total protein (50 µg) was resolved via 10%
SDS-PAGE, and subsequently transferred onto a polyvinylidene
fluoride membrane (Merck KGaA, Darmstadt, Germany). The membrane
was blocked with 5% non-fat milk in TBS containing Tween-20 (TBST)
for 1 h at 37°C and incubated with primary antibodies against the
following antigens: p53 (cat. no. sc-6243, 1:2,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), PARP-1 (cat. no. sc-7150,
1:2,000; Santa Cruz Biotechnology, Inc.), cytochrome c (cat.
no. sc-7159, 1:2,000; Santa Cruz Biotechnology, Inc.), Bcl-2
(sc-783, 1:2,000; Santa Cruz Biotechnology, Inc.) and β-actin (cat.
no. sc-7210, 1:2,000; Santa Cruz Biotechnology, Inc.) at 4°C
overnight. The secondary antibody was horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G (cat. no. 14708,
1:5,000, Cell Signaling Technology, Inc., Danvers, MA, USA) for 1 h
at 37°C subsequent to washing three times for 6 min each time using
TBST and detected using a BeyoECL Plus kit (Beyotime Institute of
Biotechnology) according to the manufacturer's protocol and
quantified using sodium Image Lab 3.0 (version 3; Bio-Rad
Laboratories, Inc.).
Statistical analysis
Results are presented as the mean ± standard
deviation. The statistical analysis was performed using the
software SPSS (version 16.0; SPSS, Inc., Chicago, IL, USA). One way
analysis of variance and Tukey's post hoc test was used to compare
two or more groups of data in order to determine statistical
significance. P<0.05 was considered to indicate a statistically
significant difference.
Results
Anticancer effects of HCPT suppress
cell viability of human osteosarcoma cells
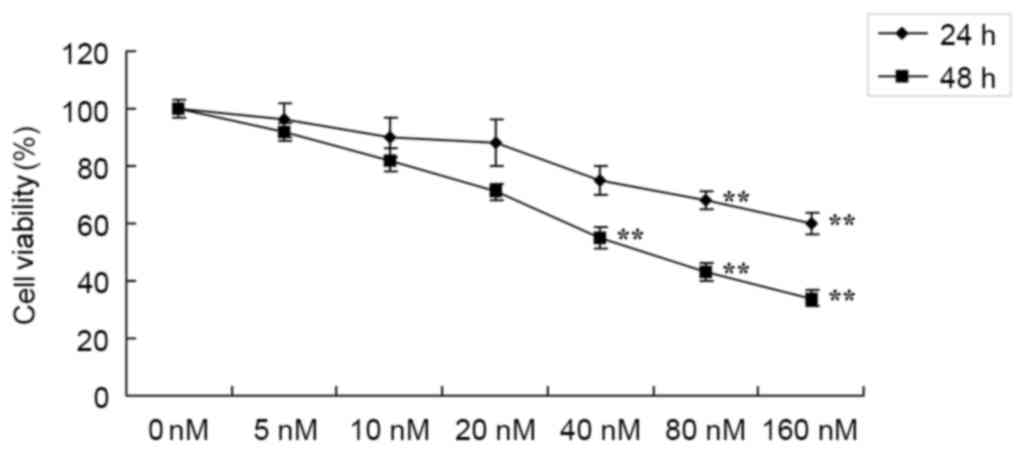
In order to investigate whether the potential
anticancer effects of HCPT suppress the cell growth of human
osteosarcoma cells, cell viability was detected using an MTT assay.
Treatment with 0–160 nM HCPT suppressed the cell viability of human
osteosarcoma MG-63 cells in a time- and dose-dependent manner
(Fig. 2). Treatment with HCPT at
40–160 nM concentrations for 48 h or 80–160 nM for 24 h effectively
suppressed the cell viability of human osteosarcoma MG-63 cells
compared with untreated cells (Fig.
2).
Anticancer effects of HCPT induce the
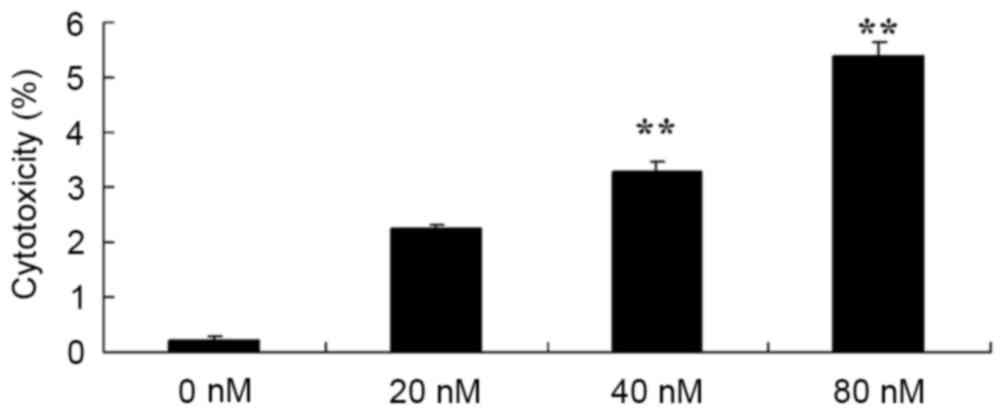
cytotoxicity of human osteosarcoma cells
In addition, the potential anticancer effects of
HCPT in terms of increasing the cytotoxicity of human osteosarcoma
cells were investigated using an LDH assay. As presented in
Fig. 3, treatment with HCPT at 40 and
80 nM concentrations for 48 h effectively induced the cytotoxicity
of human osteosarcoma MG-63 cells.
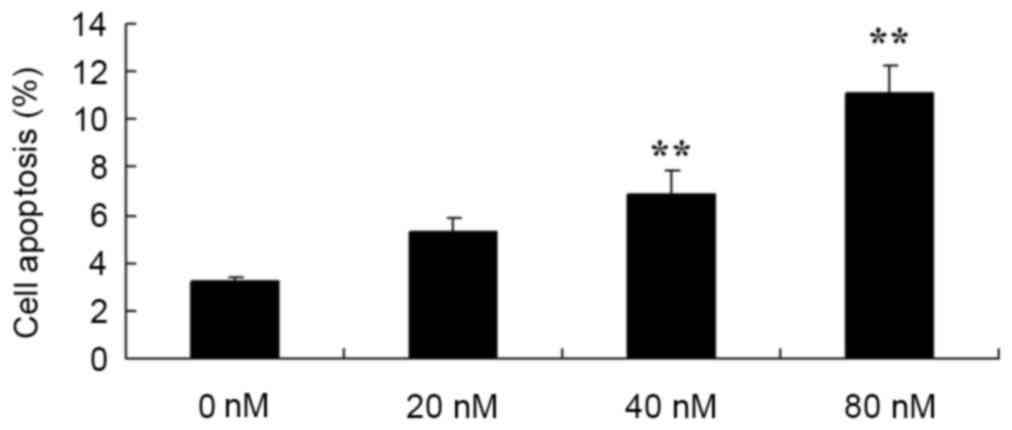
HCPT induces apoptosis in human
osteosarcoma cell
Subsequently, in order to determine the function of
the apoptosis rate in the anticancer effects of HCPT on human
osteosarcoma cells, flow cytometry was used to analyze the
apoptosis rate of human osteosarcoma MG-63 cells. Treatment with 40
and 80 nM HCPT was demonstrated to effectively induce the apoptosis
of human osteosarcoma cells in a dose dependent manner (Fig. 4).
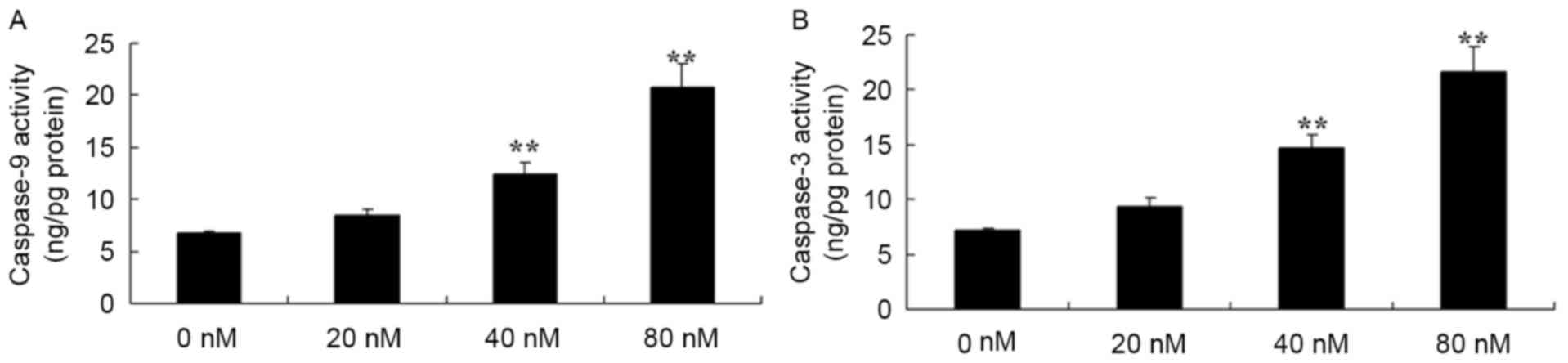
HCPT activates caspase-3 and caspase-9
in human osteosarcoma cells
HCPT activated caspase-3 and caspase-9 in human
osteosarcoma cells were investigated. It was revealed that there
were effective increases in caspase-3 and caspase-9 activity in the
human osteosarcoma MG-63 cells treated with 40 and 80 nM HCPT,
compared with 0 nM of HCPT (Fig.
5).
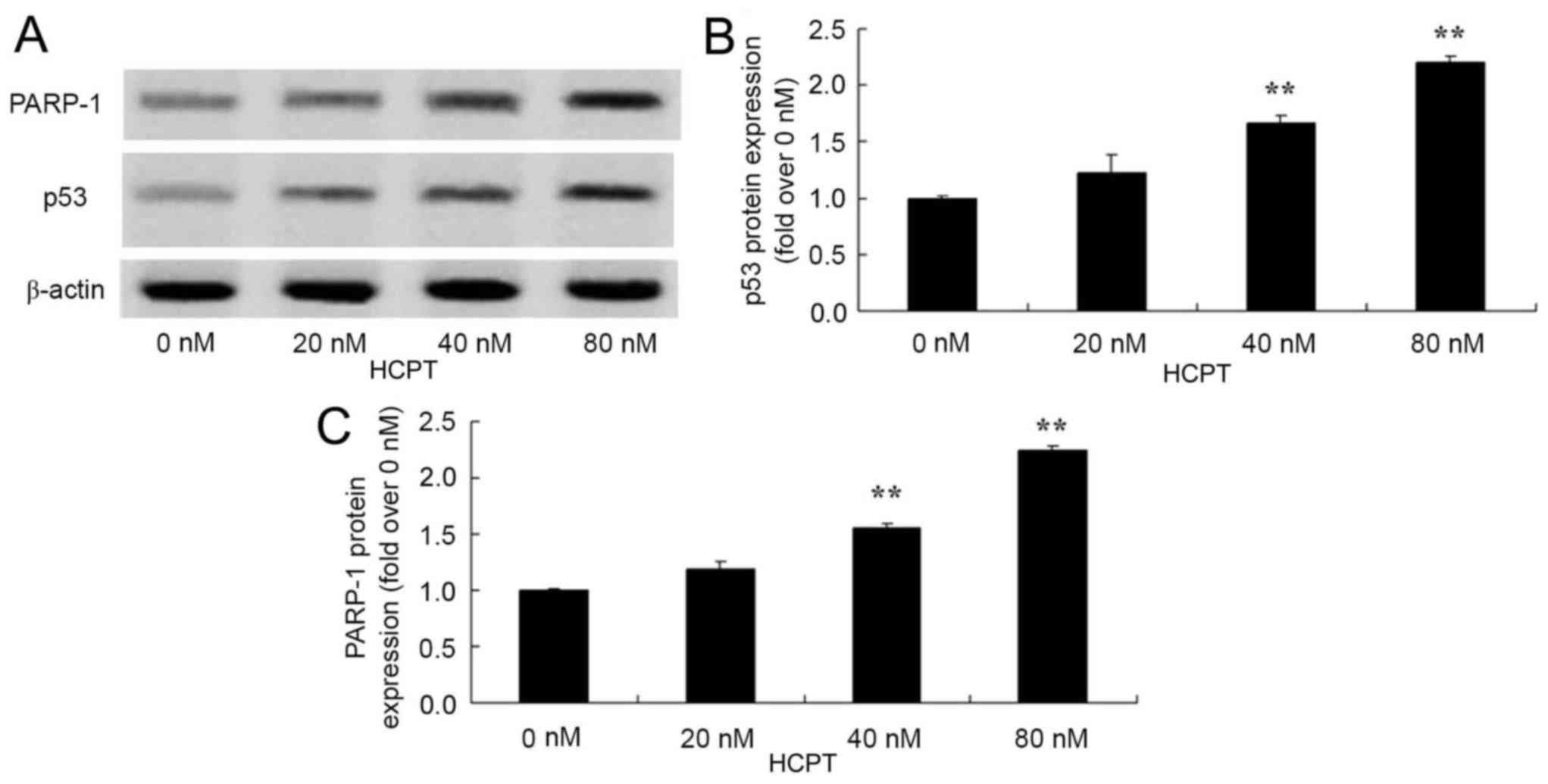
HCPT activates the p53 and PARP-1
signaling pathway of human osteosarcoma cells
In order to identify whether HCPT activates the p53
and PARP-1 signaling pathway of human osteosarcoma cells, p53 and
PARP-1 protein expression was detected using western blot analysis.
As presented in Fig. 6, HCPT (at 40
and 80 nM concentrations) effectively activated the p53 and PARP-1
protein expression in human osteosarcoma MG-63 cells.
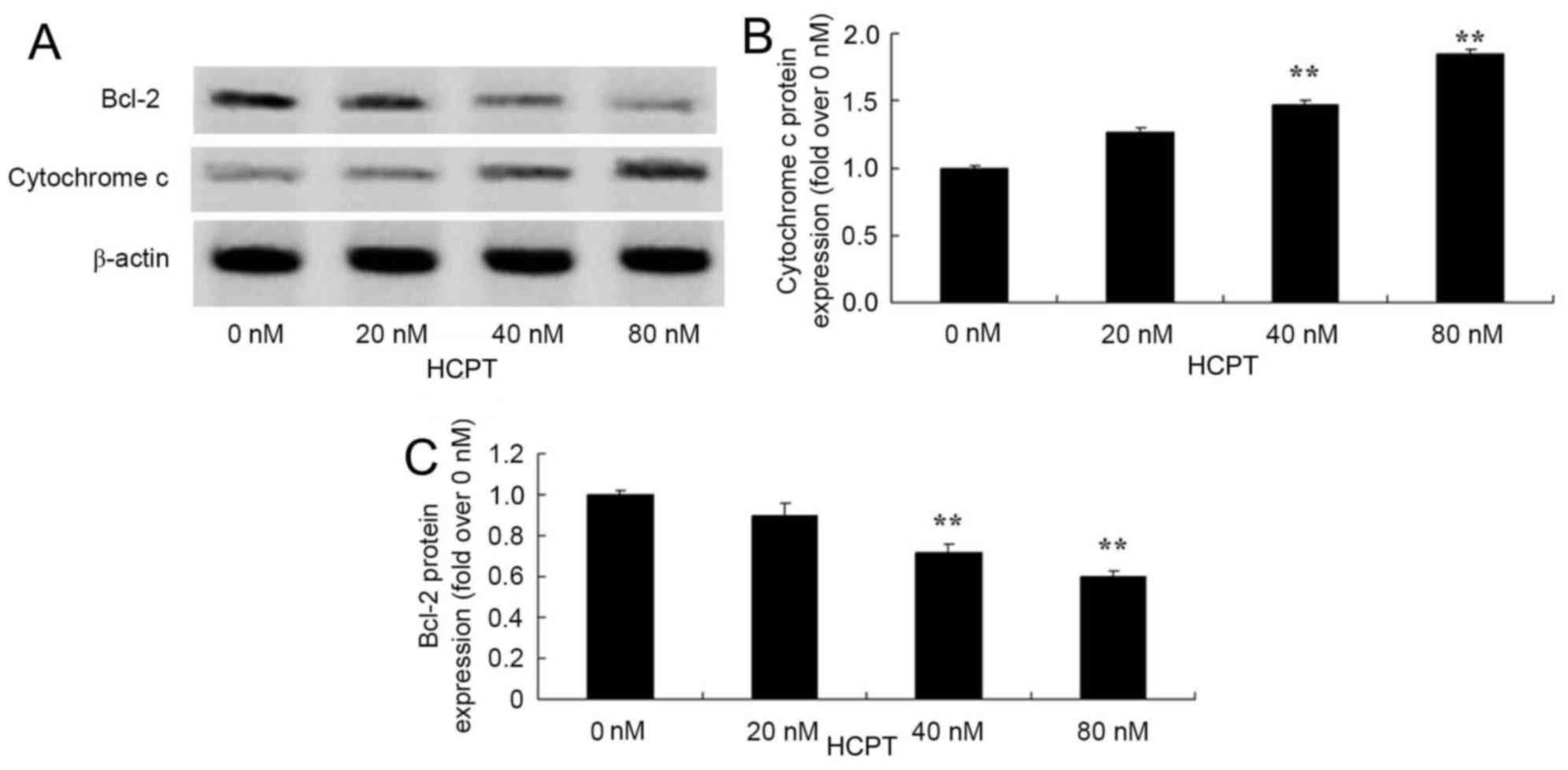
HCPT activates the cytochrome c and
Bcl-2 signaling pathway in human osteosarcoma cells
The anticancer effects of HCPT on cytochrome
c and Bcl-2 protein expression in human osteosarcoma cells
were observed. The group treated with 0 nM HCPT, 40 and 80 nM HCPT
suppressed Bcl-2 protein levels and increased cytochrome c protein
levels in human osteosarcoma MG-63 cells (Fig. 7).
Discussion
Osteosarcoma is a primary malignant tumor with a
higher risk of metastasis. Though it may occur at across all ages,
it predominantly occurs in adolescents. It is usually located in
long bones, including the distal femur and the proximal tibia
(13). Currently, therapy to treat
osteosarcoma mainly consists of neoadjuvant chemotherapy combined
with surgical resection. A chemotherapy regimen primarily consists
of Adriamycin, cis-platinum, ifosfamide and methotrexate
(14). The implementation of
neoadjuvant chemotherapy improves the prognosis of patients
significantly. For patients without metastasis, ~70% may attain
long-term survival (5). Despite this,
there are a high number of patients that experience recurrence. The
general recurrent tumor foci are long metastatic foci, but local
recurrence is not common. In the present study, HCPT was
demonstrated to effectively suppress cell viability, and to induce
cytotoxicity and the apoptosis of human osteosarcoma MG-63 cells.
Yang et al (15) suggested
that HCPT suppressed the cell growth of human breast cancers, and
further studies have demonstrated similar effects in murine
melanoma pulmonary cancer (10) and
lung cancer (16).
The p53 gene is a tumor suppressor gene and is
located in the short arm of the human no. 7 chromosome. This gene
codes for the p53 phosphoprotein. The normal function of the p53
phosphoprotein is the regulation of cell proliferation (17). In leukemia, osteosarcoma, lung cancers
and colon cancers, there may be mutations in and a deficiency of
the p53 protein. Previous research has demonstrated that the p53
protein is the most effective natural defense against tumors in the
human body (18,19). The present study provides the prospect
for a novel protein oncotherapy and protein interaction to identify
a novel and effective medical target, as p53 has previously been a
hotspot domain of tumor study (8). In
the present study, it was revealed that HCPT effectively activated
the p53 signaling pathway of human osteosarcoma MG-63 cells. Zhang
et al (9) reported that the
manner in which HCPT induced apoptosis in HepG2 cells was
associated with cell cycle arrest at the G2/M phase through p53
expression.
PARP-1 participates in DNA damage repair and
transcription regulation (20).
Furthermore, it is regarded as an important regulatory factor of
cell survival and cell death, and also participates in the
regulation of a number of transcription factors in tumorigenesis
and the inflammatory response (20).
Currently, increased expression of PARP-1 has been observed in
multiple malignant types of tumor, including human osteosarcoma
cells (20). As PARP-1 participates
in DNA damage repair, the single application of a PARP-1 inhibitor
treatment or a DNA damage drug combination may promote apoptosis
(21). A previous study has
demonstrated that drug inhibition or gene knockout PARP-1 may not
only cause the avoidance of tissue damage caused by oxidative
stress, but may also improve the prognosis of patients with cancer
(22). Additionally, in the present
study, HCPT effectively activated the PARP-1 protein expression of
human osteosarcoma MG-63 cells. Hu et al (23) observed that HCPT enhanced the
apoptosis induced by cancer therapeutic drugs through PARP-1 and
caspase-9/3 activation in androgen-independent prostate cancer
cells.
Apoptosis, also termed programmed cell death, serves
an important function in maintaining the normal physiological
equilibrium of the human body and the elimination of old cells
(24). Apoptosis is a process by
which a gene actively determines the automatic life termination of
a cell. Thus it is often termed programmed cell death (25). Apoptosis is a normal process during
the individual development of a multicellular organism. It may
maintain and resist the disturbance of various outside factors in a
stable manner and serves an important function in maturing
embryonic development, hematopoiesis and the immune system, in
maintaining normal tissues, the organic cell constant and growth
balance and also aging (26). In
previous years, the focus of studies on apoptosis had shifted from
changes in the nucleus to alterations in the mitochondrial
respiratory chain. Cytochrome c is a basic component in the
respiratory chain and serves an important function in redox and
energy metabolism. Furthermore, it has been revealed that
cytochrome c is a key substance for the mitochondrion to
initiate apoptosis (27).
Furthermore, the present study revealed that HCPT effectively
activated the cytochrome c signaling pathway of human
osteosarcoma MG-63 cells. Yuan et al (12) indicated that HCPT induces apoptosis
through the p53, cytochrome c and caspase-3 pathways of
human neuroblastoma SMS-KCNR cells.
Under normal circumstances, cytochrome c
exists in the cavity between the inner and outer mitochondrial
membranes (28). The signal
stimulation of apoptosis causes the release of cytochrome c
from the mitochondria to the cytoplasm. Once cytochrome c is
released, it may cause one of two consequences. One is that it may
combine with apoptotic peptidase activating factor-1. Under the
mediation of adenosine triphosphate/deoxyadenosine triphosphate,
the caspase-9 precursor is split into activated caspase-9 (28). The activated caspase-9 activates
caspase-3 and results in apoptosis (29). As cytochrome c is released into
the cytoplasm, intracellular cytochrome c is reduced or
absent, and therefore may result in the interruption of the
respiratory chain electron transfer chain and necrocytosis
(30). The release of cytochrome
c is the result of an increase in the permeability of the
mitochondrial outer membrane (31).
Bcl-2 proteins are primarily concentrated in the mitochondrial
outer membrane, and prevent it from releasing cytochrome c,
which inactivates the caspase in the cytoplasm, and causes
apoptosis to be blocked. Caspase-3 is one of the most important
executors of apoptosis (31).
Activated caspase-3 may degrade Bcl-2 protein and prevent it from
carrying out its anti-apoptotic effects. Once caspase-3 is
activated, the occurrence of apoptosis is irreversible (29). In the present study, it was revealed
that HCPT effectively promoted caspase-3/9 activities and inhibited
the protein expression of Bcl-2 in human osteosarcoma MG-63 cells.
Yuan et al (12) had
previously indicated that HCPT induces apoptosis through the p53,
cytochrome c and caspase-3 pathways of human neuroblastoma
SMS-KCNR cells.
In conclusion, the results of the present study
demonstrate that HCPT effectively suppresses cell viability,
induces cytotoxicity and the apoptosis of human osteosarcoma MG-63
cells, through the use of modulated caspase-3, p53 and cytochrome
c pathways. The data of the present study indicated that
HCPT may be a prognostic drug and a therapeutic target for human
osteosarcoma.
References
|
1
|
Kurzman ID, MacEwen EG, Rosenthal RC, Fox
LE, Keller ET, Helfand SC, Vail DM, Dubielzig RR, Madewell BR,
Rodriguez CO Jr, et al: Adjuvant therapy for osteosarcoma in dogs:
Results of randomized clinical trials using combined
liposome-encapsulated muramyl tripeptide and cisplatin. Clin Cancer
Res. 1:1595–1601. 1995.PubMed/NCBI
|
|
2
|
Meyers PA, Schwartz CL, Krailo M,
Kleinerman ES, Betcher D, Bernstein ML, Conrad E, Ferguson W,
Gebhardt M, Goorin AM, et al: Osteosarcoma: A randomized,
prospective trial of the addition of ifosfamide and/or muramyl
tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J
Clin Oncol. 23:2004–2011. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan TM, Charney SC, de Lorimier LP,
Garrett LD, Griffon DJ, Gordon-Evans WJ and Wypij JM: Double-blind
placebo-controlled trial of adjuvant pamidronate with palliative
radiotherapy and intravenous doxorubicin for canine appendicular
osteosarcoma bone pain. J Vet Intern Med. 23:152–160. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goldsby RE, Fan TM, Villaluna D, Wagner
LM, Isakoff MS, Meyer J, Randall RL, Lee S, Kim G, Bernstein M, et
al: Feasibility and dose discovery analysis of zoledronic acid with
concurrent chemotherapy in the treatment of newly diagnosed
metastatic osteosarcoma: A report from the Children's Oncology
Group. Eur J Cancer. 49:2384–2391. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao J, Xu H, He M, Wang Z and Wu Y: Rho
GTPase-activating protein 35 rs1052667 polymorphism and
osteosarcoma risk and prognosis. Biomed Res Int. 2014:3969472014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tóth C, Meinrath J, Herpel E, Derix J,
Fries J, Buettner R, Schirmacher P and Heikaus S: Expression of the
apoptosis repressor with caspase recruitment domain (ARC) in liver
metastasis of colorectal cancer and its correlation with DNA
mismatch repair proteins and p53. J Cancer Res Clin Oncol.
142:927–935. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deorukhkar A, Ahuja N, Mercado AL,
Diagaradjane P, Raju U, Patel N, Mohindra P, Diep N, Guha S and
Krishnan S: Zerumbone increases oxidative stress in a
thiol-dependent ROS-independent manner to increase DNA damage and
sensitize colorectal cancer cells to radiation. Cancer Med.
4:278–292. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Véquaud E, Desplanques G, Jézéquel P, Juin
P and Barillé-Nion S: Survivin contributes to DNA repair by
homologous recombination in breast cancer cells. Breast Cancer Res
Treat. 155:53–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang XW, Jiang JF and Xu B:
Differentiation-inducing action of 10-hydroxycamptothecin on human
hepatoma Hep G2 cells. Acta Pharmacol Sin. 21:364–368.
2000.PubMed/NCBI
|
|
10
|
Hu W, Zhang C, Fang Y and Lou C:
Anticancer properties of 10-hydroxycamptothecin in a murine
melanoma pulmonary metastasis model in vitro and in vivo. Toxicol
In Vitro. 25:513–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei W, Shi SJ, Liu J, Sun X, Ren K, Zhao
D, Zhang XN, Zhang ZR and Gong T: Lipid nanoparticles loaded with
10-hydroxycamptothecin-phospholipid complex developed for the
treatment of hepatoma in clinical application. J Drug Target.
18:557–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan ZF, Tang YM, Xu XJ, Li SS and Zhang
JY: 10-Hydroxycamptothecin induces apoptosis in human neuroblastoma
SMS-KCNR cells through p53, cytochrome c and caspase 3 pathways.
Neoplasma. 63:72–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gallegos-Castorena S, Martínez-Avalos A,
Mohar-Betancourt A, Guerrero-Avendaño G, Zapata-Tarrés M and
Medina-Sansón A: Toxicity prevention with amifostine in pediatric
osteosarcoma patients treated with cisplatin and doxorubicin.
Pediatr Hematol Oncol. 24:403–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Navid F, Santana VM, Neel M, McCarville
MB, Shulkin BL, Wu J, Billups CA, Mao S, Daryani VM, Stewart CF, et
al: A phase II trial evaluating the feasibility of adding
bevacizumab to standard osteosarcoma therapy. Int J Cancer.
141:1469–1477. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang Z, Luo X, Zhang X, Liu J and Jiang Q:
Targeted delivery of 10-hydroxycamptothecin to human breast cancers
by cyclic RGD-modified lipid-polymer hybrid nanoparticles. Biomed
Mater. 8:0250122013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Gan Y, Gan L, Nie S and Pan W:
PEGylated nanostructured lipid carriers loaded with
10-hydroxycamptothecin: An efficient carrier with enhanced
anti-tumour effects against lung cancer. J Pharm Pharmacol.
60:1077–1087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carvalho S, Vitor AC, Sridhara SC, Martins
FB, Raposo AC, Desterro JM, Ferreira J and de Almeida SF: SETD2 is
required for DNA double-strand break repair and activation of the
p53-mediated checkpoint. Elife. 3:e024822014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dutta S, Warshall C, Bandyopadhyay C,
Dutta D and Chandran B: Interactions between exosomes from breast
cancer cells and primary mammary epithelial cells leads to
generation of reactive oxygen species which induce DNA damage
response, stabilization of p53 and autophagy in epithelial cells.
PLoS One. 9:e975802014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Simone CB II, John-Aryankalayil M,
Palayoor ST, Makinde AY, Cerna D, Falduto MT, Magnuson SR and
Coleman CN: mRNA expression profiles for prostate cancer following
fractionated irradiation are influenced by p53 status. Transl
Oncol. 6:573–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uchiumi F, Watanabe T, Ohta R, Abe H and
Tanuma S: PARP1 gene expression is downregulated by knockdown of
PARG gene. Oncol Rep. 29:1683–1688. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Halder AK, Saha A, Saha KD and Jha T:
Stepwise development of structure-activity relationship of diverse
PARP-1 inhibitors through comparative and validated in silico
modeling techniques and molecular dynamics simulation. J Biomol
Struct Dyn. 33:1756–1779. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dawicki-McKenna JM, Langelier MF, DeNizio
JE, Riccio AA, Cao CD, Karch KR, McCauley M, Steffen JD, Black BE
and Pascal JM: PARP-1 activation requires local unfolding of an
autoinhibitory domain. Mol Cell. 60:755–768. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu H, Jiang C, Ip C, Rustum YM and Lü J:
Methylseleninic acid potentiates apoptosis induced by
chemotherapeutic drugs in androgen-independent prostate cancer
cells. Clin Cancer Res. 11:2379–2388. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Wu S, Chen Y, Zhao J, Zhang K,
Wang J and Chen S: microRNA-143 is associated with the survival of
ALDH1+CD133+ osteosarcoma cells and the chemoresistance of
osteosarcoma. Exp Biol Med (Maywood). 240:867–875. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ye Z, Jingzhong L, Yangbo L, Lei C and
Jiandong Y: Propofol inhibits proliferation and invasion of
osteosarcoma cells by regulation of microRNA-143 expression. Oncol
Res. 21:201–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon HY, Kim KS, An HK, Moon HI, Kim HJ
and Lee YC: Triptolide induces apoptosis through extrinsic and
intrinsic pathways in human osteosarcoma U2OS cells. Indian J
Biochem Biophys. 50:485–491. 2013.PubMed/NCBI
|
|
27
|
Li J, Zhang F and Wang S: A polysaccharide
from pomegranate peels induces the apoptosis of human osteosarcoma
cells via the mitochondrial apoptotic pathway. Tumour Biol.
35:7475–7482. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Z, Huang K, Fu X, Zhou Z, Cui Y and Li
H: A chemically sulfated polysaccharide derived from Ganoderma
lucidum induces mitochondrial-mediated apoptosis in human
osteosarcoma MG63 cells. Tumour Biol. 35:9919–9926. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chipoy C, Brounais B, Trichet V, Battaglia
S, Berreur M, Oliver L, Juin P, Rédini F, Heymann D and Blanchard
F: Sensitization of osteosarcoma cells to apoptosis by oncostatin M
depends on STAT5 and p53. Oncogene. 26:6653–6664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roepke M, Diestel A, Bajbouj K,
Walluscheck D, Schonfeld P, Roessner A, Schneider-Stock R and
Gali-Muhtasib H: Lack of p53 augments thymoquinone-induced
apoptosis and caspase activation in human osteosarcoma cells.
Cancer Biol Ther. 6:160–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao X, Bennett RL and May WS: c-Myc and
caspase-2 are involved in activating Bax during cytotoxic
drug-induced apoptosis. J Biol Chem. 283:14490–14496. 2008.
View Article : Google Scholar : PubMed/NCBI
|