Introduction
Non-small cell lung cancer (NSCLC) remains the
leading cause of cancer-associated mortality globally (1–3). Despite a
number of diagnostic and therapeutic advancements having been
achieved in the last thirty years the 5-year overall survival (OS)
rate remains unsatisfactory at ~16% (4,5). About one
third of patients with NSCLC present with a locally advanced
disease (at stages IIIA and B) (6).
Particularly of note, completely resected NSCLC with pathologically
confirmed N2 (pN2) stage NSCLC is a heterogeneous subgroup for
different primary tumor status, clinical nodal stage and the extent
of mediastinal lymph node (LN) involvement, with 5-year OS rates in
the range of 5 to 57% according to various prognostic factors
(7–10). Postoperative chemotherapy (POCT) has
been demonstrated by a number of studies to improve the OS rate of
patients with pN2 NSCLC and has been regarded as the gold standard
of treatment (11,12). However, the risk of locoregional
recurrence (LRR) remains as high as 20–40%, which associates
independently with worse OS (13).
Postoperative radiotherapy (PORT) holds great appeal as a means by
which to reduce LRR and improve OS. Up to now, the role of PORT
remains controversial due to the lack of definitive evidence
demonstrating a survival benefit (14–17). A
PORT meta-analysis trialists group performed a meta-analysis in the
1990s, which indicated that PORT was not associated with any
survival benefit in patients with resected pN2 NSCLC; the result
may be as a result of lagging radiation techniques and high
morbidity (18). Since the turn of
the 21st century, with improvements to modern radiation techniques,
three-dimensional conformal radiotherapy (3D-CRT) and
intensity-modulated radiation therapy (IMRT) have been widely
applied (19). Under these new
conditions, the role of PORT in patients with resected pN2 NSCLC
should be re-evaluated. A subset analysis of the Adjuvant Navelbine
International Trialist Association trial suggested a benefit in the
OS of patients with pN2 treated with PORT, regardless of the use of
POCT (20). In addition, analysis
using the Surveillance, Epidemiology, and End Results database
similarly indicated that PORT was associated with improved survival
for patients with N2 stage disease (21). However, no definitive conclusion of
the effectiveness of PORT in pN2 NSCLC may be drawn as no
prospective randomized study using modern radiation techniques in
the setting of adjuvant chemotherapy has been published thus
far.
In the present study, the role of PORT in pN2 NSCLC
and the association between clinicopathological factors and PORT
were analyzed in patients with completely resected pN2 NSCLC.
Patients and methods
Patient selection
A total of 269 consecutive patients with pN2 NSCLC
who underwent surgery at the Department of Thoracic Surgery at
Zhejiang Cancer Hospital (Hangzhou, China) between January 2009 and
December 2012 were included in the present retrospective study. The
eligibility criteria of the present study included the following:
i) Pathologically confirmed T1-3N2M0 stage IIIA according to the
American Joint Committee on Cancer (AJCC) 7th lung cancer TNM
classification (22); ii) radical
resection was performed, namely, all patients underwent either
sleeve resection, lobectomy or pneumonectomy; iii) the surgical
margin was negative; iv) all patients received mediastinal
lymphadenectomy or systematic mediastinal LN sampling; v) the
patients demonstrated an Eastern Cooperative Oncology Group (ECOG)
performance status (PS) of 0 or 1 (23); vi) patients underwent no neoadjuvant
chemotherapy or chemoradiotherapy; and vii) complete information on
tumor characteristics, pathological studies and follow-up data were
available for all patients. In addition, patients who received
sublobar resection or succumbed to postoperative complication
within 3 months were excluded. As a result of the aforementioned
selection criteria, the present study finally enrolled a total of
246 patients (175 male and 71 female; median age, 59 years, range,
38–71 years), including 213 who underwent lobectomy, 17 who
underwent pneumonectomy and 16 who underwent sleeve resection.
Among the 246 patients, 88 patients received POCT followed by PORT,
90 received adjuvant chemotherapy, 1 patient received adjuvant
radiotherapy and the remaining 67 patients did not receive any
adjuvant therapy. The Zhejiang Cancer Hospital Institutional Review
Board approved the protocols for data collection and analysis in
the present study. Clinical and pathological data was gathered
primarily on the following patient characteristics: Sex, age,
smoking history, ECOG PS, primary tumor location, extent of
surgery, histology, pT stage, number of positive N2 nodes, number
of N2 nodal stations involved, status of hilar LN, bronchial
invasion, pulmonary vascular wall invasion, visceral pleura
invasion, lymphovascular invasion and perineural invasion. Detailed
patient characteristics are presented in Table I.
 | Table I.Patient clinical characteristics. |
Table I.
Patient clinical characteristics.
| Variable | Total, n (%) | PORT, n (%) | Non-PORT, n (%) | P-value |
|---|
| Sex |
|
|
| 0.498 |
| Male | 175 (71.1) | 61 (68.5) | 114 (72.6) |
|
|
Female | 71 (28.9) | 28 (31.5) | 43 (27.4) |
|
| Age, years |
|
|
| 0.376 |
|
≤60 | 129 (52.4) | 50 (56.2) | 79 (50.3) |
|
|
>60 | 117 (47.6) | 39 (43.8) | 78 (49.7) |
|
| Smoking |
|
|
| 0.289 |
|
Yes | 149 (60.6) | 50 (56.2) | 99 (63.1) |
|
| No | 97 (39.4) | 39 (43.8) | 58 (36.9) |
|
| ECOG PS |
|
|
| 0.294 |
| 0 | 184 (74.8) | 70 (78.7) | 114 (72.6) |
|
| 1 | 62 (25.2) | 19 (21.3) | 43 (27.4) |
|
| Tumor location |
|
|
| 0.692 |
|
LUL | 49 (19.9) | 22 (24.7) | 27 (17.2) |
|
|
LLL | 42 (17.1) | 15 (16.9) | 27 (17.2) |
|
|
RUL | 66 (26.8) | 23 (25.8) | 43 (27.4) |
|
|
RML | 14 (5.7) | 5 (5.6) | 9 (5.7) |
|
|
RLL | 75 (30.5) | 24 (27.0) | 51 (32.5) |
|
| Tumor type |
|
|
| 0.266 |
|
Central | 97 (39.4) | 31 (34.8) | 66 (42.0) |
|
|
Peripheral | 149 (60.6) | 58 (65.2) | 91 (58.0) |
|
| Surgery |
|
|
| 0.232 |
|
VATS | 38 (15.4) | 17 (19.1) | 21 (13.4) |
|
|
Thoracotomy | 208 (84.6) | 72 (80.9) | 136 (86.6) |
|
| Extent of
resection |
|
|
| 0.007a |
|
Lobectomy | 229 (93.1) | 88 (98.9) | 141 (89.8) |
|
|
Pneumonectomy | 17 (6.9) | 1 (1.1) | 16 (10.2) |
|
| POCT |
|
|
|
<0.001a |
|
Yes | 178 (72.4) | 88 (98.9) | 90 (57.3) |
|
| No | 68 (27.6) | 1 (1.1) | 67 (42.7) |
|
| POCT cycles |
|
|
| 0.082 |
|
<3 | 17 (9.6) | 5 (5.7) | 12 (13.3) |
|
| ≥3 | 161 (90.4) | 83 (94.3) | 78 (86.7) |
|
POCT
Of the 246 enrolled patients, 178 (72.4%) were
administered platinum-based adjuvant chemotherapy with a median of
4 cycles (range, 2–6): 63 patients received gemcitabine (1,000
mg/m2 intravenously, on days 1 and 8) and cisplatin (25
mg/m2 intravenously, on days 1–3); 52 patients received
vinorelbine (25 mg/m2 intravenously, on days 1 and 8)
and cisplatin (25 mg/m2 intravenously, on days 1–3); 33
patients received taxane-based (135 mg/m2 intravenously,
on day 1) chemotherapy combined with cisplatin (25 mg/m2
intravenously, on days 1–3); 16 patients received pemetrexed (500
mg/m2 intravenously, on day 1) and cisplatin (25
mg/m2 intravenously, on days 1–3) and the remaining 14
patients received carboplatin-based (area under the curve = 5
intravenously, on day 1) doublet chemotherapy. The reasons for
patients not receiving adjuvant chemotherapy were mainly due to
weakness, patient refusal or physician decision.
PORT
The administration of PORT was mainly based on the
decision of the thoracic radiation oncologists. The clinical target
volume (CTV) for left-sided lung cancer includes the bronchial
stump (BS) and LN stations 2R, 2L, 4R, 4L, 5, 6, 7 and 10L to 11L,
while for right-sided lung cancer, the CTV includes the BS and LN
stations 2R, 4R, 7 and 10R to 11R, according to the 7th edition of
International Association for the Study of Lung Cancer LN map
(22).
The planning target volume (PTV) was defined as
expanding the CTV by 0.6–0.8 cm. The prescription dose was defined
as 95% of the receiving dose of PTV, with the difference in
internal target dose uniformity of <5%, and internal target
maximum dose point ≤110%. The percentage of the total normal lung
volume receiving ≤20 Gy (V20) was <25%, the mean lung dose was
<13 Gy, the spinal cord maximum dose was <45 Gy, the heart
V40 was <50% and the mean heart dose was ≤30 Gy.
Follow-up
All patients underwent regular follow-ups in the
Outpatient Department every 3 months over the first 2 years and
every 6 months after that. Each visit included a medical history,
physical examination, complete blood count, chest and upper
abdominal computed tomography (CT), brain magnetic resonance
imaging/CT and a bone scan (if deemed to be necessary due to
complaint of pain). Local recurrence was defined as disease relapse
at the BS, ipsilateral hilum and mediastinum; all other sites of
failure, including the supraclavicular fossa and contralateral
hilum, were considered to be distant metastases (24,25).
Disease progression was diagnosed with confirmed biopsy or positive
imaging findings. If disease progression was suspected, positron
emission tomography-CT was required.
Statistical analysis
A χ2 test was used to determine the
distribution of patient characteristics within the PORT group and
the non-PORT group. OS time was calculated from the first day of
treatment to mortality from any cause or last follow-up and
disease-free survival (DFS) time was calculated from the first day
of treatment to disease progression, mortality or last follow-up.
Local recurrence-free survival (LRFS) time was calculated from the
first day of treatment to local recurrence, mortality or last
follow-up. OS, DFS and LRFS rates were calculated using the
Kaplan-Meier method. To determine prognostic value, study variables
were compared with the survival measures using log-rank tests. The
prognostic factors were determined using Cox's regression model.
P<0.05 was considered to indicate a statistically significant
difference. All the analyses were performed using SPSS 21.0 (IBM
Corp., Armonk, NY, USA).
Results
Patient and tumor characteristics
The detailed patient clinical and pathological
characteristics are presented in Tables
I and II, respectively. Median
age was 59 years and the majority of patients were male (175
patients, 71.1%). The factors were comparable between the PORT
group and non-PORT group, with the exception that there were more
patients treated with lobectomy and POCT in the PORT group. Of the
246 patients, 89 (36.2%) received adjuvant PORT. Radiation was
delivered with 6 MV X-rays at 1.8–2 Gy/fraction once daily, 5
days/week, with a total dose ranging between 48.0 and 60.0 Gy, and
a median dose of 50.4 Gy. All patients who underwent PORT received
3D-CRT (40 patients) or IMRT (49 patients). The median time
interval between surgery and the start of radiotherapy for all
patients was 15.2 weeks (range, 3.4–24.8 weeks).
 | Table II.Patient pathological
characteristics. |
Table II.
Patient pathological
characteristics.
| Variable | Total, n, (%) | PORT, n (%) | Non-PORT, n
(%) | P-value |
|---|
| Histology |
|
|
|
|
| AC | 136 (55.3) | 56 (62.9) | 80 (51.0) |
|
|
Non-AC | 110 (44.7) | 33 (37.1) | 77 (49.0) | 0.070 |
| pT stage |
|
|
|
|
|
T1-2 | 210 (85.4) | 78 (87.6) | 132 (84.1) |
|
| T3 | 36 (14.6) | 11 (12.4) | 25 (15.9) | 0.447 |
| Number of N2
metastasis |
|
|
|
|
| 1 | 102 (41.5) | 37 (41.6) | 65 (41.4) |
|
| ≥2 | 144 (58.5) | 52 (58.4) | 92 (58.6) | 0.979 |
| N2 station
involved |
|
|
|
|
|
Single | 160 (65.0) | 56 (62.9) | 104 (66.2) |
|
|
Multiple | 86 (35.0) | 33 (37.1) | 53 (33.8) | 0.600 |
| Hilar LN
metastasis |
|
|
|
|
|
Yes | 112 (45.5) | 37 (41.6) | 75 (47.8) |
|
| No | 134 (54.5) | 52 (58.4) | 82 (52.2) | 0.348 |
| Bronchial
involvement |
|
|
|
|
|
Yes | 134 (54.5) | 43 (48.3) | 91 (58.0) |
|
| No | 112 (45.5) | 46 (51.7) | 66 (42.0) | 0.144 |
| Pulmonary vascular
wall invasion |
|
|
|
|
|
Yes | 55 (22.4) | 17 (19.1) | 38 (24.2) |
|
| No | 191 (77.6) | 72 (80.9) | 119 (75.8) | 0.356 |
| Visceral pleura
invasion |
|
|
|
|
|
Yes | 157 (63.8) | 55 (61.8) | 102 (65.0) |
|
| No | 89 (36.2) | 34 (38.2) | 55 (35.0) | 0.619 |
| Lymphovascular
invasion |
|
|
|
|
|
Yes | 100 (40.7) | 35 (39.3) | 65 (41.4) |
|
| No | 146 (59.3) | 54 (60.7) | 92 (58.6) | 0.750 |
| Perineural
invasion |
|
|
|
|
|
Yes | 51 (20.7) | 19 (21.3) | 32 (20.4) |
|
| No | 195 (79.3) | 70 (78.7) | 125 (79.6) | 0.857 |
Survival analysis
The median follow-up time from the end of treatment
was 38.3 months (range, 3.8–83.1 months). A total of 160 patients
(65.0%) experienced disease progression, of which 133 patients
succumbed, during follow-up. The 1-, 3- and 5-year OS rates in the
PORT group were 98.9, 71.3 and 54.9%, respectively, whereas the
non-PORT group exhibited 1-, 3- and 5-year OS rates of 93.0, 58.4
and 36.7%, respectively. A statistically significantly difference
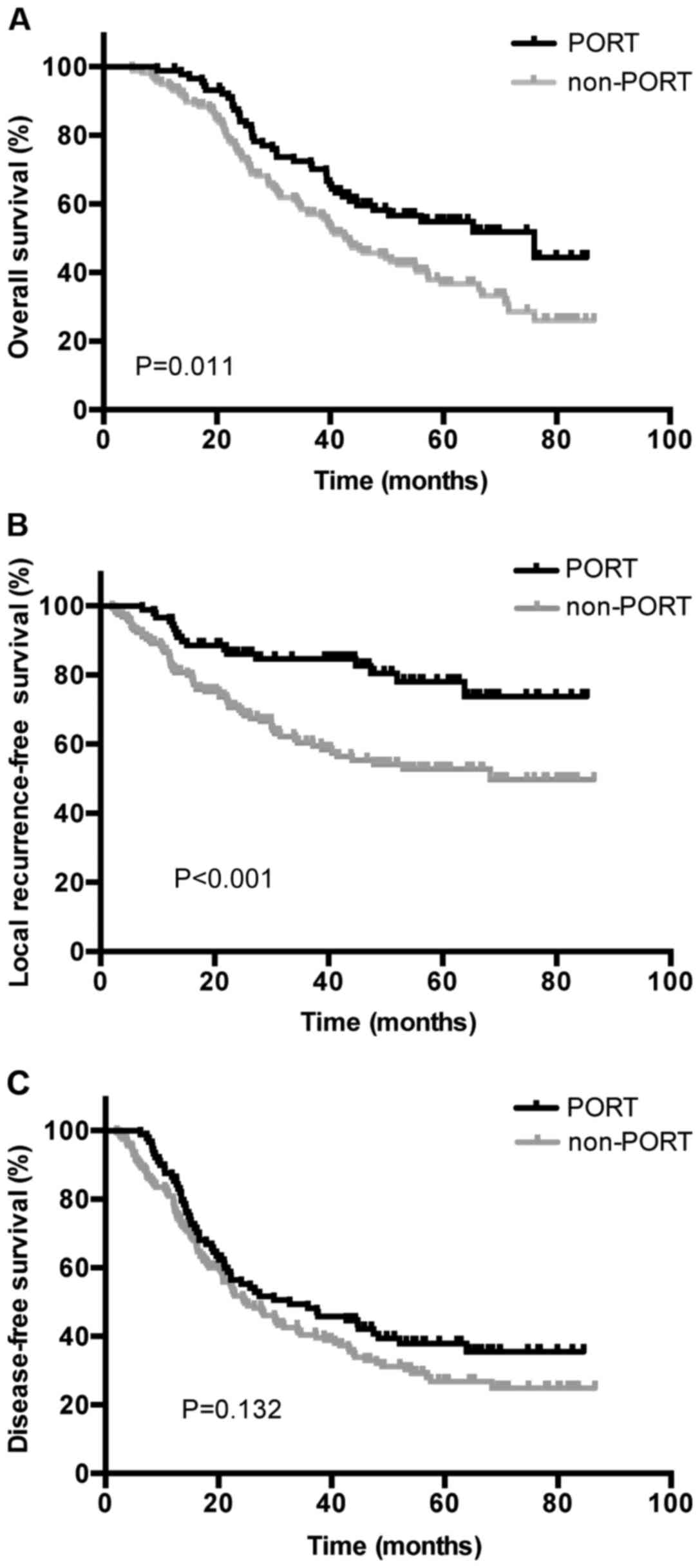
was indicated between the two groups (P=0.011; Fig. 1A). A total of 65 (26.4%) patients were
diagnosed with local recurrence, and 16 with simultaneous local and
distant progression during follow-up, with 1-, 3- and 5-year LRFS
rates of 95.5, 84.6 and 78.0%, respectively, in the PORT group, and
86.6, 70.6 and 52.8%, respectively, in the non-PORT group
(P<0.001; Fig. 1B). Additionally,
79 (32.1%) patients were diagnosed with distant metastasis during
follow-up, combined with 16 patients demonstrating simultaneous
local and distant progression. The 1-, 3- and 5-year DFS rates were
86.5, 55.2 37.9%, respectively, in the PORT group, and 80.9, 40.3
and 26.8%, respectively, in the non-PORT group (P=0.132; Fig. 1C). Distant metastasis occurred in the
lungs (n=36), supraclavicular fossa or contralateral hilum (n=22),
bone (n=13), brain (n=17), adrenal glands (n=8), liver (n=6) and
other locations (n=3).
Distinct treatment strategies were also
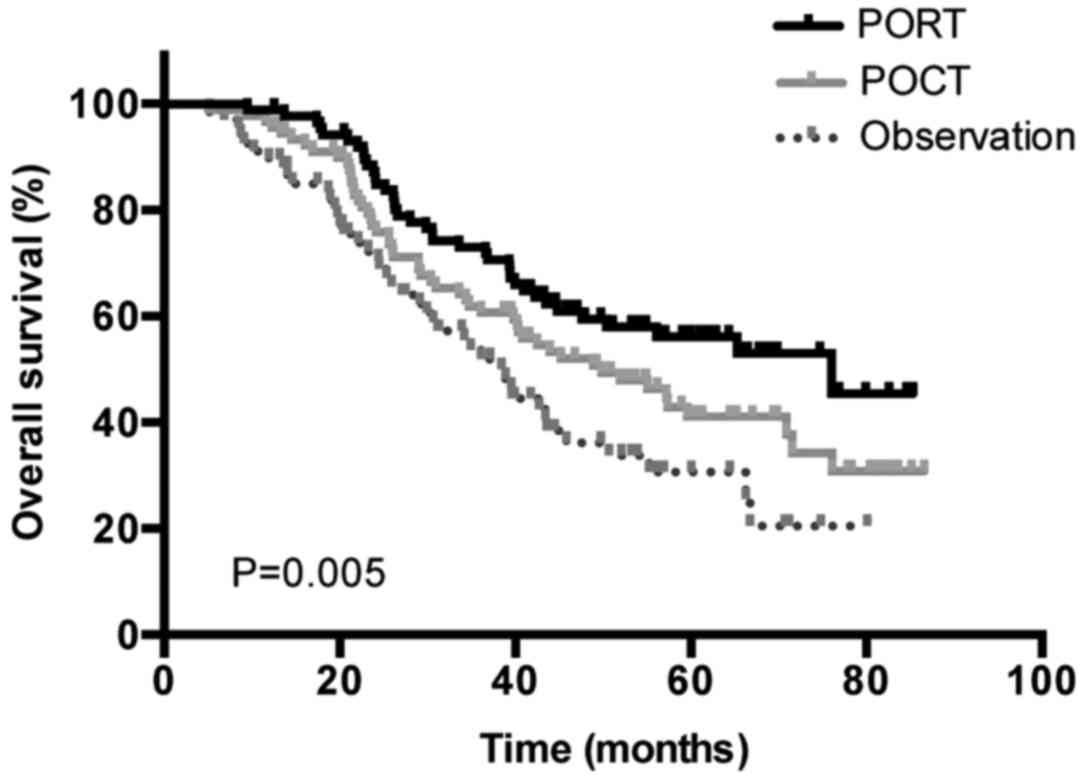
investigated. The median OS times were as follows: For patients who
underwent surgery followed by POCT and PORT, 76.03 months [95%
confidence interval (CI), 43.99–108.74]; for patients who underwent
surgery followed by POCT, 49.83 months (95% CI, 34.20–65.47); and
finally for patients who underwent surgery alone, 38.87 months (95%
CI, 32.65–45.09) (P=0.005; Fig.
2).
Univariate analysis
Univariate analysis was performed to determine the
association between clinical and pathological factors, PORT and
POCT treatments, and 5-year OS, DFS and LRFS rates. Results are
presented in Table III. OS rates
were identified to be significantly increased in patients with
peripheral tumor (P=0.029), pT1-2 (P=0.015), 1 N2 LN metastasis
(P=0.001), single N2 station metastasis (P=0.030), no bronchial
involvement (P=0.025), and use of PORT (P=0.011) and POCT
(P=0.003). Furthermore, pT1-2 (P=0.007), 1 N2 LN metastasis
(P<0.001), single N2 station metastasis (P<0.001), negative
hilar LN metastasis (P=0.007) and no bronchial involvement
(P=0.044) were associated with improved DFS rates. In addition,
LRFS rates were significantly increased in females (P=0.036), ECOG
PS=0 (P=0.024), peripheral tumor (P=0.015), lobectomy (P=0.005), 1
N2 LN metastasis (P=0.045), single N2 station metastasis (P=0.035),
no bronchial involvement (P=0.029), and use of PORT (P<0.001)
and POCT (P=0.002).
 | Table III.Univariate analysis of prognostic
factors for OS, DFS and LRFS. |
Table III.
Univariate analysis of prognostic
factors for OS, DFS and LRFS.
| Variables | 5-year OS, % | P-value | 5-year DFS, % | P-value | 5-year LRFS, % | P-value |
|---|
| Sex |
| 0.234 |
| 0.337 |
| 0.036a |
|
Male | 42.9 |
| 30.1 |
| 57.4 |
|
|
Female | 44.5 |
| 33.9 |
| 74.8 |
|
| Age, years |
| 0.917 |
| 0.641 |
| 0.078 |
|
≤60 | 43.0 |
| 31.7 |
| 71.1 |
|
|
>60 | 43.4 |
| 30.3 |
| 51.8 |
|
| Smoking
history |
| 0.474 |
| 0.534 |
| 0.353 |
|
Yes | 43.2 |
| 33.8 |
| 59.8 |
|
| No | 43.6 |
| 28.6 |
| 68.7 |
|
| ECOG PS |
| 0.290 |
| 0.667 |
| 0.024a |
| 0 | 44.8 |
| 32.6 |
| 67.8 |
|
| 1 | 38.9 |
| 28.4 |
| 50.3 |
|
| Tumor location |
| 0.461 |
| 0.753 |
| 0.543 |
|
LUL | 44.4 |
| 33.7 |
| 58.3 |
|
|
LLL | 28.6 |
| 31.9 |
| 59.0 |
|
|
RUL | 45.0 |
| 22.3 |
| 58.2 |
|
|
RML | 32.3 |
| 23.4 |
| 72.9 |
|
|
RLL | 49.6 |
| 38.7 |
| 67.0 |
|
| Tumor type |
| 0.029a |
| 0.542 |
| 0.015a |
|
Central | 38.6 |
| 32.7 |
| 53.0 |
|
|
Peripheral | 46.3 |
| 30.0 |
| 68.4 |
|
| Surgery method |
| 0.357 |
| 0.630 |
| 0.559 |
|
VATS | 49.3 |
| 24.9 |
| 68.0 |
|
|
Thoracotomy | 42.1 |
| 32.2 |
| 61.2 |
|
| Extent of
resection |
| 0.103 |
| 0.135 |
| 0.005a |
|
Lobectomyb | 44.6 |
| 32.1 |
| 64.3 |
|
|
Pneumonectomy | 18.4 |
| 17.2 |
| 37.1 |
|
| POCT |
| 0.003a |
| 0.387 |
| 0.002a |
|
Yes | 47.9 |
| 32.8 |
| 68.6 |
|
| No | 30.0 |
| 25.6 |
| 44.0 |
|
| POCT cycles |
| 0.280 |
| 0.389 |
| 0.551 |
|
1–2 | 47.1 |
| 30.0 |
| 64.7 |
|
|
3–4 | 48.2 |
| 34.1 |
| 69.1 |
|
| Histology |
| 0.354 |
| 0.921 |
| 0.105 |
| AC | 43.5 |
| 28.5 |
| 67.1 |
|
|
Non-AC | 42.8 |
| 34.5 |
| 56.9 |
|
| pT stage |
| 0.015a |
| 0.007a |
| 0.161 |
|
T1-2 | 46.4 |
| 34.2 |
| 63.5 |
|
| T3 | 25.1 |
| 11.8 |
| 54.5 |
|
| Number of N2
metastasis |
| 0.001a |
|
<0.001a |
| 0.045a |
| 1 | 53.9 |
| 45.1 |
| 68.7 |
|
| ≥2 | 35.7 |
| 21.1 |
| 57.5 |
|
| N2 station
involved |
| 0.030a |
|
<0.001a |
| 0.035a |
|
Single | 49.4 |
| 38.9 |
| 66.6 |
|
|
Multiple | 31.1 |
| 16.5 |
| 53.9 |
|
| Hilar LN
metastasis |
| 0.055 |
| 0.007a |
| 0.251 |
|
Yes | 36.7 |
| 23.3 |
| 59.8 |
|
| No | 49.0 |
| 37.9 |
| 64.4 |
|
| Bronchial
involvement |
| 0.025a |
| 0.044a |
| 0.029a |
|
Yes | 37.9 |
| 27.4 |
| 58.2 |
|
| No | 50.0 |
| 35.3 |
| 67.4 |
|
| Pulmonary vascular
wall invasion |
| 0.380 |
| 0.314 |
| 0.268 |
|
Yes | 31.9 |
| 26.0 |
| 58.6 |
|
| No | 46.6 |
| 32.5 |
| 63.8 |
|
| Visceral pleural
invasion |
| 0.213 |
| 0.836 |
| 0.195 |
|
Yes | 43.3 |
| 29.9 |
| 65.9 |
|
| No | 44.3 |
| 33.1 |
| 54.8 |
|
| Lymphovascular
invasion |
| 0.154 |
| 0.364 |
| 0.662 |
|
Yes | 35.0 |
| 31.0 |
| 64.7 |
|
| No | 48.5 |
| 31.3 |
| 61.4 |
|
| Perineural
invasion |
| 0.991 |
| 0.971 |
| 0.612 |
|
Yes | 46.8 |
| 36.1 |
| 61.4 |
|
| No | 42.5 |
| 29.8 |
| 62.3 |
|
| PORT |
| 0.011a |
| 0.132 |
|
<0.001a |
|
Yes | 54.9 |
| 37.9 |
| 78.0 |
|
| No | 36.7 |
| 26.8 |
| 52.8 |
|
Multivariate analysis
Based on the results of the univariate analysis, a
multivariate analysis using Cox's regression model was performed to
identify independent prognostic factors regarding survival and
disease control. As presented in Table
IV, the use of PORT (HR, 0.755; 95% CI, 0.498–0.986; P=0.047),
the use of POCT (HR, 0.645; 95% CI, 0.420–0.988; P=0.044),
bronchial involvement (HR, 1.453; 95% CI, 1.002–2.107; P=0.049) and
≥2 N2 metastases (HR, 1.969; 95% CI, 1.228–3.157; P=0.005) were
identified to be significantly independent predictors of OS.
Bronchial involvement (HR, 1.419; 95% CI, 1.013–1.987; P=0.042) and
≥2 N2 metastases (HR, 1.807; 95% CI, 1.173–2.783; P=0.007) were
associated with significantly worse DFS, and only PORT (HR, 0.488;
95% CI, 0.271–0.881; P=0.017) was an independent predictor of LRFS.
Subgroup survival analysis was then performed for all patients
based on the status of bronchial involvement and number of N2
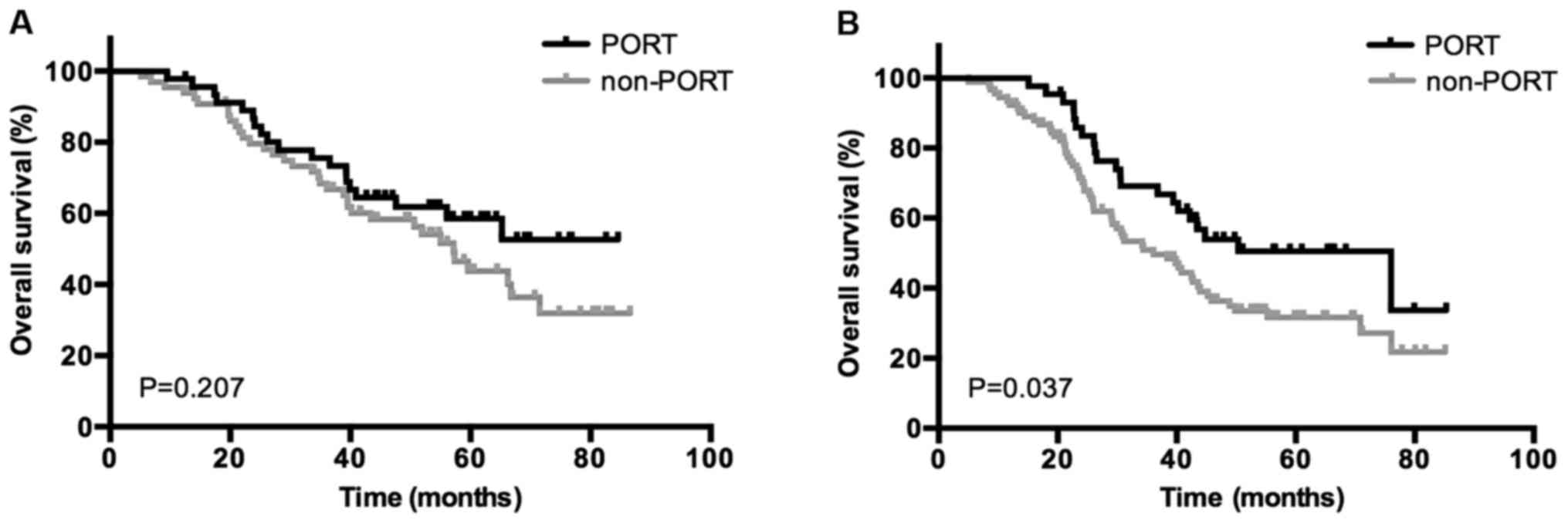
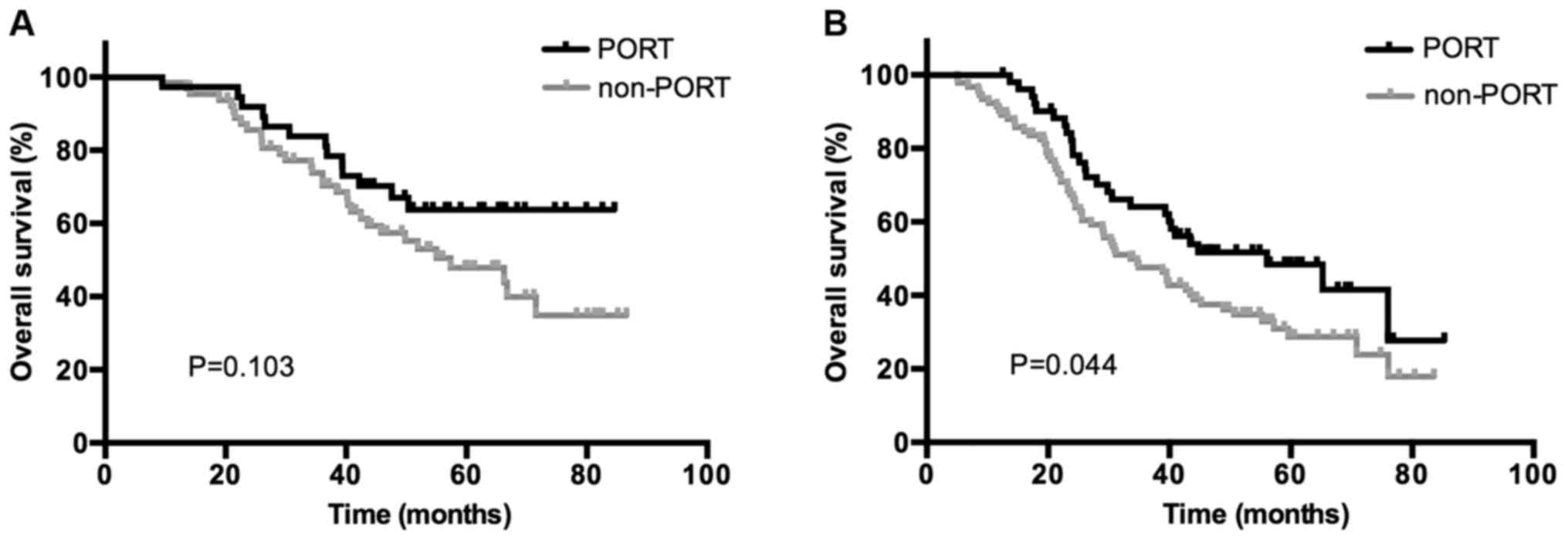
metastases. The use of PORT was associated with a significantly
increased OS rate in patients who were positive for bronchial
involvement (P=0.037) and ≥2 N2 LN metastases (P=0.044); however,
no association between patients with negative bronchial involvement
(P=0.207) or 1 N2 metastasis (P=0.103) was indicated. Kaplan-Meier
curves of the association between PORT and OS according to the
status of bronchial involvement and number of N2 metastasis are
presented in Figs. 3 and 4, demonstrating an improved OS rate with
PORT only in the subgroup of patients with positive bronchial
involvement and ≥2 N2 LN metastases.
 | Table IV.Multivariate analyses of prognostic
factors for OS, DFS and LRFS. |
Table IV.
Multivariate analyses of prognostic
factors for OS, DFS and LRFS.
|
| Overall
survival | Disease-free
survival | Local
recurrence-free survival |
|---|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Female | 0.902 | 0.598–1.362 | 0.624 | 0.828 | 0.571–1.202 | 0.321 | 0.669 | 0.377–1.185 | 0.168 |
| PS=1 | 1.046 | 0.702–1.558 | 0.826 | 0.988 | 0.683–1.430 | 0.950 | 1.449 | 0.894–2.346 | 0.132 |
| Peripheral
tumor | 0.825 | 0.415–1.140 | 0.053 | 0.925 | 0.644–1.329 | 0.642 | 0.647 | 0.383–1.076 | 0.093 |
| Pneumonectomy | 0.598 | 0.288–1.244 | 0.169 | 0.770 | 0.382–1.550 | 0.464 | 1.200 | 0.508–2.836 | 0.678 |
| pT3 stage | 1.426 | 0.855–2.377 | 0.174 | 1.330 | 0.961–1.841 | 0.085 | 0.946 | 0.607–1.473 | 0.805 |
| Number of N2
metastasis ≥2 | 1.969 | 1.228–3.157 | 0.005a | 1.807 | 1.173–2.783 | 0.007a | 1.235 | 0.663–2.301 | 0.506 |
| Multiple N2
stations involved | 0.978 | 0.618–1.550 | 0.926 | 1.255 | 0.827–1.904 | 0.286 | 1.618 | 0.881–2.969 | 0.121 |
| Hilar LN
metastasis | 1.298 | 0.894–1.886 | 0.171 | 1.319 | 0.937–1.858 | 0.113 | 1.102 | 0.679–1.787 | 0.694 |
| Bronchial
involvement | 1.453 | 1.002–2.107 | 0.049a | 1.419 | 1.013–1.987 | 0.042a | 1.496 | 0.916–2.444 | 0.108 |
| POCT | 0.645 | 0.420–0.988 | 0.044a | 0.983 | 0.660–1.463 | 0.908 | 0.735 | 0.437–1.236 | 0.245 |
| PORT | 0.755 | 0.498–0.986 | 0.047a | 0.811 | 0.561–1.171 | 0.263 | 0.488 | 0.271–0.881 | 0.017a |
Discussion
The present study demonstrated that the use of PORT
improved the OS rate (P=0.011) and LRFS rate (P<0.001) in
patients with completely resected pN2 NSCLC compared with that in
patients who were not treated with PORT. Results revealed that the
optimal strategy for the treatment of postoperative pN2 NSCLC is
adjuvant chemotherapy followed by radiotherapy. The median OS times
of three treatment strategies, namely surgery followed by POCT and
PORT, surgery followed by POCT or surgery alone, were 76.03 months
(95% CI, 43.99–108.74), 49.83 months (95% CI, 34.20–65.47) and
38.87 months (95% CI, 32.65–45.09), respectively (P=0.005).
Patients with completely resected NSCLC and pN2 disease are
extremely heterogeneous, and the treatment strategy is complex and
variable, with survival rates ranging between 7 and 36% (9,26,27). With the wide use of modern radiation
techniques and adequate radiation dosages, a number of previous
retrospective studies have demonstrated that PORT may improve the
survival rates of patients with completely resected pN2 NSCLC
(28–30).
Corso et al (31) retrospectively analyzed a total of
30,552 cases of stage II–IIIA R0 resection of NSCLC from the
National Cancer Database of data gathered between 1998 and 2006. A
total of 3,430 (11.2%) patients received PORT, including 1,660 N2
patients. PORT was administered using 3D-CRT or IMRT. The results
demonstrated that the 5-year survival rates in patients with pN0
and N1 with PORT were worse than those the patients with pN0 and N1
without PORT, at 48 vs. 37.7% (P<0.001), and 39.4 vs. 34.8%
(P<0.001), respectively. Conversely, pN2 patients with PORT
experienced a significantly improved 5-year survival rate compared
with those without PORT (P<0.001).
Notably, to the best of our knowledge, all previous
studies into the subject have been retrospective thus far, and
prospective randomized studies are required to verify the
conclusions. Lung ART, conducted by the Adjuvant Radiotherapy Lung
Study Group, is an ongoing randomized controlled phase III study
for comparing PORT with non-PORT in resected NSCLC with N2 using a
modern radiotherapy technique. The research predicts to increase
3-year DFS rate by 10% (32).
Previous studies had reported that a number of
pathological factors are associated with survival rate, including
visceral pleural invasion (33),
vascular invasion (34) and
perineural invasion (35). In the
present study, it was identified that bronchial involvement was an
independent predictor of OS and DFS rates, however, the status of
pulmonary vascular wall invasion, visceral pleural invasion,
lymphovascular invasion and perineural invasion demonstrated no
significant association with survival rate. In addition, the status
of bronchial involvement was able to predict the efficacy of PORT.
However, improved OS with PORT was only demonstrated in the
subgroup of patients with positive bronchial involvement. To the
best of our knowledge, no previous study has revealed the
association between the status of bronchial involvement and the
effect of PORT and prognosis. The risk of local relapse may be
increased in the patients with positive bronchial involvement and
PORT serves a crucial function in this subset. Additional research
should be performed to verify the association between the
pathological factors and the survival outcome and the efficacy of
PORT in locally advanced NSCLC.
The number of LN metastases has been demonstrated to
be a significant prognostic factor in a number of types of solid
cancer and is also incorporated in the definition of pN stage in
numerous types of cancer in the current TNM classification system,
including breast, gastric and esophageal cancer (36). Notably, the prognostic value of the
number of LN metastases in NSCLC has also been investigated in a
number of studies, in which results have indicated that the number
of LN metastases may be a superior prognostic indicator compared
with the current location-based pN classification. In addition, the
significance of the number of metastatic LNs appeared to be more
prominent in patients with pN2 compared with that in patients with
pN1 (37,38). In the present study, univariate
analysis demonstrated that patients who developed only 1 N2 LN
metastasis experienced a significant improvement compared with
multiple N2 metastases, not only in terms of OS rate, but also for
DFS and LRFS rates. Multivariate analyses indicated that the number
of metastatic LNs was a prognostic indicator of OS and DFS rates.
Additionally, PORT treatment demonstrated an improved OS rate in
the subgroup of patients with ≥2 N2 LN metastases compared with
that in patients not treated with PORT, however, no significant
difference was indicated in patients with 1 N2 LN metastasis. The
results of the present study were in agreement with the
aforementioned studies and indicated that PORT treatment improved
survival rates in patients with multiple N2 LN metastases.
The univariate analysis performed in the present
study demonstrated that multiple N2 station involvement was
associated with a significantly poorer outcome not only in terms of
OS rate, but also for DFS and LRFS rates (P=0.030, P<0.001,
P=0.035, respectively). However, multivariate analyses did not
indicate its value as a prognostic factor in OS, DFS or LRFS. In
addition, the number of N2 station involvements was unable to
predict the efficacy of PORT, and there were no significant
differences between the PORT and non-PORT groups in either single
or multiple N2 station-involved subsets.
The present study demonstrates several limitations
owing to the retrospective nature of the analysis. First, the
patients all came from a single hospital and the number of cases
was limited, which may confer selection bias. Secondly, adjuvant
chemotherapy has been the standard treatment of IIIA NSCLC,
however, only 72.4% of patients in the study accepted chemotherapy
for various reasons, and almost all of PORT administrated was in a
POCT setting, which may cause survival bias when analyzing the
benefit of adjuvant radiotherapy. Thirdly, the majority of the
enrolled patients were not tested for epidermal growth factor
receptor (EGFR), anaplastic lymphoma kinase or B-RAF gene status.
When the disease progressed, 28 patients were treated with EGFR
inhibitors or other targeted therapies, which may exhibit distinct
influences on the final OS rates. Finally, selected factors were
based on the clinicopathological information available; treatment
of NSCLC has already entered the molecular era and combining the
clinicopathological factors and molecular biomarkers may be more
relevant when analyzing the survival rates and effects of PORT.
In conclusion, the present study demonstrated that
PORT may improve LRFS and OS rates in patients with resectable pN2
NSCLC. Adjuvant chemotherapy followed by radiotherapy was the
optimal adjuvant treatment strategy. PORT, POCT, bronchial
involvement status and number of N2 metastases were identified to
be significant independent predictors of OS rate. Bronchial
involvement and ≥2 N2 metastases were significantly associated with
poorer DFS rates, and only PORT was an independent predictor of
LRFS rate. PORT was associated with a significant increase in OS
rates in patients with bronchial involvement and ≥2 N2 LN
metastases. Further prospective studies to validate these results
in a pN2 population are warranted.
Acknowledgements
The present study was supported by the Key Project
of Zhejiang Provincial Natural Science Foundation (grant no.
LZ13H16003), and the Zhejiang Medical Science and Technology
Foundation (grant no. 201480784).
References
|
1
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK,
Govindan R, et al: Non-small cell lung cancer. J Natl Compr Canc
Netw. 10:1236–1271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Govindan R,
Grannis FW Jr, et al: Non-small cell lung cancer, version 2.2013. J
Natl Compr Canc Netw. 11:645–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goeckenjan G, Sitter H, Thomas M,
Branscheid D, Flentje M, Griesinger F, Niederle N, Stuschke M, Blum
T, Deppermann KM, et al: Prevention, diagnosis, therapy, and
follow-up of lung cancer: Interdisciplinary guideline of the German
Respiratory Society and the German Cancer Society. Pneumologie.
65:39–59. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alberg AJ, Brock MV, Ford JG, Samet JM and
Spivack SD: Epidemiology of lung cancer: Diagnosis and management
of lung cancer, 3rd ed: American College of Chest Physicians
evidence-based clinical practice guidelines. Chest. 143 Suppl
5:e1S–e29S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayat MJ, Howlader N, Reichman ME and
Edwards BK: Cancer statistics, trends, and multiple primary cancer
analyses from the surveillance, epidemiology, and end results
(SEER) program. Oncologist. 12:20–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mountain CF: Revisions in the
international system for staging lung cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorent N, De Leyn P, Lievens Y, Verbeken
E, Nackaerts K, Dooms C, Van Raemdonck D, Anrys B and Vansteenkiste
J: Leuven Lung Cancer Group: Long-term survival of surgically
staged IIIA-N2 non-small cell lung cancer treated with a surgical
combined modality approach: Analysis of a 7-year prospective
experience. Ann Oncol. 15:1645–1653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Casali C, Stefani A, Natali P, Rossi G and
Morandi U: Prognostic factors in surgically resected N2 non-small
cell lung cancer: The importance of patterns of mediastinal lymph
nodes metastases. Eur J Cardiothorac Surg. 28:33–38. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andre F, Grunenwald D, Pignon JP, Dujon A,
Pujol JL, Brichon PY, Brouchet L, Quoix E, Westeel V and Le
Chevalier T: Survival of patients with resected N2 non-small cell
lung cancer: Evidence for a subclassification and implications. J
Clin Oncol. 18:2981–2989. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suzuki K, Nagai K, Yoshida J, Nishimura M,
Takahashi K and Nishiwaki Y: The prognosis of surgically resected
N2 non-small cell lung cancer: The importance of clinical N status.
J Thorac Cardiovasc Surg. 118:145–153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Douillard JY, Rosell R, De Lena M,
Carpagnano F, Ramlau R, Gonzáles-Larriba JL, Grodzki T, Pereira JR,
Le Groumellec A, Lorusso V, et al: Adjuvant vinorelbine plus
cisplatin versus observation in patients with completely resected
stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine
International Trialist Association [ANITA]): A randomised
controlled trial. Lancet Oncol. 7:719–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J: International Adjuvant
Lung Cancer Trial Collaborative Group: Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Le Péchoux C: Role of postoperative
radiotherapy in resected non-small cell lung cancer: A reassessment
based on new data. Oncologist. 16:672–681. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
No authors listed: Postoperative
radiotherapy in non-small-cell lung cancer: Systematic review and
meta-analysis of individual patient data from nine randomised
controlled trials. PORT Meta-analysis Trialists Group. Lancet.
352:257–263. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bekelman JE, Rosenzweig KE, Bach PB and
Schrag D: Trends in the use of postoperative radiotherapy for
resected non-small-cell lung cancer. Int J Radiat Oncol Biol Phys.
66:492–499. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Uno T, Sumi M, Kihara A, Numasaki H,
Kawakami H, Ikeda H, Mitsumori M and Teshima T: Japanese PCS
Working Subgroup of Lung Cancer: Postoperative radiotherapy for
non-small-cell lung cancer: Results of the 1999–2001 patterns of
care study nationwide process survey in Japan. Lung Cancer.
56:357–362. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lally BE, Zelterman D, Colasanto JM,
Haffty BG, Detterbeck FC and Wilson LD: Postoperative radiotherapy
for stage II or III non-small-cell lung cancer using the
surveillance, epidemiology, and end results database. J Clin Oncol.
24:2998–3006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Le Péchoux C, Dunant A, Pignon JP, De
Ruysscher D, Mornex F, Senan S, Casas F, Price A and Milleron B:
Need for a new trial to evaluate adjuvant postoperative
radiotherapy in non-small cell lung cancer patients with N2
mediastinal involvement. J Clin Oncol. 25:e10–e11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Yu XL, Zheng GF and Zhao F:
Intensity-modulated radiotherapy and volumetric-modulated arc
therapy have distinct clinical advantages in non-small cell lung
cancer treatment. Med Oncol. 32:942015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Douillard JY, Rosell R, De Lena M, Riggi
M, Hurteloup P and Mahe MA: Adjuvant Navelbine International
Trialist Association: Impact of postoperative radiation therapy on
survival in patients with complete resection and stage I, II, or
IIIA non-small cell lung cancer treated with adjuvant chemotherapy:
The adjuvant Navelbine international trialist association (ANITA)
randomized trial. Int J Radiat Oncol Biol Phys. 72:695–701. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lally BE, Zelterman D, Colasanto JM,
Haffty BG, Detterbeck FC and Wilson LD: Postoperative radiotherapy
for stage II or III non-small cell lung cancer using the
surveillance, epidemiology, and end results database. J Clin Oncol.
24:2998–3006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rusch VW, Asamura H, Watanabe H, Giroux
DJ, Rami-Porta R and Goldstraw P: Members of IASLC Staging
Committee: The IASLC lung cancer staging project: A proposal for a
new international lymph node map in the forthcoming seventh edition
of the TNM classification for lung cancer. J Thorac Oncol.
4:568–577. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Higgins KA, Chino JP, Berry M, Ready N,
Boyd J, Yoo DS and Kelsey CR: Local failure in resected N1 lung
cancer: Implications for adjuvant therapy. Int J Radiat Oncol Biol
Phys. 83:727–733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Varlotto JM, Yao AN, DeCamp MM,
Ramakrishna S, Recht A, Flickinger J, Andrei A, Reed MF, Toth JW,
Fizgerald TJ, et al: Nodal stage of surgically resected non-small
cell lung cancer and its effect on recurrence patterns and overall
survival. Int J Radiat Oncol Biol Phys. 91:765–773. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim KJ, Ahn YC, Lim DH, Han J, Park K,
Park JO, Kim K, Kim J and Shim YM: Analyses on prognostic factors
following tri-modality therapy for stage IIIa non-small cell lung
cancer. Lung Cancer. 55:329–336. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Casali C, Stefani A, Natali P, Rossi G and
Morandi U: Prognostic factors in surgically resected N2 non-small
cell lung cancer: The importance of patterns of mediastinal lymph
nodes metastasis. Eur J Cardiothorac Surg. 28:33–38. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou B, Xu Y, Li T, Li W, Tang B, Zhou L,
Li L, Liu Y, Zhu J, Huang M, et al: A multicenter retrospective
analysis of survival outcome following postoperative
chemoradiotherapy in non-small-cell lung cancer patients with N2
nodal disease. Int J Radiat Oncol Biol Phys. 77:321–328. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Patel SH, Ma Y, Wernicke AG, Nori D, Chao
KS and Parashar B: Evidence supporting contemporary post-operative
radiation therapy (PORT) using linear accelerators in N2 lung
cancer. Lung Cancer. 84:156–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Billiet C, Decaluwé H, Peeters S,
Vansteenkiste J, Dooms C, Haustermans K, De Leyn P and De Ruysscher
D: Modern post-operative radiotherapy for stage III non-small cell
lung cancer may improve local control and survival: A
meta-analysis. Radiother Oncol. 110:3–8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Corso CD, Rutter CE, Wilson LD, Kim AW,
Decker RH and Husain ZA: Re-evaluation of the role of postoperative
radiotherapy and the impact of radiation dose for non-small-cell
lung cancer using the National Cancer Database. J Thora Oncol.
10:148–155. 2015. View Article : Google Scholar
|
|
32
|
Finn CF, Pechoux CL, Edwards J and Lunt C:
189: Lung ART: Phase III study comparing post-operative conformal
radiotherapy to no post-operative radiotherapy in patients with
completely resected non-small cell lung cancer and mediastinal N2
involvement. Lung Cancer. 87 Suppl 1:S70–S71. 2015. View Article : Google Scholar
|
|
33
|
Ou SH, Zell JA, Ziagos A and Anton-Culver
H: Prognostic significance of the non-size-based AJCC T2
descriptors: Visceral pleura invasion, hilar atelectasis, or
obstructive pneumonia in stage IB non-small cell lung cancer is
dependent on size. Chest. 133:662–669. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsuchiya T, Hashizume S, Akamine S,
Muraoka M, Honda S, Tsuji K, Urabe S, Hayashi T, Yamasaki N and
Nagayasu T: Upstaging by vessel invasion improves the pathology
staging system of non-small cell lung cancer. Chest. 132:170–177.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yilmaz A, Duyar SS, Cakir E, Aydin E,
Demirag F, Karakaya J, Yazici U and Erdogan Y: Clinical impact of
visceral pleural, lymphovascular and perineural invasion in
completely resected non-small cell lung cancer. Eur J Cardiothorac
Surg. 40:664–670. 2011.PubMed/NCBI
|
|
36
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wei S, Asamura H, Kawachi R, Sakurai H and
Watanabe S: Which is the better prognostic factor for resected
non-small cell lung cancer: The number of metastatic lymph nodes or
the currently used nodal stage classification? J Thorac Oncol.
6:310–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee JG, Lee CY, Park IK, Kim DJ, Park SY,
Kim KD and Chung KY: Number of metastatic lymph nodes in resected
non-small cell lung cancer predicts patient survival. Ann Thorac
Surg. 85:211–215. 2008. View Article : Google Scholar : PubMed/NCBI
|