Introduction
Esophageal carcinoma is one of the most aggressive
malignancies with poor prognosis and has been defined as two major
histologic types: Esophageal squamous cell carcinoma (ESCC) and
esophageal adenocarcinoma (1). ESCC
is the fourth most frequently diagnosed cancer and the fourth
leading cause of cancer-associated mortality in China (2). Lymph node metastasis is one of the
biggest challenges in treatment and survival of this type of
cancer. Further therapeutic improvements are required and a focus
on targeted therapy is warranted. This has led to increased
interest in the prognostic and therapeutic value of tumor
biomarkers that are known to serve a key role in carcinogenesis and
progression.
Evidence indicates that epithelial-mesenchymal
transition (EMT) serves a key role in a variety of diseases,
including cardiovascular disease and cancer (3). EMT is of importance during the processes
of tumorigenesis and metastasis; EMT has been reported to reduce
the adhesion and increase the motility of epithelial tumor cells
(4). The underlying mechanism is
essential to early-phase cancer metastasis. Numerous signaling
pathways, including transforming growth factor-β (TGF-β) and Notch,
participate in the progression of EMT and the target genes are
responsible for activation of the mesenchymal phenotype (5).
Cluster of differentiation 147 (CD147), also known
as basigin, is highly expressed on the surface of carcinoma cells,
but not on that of normal cells (6).
CD147 is a heavily glycosylated type I transmembrane glycoprotein;
its overexpression of CD147 was significantly associated with
various malignant tumors (7). In
addition, a report demonstrated that CD147 served an important role
in EMT and regulated diverse signaling pathways (8). Pituitary tumor transforming gene (PTTG)
is an oncogene that is highly expressed in a variety of tumor
tissues (9). The overexpression of
PTTG stimulated the expression of matrix metalloproteinase-2 and
enhanced EMT process in oral squamous cell carcinoma (10). CD44v6, a splice variant of CD44, is
highly associated with tumor invasion and metastasis and is
reported to be a key biomarker for certain types of metastatic
cancer (11). A prior study reported
that CD44v6 contributed to autophagy, EMT and the activation of
numerous pathways in colon cancer (12). However, the association between EMT
and ESCC is not fully understood. Further investigation to identify
EMT-associated proteins in ESCC is required; these proteins may be
associated with adverse patient prognosis and an improved
understanding of them could contribute to the development of
therapeutic strategies for patients with ESCC.
In the present study, the expression levels of
CD147, PTTG and CD44v6 in ESCC tissues were analyzed; the
correlation between the expressions of these EMT-associated
molecules was also assessed. Furthermore, Kaplan-Meier analysis was
performed to investigate the prognostic value of CD147, PTTG and
CD44v6 in patients with ESCC.
Materials and methods
Pathological specimens
A tissue microarray, purchased from Shanghai Outdo
Biotech Co., Ltd. (cat. no. HEso-Squ172Sur-01; Shanghai, China)
contained 86 cases totally from patients who underwent surgery at
hospitals between July 2006 and September 2008. Tissue specimens
were collected from 64 males and 22 females, with a median age of
65 years (range 41–81). Excluding those who were lost to follow-up
or lacked pathological information, the samples from 76 patients
were enrolled in the present study, including 57 male patients and
19 female patients with a median age of 65 years ranging from 41 to
81 years. Additionally, 72 paired adjacent normal tissues in the
same microarray were used for comparison in the same manner. All
patients had a single tumor and no distant metastasis and the
clinical stages were classified by the American Joint Committee on
Cancer system (AJCC) (13).
Immunohistochemical staining
The staining procedure was performed by standard
streptavidin-horseradish peroxidase complex method. The slides were
dried in an incubator at 65°C overnight, and following
deparaffinization via 100% xylene for 15 min and hydration using
100% ethanol for 5 min, 95% ethanol for 2 min, 80% ethanol for 2
min and 75% ethanol for 2 min. Tissue sections were incubated with
3% hydrogen peroxide in methanol at room temperature for 30 min to
block endogenous peroxidase activity. Briefly, the anti-PTTG (1:50
dilution; cat. no. 12575-1-AP; ProteinTech Group, Inc., Chicago,
IL, USA), anti-CD44v6 (pre-diluted; cat. no. 2M-0052; OriGene
Technologies, Inc., Beijing, China), and anti-CD147 (CD147
Diagnostic kit; cat. no. CL001-01; Jiangsu Pacific-Meinuoke
Biopharmaceutical Co., Ltd., Jiangsu, China) antibodies were
applied and incubated at 4°C overnight, followed by washing with
PBS. The sections were incubated with a secondary antibody
(ready-to-use kit; cat. no. SP9000; goat anti-mouse IgG; OriGene
Technologies, Inc.), for 30 min at room temperature. Signals were
developed with 3,3-diaminobenzine substrate (1:200; cat no.
ZLI-9018; OriGene Technologies, Inc.) for 2 min and counterstained
with hematoxylin (ready-to-use; cat. no. BA-4041; Baso Diagnostic,
Inc.) for 10 min at room temperature.
Evaluation of immunohistochemical
staining
Protein expression determined by immunohistochemical
staining was evaluated using an Olympus microscope (Olympus
Corporation, Tokyo, Japan). The sections were carefully diagnosed
under light microscope and five random fields of view were examined
at magnification, ×200 and ×400. Staining for these biomarkers was
classified by the staining intensity and the percentage of positive
tumor cells. The percentage of positively stained cells was counted
for each tissue section. According to the degree of staining, the
expression was defined as follows: - (negative), + (weak intensity;
5–30% positive tumor cell), ++ (moderate intensity; 30–70% positive
tumor cell) and +++ (strong intensity; >70% positive tumor
cell).
Statistical analysis
Associations between immunohistochemical staining
patterns and clinicopathological parameters were evaluated by
χ2 tests. The correlation between the expression of
these biomarkers was analyzed by Spearman's correlation
coefficient. Kaplan-Meier statistical analysis and a log-rank test
were used to assess the survival curve and compare between
subgroups. Statistical analyses were performed by SPSS 19.0 (IBM
Corp., Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics of the selected
patients with ESCC
The age of patients ranged between 41 and 81-years,
the mean age was 65. Of the patients, there were 57 males and 19
females. By the time of the final follow-up in September 2013, 52
patients had succumbed to mortality, during a median follow-up time
of 30.5 months (range, 0–85.0 months).
Association between EMT-associated
molecule expression and clinicopathological parameters
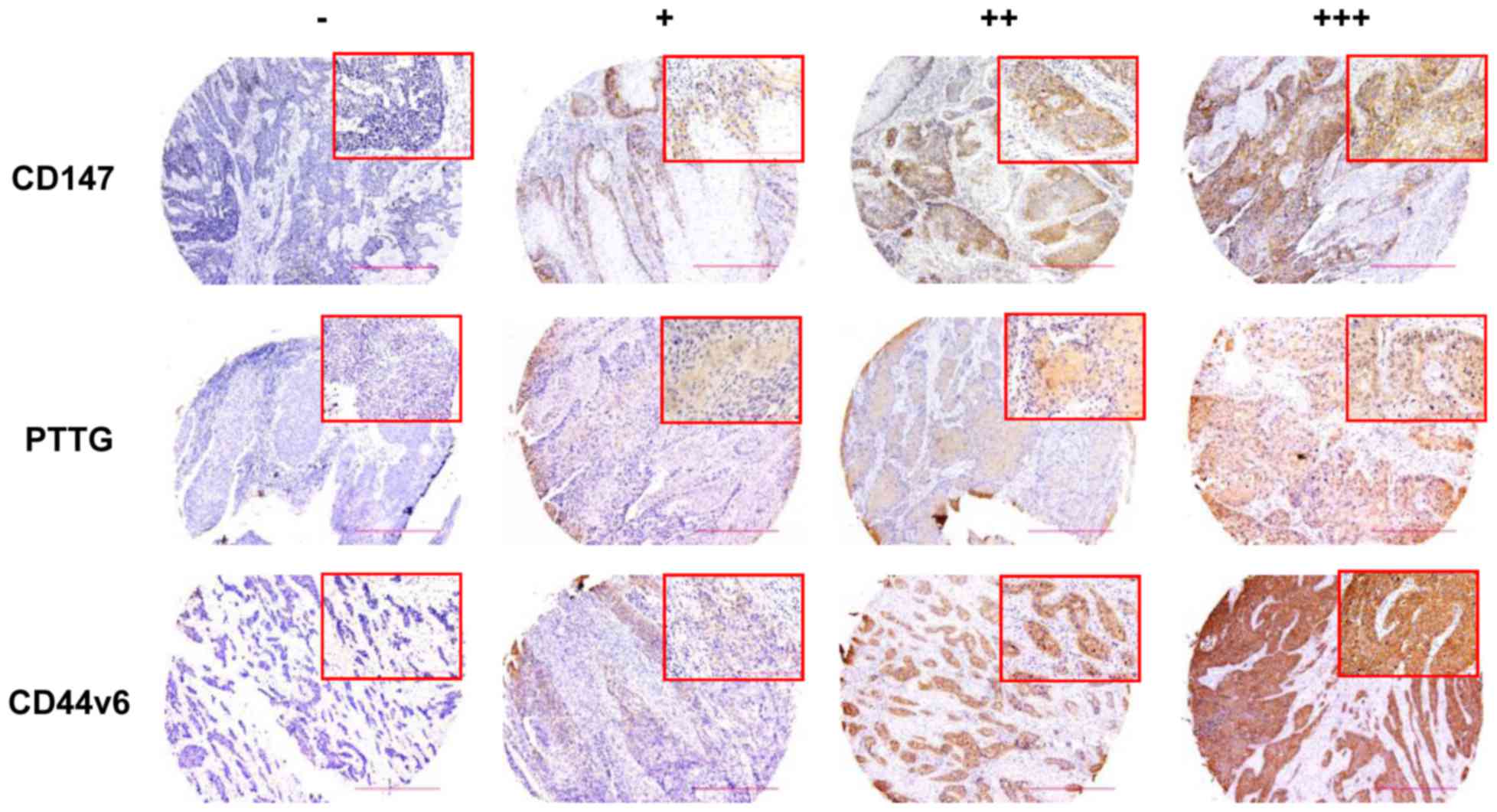
In the present study, CD147 expression was detected
in 69 samples (90.8%) and was enriched on the surface of the cancer
cells. However, in adjacent normal tissues, staining for CD147
antibody was almost negative (data not shown). Representative
histological pictures are presented in Fig. 1. Statistical analysis revealed that
increased CD147 expression levels were associated with patient sex
(P=0.003), AJCC clinical stage (P=0.037) and lymph node metastasis
(P=0.025; Table I). PTTG expression
was detected in 52 cancer tissues (68.4%) and was also observed in
the nucleus and plasma of cancer cells (Fig. 1). Overexpression of PTTG was
significantly associated with histological grade (P=0.035; Table I), but not with lymph node metastasis
or AJCC clinical stage. Furthermore, the staining for CD44v6
(Fig. 1), mainly in the cell
membrane, appeared in 59 carcinoma tissues (77.6%). However, the
overexpression of CD44v6 within tumor tissues was not associated
with histological grade, lymph node metastasis and AJCC clinical
grade.
 | Table I.Expression of CD147, PTTG and CD44v6
in esophageal squamous cell carcinoma. |
Table I.
Expression of CD147, PTTG and CD44v6
in esophageal squamous cell carcinoma.
|
|
| CD147 | PTTG | CD44v6 |
|---|
|
|
|
|
|
|
|---|
| Variable | Patients, n | Negative | Positive | P-value | Negative | Positive | P-value | Negative | Positive | P-value |
|---|
| All patients | 76 | 7 | 69 |
| 24 | 52 |
| 17 | 59 |
|
| Sex |
|
|
| 0.003a |
|
| 0.569 |
|
| 0.874 |
| Male | 57 | 2 | 55 |
| 17 | 40 |
| 13 | 44 |
|
|
Female | 19 | 5 | 14 |
| 7 | 12 |
| 4 | 15 |
|
| Age, years |
|
|
| 0.264 |
|
| 0.406 |
|
| 0.21 |
|
<65 | 37 | 2 | 35 |
| 10 | 27 |
| 6 | 31 |
|
| ≥65 | 39 | 5 | 34 |
| 14 | 25 |
| 11 | 28 |
|
| AJCC clinical
stage |
|
|
| 0.037 a |
|
| 0.52 |
|
| 0.292 |
| I | 6 | 0 | 6 |
| 1 | 5 |
| 1 | 12 |
|
| II | 41 | 7 | 34 |
| 15 | 26 |
| 5 | 29 |
|
| III | 29 | 0 | 29 |
| 8 | 21 |
| 6 | 41 |
|
| Lymph node
metastasis |
|
|
| 0.025 a |
|
| 0.79 |
|
| 0.335 |
|
Positive | 30 | 0 | 30 |
| 10 | 20 |
| 5 | 25 |
|
|
Negative | 46 | 7 | 39 |
| 14 | 32 |
| 12 | 34 |
|
| Histological
grade |
|
|
| 0.191 |
|
| 0.035a |
|
| 0.827 |
| I | 24 | 1 | 23 |
| 4 | 20 |
| 5 | 19 |
|
| II | 38 | 3 | 35 |
| 12 | 26 |
| 8 | 30 |
|
|
III | 14 | 3 | 11 |
| 8 | 6 |
| 4 | 10 |
|
Correlation between CD147, PTTG and
CD44v6 expression
In the present study, the correlation between the
expression of CD147, PTTG and CD44v6 was assessed using Spearman's
correlation analysis. CD147 expression was significantly correlated
with the expression of PTTG (Table
II) and CD44v6 (Table III).
There was also a significant correlation between PTTG and CD44v6
expression (R=0.527, P=0.001; Table
IV).
 | Table II.Correlation between CD147 and PTTG
expression. |
Table II.
Correlation between CD147 and PTTG
expression.
|
|
| PTTG | Spearman's
correlation |
|---|
|
|
|
|
|
|---|
|
|
| − | + | ++ | +++ | R-value | P-value |
|---|
| CD147 | − | 7 | 0 | 0 | 0 | 0.369 | 0.001 |
|
| + | 12 | 24 | 3 | 4 |
|
|
|
| ++ | 4 | 6 | 6 | 0 |
|
|
|
| +++ | 1 | 5 | 4 | 0 |
|
|
 | Table III.Correlation between CD147 and CD44v6
expression. |
Table III.
Correlation between CD147 and CD44v6
expression.
|
|
| CD44v6 | Spearman's
correlation |
|---|
|
|
|
|
|
|---|
|
|
| − | + | ++ | +++ | R-value | P-value |
|---|
| CD147 | − | 4 | 2 | 1 | 0 | 0.320 | 0.005 |
|
| + | 9 | 17 | 11 | 6 |
|
|
|
| ++ | 2 | 4 | 2 | 8 |
|
|
 | Table IV.Correlation between PTTG and CD44v6
expression. |
Table IV.
Correlation between PTTG and CD44v6
expression.
|
|
| PTTG | Spearman's
correlation |
|---|
|
|
|
|
|
|---|
|
|
| − | + | ++ | +++ | R-value | P-value |
|---|
| CD44v6 | − | 13 | 4 | 0 | 0 | 0.527 | <0.001 |
|
| + | 7 | 14 | 2 | 2 |
|
|
|
| ++ | 2 | 10 | 5 | 0 |
|
|
|
| +++ | 2 | 7 | 6 | 2 |
|
|
Effects of CD147, PTTG and CD44v6
expression on OS rate in patients with ESCC
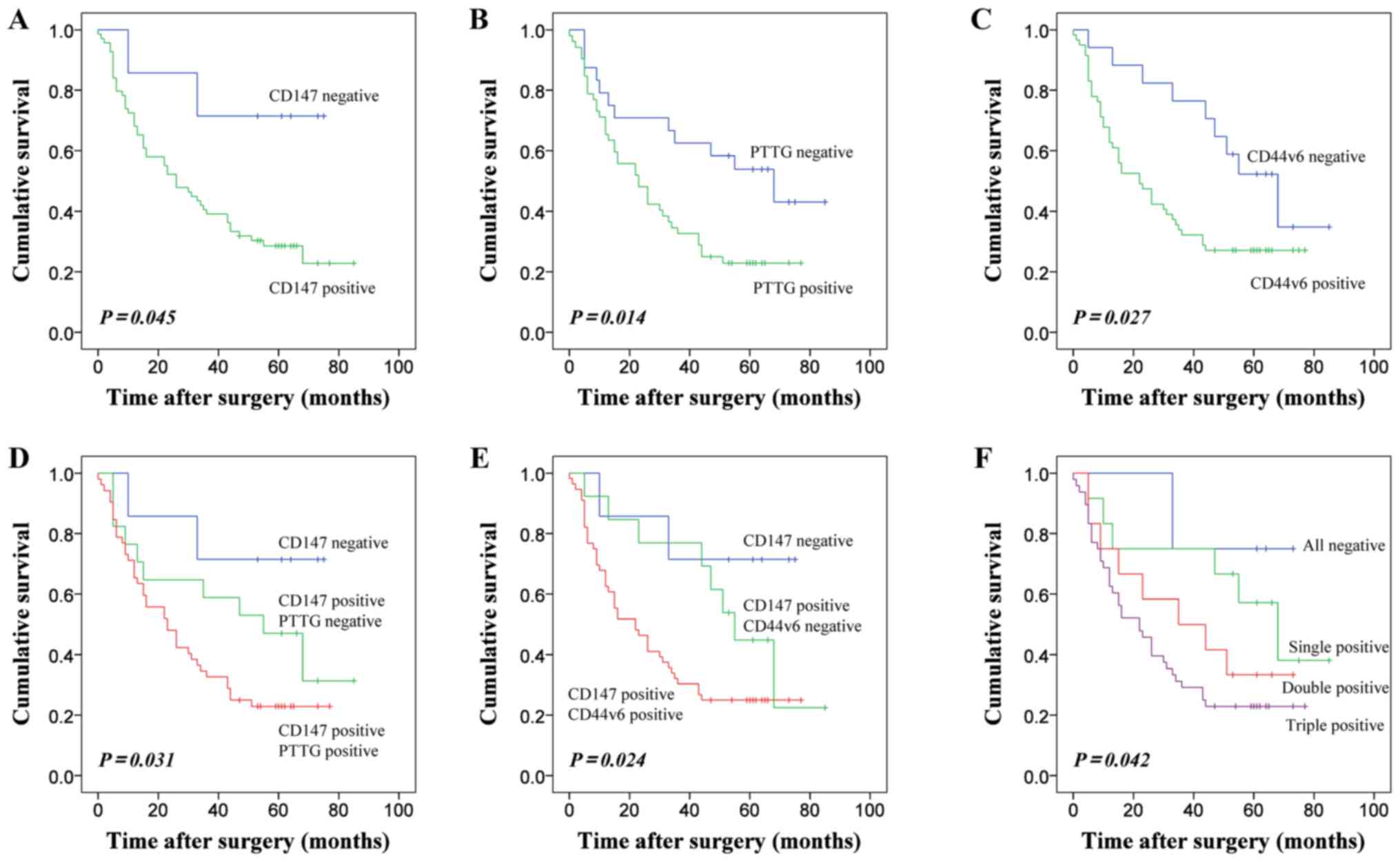
Kaplan-Meier curves were generated for patients with
ESCC categorized patients according to the expression of CD147,
PTTG and CD44v6. Patients negative for CD147 expression exhibited
longer OS rate than patients with CD147 positive expression, a
difference that was statistically significant (Fig. 2A). The results also indicated that the
duration of survival was significantly associated with positive
PTTG expression (Fig. 2B).
Additionally, the patients with positive CD44v6 expression
experienced a poorer survival rate (Fig.
2C). As CD147 expression was significantly associated with the
expression of PTTG and CD44v6, patients were divided into subgroups
according to the combined expression of these EMT-associated
markers to detect whether the prediction of ESSC prognosis was more
accurate. Regarding co-expression, patients with positive staining
for CD147 and PTTG demonstrated lower OS rates (Fig. 2D). Furthermore, the combination of
CD147 and CD44v6 expression suggested that the overall survival of
these subgroups had a statistical difference (Fig. 2E). Positive expression of CD147, PTTG
and CD44v6 was associated with the poorest patient prognosis
(Fig. 2F).
Discussion
In the present study, the expression levels of
CD147, PTTG and CD44v6 protein were investigated in a tissue
microarray of samples from 76 patients with ESCC. The results
demonstrated that the overexpression of these three proteins was
significantly associated with various pathological characteristics
and may be considered as a predictor of shorter OS times in
patients with ESSC.
CD147 is overexpressed in various tumor cells and
may promote tumor invasion and lymph node metastasis (14). As a target gene of the TGF-β signaling
pathway, CD147 induces EMT and may be a potential target for the
treatment of HCC (15). In the
present study, CD147 was reportedly increased in human ESCC. The
overexpression of CD147 was associated with advanced with the AJCC
clinical stages and lymph node metastasis, consistent with previous
data reported by Zhu et al (16). Furthermore, when these features were
subjected to Kaplan-Meier analysis, the expression of CD147 was
associated with lower OS rates.
PTTG is an oncogene that is regulated by the
β-catenin signaling pathway in human ESCC (17). PTTG has been suggested to accelerate
the induction of EMT in lung cancer by regulating integrin
αvβ3 and adhesion-complex proteins, such as
talin, paxillin, vinculin and actinin (18). EMT is also induced by PTTG in human
ovarian epithelial cancer cells via the regulation of TGF-β and
E-cadherin (19). A previous study
demonstrated that the overexpression of PTTG may contribute to the
malignant progression of ESCC and serve as an independent
prognostic factor of OS time in ESCC (20). PTTG enhances the metastatic potential
of breast cancer cells by inducing CD147 via the activation of the
focal adhesion kinase/protein kinase B/mechanistic target of
rapamycin signaling pathway (21).
The findings of the present study demonstrated that the level of
PTTG expression was associated with the duration of survival and
the histological grade of the disease. However, the poorer OS times
of individuals with double-positive expression of CD147 and PTTG
indicated that these proteins may collectively participate in the
regulation of tumorigenesis and metastasis in ESCC; thus, further
investigation into the underlying mechanism is required.
CD44v6 expression is associated with higher tumor
stage and metastatic potential in numerous types of tumors. In
colorectal cancer, high expression levels of CD44v6 revealed a
significant inverse correlation with E-cadherin expression and a
positive correlation with vimentin expression (22). However, the role of CD44v6 expression
was controversial in certain other tumor types, including oral
squamous cell carcinoma (23). The
results of the present study indicated that the overexpression of
CD44v6 was a useful marker for patient prognosis; the combination
of CD44v6 and CD147 was associated with reduced OS rate.
EMT has been considered to be a critical step that
promotes the early phase of cancer metastasis. EMT-activating
signaling pathways and downstream proteins are responsible for
promoting EMT (24). In a recent
study, PTTG promoted invasion in a human breast cancer cell line by
upregulating CD147 (17). In
addition, CD147, as a novel upstream activator of signal transducer
and activator of transcription 3 (STAT3), interacts with CD44 and
serves as a critical role in the development of pancreatic cancer
(25). Furthermore, the present study
revealed that patients with tumors that concurrently expressed
these three markers exhibited an inferior OS rate. This result
indicated that the increased expression levels of EMT-associated
proteins in ESCC may substantially alter the prognosis of patients.
In addition, the identification of distinct prognostic subgroups
can be beneficial for the accuracy of ESSC prognosis in the
clinical practice.
In conclusion, the overexpression of CD147, PTTG and
CD44v6, individually, was associated with the development of ESSC
and was significantly associated with OS rate, which indicated that
these proteins are potential biomarkers for prognosis.
Additionally, the significant correlation between CD147, PTTG and
CD44v6 expression indicated that these molecules may collectively
participate in the regulation of EMT-associated signaling pathways
in ESCC. CD147, PTTG and CD44v6 may be considered as potential
targets for the development of anticancer therapies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31571434) and the
National Basic Research Program of China (grant no.
2015CB553701)
References
|
1
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chua KN, Poon KL, Lim J, Sim WJ, Huang RY
and Thiery JP: Target cell movement in tumor and cardiovascular
diseases based on the epithelial-mesenchymal transition concept.
Adv Drug Deliv Rev. 63:558–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weidle UH, Scheuer W, Eggle D, Klostermann
S and Stockinger H: Cancer-related issues of CD147. Cancer Genomics
Proteomics. 7:157–169. 2010.PubMed/NCBI
|
|
7
|
Toole BP: Emmprin (CD147), a cell surface
regulator of matrix metalloproteinase production and function. Curr
Top Dev Biol. 54:371–389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ru NY, Wu J, Chen ZN and Bian H:
HAb18G/CD147 is involved in TGF-β-induced epithelial-mesenchymal
transition and hepatocellular carcinoma invasion. Cell Biol Int.
39:44–51. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vlotides G, Eigler T and Melmed S:
Pituitary tumor-transforming gene: Physiology and implications for
tumorigenesis. Endocr Rev. 28:165–186. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang E, Liu S, Xu Z, Huang S, Tan X, Sun
C and Lu L: Pituitary tumor-transforming gene 1 (PTTG1) is
overexpressed in oral squamous cell carcinoma (OSCC) and promotes
migration, invasion and epithelial-mesenchymal transition (EMT) in
SCC15 cells. Tumour Biol. 35:8801–8811. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orian-Rousseau V: CD44, a therapeutic
target for metastasising tumours. Eur J Cancer. 46:1271–1277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lv L, Liu HG, Dong SY, Yang F, Wang QX,
Guo GL, Pan YF and Zhang XH: Upregulation of CD44v6 contributes to
acquired chemoresistance via the modulation of autophagy in colon
cancer SW480 cells. Tumour Biol. 37:8811–8824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rice TW, Gress DM, Patil DT, Hofstetter
WL, Kelsen DP and Blackstone EH: Cancer of the esophagus and
esophagogastric junction-Major changes in the American Joint
Committee on Cancer eighth edition cancer staging manual. CA Cancer
J Clin. 67:304–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan L, Zucker S and Toole BP: Roles of the
multifunctional glycoprotein, emmprin (basigin; CD147), in tumour
progression. Thromb Haemost. 93:199–204. 2005.PubMed/NCBI
|
|
15
|
Wu J, Ru NY, Zhang Y, Li Y, Wei D, Ren Z,
Huang XF, Chen ZN and Bian H: HAb18G/CD147 promotes
epithelial-mesenchymal transition through TGF-β signaling and is
transcriptionally regulated by Slug. Oncogene. 30:4410–4427. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu S, Li Y, Mi L, Zhang Y, Zhang L, Gong
L, Han X, Yao L, Lan M, Chen Z and Zhang W: Clinical impact of
HAb18G/CD147 expression in esophageal squamous cell carcinoma. Dig
Dis Sci. 56:3569–3576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou C, Liu S, Zhou X, Xue L, Quan L, Lu
N, Zhang G, Bai J, Wang Y, Liu Z, et al: Overexpression of human
pituitary tumor transforming gene (hPTTG), is regulated by
beta-catenin/TCF pathway in human esophageal squamous cell
carcinoma. Int J Cancer. 113:891–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shah PP, Fong MY and Kakar SS: PTTG
induces EMT through integrin αVβ3-focal adhesion kinase signaling
in lung cancer cells. Oncogene. 31:3124–3135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shah PP and Kakar SS: Pituitary tumor
transforming gene induces epithelial to mesenchymal transition by
regulation of Twist, Snail, Slug, and E-cadherin. Cancer Lett.
311:66–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Yang Y, Chen L, Zheng D and Ma J:
Overexpression of pituitary tumor transforming gene (PTTG) is
associated with tumor progression and poor prognosis in patients
with esophageal squamous cell carcinoma. Acta Histochem.
116:435–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao H, Zhong F, Xie J, Peng J and Han Z:
PTTG promotes invasion in human breast cancer cell line by
upregulating EMMPRIN via FAK/Akt/mTOR signaling. Am J Cancer Res.
6:425–439. 2016.PubMed/NCBI
|
|
22
|
Saito S, Okabe H, Watanabe M, Ishimoto T,
Iwatsuki M, Baba Y, Tanaka Y, Kurashige J, Miyamoto Y and Baba H:
CD44v6 expression is related to mesenchymal phenotype and poor
prognosis in patients with colorectal cancer. Oncol Rep.
29:1570–1578. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kunishi M, Kayada Y and Yoshiga K:
Down-regulated expression of CD44 variant 6 in oral squamous cell
carcinomas and its relationship to regional lymph node metastasis.
Int J Oral Maxillofac Surg. 26:280–283. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li L, Tang W, Wu X, Karnak D, Meng X,
Thompson R, Hao X, Li Y, Qiao XT, Lin J, et al: HAb18G/CD147
promotes pSTAT3-mediated pancreatic cancer development via CD44s.
Clin Cancer Res. 19:6703–6715. 2013. View Article : Google Scholar : PubMed/NCBI
|