Introduction
The prostate stem cell antigen (PSCA) gene encodes a
glycosylphosphatidylinositol (GPI)-anchored membrane protein
(1). Although its biological function
is unknown, it is thought that PSCA is localized to lipid rafts on
the outer surface of the cell membrane, a special microdomain
enriched in glycosphingolipids, cholesterol and other lipidated
proteins, and has certain functions in subcellular signal
transduction (2). Originally, PSCA
was identified as a gene upregulated in prostate cancer (3), and later its upregulation was
demonstrated in other types of tumor, including urinary bladder
cancer, renal cell carcinoma, hydatidiform mole and ovarian
mucinous tumor, where PSCA is thought to be involved in tumor
progression (1). However,
downregulation of the gene was reported in gastric and gallbladder
cancer, where it may act as a tumor suppressor (4,5). As
another pattern of its expression in cancer, PSCA is not expressed
in the ductal cells of the normal pancreas or the epithelial cells
in the normal lung, however, it is expressed in their malignant
counterparts, pancreatic cancer and non-small cell lung cancer
(6,7).
It was previously reported that PSCA expression was
almost absent in the normal human brain but was detected in glioma,
and it may represent a suitable target for immunotherapy of gliomas
(8). Additionally, PSCA protein was
recently detected in human brain cortex by western blot analyses
(9). PSCA expression in the brain was
also reported in other animals, including in the cerebellum,
telencephalon and autonomic ganglia of chick embryos and adult
mice, and in the frontal cortex and hippocampus of rats (10,11).
However, the detail of its expression in the human brain is yet to
be elucidated.
The current study aimed to examine PSCA expression
sites in the normal human brain. Immunohistochemistry was performed
using a mouse monoclonal anti-PSCA antibody, which revealed its
expression in several types of normal cells in the brain. The
expression status in their malignant counterparts was also
examined.
Materials and methods
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA samples isolated from normal human brain
tissues were obtained from Clontech Laboratories, Inc. (Mountain
View, CA, USA), which include frontal lobe (cat. no. 636563),
temporal lobe (cat. no. 636564), parietal lobe (cat. no. 636571),
occipital lobe (cat. no. 636570), paracentral gyrus (cat. no.
636574), postcentral gyrus (cat. no. 636573), insula (cat. no.
636568), corpus callosum (cat. no. 636567), putamen (cat. no.
636575), substantia nigra (cat. no. 636560), nucleus ambiguous
(cat. no. 636569), hippocampus (cat. no. 636593), cerebellum (cat.
no. 636535), pons (cat. no. 636572), medulla oblongata (cat. no.
636562) and stomach RNA (BioChain Institute Inc., Newark, CA, USA).
The templates cDNA were synthesized using the ThermoScript RT-PCR
system (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). qPCR was performed using a TaqMan Gene Expression assay
(assay ID Hs00194665_m1 for PSCA; Applied Biosystems; Thermo Fisher
Scientific, Inc.; cat. no. 4326317E for GAPDH), which was conducted
for 40 cycles of 95°C for 15 sec and 60°C for 60 sec, using ABI
PRISM 7900HT Sequence Detection system (Thermo Fisher Scientific,
Inc.). The relative transcript level was calculated using the
2−∆∆Cq method (12) with
GAPDH transcript as a reference, as it had been demonstrated
in our previous studies that GAPDH is an excellent reference
for quantification of the PSCA expression (4,5). For gel
electrophoresis, RT-PCR was conducted for 35 cycles of 94°C for 60
sec, 5894 for 60 sec and 60°C for 60 sec using the following primer
pairs: PSCA, 5′-TGGAGAACTGCACCCAGCT-3′ and
5′-GACTTGCGTTAGGATGTGCC-3′; ACTB, 5′-TCATCACCATTGGCAATGAG-3′
and 5′-CACTGTGTTGGCGTACAGGT-3′.
Immunohistochemistry
Embedded normal brain tissue specimens on glass were
purchased from BioChain Institute, Inc. (cat. nos. T8234564,
T8234444, T8234445, T2234043 and T2234044). The PSCA expression in
brain tumor tissues was examined using two tissue arrays: Brain
disease spectrum (brain cancer progression) tissue array (cat. no.
CNS801; US Biomax, Inc., Rockville, MD, USA), and multiple brain
cancer and normal adjacent tissue array (cat. no. GL1001; US
Biomax, Inc.). Immunohistochemistry was performed with mouse
monoclonal anti-PSCA antibod, which was produced in our previous
study (4) and whose specificity had
been demonstrated previously (4,5), and
rabbit anti-PCNA antibody (cat. no. sc-7907; Santa Cruz
Biotechnology, Dallas, Texas, USA). The sections were incubated at
4°C overnight with both antibodies (each 1:50 dilution)
simultaneously, and then with an alkaline phosphatase-conjugated
anti-mouse IgG antibody (cat. no. 414241; NICHIREI Biosciences,
Inc., Tokyo, Japan) and peroxidase-conjugated anti-rabbit IgG
antibody (cat. no. 424141; NICHIREI Biosciences, Inc.), at original
concentration, for 30 min at room temperature. PSCA protein was
visualized using Alkaline Phosphatase Substrate kit III (Vector
Laboratories, Inc., Burlingame, CA, USA) and PCNA by VECTOR NovaRED
Substrate kit for Peroxidase (Vector Laboratories, Inc.). For
immunocytochemistry, using SuperFect Transfection Reagent (Qiagen,
Inc., Valencia, CA, USA), a pcDNA3.1 construct for expressing
PSCA sense strand or that expressing PSCA antisense
was introduced into HSC-57 gastric cancer cell line, not into brain
tissue-derived cell lines, as it is the cell line in which no
PSCA expression had been demonstrated in our previous
studies (4,5). HSC-57 was established and provided by
Dr. Kazuyoshi Yanagihara (4) and
maintained in Dulbecco's modified Eagle's medium supplemented with
10% bovine serum at 37°C under an atmosphere of 5% CO2.
The immunocytochemistry was performed in the same manner as the
immunohistochemistry, after fixing the cells on the chamber slides
using 4% paraformaldehyde.
Results
PSCA is expressed in neural and
choroid plexus cells in the normal human brain
Initially, PSCA expression in normal human brain
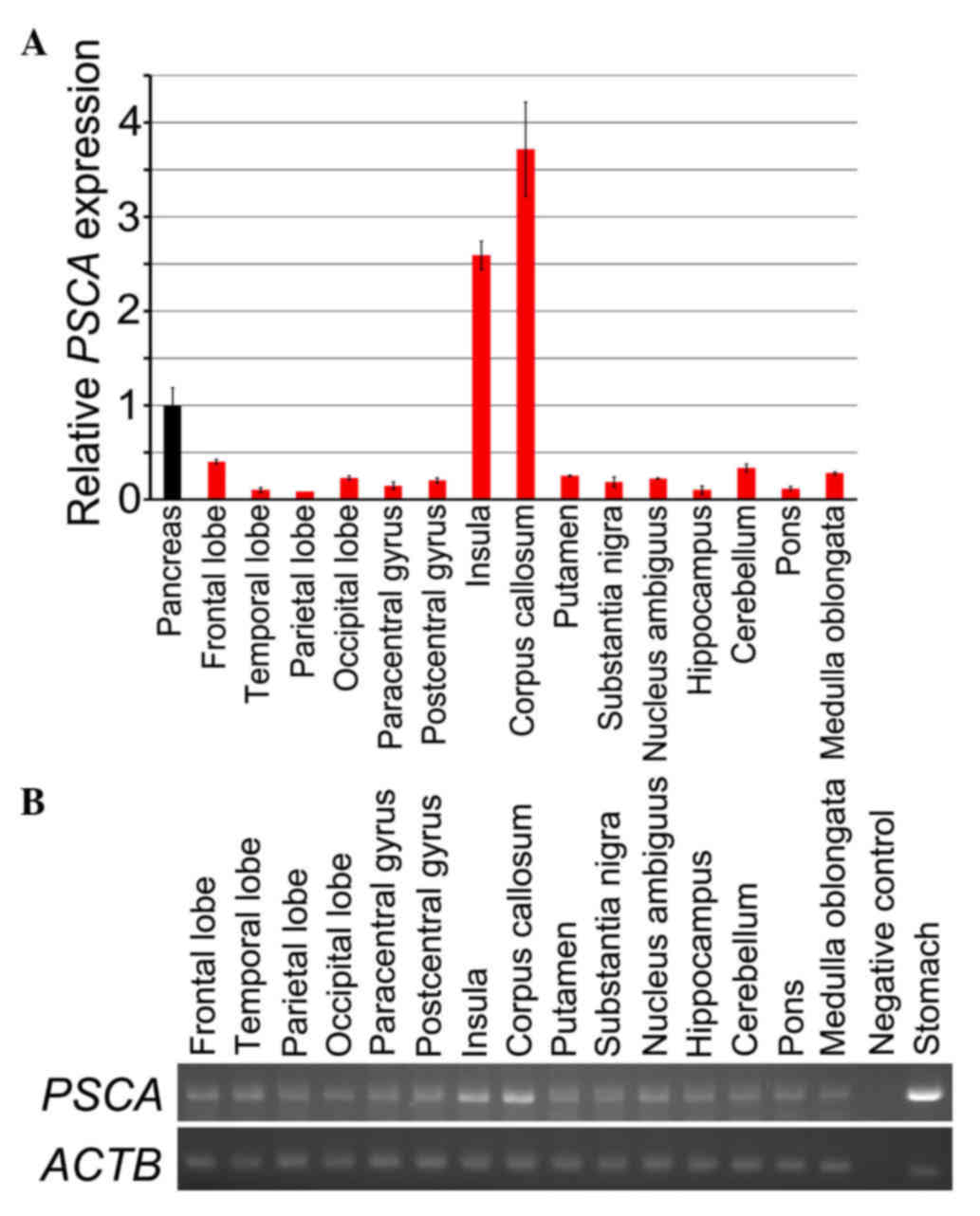
tissues was examined by RT-qPCR (Fig.
1A). PSCA transcripts were detected in all the tissues
examined, however the expression level was weak; the mean ∆Cq
values of the samples were <30, except in the insula and corpus
callosum, exhibiting a far lower expression compared with the level
in the stomach (data not shown). Consequently, the amount of the
PSCA transcripts in each tissue is demonstrated in Fig. 1A, in comparison with the amount in the
pancreas, which expresses PSCA at a lower level than the
stomach (13). However, analyses of a
standard curve for PSCA amplification revealed that the
expression level of the tissue was so low that it may be out of the
range in which the Cq values precisely reflect the difference in
the amount of the transcripts among the tissues. Consequently, the
result may be lacking in precise quantitativity. Amplified product
was also observed for all the samples by agarose gel
electrophoresis (Fig. 1B).
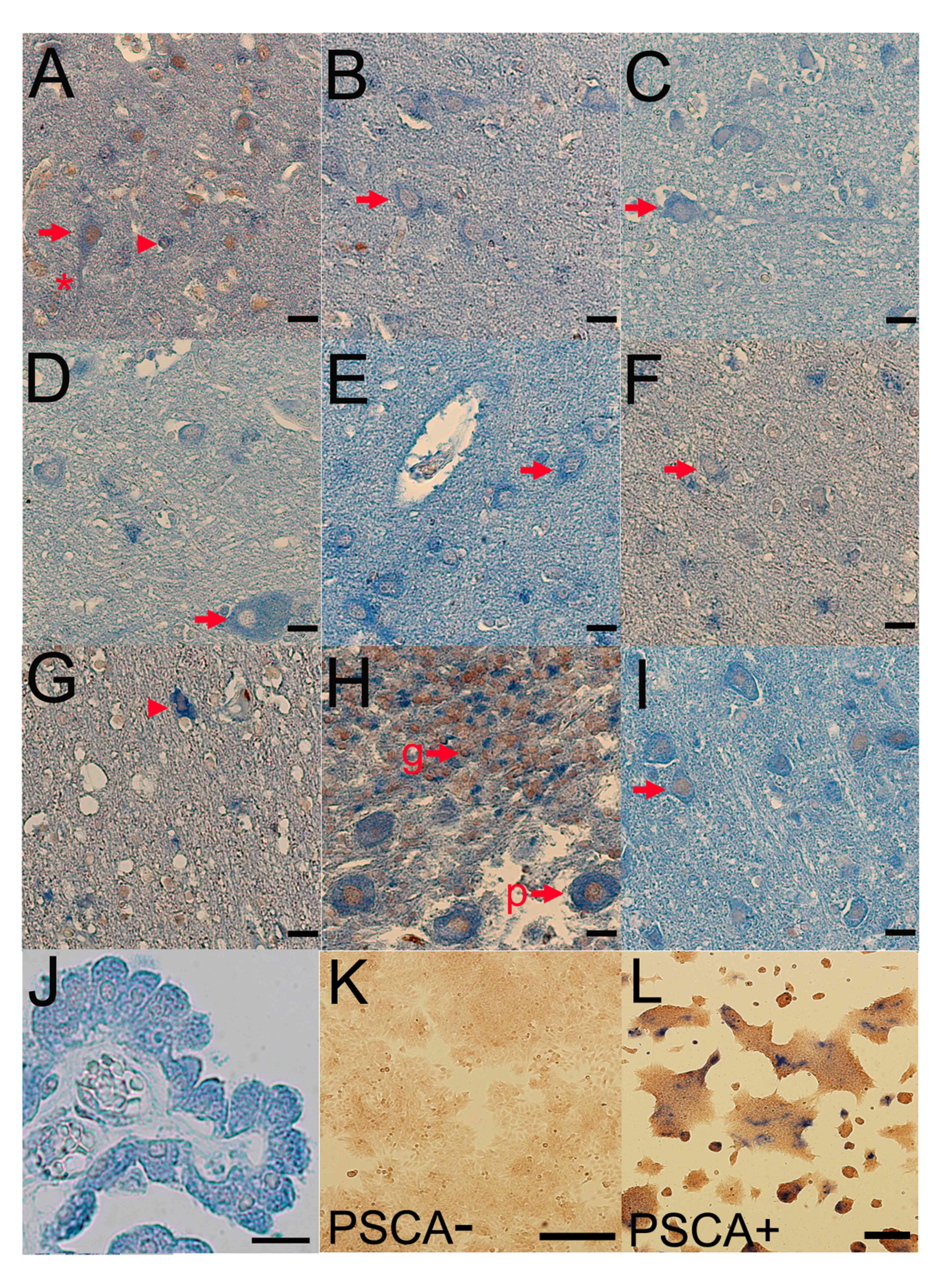
Subsequently, double-staining immunohistochemistry
was performed for PSCA and PCNA (proliferating cell nuclear
antigen), which detected PSCA proteins in the frontal lobe
(Fig. 2A), precentral gyrus (Fig. 2B), postcentral gyrus (Fig. 2C), temporal lobe (Fig. 2D), parietal lobe (Fig. 2E), occipital lobe (Fig. 2F), corpus callosum (Fig. 2G), celleberum (Fig. 2H) and pons (Fig. 2I) of the normal human brain (Fig. 2). The PSCA staining was predominantly
observed in the perikaryon, a cell body consisting of a nucleus and
the surrounding cytoplasm, but also in the dendrites of neuronal
cells (asterisk in Fig. 2A). The
expression level was weak, compared with that in gastric and
gallbladder epithelium (4,5), which reduced the signal/noise ratio in
this immunohistochemical expression analysis. In addition to the
neuronal cells, PSCA expression was also detected in choroid plexus
cells (Fig. 2J). PSCA dependent
staining was confirmed by imunocytochemistry using a gastric cancer
cell line with and without PSCA expression (Fig. 2K and L).
PSCA is expressed in brain tumors
As PSCA exhibits an organ-dependent expression
pattern in cancer (1), its expression
status was investigated in brain tumors using immunohistochemistry
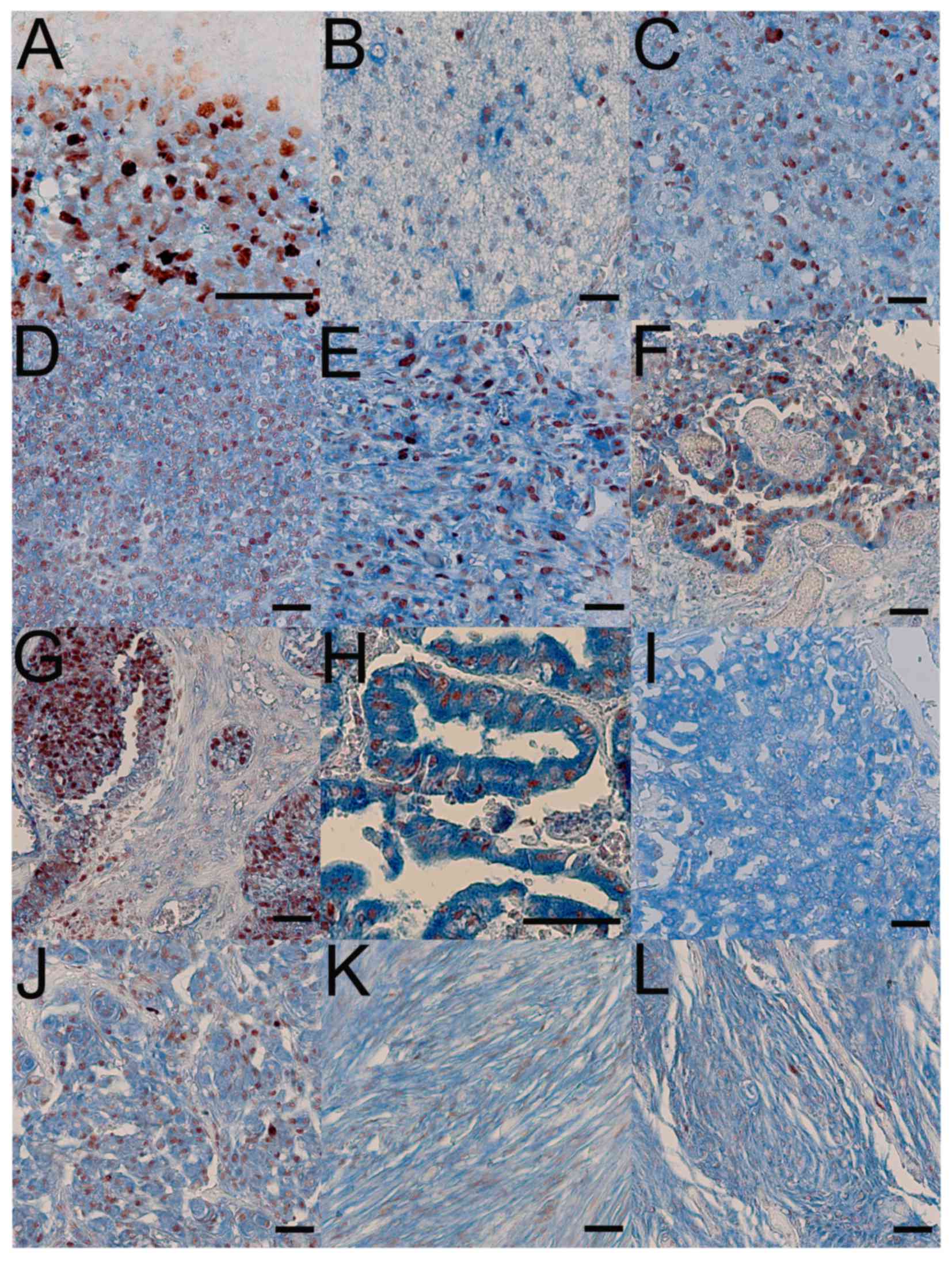
(Fig. 3 and Table I). The current study detected PSCA
expression in medulloblastoma (Fig.
3A), and, in 8 of 14 cases, the staining was clearly stronger
than in normal neuronal cells in the cerebral cortex.
 | Table I.Results of immunohistochemical
analysis of prostate stem cell antigen expression in brain
tumors. |
Table I.
Results of immunohistochemical
analysis of prostate stem cell antigen expression in brain
tumors.
| Cancer type | Tumor | Cases | Expression
(%)a |
|---|
| Medulloblastoma | 14 | 14 | 100.0 |
| Astrocytoma |
|
|
|
| I | 16 | 12 | 75.0 |
| II | 49 | 41 | 83.7 |
| III | 6 | 6 | 100.0 |
| IV | 9 | 9 | 100.0 |
|
Oligodendroglioma |
|
|
|
| II | 6 | 5 | 83.3 |
| III | 2 | 2 |
|
| Ependymoma |
|
|
|
| II | 3 | 3 | 100.0 |
| III | 3 | 3 | 100.0 |
| Ependymoblastoma |
|
|
|
| IV | 2 | 2 |
|
| Choroid plexus |
|
|
|
|
Papilloma | 2 | 2 |
|
| Papillary
carcinoma | 1 | 1 |
|
| Meningioma |
|
|
|
|
Meningotheliomatous | 3 | 3 | 100.0 |
|
Fibrous | 15 | 15 | 100.0 |
|
Transitional | 10 | 10 | 100.0 |
As previously demonstrated in another study
(8), PSCA expression was observed in
glioma, including astrocytoma (Fig.
3B-D) and oligodedroglioma (Fig.
3E), and its positive staining rate in astrocytoma is
associated with stage-progression; 75% in grade I, 83.7% in grade
II, 100% in grade III and IV. In addition to glioma, PSCA
expression was also exhibited in ependymoma (Fig. 3F) and ependymoblastoma (Fig. 3G), and a relatively strong PSCA
expression was revealed in choroid plexus papilloma (Fig. 3H) and papillary carcinoma (Fig. 3I), although this seems to reflect the
expression status in normal choroid plexus cells (Fig. 2J).
Furthermore, PSCA expression was observed in
meningioma (Fig. 3J-L). It appeared
to be upregulated in all the specimens, as PSCA expression was not
detected in the normal meninges in the current immunohistochemistry
analysis (data not shown). However, the relative level of the PSCA
signal in meningioma compared with in normal meninges was not
evaluated because the tissue array contained no reference for
meningeal expression.
Discussion
The current study demonstrated PSCA expression in
the neuronal cells of the brain, to the best of our knowledge, for
the first time. However, its function in human brain and the brain
of other animals is unknown. Although unknown within the central
nervous system, the function of PSCA in neuronal cells is well
elucidated in the peripheral ganglion of the chick. In chick
ciliary ganglion, α7-containing nicotinic acetylcholine receptors
have a role in the induction of the developmentally regulated cell
death of neuronal cells, and PSCA has an antagonistic function
against the cell death induction and rescues neuronal cells from
cell death (10). In the development
of the ganglion, PSCA expression is also developmentally regulated.
A previous study reported developmental regulation of PSCA
expression in the rat brain, however, in the study, the expression
was examined by RT-PCR analyses on brain tissues, not by
immunohistochemistry to reveal the precise cell types with PSCA
expression (11). Notably, PSCA
knockout mice were viable and exhibited no gross abnormality
(14).
The PSCA expression in the choroid plexus is
notable, as it is a type of ependymal cells specialized for
cerebrospinal fluid production. PSCA is thought to have a role in
cell differentiation and proliferation in certain epithelial
tissues, including gastric epithelium. However, its expression in
gallbladder epithelium, which consists of a single layer of the
cells and functions in absorbing water and electrolytes for bile
condensation, suggests that it may have a simple function in the
gallbladder. PSCA may also have a simple function, such as fluid
import and export in the choroid plexus.
To the best of our knowledge, the current study is
the first to demonstrated that PSCA is expressed in medulloblastoma
and meningioma. As reported in a previous study (8), the PSCA expression status is positively
correlated to the grade of astrocytoma (Table I).
The results of the current study suggest that, in
the brain tumors, PSCA has a tumor-promoting function and may be a
promising therapeutic target. However, it is also important to note
that PSCA is expressed in the normal brain, because it is
considered as a target molecule in for treatment of pancreatic and
prostate cancers, in addition to brain tumors (15,16). Thus,
clinical applications targeting PSCA have a potential risk of
leading to adverse events in the central nervous system.
Acknowledgements
This study was supported by a JST grant for the
personalized medicine project and by JSPS KAKENHI (grant no.
23501327).
Glossary
Abbreviations
Abbreviations:
|
PSCA
|
prostate stem cell antigen
|
References
|
1
|
Saeki N, Gu J, Yoshida T and Wu X:
Prostate stem cell antigen: A Jekyll and Hyde molecule? Clin Cancer
Res. 16:3533–3538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharom FJ and Radeva G: GPI-anchored
protein cleavage in the regulation of transmembrane signals.
Subcell Biochem. 37:285–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reiter RE, Gu Z, Watabe T, Thomas G,
Szigeti K, Davis E, Wahl M, Nisitani S, Yamashiro J, Le Beau MM, et
al: Prostate stem cell antigen: A cell surface marker overexpressed
in prostate cancer. Proc Natl Acad Sci USA. 95:pp. 1735–1740. 1998;
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Study Group of Millennium Genome Project
for Cancer, ; Sakamoto H, Yoshimura K, Saeki N, Katai H, Shimoda T,
Matsuno Y, Saito D, Sugimura H, Tanioka F, et al: Genetic variation
in PSCA is associated with susceptibility to diffuse-type gastric
cancer. Nat Genet. 40:730–740. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ono H, Hiraoka N, Lee YS, Woo SM, Lee WJ,
Choi IJ, Saito A, Yanagihara K, Kanai Y, Ohnami S, et al: Prostate
stem cell antigen, a presumable organ-dependent tumor suppressor
gene, is down-regulated in gallbladder carcinogenesis. Genes
Chromosomes Cancer. 51:30–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Argani P, Rosty C, Reiter RE, Wilentz RE,
Murugesan SR, Leach SD, Ryu B, Skinner HG, Goggins M, Jaffee EM, et
al: Discovery of new markers of cancer through serial analysis of
gene expression: Prostate stem cell antigen is overexpressed in
pancreatic adenocarcinoma. Cancer Res. 61:4320–4324.
2001.PubMed/NCBI
|
|
7
|
Kawaguchi T, Sho M, Tojo T, Yamato I, Nomi
T, Hotta K, Hamada K, Suzaki Y, Sugiura S, Kushibe K, et al:
Clinical significance of prostate stem cell antigen expression in
non-small cell lung cancer. Jpn J Clin Oncol. 40:319–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geiger KD, Hendruschk S, Rieber EP,
Morgenroth A, Weigle B, Juratli T, Senner V, Schackert G and Temme
A: The prostate stem cell antigen represents a novel
glioma-associated antigen. Oncol Rep. 26:13–21. 2011.PubMed/NCBI
|
|
9
|
Jensen MM, Arvaniti M, Mikkelsen JD,
Michalski D, Pinborg LH, Härtig W and Thomsen MS: Prostate stem
cell antigen interacts with nicotinic acetylcholine receptors and
is affected in Alzheimer's disease. Neurobiol Ageing. 36:1629–1638.
2015. View Article : Google Scholar
|
|
10
|
Hruska M, Keefe J, Wert D, Tekinay AB,
Hulce JJ, Ibañez-Tallon I and Nishi R: Prostate stem cell antigen
is an endogenous lynx1-like prototoxin that antagonizes
alpha7-containing nicotinic receptors and prevents programmed cell
death of parasympathetic neurons. J Neurosci. 29:14847–14854. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thomsen MS, Cinar B, Jensen MM, Lyukmanova
EN, Shulepko MA, Tsetlin V, Klein AB and Mikkelsen JD: Expression
of the Ly-6 family proteins Lynx1 and Ly6H in the rat brain is
compartmentalized, cell-type specific, and developmentally
regulated. Brain Struct Funct. 219:1923–1934. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ono H, Yanagihara K, Sakamoto H, Yoshida T
and Saeki N: Prostate stem cell gene is expressed in islets of
pancreas. Anat Cell Biol. 45:149–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Moore ML, Teitell MA, Kim Y, Watabe T,
Reiter RE, Witte ON and Dubey P: Deletion of PSCA increases
metastasis of TRAMP-induced prostate tumors without altering
primary tumor formation. Prostate. 68:139–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wente MN, Jain A, Kono E, Berberat PO,
Giese T, Reber HA, Friess H, Büchler MW, Reiter RE and Hines OJ:
Prostate stem cell antigen is a putative target for immunotherapy
in pancreatic cancer. Pancreas. 31:119–125. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morris MJ, Eisenberger MA, Pili R,
Denmeade SR, Rathkopf D, Slovin SF, Farrelly J, Chudow JJ, Vincent
M, Scher HI and Carducci MA: A phase I/IIA study of AGS-PSCA for
castration-resistant prostate cancer. Ann Oncol. 23:2714–2719.
2012. View Article : Google Scholar : PubMed/NCBI
|