Introduction
Ovarian cancer remains one of the leading causes of
cancer-associated mortality, representing a serious threat to the
health and lives of women globally (1). However, the exact molecular mechanisms
leading to ovarian tumorigenesis remain to be fully elucidated.
Despite advances in the diagnostic and therapeutic methods
associated with ovarian cancer, there have been no significant
changes in the 5-year survival rate of patients due to a lack of
specific symptoms in the early stage and chemotherapy resistance
(2). Therefore, it is important to
identify early diagnostic markers and novel therapeutic targets for
the treatment of ovarian cancer.
Enhancer of zeste homolog 2 (EZH2), an epigenetic
regulator, functions as a histone methyltransferase specific to
histone H3 lysine 27 (H3K27), and is an important component of
polycomb repressive complex 2. Studies have reported that EZH2 is
involved in the progression and development of several types of
cancer via its effects on promoting cell proliferation, migration
and invasion (3). Ectopic expression
of EZH2 has been observed in pancreatic cancer (4), breast cancer (5) and colon cancer (6), and it is significantly associated with
the poor prognosis of patients. Our previous study indicated that
the mRNA and protein levels of EZH2 were significantly increased in
ovarian cancer, compared with those in normal tissues. Furthermore,
the inhibition of EZH2 repressed the proliferation and migration of
cancer cells in vitro and in vivo (7).
P16 is a well-known cell cycle regulator, which
controls the G1-to-S transition by inhibiting cyclin-dependent
kinases 4 and 6, and is a critical tumor suppressor (8). Aberrant expression of p16 may interfere
with the normal cell cycle, induce uncontrolled cell proliferation
and, finally, result in tumorigenesis (9). Cui et al demonstrated that DNA
methylation at the p16 promoter, particularly at the CpG islands,
directly inactivated its transcription and facilitated cancer
migration and invasion due to the inhibition of p16 (10). In addition, the upregulation of p16 in
ovarian cancer was demonstrated to decrease the translation of
eukaryotic translation elongation factor 1α2 protein and reduce the
proliferation of tumor cells, including PA-1, SKOV3 and OVCAR8
cells, in vitro (11). Kong
et al reported that the silencing of EZH2 using short
hairpin RNA (shRNA) increased the mRNA and protein levels of p16 in
gastric cancer cells (12).
Therefore, it has been hypothesised that p16 may be one of the
target genes of EZH2 in ovarian cancer. The present study aimed to
elucidate the function of EZH2 in the regulation of p16, and its
functions in the progression of ovarian cancer.
Materials and methods
Cell culture
Human A2780 and SKOV3 ovarian cancer cell lines were
purchased from the China Center for Type Culture Collection (Wuhan,
China). The two cell lines were grown in RPMI-1640 media (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) and maintained in 5% CO2 at 37°C in incubators
with suitable humidity.
Lentivirus construction and
transduction
The specific shRNA-targeted EZH2 was synthesised
according to the EZH2 gene sequences obtained from GenBank
(accession no. NM_004456). Following an annealing reaction in a
polymerase chain reaction (PCR) instrument, in which the
complementary DNA fragments of shEZH2 and shNC were dissolved in
annealing buffer (Beyotime Institute of Biotechnology Co., Ltd.,
Shanghai, China) and placed in a water bath at 90°C for 15 min,
then cooled to room temperature, the shEZH2 and negative control
(shNC) fragments were cloned into the shRNA expression vector
(GeneChem Co., Ltd., Shanghai, China) and used for lentiviral
packaging. Lentiviral transduction of the A2780 and SKOV3 cells was
performed according to the manufacturer's protocol. The sequences
of EZH2 siRNA oligonucleotides were as follows:
5′-GAAATCTTAAACCAAGAAT-3′. The sequences of NC siRNA
oligonucleotides were as follows: 5′-TTCTCCGAACGTGTCACGT-3′.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR) analysis
Total RNAs of the A2780 and SKOV3 were extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The RT reaction was then
performed using a PrimeScript™ RT reagent kit (Takara Bio, Inc.,
Kyoto, Japan). The EZH2 primer sequences were as follows:
5′-TTGTTGGCGGAAGCGTGTAAAATC-3′ for the forward primer and
5′-TCCCTAGTCCCGCGCAATGAGC-3′ for the reverse primer. The p16 primer
sequences were as follows: 5′-CCTTTGGTTATCGCAAGCTG-3′ for the
forward primer and 5′-CCCTGTAGGACCTTCGGTGA-3′ for the reverse
primer. The β-actin primer sequences were as follows:
5′-GTCCACCGCAAATGCTTCTA-3′ for the forward primer and
5′-TGCTGTCACCTTCACCGTTC-3′ for the reverse primer. An Applied
Biosystems 7300 Real-time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was used for the RT-qPCR analysis. Each
reaction sample was composed of 10 µl of SYBR-Green Real-time PCR
master mix (Rox; Roche Diagnostics GmbH, Mannheim, Germany), 7.3 µl
of RNase-free water, 0.6 µl of 10 µM primer and 1.5 µl of cDNA
sample. The reactions were performed according to the following
cycling conditions: Initial denaturation at 95°C for 10 min,
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
relative mRNA levels of EZH2 and p16 were calculated using the
comparative quantification cycle (Cq) method (2−ΔΔCq)
(13) normalised by the expression of
β-actin. All experiments were repeated three times.
Protein isolation and western blot
analysis
The A2780 and SKOV3 cell lysates were collected
using radioimmunoprecipitation assay buffer (EMD Millipore,
Billerica, MA, USA). The total proteins (40 µg) were boiled in a
water bath for 5 min following detection of the protein
concentrations of each sample using a bicinchoninic acid assay
(Beyotime Institute of Biotechnology Co., Ltd.). Following this,
the denatured proteins were separated by 10% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis. The proteins were then
transferred onto a polyvinylidene difluoride membrane. The membrane
was blocked for 2 h with freshly prepared 5% bovine serum albumin
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China), and the membrane then was incubated with rabbit anti-EZH2
polyclonal antibody (1:1,000 dilution; cat. no. ab3748; Abcam,
Cambridge, MA, USA) or rabbit anti-p16 monoclonal antibody (1:2,000
dilution; cat. no. ab51243; Abcam) overnight at 4°C. The
fluorescent dye-conjugated anti-rabbit secondary antibody (1:15,000
dilution; cat. no. 5366; Cell Signaling Technology, Danvers, MA,
USA) was used for detecting the primary antibodies for 1 h at room
temperature. Following washing three times with TBST, the protein
bands were visualised using an Odyssey infrared fluorescence
scanning imaging system (Odyssey; LI-COR Biosciences, Lincoln, NE,
USA).
Cell proliferation assay
A CCK-8 kit (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) was used to examine the proliferation ability of
the A2780 and SKOV3 cells following the downregulation of EZH2. In
brief, 48 h following lentiviral transduction, the ovarian cancer
cells (3,000/well) were seeded into 96-well plates with six
replicates and cultured at 37°C in a 5% CO2 atmosphere.
Every 24 h for 4 consecutive days, CCK-8 was added to one of the
96-well plates and incubated for 1 h, following which the optical
densities of each well were detected at 450 nm with a microplate
reader. Cell growth curves were drawn according to the average
optical densities of each day.
Cell migration assay
A Transwell migration assay was performed to analyse
the migration capability of the ovarian cancer cells. The A2780 and
SKOV3 cells were resuspended with 100 µl of serum-free RPMI-1640
medium at a density of 1×106 cells/well, and then seeded
into the upper chambers of the Transwell inserts (Corning Costar,
Cambridge, MA, USA). The lower chambers were filled with 600 µl
complete medium. Following incubation for 24 h, cells adhering to
the lower surface of the membrane were fixed with methanol and
stained with 0.1% crystal violet. The numbers of migrated cells
were counted in five randomly selected high-power fields
(magnification, ×400) under an optical microscope. The experiment
was repeated three times, and the average numbers of cells were
used for assessing the migration ability.
In vivo growth of ovarian cancer in a
xenograft model
For the cancer formation experiment, the present
study used A2780 cells, which can be readily used for stable
transfection. Briefly, 10 five-week-old female nude mice were
randomly divided into two groups, with each group containing five
nude mice. The BALB/C nude mice were obtained from the Animal
Experimental Centre of Guangxi Medical University. The room
temperature was between 24–25°C, and a 12-h light-dark diurnal
cycle was used. The mice were housed under specific pathogen-free
conditions in an animal facility with free access to a rodent diet,
including sunflower seeds and egg yolks, which were sterilized.
Access to water was ad libitum. The mice in each group were
subcutaneously injected with 1×106 shEZH2-A2780 cells or
shNC-A2780 cells into the dorsal flank. The tumor volumes were
measured every week, and the xenografts were removed for further
analysis when the mice had developed symptoms of cachexia. Each
tumor was analysed for mRNA levels of EZH2 and p16 via RT-qPCR
analysis. The present study was approved by the Animal Care and
Welfare Committee of the Department of Laboratory Animal Science of
Guangxi Medical University (Nanning, China).
Statistical analyses
Data processing was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). Significant differences
among multiple groups were evaluated using one-way analysis of
variance, and the Student's t-test was used for analysing
differences between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibition of EZH2 enhances the
expression of p16 in A2780 and SKOV3 cells
To investigate the expression of p16 following the
downregulation of EZH2, the present study selectively decreased the
expression of EZH2 in the A2780 and SKOV3 ovarian cancer cell lines
via an EZH2 shRNA lentiviral expression vector. The EZH2
interference efficiency and levels of p16 were then detected using
RT-qPCR and western blot analyses. The shNC lentiviral expression
vector was used as a negative control, and the untransduced cells
were used as a blank control. The results of the RT-qPCR analysis
indicated that the mRNA expression level of EZH2 in the
shEZH2-A2780 cells was 27.14±3.6% of that in the blank control,
which was significantly lower than the results for shNC-A2780 and
untransduced A2780 cells (P<0.001). By contrast, the mRNA
expression of p16 increased to 131.78±8.0% in the shEZH2-A2780
cells, compared with that in the untransduced A2780 cells
(P=0.001), whereas no significant difference was identified between
levels in the shNC-A2780 cells and the blank control (P>0.05;
Fig. 1A and B). In the shEZH2-SKOV3
cells, the mRNA level of EZH2 was reduced to 26.34±3.42%
(P<0.001). The mRNA expression of p16 was increased to
131.31±2.61% following EZH2-knockdown (P<0.001). No significant
differences were observed in the mRNA levels of EZH2 and p16
between the shNC-SKOV3 and untransduced SKOV3 cells (P>0.05;
Fig. 1A and B).
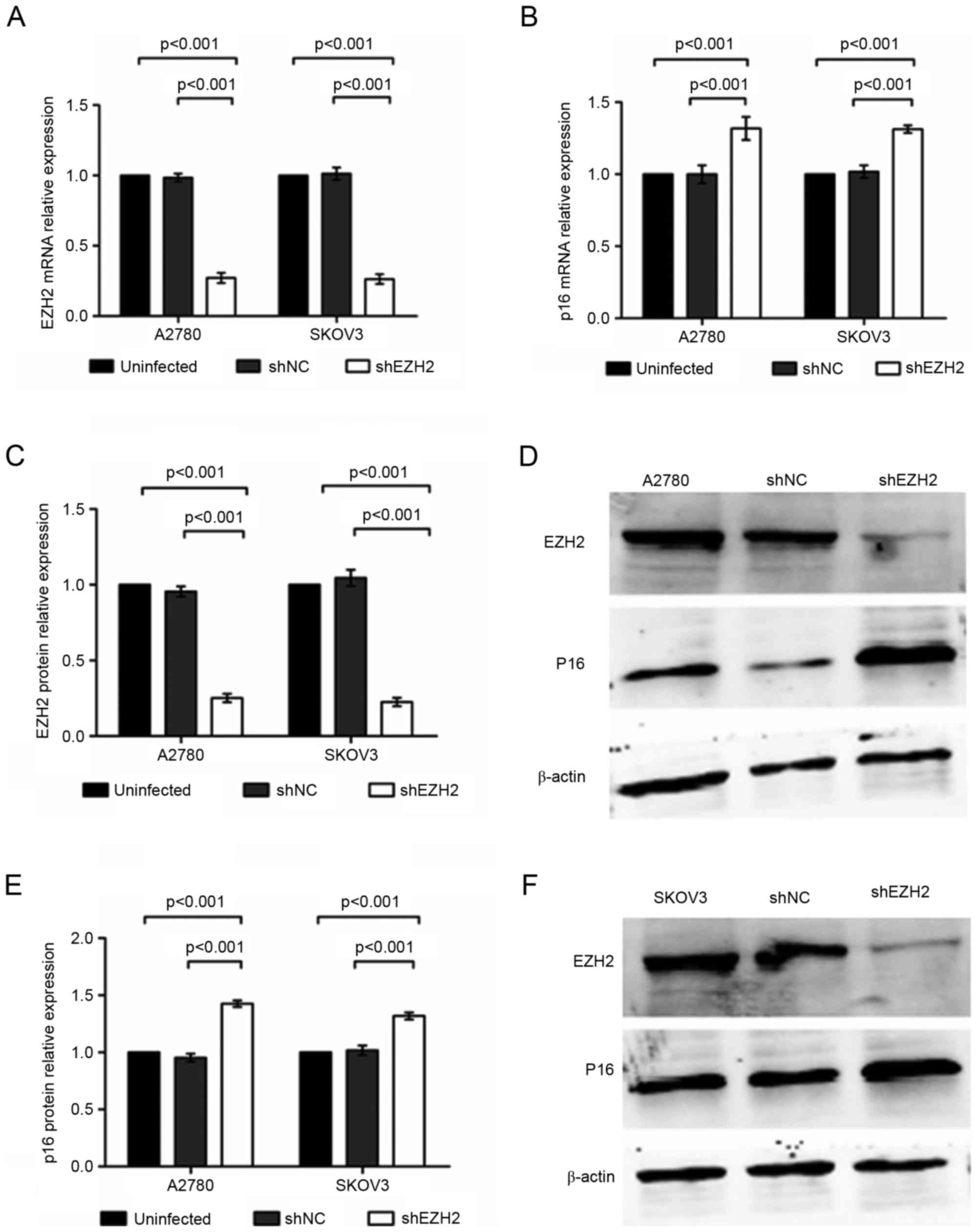 | Figure 1.Inhibition of EZH2 enhances the
expression of p16 in A2780 and SKOV3 cells. (A) mRNA expression of
EZH2 in A2780 and SKOV3 cells, detected using RT-qPCR analysis. (B)
mRNA expression of P16 in A2780 and SKOV3 cells, detected using
RT-qPCR analysis. (C) Protein expression of EZH2 in A2780 and SKOV3
cells, detected using western blot analysis. (D) Protein expression
of EZH2 and p16 in A2780, shNC-A2780 and shEZH2-A2780 cells. (E)
Protein expression of P16 in A2780 and SKOV3 cells, detected using
western blot analysis. (F) Protein expression of EZH2 and p16 in
SKOV3, shNC-SKOV3 and shEZH2-SKOV3 cells. EZH2, enhancer of zeste
homolog 2; sh, short hairpin RNA; NC, negative control; RT-qPCR,
reverse transcription-quantitative polymerase chain reaction. |
The results of the western blot analysis confirmed
that the protein level of EZH2 was reduced to 25.15±2.74% in the
shEZH2-A2780 cells, which was significantly lower, compared with
levels in the shNC-A2780 and untransduced A2780 cells (P<0.001).
In addition, the protein level of p16 in the shEZH2-A2780 cells was
increased to 142.55±2.82% following EZH2-knockdown (P<0.001). No
statistically significant difference was revealed between the
shNC-A2780 cells and the blank control (P>0.05; Fig. 1C-E). In the shEZH2-SKOV3 cells, the
protein expression of EZH2 was 22.60±2.78% of that in the blank
control, which was reduced, compared with the expression in the
negative and blank controls (P<0.001; Fig. 1C and F). By contrast, EZH2-knockdown
increased the protein level of p16 to 131.87±3.07% in the SKOV3
cells (P<0.001), whereas no significant differences were
observed in the protein levels of p16 between the shNC-SKOV3 and
untransduced SKOV3 cells exhibited no significant differences
(P>0.05; Fig. 1E and F). These
results revealed that the inhibition of EZH2 enhanced the
expression mRNA and protein expression levels of p16 in the A2780
and SKOV3 ovarian cancer cell lines.
EZH2-knockdown reduces the
proliferation capability of ovarian cancer cells in vitro
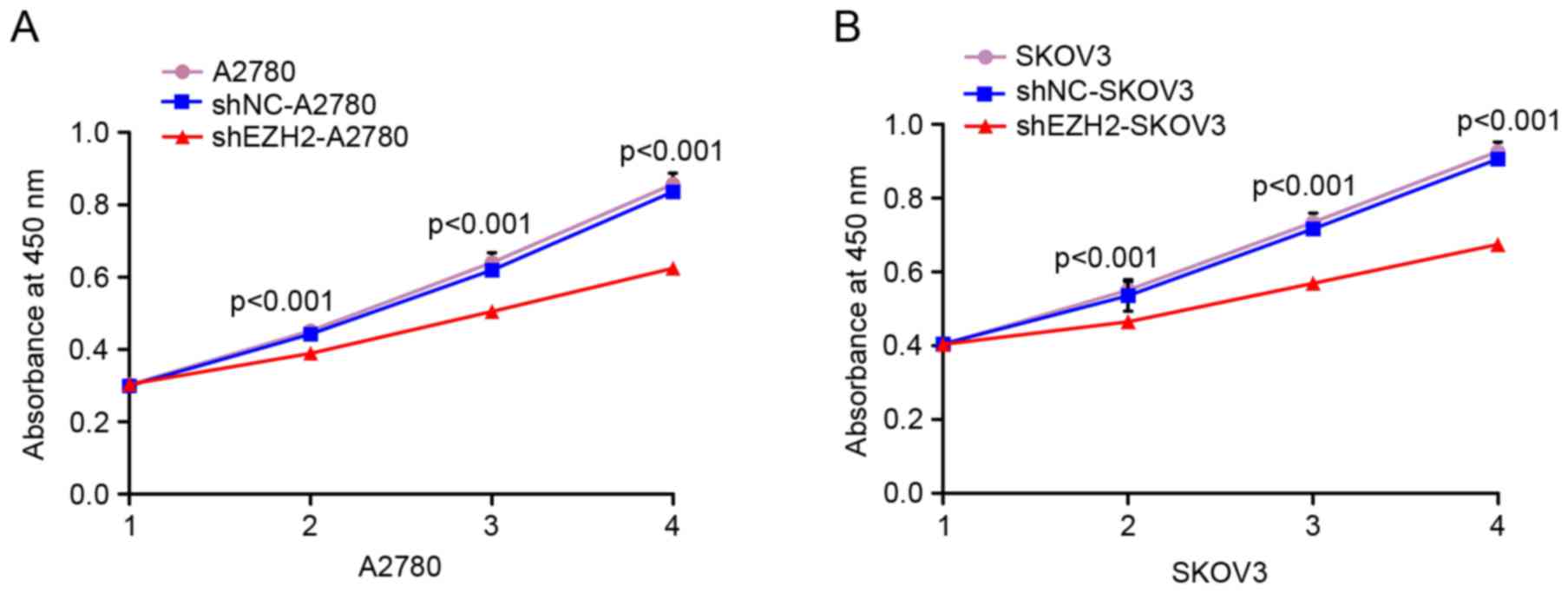
A CCK-8 assay was used for evaluating the effect of
the inhibition of EZH2 on cell proliferation. The cell growth
curves in the CCK-8 assay results demonstrated that, compared with
the negative and blank controls of the A2780 and SKOV3 cells, the
absorbance values of the shEZH2-A2780 and shEZH2-SKOV3 cells were
reduced on days 2, 3 and 4. These differences were statistically
significant (P<0.001 vs. negative control; P<0.001 vs. blank
control; Fig. 2A and B). This result
indicated that the proliferation of ovarian cancer cells was
decreased following EZH2-knockdown.
EZH2-silencing decreases ovarian
cancer cell migration in vitro
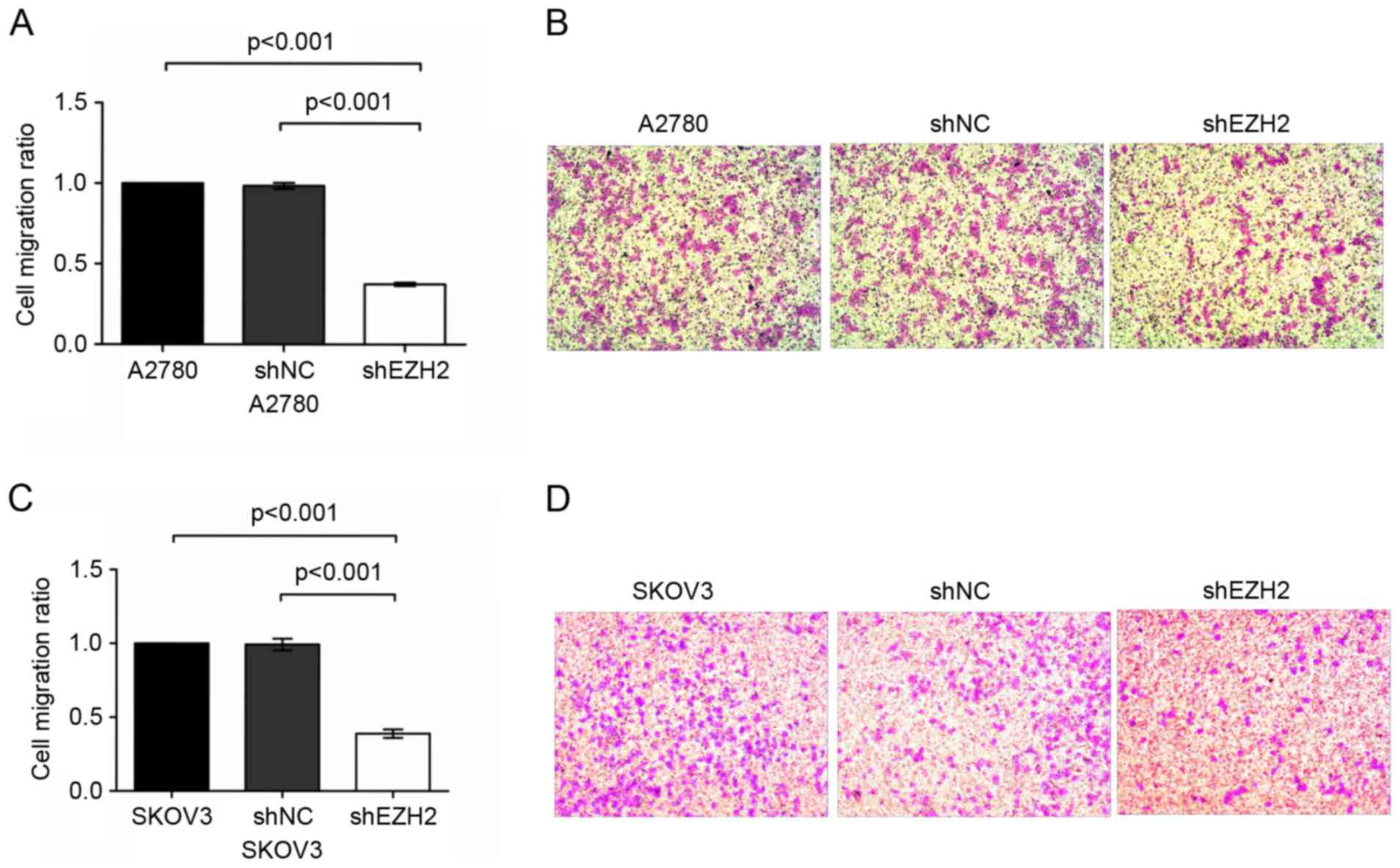
To evaluate the migration capability of the A2780
and SKOV3 cells, a Transwell migration assay was performed
following the inhibition of EZH2. It was observed that the number
of migrated shEZH2-A2780 cells was significantly lower, compared
with the number of migrated untransduced A2780 cells (P<0.001;
Fig. 3A and B). However, no
significant difference was revealed between the shNC-A2780 cells
and the blank controls (P>0.05).
In the SKOV3 ovarian cancer cell line, the number of
migrated shEZH2-SKOV3 cells was markedly reduced, which was
statistically significant, compared with the number of migrated
shNC-SKOV3 and untransduced SKOV3 cells (P<0.001 vs. shNC-SKOV3
cells and P<0.001 vs. untransduced SKOV3 cells; Fig. 3C and D). These data demonstrated that
EZH2-silencing was involved in regulating the migration of ovarian
cancer cells.
EZH2 depletion suppresses ovarian
tumor formation in vivo
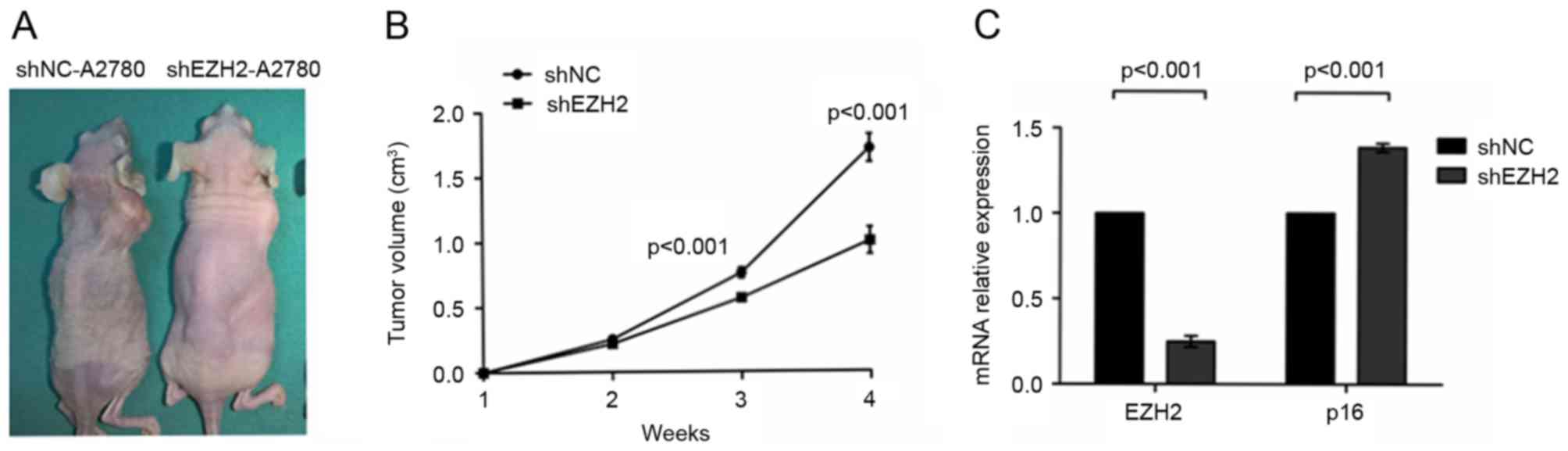
The present study further examined the function of
EZH2 in tumor development by inducing the growth of A2780 ovarian
cancer cells in a xenograft model. In brief, shNC-A2780 or
shEZH2-A2780 cells were injected into the dorsal flanks of five
nude mice each, and the tumor sizes of each nude mouse were
measured continuously for 4 weeks. After the 4 weeks, it was found
that the growth of tumors in the shEZH2-A2780 group was suppressed
(P<0.001; Fig. 4A and B). The
results of the RT-qPCR analysis revealed that the mRNA expression
of p16 in the shEZH2-A2780 cells was increased to 121.44±6.41%,
compared with shNC-A2780, and this difference was statistically
significant (P<0.001; Fig. 4C).
These data indicated that EZH2 depletion suppressed ovarian cancer
formation in vivo.
Discussion
Ovarian cancer is one of three types of malignant
tumor of the female reproductive system; it is also the most life
threatening gynaecological malignancy. Patients who are diagnosed
with advanced ovarian cancer are more likely to have a poor
prognosis; however, the exact pathogenesis of ovarian cancer
remains to be fully elucidated. Tumorigenesis involves oncogene
activation and the inhibition of tumor suppressor genes depending
on different physiological and pathological processes. Gene
expression is regulated by epigenetic mechanisms through DNA
methylation (14), histone
modifications (15), chromatin
remodelling (16) and non-coding RNA
regulation (17). Epigenetic
alterations may result in aberrant gene expression, eventually
promoting tumor development.
EZH2 is a critical histone methyltransferase, which
inhibits transcription by catalysing the trimethylation of H3K27 at
the promoters of target genes (18,19).
Several studies have revealed that EZH2 functions as an oncogene
and contributes to cancer genesis by promoting cell proliferation,
invasion and tumor drug resistance (3–21). In the
case of prostate cancer, Zhang et al revealed that EZH2
epigenetically silenced the expression of proapoptotic genes,
namely microRNA (miR)-31 and miR-205, and eventually reduced tumor
cell apoptosis (22). The protein
level of EZH2 was higher in colon cancer, compared with that in
paracancer tissues and was associated with a poor prognosis for
patients. The downregulation of EZH2 by specific siRNA has been
revealed to significantly decrease the proliferation and migration
ability of colon cancer cells in vitro (6). Zhou et al confirmed that the high
expression of EZH2 was involved in maintaining the resistance of
A549/DDP cells and AGS/DDP cells to cisplatin, and was partly
mediated by its epigenetic regulation of the multidrug resistance 1
gene (21). The present study
indicated that the proliferation and migration of ovarian cancer
cells were significantly suppressed in vitro and in
vivo following the downregulation of EZH2, which was consistent
with our previous study (7).
As a member of the INK family, p16 is important in
several physiological and pathological processes, including cell
cycle control, tumor suppression and the induction of apoptosis
(23). Loss of the expression of p16
often leads to cell cycle dysregulation, the increase of mitosis,
and finally tumorigenesis. In a nude mouse model, Chang et
al reported that orthotopic pancreatic cancer was induced by
the inactivation of p16 (24). In the
present study, it was found that the increased expression of p16,
which was induced by the inhibition of EZH2, suppressed the growth
of ovarian cancer cells in vitro and in vivo, and
reduced the migration ability of tumor cells in vitro. These
results indicated that p16 may act as a tumor suppressor in the
progression of ovarian cancer. However, previous studies have
demonstrated the tumor-promoting function of p16 by revealing that
viral protein E7 encoded by human papilloma virus (HPV) may
indirectly increase the expression of p16, and the two are involved
in the development of HPV-induced cervical cancer (25). Therefore, it appears that p16 has
different effects on different tissues.
A previous study based on lymphoma revealed that the
EZH2 protein is recruited at the promoter region of the p16 gene,
which is accompanied by a high level of H3K27me3 at the p16
promoter; these results revealed that EZH2 maintained malignant
tumor phenotypes (26). In the
present study, the expression of EZH2 was selectively inhibited in
ovarian cancer cells via an EZH2 shRNA lentiviral vector. The
results of subsequent RT-qPCR and western blot analyses indicated
that the mRNA and protein levels of p16 were increased following
the downregulation of EZH2, which suggested that the p16 gene may
be epigenetically regulated by EZH2 in ovarian cancer cells.
In conclusion, EZH2 depletion promoted the
expression of p16, which may contribute to the repression of
ovarian cancer proliferation in vitro and in vivo,
and to the inhibition of cell migration in vitro. The
results of the present study indicated the effect of EZH2 on the
epigenetic regulation of p16 in ovarian cancer, suggesting a novel
therapeutic approach for ovarian cancer treatment.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (grant no. 81360389) and the National
Natural Science Foundation of Guangxi (grant no.
2015jjAA40440).
References
|
1
|
Jelovac D and Armstrong DK: Recent
progress in the diagnosis and treatment of ovarian cancer. CA
Cancer J Clin. 61:183–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katsumata N: Dose-dense approaches to
ovarian cancer treatment. Curr Treat Options Oncol. 16:212015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamaguchi H and Hung MC: Regulation and
role of EZH2 in cancer. Cancer Res Treat. 46:209–222. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han T, Jiao F, Hu H, Yuan C, Wang L, Jin
ZL, Song WF and Wang LW: EZH2 promotes cell migration and invasion
but not alters cell proliferation by suppressing E-cadherin, partly
through association with MALAT-1 in pancreatic cancer. Oncotarget.
7:11194–11207. 2016.PubMed/NCBI
|
|
5
|
Guo S, Li X, Rohr J, Wang Y, Ma S, Chen P
and Wang Z: EZH2 overexpression in different immunophenotypes of
breast carcinoma and association with clinicopathologic features.
Diagn Pathol. 11:412016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Y, Lin C, Liao G, Liu S, Ding J,
Tang F, Wang Z, Liang X, Li B, Wei Y, et al: MicroRNA-506
suppresses tumor proliferation and metastasis in colon cancer by
directly targeting the oncogene EZH2. Oncotarget. 6:32586–32601.
2015.PubMed/NCBI
|
|
7
|
Guo J, Cai J, Yu L, Tang H, Chen C and
Wang Z: EZH2 regulates expression of p57 and contributes to
progression of ovarian cancer in vitro and in vivo. Cancer Sci.
102:530–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rayess H, Wang MB and Srivatsan ES:
Cellular senescence and tumor suppressor gene p16. Int J Cancer.
130:1715–1725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao R, Choi BY, Lee MH, Bode AM and Dong
Z: Implications of genetic and epigenetic alterations of CDKN2A
(p16INK4a) in cancer. EBioMedicine. 8:30–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui C, Gan Y, Gu L, Wilson J, Liu Z, Zhang
B and Deng D: P16-specific DNA methylation by engineered zinc
finger methyltransferase inactivates gene transcription and
promotes cancer metastasis. Genome Biol. 16:2522015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee MH, Choi BY, Cho YY, Lee SY, Huang Z,
Kundu JK, Kim MO, Kim DJ, Bode AM, Surh YJ and Dong Z: Tumor
suppressor p16(INK4a) inhibits cancer cell growth by downregulating
eEF1A2 through a direct interaction. J Cell Sci. 126:1744–1752.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong R, Zhang EB, Yin DD, You LH, Xu TP,
Chen WM, Xia R, Wan L, Sun M, Wang ZX, et al: Long noncoding RNA
PVT1 indicates a poor prognosis of gastric cancer and promotes cell
proliferation through epigenetically regulating p15 and p16. Mol
Cancer. 14:822015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu XS, Wu H, Ji X, Stelzer Y, Wu X,
Czauderna S, Shu J, Dadon D, Young RA and Jaenisch R: Editing DNA
methylation in the mammalian genome. Cell. 167:233–247.e17. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brunmeir R, Lagger S, Simboeck E, Sawicka
A, Egger G, Hagelkruys A, Zhang Y, Matthias P, Miller WJ and Seiser
C: Epigenetic regulation of a murine retrotransposon by a dual
histone modification mark. PLoS Genet. 6:e10009272010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou CY, Johnson SL, Gamarra NI and
Narlikar GJ: Mechanisms of ATP-dependent chromatin remodeling
motors. Annu Rev Biophys. 45:153–181. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Engreitz JM, Ollikainen N and Guttman M:
Long non-coding RNAs: Spatial amplifiers that control nuclear
structure and gene expression. Nat Rev Mol Cell Biol. 17:756–770.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chinaranagari S, Sharma P and Chaudhary J:
EZH2 dependent H3K27me3 is involved in epigenetic silencing of ID4
in prostate cancer. Oncotarget. 5:7172–7182. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luo H, Jiang Y, Ma S, Chang H, Yi C, Cao
H, Gao Y, Guo H, Hou J, Yan J, et al: EZH2 promotes invasion and
metastasis of laryngeal squamous cells carcinoma via
epithelial-mesenchymal transition through H3K27me3. Biochem Biophys
Res Commun. 479:253–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benard A, Goossens-Beumer IJ, van Hoesel
AQ, Horati H, Putter H, Zeestraten EC, van de Velde CJ and Kuppen
P: Prognostic value of polycomb proteins EZH2, BMI1 and SUZ12 and
histone modification H3K27me3 in colorectal cancer. PLoS One.
9:e1082652014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou W, Wang J, Man WY, Zhang QW and Xu
WG: siRNA silencing EZH2 reverses cisplatin-resistance of human
non-small cell lung and gastric cancer cells. Asian Pac J Cancer
Prev. 16:2425–2430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Padi SK, Tindall DJ and Guo B:
Polycomb protein EZH2 suppresses apoptosis by silencing the
proapoptotic miR-31. Cell Death Dis. 5:e14862014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Khalaf HH and Aboussekhra A: p16(INK4A)
positively regulates p21(WAF1) expression by suppressing
AUF1-dependent mRNA decay. PLoS One. 8:e701332013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang Z, Ju H, Ling J, Zhuang Z, Li Z,
Wang H, Fleming JB, Freeman JW, Yu D, Huang P and Chiao PJ:
Cooperativity of oncogenic K-Ras and downregulated p16/INK4A in
human pancreatic tumorigenesis. PLoS One. 9:e1014522014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McLaughlin-Drubin ME, Park D and Munger K:
Tumor suppressor p16INK4A is necessary for survival of cervical
carcinoma cell lines. Proc Natl Acad Sci USA. 110:pp. 16175–16180.
2013; View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qi W, Chan H, Teng L, Li L, Chuai S, Zhang
R, Zeng J, Li M, Fan H, Lin Y, et al: Selective inhibition of Ezh2
by a small molecule inhibitor blocks tumor cells proliferation.
Proc Natl Acad Sci USA. 109:pp. 21360–21365. 2012; View Article : Google Scholar : PubMed/NCBI
|