Introduction
125I particles are capable of releasing
X-ray and gamma-rays in the process of decay, through in
vivo implantation for continuous internal radiation therapy.
These particles have the ability to inhibit tumor cell
proliferation and induce apoptosis. 125I has a low dose
rate, high relative biological effect, appropriate radius of
killing, and is used widely in the treatment of a variety of
malignant tumors, showing obvious advantages in the local control
and survival rate of patients (1,2). With the
rapid development of molecular biology, modern advanced biology
techniques have been used to study the mechanism of tumorigenesis
and prognosis, and the treatment of malignant tumors with
125I particles is no exception. Through a lot of basic
research, the molecular mechanism of 125I particles in
radiotherapy for malignant tumor has achieved certain results in
the induction of tumor cell apoptosis and cell cycle arrest,
intracellular signal transduction and inhibition of tumor
angiogenesis. In patients with recurrent bladder cancer, the
efficacy of the general chemotherapy regimen is limited (3). In this study, we implanted
125I radioactive particles into the tumor, and observed
the clinical efficacy of this treatment modality during recurrent
bladder cancer.
Materials and methods
General
A total of 16 patients with recurrent bladder cancer
treated with 125I, there were 13 males and 3 females,
aged 47–69 years, and the average was 59.2±3.28 years. Of the 16
cases, 10 cases were diagnosed as transitional cell carcinoma, 1
case was squamous cell carcinoma, and 5 cases were hamartoma. All
16 patients had hematuria, urinary frequency and urgency symptoms,
and 1 patient had dysuria. In the chemotherapy group, there were 14
males and 2 females, aged 43–65 years, with an average age of
58.3±2.33 years. Of these, 8 cases were transitional cell
carcinoma, 4 cases were squamous cell carcinoma, and 4 cases were
hamartoma. All 16 patients had hematuria, urinary frequency and
urgency symptoms. There was no significant difference in age
between the two groups. There was no significant difference in
pathological types and the number of complications by the
Chi-square test (P>0.05) (Table
I).
 | Table I.Comparison of general clinical
data. |
Table I.
Comparison of general clinical
data.
| Variables | No. of cases | Sex
(male/female) | Age (years) | Transitional cell
carcinoma | Squamous cell
carcinoma | Hamartoma |
|---|
| 125I
group | 16 | 13/3 | 59.2±3.28 | 10 | 1 | 5 |
| Chemotherapy
group | 16 | 14/2 | 58.3±2.33 | 8 | 4 | 4 |
| T/χ2
value | – | 0.43 | 0.22 | 0.83 |
|
|
| P-value | – | 0.59 | 0.78 | 0.18 |
|
|
The present study has been approved by the Ethics
Committee of The First Affiliated Hospital of Xiamen University
(Fujian, China). Informed consents were signed by the patients
and/or guardians.
Instruments
The instruments used for the study were:
3D-stereotactic radiotherapy planning system for tumor tissue (TPS,
jointly designed and developed by the China Institute of Atomic
Energy and Beijing Bo Intel System Engineering Co., Ltd., Beijing,
China). Plant equipments included planting needles, planting guns,
templates and B ultrasonic. The radioactive 125I
particle source was obtained from the American SynQor Inc.
(Boxborough, MA, USA), and the activity of each particle was
0.40–0.50 mCi, with a half-life of 60.1 days; gamma-ray energy of
27,35 kEV; and tissue penetration distance of 1.7 cm.
Methods
To determine the range of tumor surface markers
measured by B ultrasound, combined with the TPS planning system,
the grid was drawn using the pitch 1.0 cm square matrix. Each
intersection of the grid served as the access channel. After local
routine disinfection, an ultrasonic probe navigation frame was
fixed to the edge of the tumor. From this point, the needle was
taken up to 1 cm from the edge of the tumor, the first particle was
planted, and the needle was withdrawn 1 cm. The second particle was
planted, up to 1 cm from the edge of the tumor, the last particle
of the channel was planted, and the replacement of the channel
continued to grow. A postoperative indwelling catheter was left,
with regular bladder irrigation, antibiotics were taken to prevent
infection, and hemostasis and other symptomatic treatments were
taken. Tumor sites and the number of implants of 125I
group are presented in Table II.
 | Table II.Tumor location and number of implanted
particles. |
Table II.
Tumor location and number of implanted
particles.
| No. | Age | Tumor location | Tumor size
(cm3) | No. of particles |
|---|
| 1 | 58 | Right anterior wall
of bladder | 21.2 | 40 |
| 2 | 57 | Right posterior wall
of bladder | 9.2 | 20 |
| 3 | 54 | Right anterior wall
of bladder | 9.8 | 20 |
| 4 | 59 | Right anterior wall
of bladder | 4.8 | 9 |
| 5 | 53 | Left posterior wall
of bladder | 10.2 | 20 |
| 6 | 50 | Left posterior wall
of bladder | 11.3 | 20 |
| 7 | 49 | Posterior wall and
right wall of bladder | 20.3 | 40 |
| 8 | 63 | Right anterior wall
of bladder | 12.5 | 20 |
| 9 | 52 | Right posterior wall
of bladder | 3.8 | 9 |
| 10 | 53 | Right anterior wall
of bladder |
4.4 | 9 |
| 11 | 54 | Posterior wall and
right wall of bladder |
5.4 | 9 |
| 12 | 59 | Posterior wall and
right wall of bladder |
5.7 | 9 |
| 13 | 61 | Right wall of
bladder |
7.8 | 10 |
| 14 | 60 | Left and right
posterior wall of bladder |
9.8 | 20 |
| 15 | 58 | Right posterior
wall of bladder |
9.6 | 20 |
| 16 | 69 | Posterior wall and
right wall of bladder |
3.5 | 9 |
Chemotherapy regimen
Both groups of patients were treated with internal
iliac artery chemotherapy for the first time within 2 weeks after
surgery, and the second internal iliac artery chemotherapy was
performed within 1 month after surgery in the two groups. The
chemotherapeutic drugs Pharmorubcin 30 mg/m2 (surface
area) was taken each time. The Seldinger technique was used to
puncture one side of the femoral artery, and internal iliac artery
angiography was taken on both sides to observe the blood supply of
the bladder. The catheter was placed into the internal iliac artery
of the affected side, and 2/3 of the drug was injected. Then the
catheter was placed into the contralateral internal iliac artery,
and the remaining 1/3 of the drug was injected. Following
chemotherapy, hydration, alkalization and hepatoprotective
treatments were taken.
Curative effect judgment
According to the improvement of clinical symptoms,
the changes of tumor size by the re-examination of B-ultrasound or
CT and CT review after 17 months, the curative effect was
evaluated.
Statistical analysis
The collected data were processed using SPSS 15.0
statistical software (SPSS, Inc., Chicago, IL, USA). Measurement
data were presented as mean ± SD. An independent sample t-test was
used for comparison between groups. Countable data were expressed
by case number or constituent ratio, and tested using the
Chi-square test. The log-rank test and COX multivariate analysis
were used to analyze the prognosis of the patients. A P<0.05 was
considered to indicate a statistically significant analysis.
Results
Changes of tumor volume before and
after treatment
We analyzed the tumor volume of the two groups
before and after treatment by CT. The results showed that the tumor
volume of the two groups of patients after treatment was
significantly reduced. The tumor volume of 125I was
9.33±5.27 before treatment, while it reduced to 3.44±1.23 after
treatment. The tumor volume of patient in chemotherapy group was
9.21±1.23 before treatment and 6.57±2.17 after treatment. However,
compared with the chemotherapy group, the tumor volume of the
125I group was significantly reduced (P<0.05)
(Table III).
 | Table III.Changes of tumor volume before and
after treatment. |
Table III.
Changes of tumor volume before and
after treatment.
| Variables | Number of
cases | Before
treatment | After
treatment | T-value | P-value |
|---|
| 125I
group | 16 | 9.33±5.27 | 3.44±1.23 | 2.18 | 0.02 |
| Chemotherapy
group | 16 | 9.21±1.23 | 6.57±2.17 | 3.28 | 0.012 |
| T-value | – | 0.27 | 3.49 | – | – |
| P-value | – | 0.79 | 0.003 | – | – |
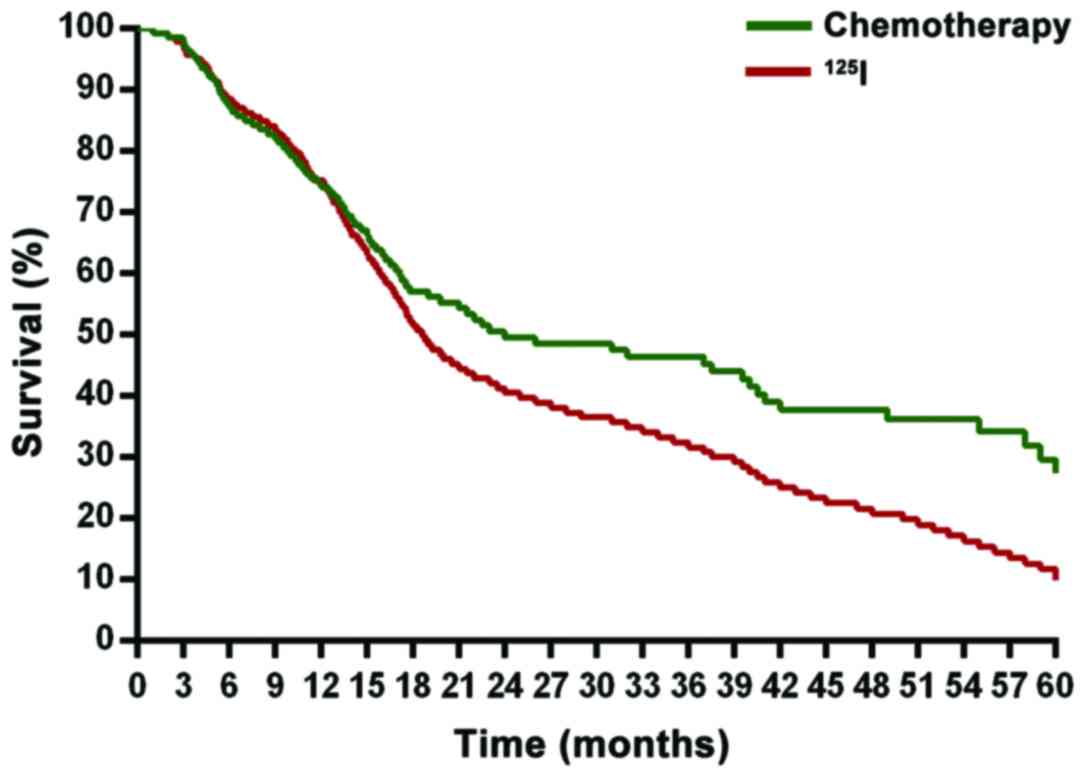
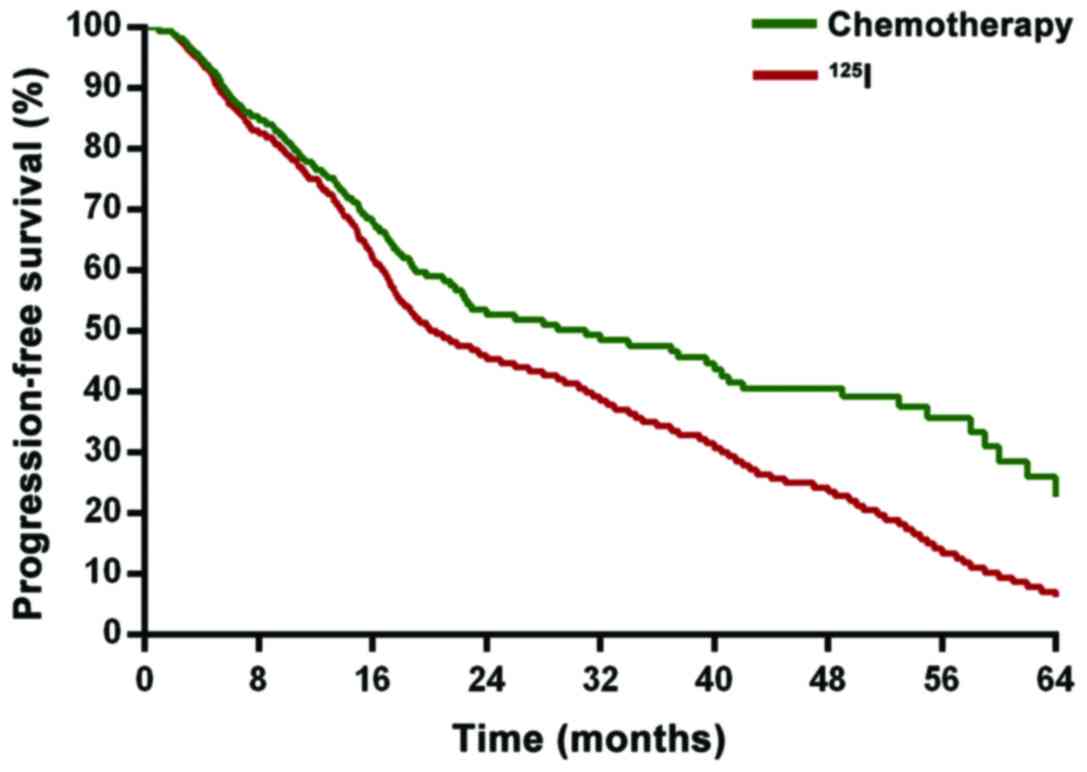
Comparison of survival prognosis
Concerning the survival prognosis of the two groups,
we found that compared with the chemotherapy group, the
125I group had a significantly higher survival rate of
five years (P<0.05). Disease-free survival was significantly
prolonged (P<0.05). The difference was statistically significant
(P<0.05) (Figs. 1 and 2).
Discussion
Bladder cancer is one of the most common malignant
tumors in the urinary system. The key to treatment is to control
the recurrence and metastasis of the local tumor through
multidisciplinary efforts (4–6). For nearly half a century, the academic
community has made noteworthy progress in the comprehensive
treatment of surgery, radiotherapy and chemotherapy. However, the
recurrence rate within a few years after tumor resection is 50–80%
and invasive evolution accounts for 50–80% in recurrent cases
(7). Although radical cystectomy is
widely accepted, there is currently no evidence that surgery is
more effective than radiotherapy. Furthermore, radiotherapy may
retain the bladder, especially in improving the quality of life of
patients as it has greater advantages (8). Although there are certain effects of
external radiotherapy, the loss of normal tissue is serious and the
quality of life is also reduced. Moreover, it has side effects and
high equipment costs.
Brachytherapy was performed by implanting
radioactive sources into the hollow organs (intracavitary
radiotherapy) or directly implanted in the tumor tissue
(interstitial brachytherapy). A short range of treatment ranging
from approximately 5 mm to 5 cm characterizes it. Additionally, the
radiation dose is mainly concentrated in a small part of the tumor
tissue and surrounding normal tissue (9). High-dose irradiation can be obtained in
the treatment of brachytherapy and the surrounding normal tissues
may be well protected (10).
Brachytherapy is one of main treatments of bladder cancer and
prostate cancer (11). The physical
half-life of the radioactive element 125I particles is
59.6 days, with the energy of 27.4–31.5 Kev X-rays and 35.5 Kev
gamma-rays (12). When the
125I particles were implanted into the tumor focus and
the lymphatic system, the particles were scattered with low energy
X-rays and gamma-rays. Tumor proliferation was significantly
reduced by continuous irradiation (13). At the same time, continuous low energy
irradiation inhibits tumor mitosis, with the tumor cell
concentration in the G2 phase, resulting in tumor cell killing by
radiation effect to the maximum, to effectively inhibit tumor cell
proliferation and cytothesis, in order to achieve the purpose of
cure (14). Furthermore,
125I particles are not involved in metabolism, and cause
little damage to the patients and medical staff (15). In particular, according to the
intraoperative situation, targeted at a certain location, tumor
cells are directly killed at different stages of the cell cycle
(16). However, conventional external
irradiation is short-time high-intensity fractionated irradiation,
which could kill the M cells. This is often terminated because the
patient could not tolerate the radiation reaction (17). Interstitial implantation of
radioactive 125I particles brachytherapy is a minimally
invasive treatment technology developed in previous years, showing
a good therapeutic effect in the clinical practice of treating
multiple cancers with high efficiency and few side effects
(18).
Brachytherapy is primarily used for the treatment of
bladder and prostate cancer (19).
However, to the best of our knowledge, there is no report on the
application of 125I particles implantation in the
treatment of bladder cancer (20).
Our results have shown that 125I particles were
implanted into the lesion or in the tissue for the treatment of
bladder cancer, and the local recurrence rate was low. At the same
time, the unresectable cancer tissue or the remaining tissue was
directly radiated (21). Our study
found that tumor volume measurement and statistical analysis in the
CT of the two groups of patients before and after treatment were
significantly reduced as compared with prior to treatment. However,
compared with the chemotherapy group, the tumor volume of
125I group was significantly reduced. Following a
comparison of the survival prognosis of the two groups, we found
that compared with the chemotherapy group, the 125I
group had a significantly higher survival rate of 5 years
(P<0.05). Disease-free survival was significantly prolonged
(P<0.05). The results showed that 125I particle
radiation therapy has good clinical efficacy in clinical life
therapy and survival prognosis of recurrent bladder cancer
patients.
125I particle implantation-assisted
radiotherapy for the treatment of bladder cancer is optimal
compared to in vitro irradiation treatment, and especially
applicable to patients for whom surgery is not possible.
125I particles are capable of killing the tumor cells
directly, and are not affected by the oxygen content in tumor cells
(22,23). Owing to the accurate localization, the
local effective dose is large, but the radiation distance is small,
the radiation damage is slight, and there is no obvious adverse
effect on the surrounding tissue. Therefore, the side effects are
small and have no significant influence on the quality of life
(24). We believe that the treatment
of bladder cancer by 125I particles brachytherapy is
more advantageous than that of in vitro irradiation. It has
the following advantages (25–29): i)
The radioactive source is small, the half-life is short (only 60
days), the energy is low, and the killing radius is not more than 2
cm; ii) the operation is simple, the 125I particle
source may be implanted into the tissue under direct vision, the
accuracy of the treatment is improved, and the range of the
surgical injury is reduced; iii) the accuracy rate of the cancer
tissue is high, and the continuous emission of pure gamma-ray can
effectively hit the mitotic phase of the cancer cell. The
continuous low-dose irradiation inhibits the mitosis of the tumor
cells, and the curative effect is good; and iv) it may effectively
improve the dose distribution ratio of tumor and normal tissue.
In conclusion, we believe that 125I
radioactive particles in the treatment of bladder cancer may not
only improve the symptoms of patients with bladder cancer in the
short term, rapidly reduce the tumor, but also continuously kill
residual tumor and prevent recurrence of the tumor.
References
|
1
|
Ohga S, Nakamura K, Shioyama Y, Tatsugami
K, Sasaki T, Nonoshita T, Yoshitake T, Asai K, Hirata H, Naito S
and Honda H: Acute urinary morbidity after a permanent
125I implantation for localized prostate cancer. J
Radiat Res. 55:1178–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martens C, Pond G, Webster D, McLean M,
Gillan C and Crook J: Relationship of the International Prostate
Symptom score with urinary flow studies, and catheterization rates
following 125I prostate brachytherapy. Brachytherapy.
5:9–13. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arias F, Dueñas M, Martínez E, Domínguez
MA, Illarramendi JJ, Villafranca E, Tejedor M, Molina F, Meiriño R
and Valerdi JJ: Radical chemoradiotherapy for elderly patients with
bladder carcinoma invading muscle. Cancer. 80:115–120. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li Y, Lin K, Yang Z, Han N, Quan X, Guo X
and Li C: Bladder cancer stem cells: Clonal origin and therapeutic
perspectives. Oncotarget. 8:66668–66679. 2017.PubMed/NCBI
|
|
5
|
Radford IR and Aldridge DR: Importance of
DNA damage in the induction of apoptosis by ionizing radiation:
Effect of the scid mutationand DNA ploidy on the radiosensifivity
of murine lymphoid cell lines. Int J Radiat Biol. 75:143–153. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohga S, Nakamura K, Shioyama Y, Tatsugami
K, Sasaki T, Nonoshita T, Yoshitake T, Asai K, Hirata H, et al:
Acute urinary morbidity after a permanent 125I
implantation for localized prostate cancer. J Radiat Res.
55:1178–1183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Prada PJ, Hevia M, Juan G, Abascal JM, de
la Rúa A, Abascal R, Fernández J and Rodríguez R: I125 Low dose
rate brachytherapy in localized prostate cancer. Preliminary
results after 5 years. Arch Esp Urol. 58:213–226. 2005.(In
Spanish). PubMed/NCBI
|
|
8
|
Lucerna M, Pomyje J, Mechtcheriakova D,
Kadl A, Gruber F, Bilban M, Sobanov Y, Schabbauer G, Breuss J,
Wagner O, et al: Sustained expression of early growth response
protein-1 blocks angiogenesis and tumor growth. Cancer Res.
66:6708–6713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nowak AK, Chow PKH and Findlay M: Systemic
therapy for advanced hepatocellular carcinoma: A review. Eur J
Cancer. 40:1474–1484. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Ji P, Liu J, Broaddus RR, Xue F
and Zhang W: Centrosome-associated regulators of the G(2)/M
checkpoint as targets for cancer therapy. Mol Cancer. 8:82009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao XF, He XT, Ji L, Xiao J and Lv J:
Effects of neoadjuvant radiochemotherapy on pathological staging
and prognosis for locally advanced esophageal squamous cell
carcinoma. Dis Esophagus. 22:477–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu MY, Wu XY, Li QS and Zheng RM:
Expression of Egr-1 gene and its correlation with the oncogene
proteins in non-irradiated and irradiated esophageal squamous cell
carcinoma. Dis Esophagus. 19:267–272. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gassmann M, Chilov D and Wenger RH:
Regulation of the hypoxia-inducible factor-1 alpha. ARNT is not
necessary for hypoxic induction of HIF-1 alpha in the nucleus. Adv
Exp Med Biol. 475:87–99. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sjöström A, Bue P, Malmstrom P and
Carlsson J: Binding of 125I after administration of
125I-EGF-dextran, 125I-EGF or 125I to human bladder cancer
spheroids. Int J Oncol. 17:559–564. 2000.PubMed/NCBI
|
|
15
|
Sjöström A, Bue P, Malmström PU, Nilsson S
and Carlsson J: Binding, internalization and degradation of
EGF-dextran conjugates in two human bladder-cancer cell lines. Int
J Cancer. 70:383–389. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mariani G, Collecchi P, Baldassarri S, Di
Luca L, Buralli S, Fontanini G, Baranowska-Kortylewicz J, Adelstein
SJ and Kassis AI: Tumor uptake and mitotic activity pattern of
5-[125I]iodo-2′- deoxyuridine after intravesical infusion in
patients with bladder cancer. J Nucl Med. 37:16–19. 1996.PubMed/NCBI
|
|
17
|
van den Abbeele AD, Tutrone RF, Berman RM,
Baranowska-Kortylewicz J, Barclay PD, Richie JP, Adelstein SJ and
Kassis AI: Tumor-targeting potential of radioiodinated
iododeoxyuridine in bladder cancer. J Nucl Med. 37:315–320.
1996.PubMed/NCBI
|
|
18
|
Colpi GM, Fanciullacci F, Beretta G, Negri
L and Zanollo A: Evoked sacral potentials in subjects with true
premature ejaculation. Andrologia. 18:583–586. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xin ZC, Choi YD, Rha KH and Choi HK:
Somatosensory evoked potentials in patients with primary premature
ejaculation. J Urol. 158:451–455. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xin ZC, Chung WS, Choi YD, Seong DH, Choi
YJ and Choi HK: Penile sensitivity in patients with primary
premature ejaculation. J Urol. 156:979–981. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Smith K, Fennelly JA, Neal DE, Hall RR and
Harris AL: Characterization and quantitation of the epidermal
growth factor receptor in invasive and superficial bladder tumors.
Cancer Res. 49:5810–5815. 1989.PubMed/NCBI
|
|
22
|
Tamura K, Araki F and Ohno T: SU-F-T-46:
The effect of inter-seed attenuation and tissue composition in
prostate 125I brachytherapy dose calculations. Med Phys.
43:3471–3472. 2016. View Article : Google Scholar
|
|
23
|
Tan Q, Qin Q, Yang W, Lian B, Mo Q and Wei
C: Combination of 125I brachytherapy and chemotherapy for
unresectable recurrent breast cancer: A retrospective control
study. Medicine (Baltimore). 95:e53022016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jeon J, Shim HE, Mushtaq S, Choi MH, Park
SH, Choi DS and Jang BS: An optimized protocol for the efficient
radiolabeling of gold nanoparticles by using a
125I-labeled azide prosthetic group. J Vis Exp.
e547592016.
|
|
25
|
Jiao D, Wu G, Ren J and Han X:
Radiofrequency ablation versus 125I-seed brachytherapy
for painful metastases involving the bone. Oncotarget.
7:87523–87531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Becker S, Schlederer T, Kramer MF, Haack
M, Vrtala S, Resch Y, Lupinek C, Valenta R and Gröger M: Real-life
study for the diagnosis of house dust mite allergy - the value of
recombinant allergen-based IgE serology. Int Arch Allergy Immunol.
170:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheikholeslami S, Nedaie HA, Sadeghi M,
Pourbeigy H, Shahzadi S, Zehtabian M, Hasani M and Meigooni AS:
Monte Carlo calculations and experimental measurements of the
TG-43U1-recommended dosimetric parameters of 125I (Model
IR-Seed2) brachytherapy source. J Appl Clin Med Phys. 17:430–441.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Asadi S, Vaez-Zadeh M, Vahidian M,
Marghchouei M and Masoudi SF: Ocular brachytherapy dosimetry for
103Pd and 125I in the presence of gold
nanoparticles: A Monte Carlo study. J Appl Clin Med Phys. 17:90–99.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sugawara A1, Nakashima J, Shigematsu N,
Kunieda E and Kubo A: Prediction of seed migration after
transperineal interstitial prostate brachytherapy with I-125 free
seeds. Brachytherapy. 8:52–56. 2009. View Article : Google Scholar : PubMed/NCBI
|