Introduction
Cervical cancer is one of the most common
gynecological malignancies. Based on a recent statistic, more than
520,000 cases are newly reported annually (1). Traditional treatments for cervical
cancer consist of surgery, chemotherapy and radiotherapy. Despite
great advances in surgery and chemo-radiotherapy for the last
decades, the long-term prognosis for patients suffering from
cervical cancer remains still poor with a 5-year survival rate of
less than 30% (2). The poor outcome
of cervical cancer patients is largely due to the property of
metastasis or recurrence (3). In
fact, it has been well documented that a common feature of cervical
cancer is an uncontrolled cell growth and metastasis (4). Thus, it is mandatory to find novel
markers for the early diagnosis and treatment of cervical
cancer.
With the rapid development of genome and
transcriptome sequencing technologies and implement of genomics
consortiums such as ENCODE and FANTOM, the classic view of the
transcriptome landscape and its mRNA-centric paradigm for
transcript annotation has undergone a fundamental change (5,6). It has
now been well established that more than 90% of the genome can be
transcribed with only <2% being subsequently translated. This
means that the vast majority of genome serves as the template for
the transcription of noncoding RNAs (ncRNAs) (7).
Long noncoding RNAs (lncRNAs) are a newly emerged
class of noncoding RNA containing more than 200 nucleotides that
are widely transcribed in the genome. Unlike other noncoding RNAs
such as microRNAs, lncRNAs involvement in human diseases remains
largely uncovered. Current knowledge has implicated that lncRNAs
may widely participate in multiple intracellular and extracellular
activities, including gene transcription, mRNA splice and
tumorigenesis (8,9). Emerging evidence have assessed the
functional roles of specific lncRNA in cervical cancer so far. For
instance, the lncRNA TUG1 was found to be upregulated and promoted
cervical cancer proliferation and migration (10). Expression quantitative trait loci in
lncRNA PAX8-AS1 are associated with decreased risk of cervical
cancer (11). LncRNA CCAT2 promoted
the proliferation and survival of cervical cancer (12).
BLACAT1 (also known as linc-UBC1) is one of the few
well-known lincRNAs (one class of lncRNAs), with a length of 2,616
bp. BLACAT1 was firstly characterized in bladder cancers and exerts
a functional role in recruiting and binding to polycomb repressive
complex 2 (PRC2) (13). Recent
studies have suggested that BLACAT1 exhibited tumor pro-oncogenic
activity in gastric cancer (14), and
may also serve as a negative predictor for prognosis in patients
with gastric cancer (14) and
colorectal cancer (15). These
pioneer studies implied that BLACAT1 might be functional in solid
tumor. However, whether BLACAT1 has any functional role in cervical
cancer remains to be elucidated.
The present study sought to examine the expression
profile of BLACAT1 in cervical cancer and then assess the
biological roles of BLACAT1 in cervical cancer. Our results showed
that BLACAT1 was upregulated in cervical cancer and its
upregulation remarkably promoted cell proliferation and migration.
Our data might provide novel evidence for development of
therapeutic strategies against cervical cancer in clinic.
Materials and methods
Human samples
The present study was approved by the Ethic
Committee of Tianjin Hospital. Cervical cancer tissues from 100
patients (age range, 45–72 years; mean age, 55 years; male:
Female=63:37) admitted to the Department of Gynaecology and
Obstetrics, Tianjin Hospital between April 2015 and May 2016 were
collected via surgical resection and frozen into liquid nitrogen
immediately and then stored at −80°C. Matched adjacent
non-cancerous tissues were also obtained. All patients showed their
full intentions to participate in the present study and a written
consent form was obtained from each patient.
Cell culture and transfection
Primary normal cervical epithelial cells (NCECs)
were from non-cancerous cervical tissues, and were purchased from
American Type Tissue Collection (ATCC, Massachusetts, USA).
Cervical cancer cell lines Siha, CaSki, Hela, ME180 and C33A were
commercially from the Cell Bank of Chinese Academy of Sciences
(Shanghai, China). All of the cell lines were cultured in DMEM
(Gibco, Grand Island, NY, USA) supplied with 10% fetal bovine serum
(FBS; Gibco) and 1% antibiotics (penicillin/streptomycin). The
cells were incubated in a 37°C incubator with 5% CO2.
Specific shRNA against BLACAT1 (shBLACAT1) and control shRNA (shNC)
were designed and synthesized by Genepharm Co. (Shanghai, China).
The shRNA sequence against BLACAT1 is
5′-AGGCUGGUUUCUGCCCUCAUCCUUU-3′, and the control sequence is
5′-UUUCUCCGAACGUGUCACGUTT-3′. The transfections were performed by
Lipofectamine 2000 (Invitrogen, NY, USA) according to the
manufactures' instructions. Six hours after transfection, the
culture medium was replaced with fresh DMEM.
RNA isolation and RT-qPCR
Total RNAs from human tissues and cultured cells
were extracted with TRIzol® reagent (Takara Bio, Inc.,
Otsu, Japan) in a dilution of 1 ml for each well of a six-well
plate. The RNA quality and concentration were determined by
collecting the absorbance using the Nanodrop 2000 spectrophotometer
(Thermo Fisher Scientific, Inc., Beijing, China) at 260 and 280 nm.
Reverse transcription (RT) of first-strand cDNAs was conducted by
PrimeScript RT Master Mix (Perfect Real Time; Takara Bio, Inc.)
according to the manufacturer's protocol. All PCR reactions were
performed in an ABI PRISM 7900 Real-Time system (Applied
Biosystems; Thermo Fisher Scientific, Inc.) with the
SYBR® Premix Ex Taq™ kit (Takara Bio, Inc).
The thermocycling protocol was listed as follows: Initial
denaturation at 95°C for 2 min, followed by 35 repeats of the
three-step cycling program consisting of 30 sec at 95°C
(denaturation), 1 min at 53°C (primer annealing) and 30 sec at 72°C
(elongation), followed by a final extension step for 10 min at
72°C. The primer sequences used for qPCR are listed below: BLACAT1:
Sense, 5′-GTCTCTGCCCTTTTGAGCCT-3′ and antisense,
5′-GTGGCTGCAGTGTCATACCT-3′; GAP DH: Sense: 5′-GGGAAACTGTGGCGTGAT-3′
and antisense, 5′-GAGTGGGTGTCGCTGTTGA-3′. The housekeeping gene
GAPDH was used as the internal control. Primers were purchased from
Shenggong Co. (Shanghai, China). All quantitative data were
normalized to GAPDH using the 2−ΔΔCq method (16).
Colony formation assay
ME180 and C33A cells were transfected with shNC or
shBLACAT1 and cultivated in six-well plates at a density of 200
cells/well. After 2 weeks in 37°C incubator, the cell colonies that
contained >50 cells were counted by staining with 0.5% crystal
violet for 10 min and observation under a light microscope with a
magnification of 200× (Nikon, Tokyo, Japan).
Cell cycle analysis
Prior to tests, ME180 and C33A cells were
transfected with shRNAs with or without BLACAT1 knockdown for 72 h.
Next, cells were collected by low speed centrifuge (840 g, 5 min,
4°C) and fixed by pre-cold ethanol (70%) for 10 min on ice. The
cells were washed and re-suspended in pre-cold PBS and incubated at
37°C for 30 min with 10 mg/ml RNase and 1 mg/ml propidium iodide
(PI) (Sigma-Aldrich, St Louis, MO, USA). The percentage of cells in
each cell cycle phase was determined using the Cell Quest
acquisition software (BD Biosciences, San Diego, CA, USA). Cell
proliferation assay. Both ME180 and C33A cells were seeded in a
96-well plate at a concentration of 1×103/well. After
incubation for 24 h, cells were transfected with shRNA against
BLACAT1 or control shRNA. Cell proliferation was examined every day
in the consecutive 5 days using a CellTiter 96 AQueous
Non-Radioactive Cell Proliferation kit (Promega Corp., Madison, WI,
USA) following the manufacturer's protocol. The cell proliferation
rate was determined by measuring the absorbance at 490 nm using a
microplate reader (Tecan, Männedorf, Switzerland).
Transwell assay
For cell migration assays, ME180 and C33A cells were
trypsinized and collected by low-speed centrifugation (840 g, 4°C,
and 5 min) with serum-free medium. A total of 1×104
cells (~150 µl) were spread into the upper chamber. The lower
chamber was filled with 600 µl medium containing 10% FBS.
Afterwards, the plate was incubated in the 37°C incubator and the
cells are allowed to grow freely. At 24 h post-seeding, the
membrane was fixed with pre-cooled methanol and stained with
crystal violet (1%) for 5 min at room temperature. Cell migration
was assessed by counting the cells that had migrated through the
membrane. Five random fields were selected and images captured
under a Nikon light microscope (Nikon) at a magnification of 100×.
For cell invasion assays, the membrane was pre-coated with Matrigel
(Corning Inc., Corning, NY, USA) for 6 h at 37°C incubator.
Wound-healing assay
ME180 and C33A cells were transfected with shRNA
against BLACAT1 or control shRNA, and were then cultured in DMEM in
a six-well culture plate at a density of 5×105
cells/well and allowed to grow to a confluence of 90% overnight.
The culture medium was replaced with serum-free DMEM. A line was
scratched in the single cell layer using a 10 µl pipette tip and
the cells were then washed with PBS three times. Following
incubation for 12 h, images of the migrating cells were observed
and images captured using a Nikon light microscope.
Western blot analysis
Protein expression in the process of EMT and cell
cycle were evaluated by western blot analysis. Briefly, total
proteins from ME180 and C33A cells were collected by lysis buffer
(NP-40; Beyotime, Nantong, China) on ice and quantified using
Bio-Rad protein assay reagent (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Equal amounts of protein (50 µg) were loaded
onto 10% sodium dodecyl sulfate-polyacrylamide gel for
electrophoresis and then transferred to a nitrocellulose membrane
(NC, Millipore, MA, USA). The membrane was blocked for 1 h with 5%
skimmed milk at room temperature and then incubated with primary
antibodies overnight at 4°C. The primary antibodies against Cyclin
B1 (sc-70898, 1:1,000), CDC25C (sc-327, 1:1,000), E-Cadherin
(sc-71009, 1:1,000), N-Cadherin (sc-53488, 1:1,000) and GAPDH
(sc-32233, 1:1,000) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). After washing with TBST for 4 times, the
membrane was then incubated with secondary goat-anti-rabbit
(sc-2004) or goat-anti-mouse (sc-2005) antibody (Santa Cruz
Biotechnology, Inc.) for 1 h at 37°C with a dilution of 1:1,000.
Finally, the proteins were quantified using ECL Prime Western
Blotting Detection reagent (GE Healthcare, Parsippany, NJ, USA) and
an ImageQuant LAS 4000 Mini Biomolecular Imager (GE
Healthcare).
Statistically analysis
In vitro experiments were repeated at least
three times in triplicate to yield reproducible results. All data
were presented as the mean ± standard deviation (SD). Student's
t-test analysis was used for the comparison between two groups.
Data were analyzed using Prism 6 (GraphPad Software Inc., San
Diego, CA, USA). P<0.05 was considered to indicate a
statistically significant difference
Results
Long noncoding RNA BLACAT1 is
upregulated in human cervical cancer in vivo and in vitro
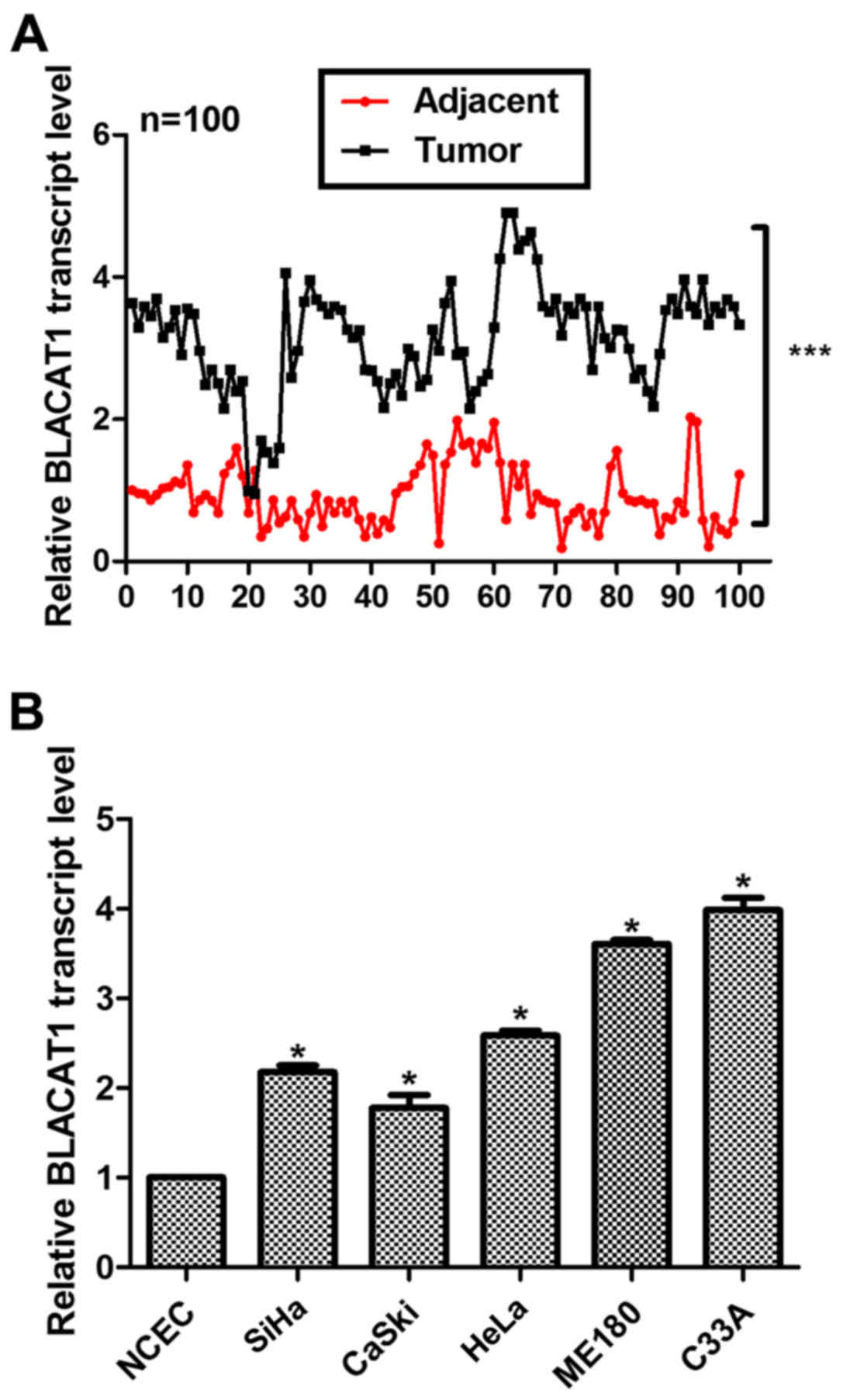
A total of 100 cervical cancer patients were
involved and both their tumor tissues and adjacent non-cancerous
tissues were subjected for RT-PCR analysis after dissection. As
shown in Fig. 1A, the relative
transcript level of BLACAT1 was significantly higher in most of the
tumor tissues (99/100) as compared with their counterparts
(***P<0.0001). Primary normal cervical epithelial cells (NCECs)
were from non-cancerous cervical tissues and used as a control. It
was shown that the relative transcript level of BLACAT1 was
remarkably upregulated in all of the cervical cancer cell lines, of
which the ME180 and C33A cells showed the highest expression of
BLACAT1 (Fig. 1B). Therefore, these
two cell lines were selected for subsequent analysis. Our data
suggested that long noncoding RNA BLACAT1 was highly upregulated in
human cervical cancer.
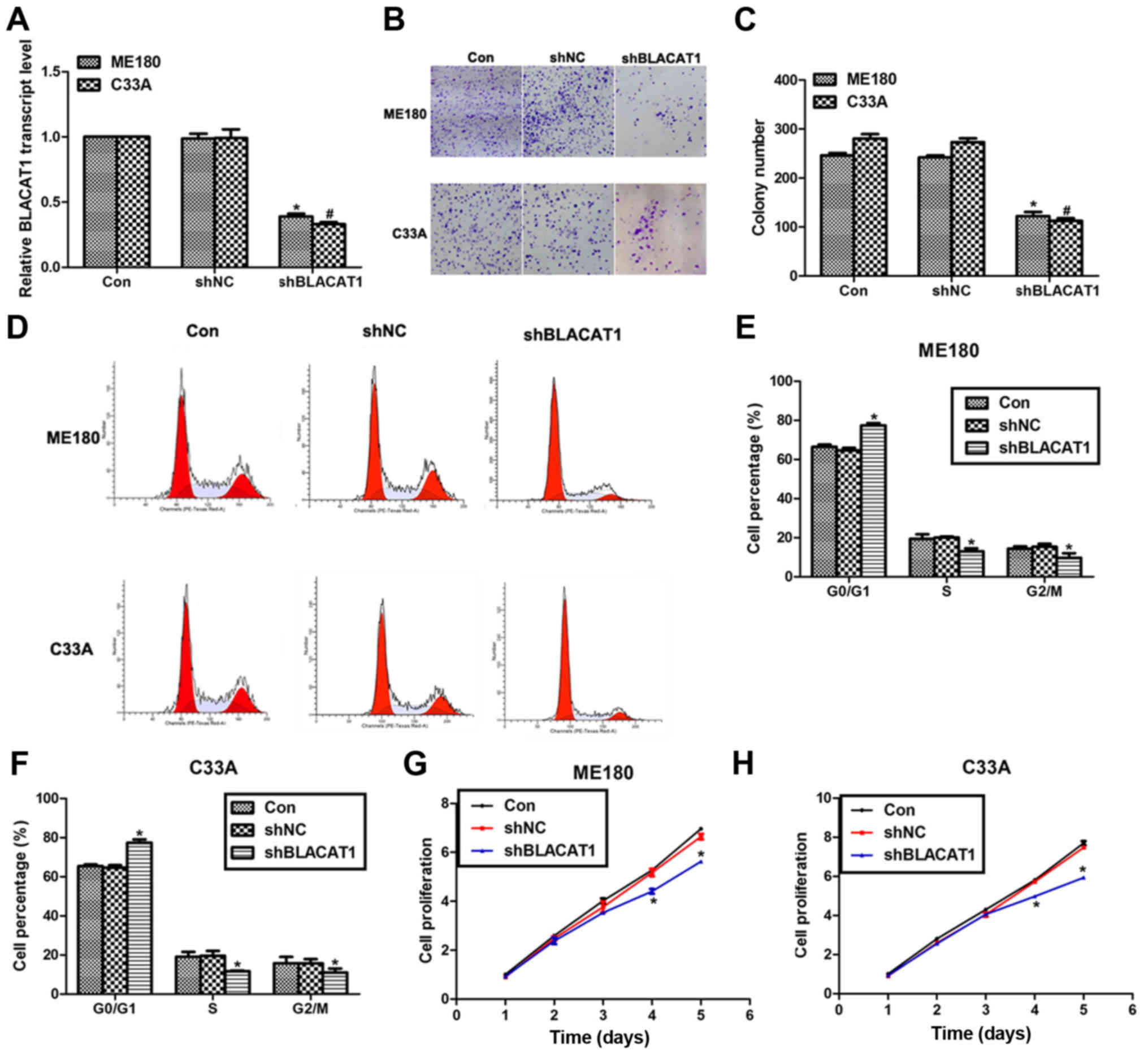
Knockdown of BLACAT1 inhibits cell
proliferation in human cervical cancer in vitro
To explore the effects of BLACAT1 on human cervical
cancer, specific shRNA were used to knock down the expression of
BLACAT1 in ME180 and C33A cells. As shown in Fig. 2A, transfection of shBLACAT1 decreased
the expression of BLACAT1 by more than 50% in both ME180 and C33A
cells. Colony formation assay showed that approximate 250 colonies
for control ME180 cells and 280 colonies for control C33A cells
were formed; however, only 125 colonies for ME180 cells and 116
colonies for C33A cells were observed upon shBLACAT1 transfection
(Fig. 2B and C). Moreover, knockdown
of BLACAT1 in ME180 and C33A cells arrested cell cycle in G0/G1
phase, as evidenced by findings that the cell percentage in G0/G1
phase was increased by 13% for ME180 cells and 15% for C33A cells.
Meanwhile, the cell percentage in S phase and G2/M phase was
decreased accordingly for both cell lines (Fig. 2D-F). Cell proliferation was also
determined in both cell lines upon shBLACAT1 transfection. There
were no notable differences among three groups in the former three
days in ME180 and C33A cells; however, on the fourth day, the cell
proliferative rate was decreased by 18% for ME180 cells and 21% for
C33A cells. The inhibitive effects were more obvious on the fifth
day by the transfection of shBLACAT1 in both cell lines (Fig. 2G and H). All of these data suggested
that knockdown of BLACAT1 in ME180 and C33A cells inhibited cell
proliferation in vitro.
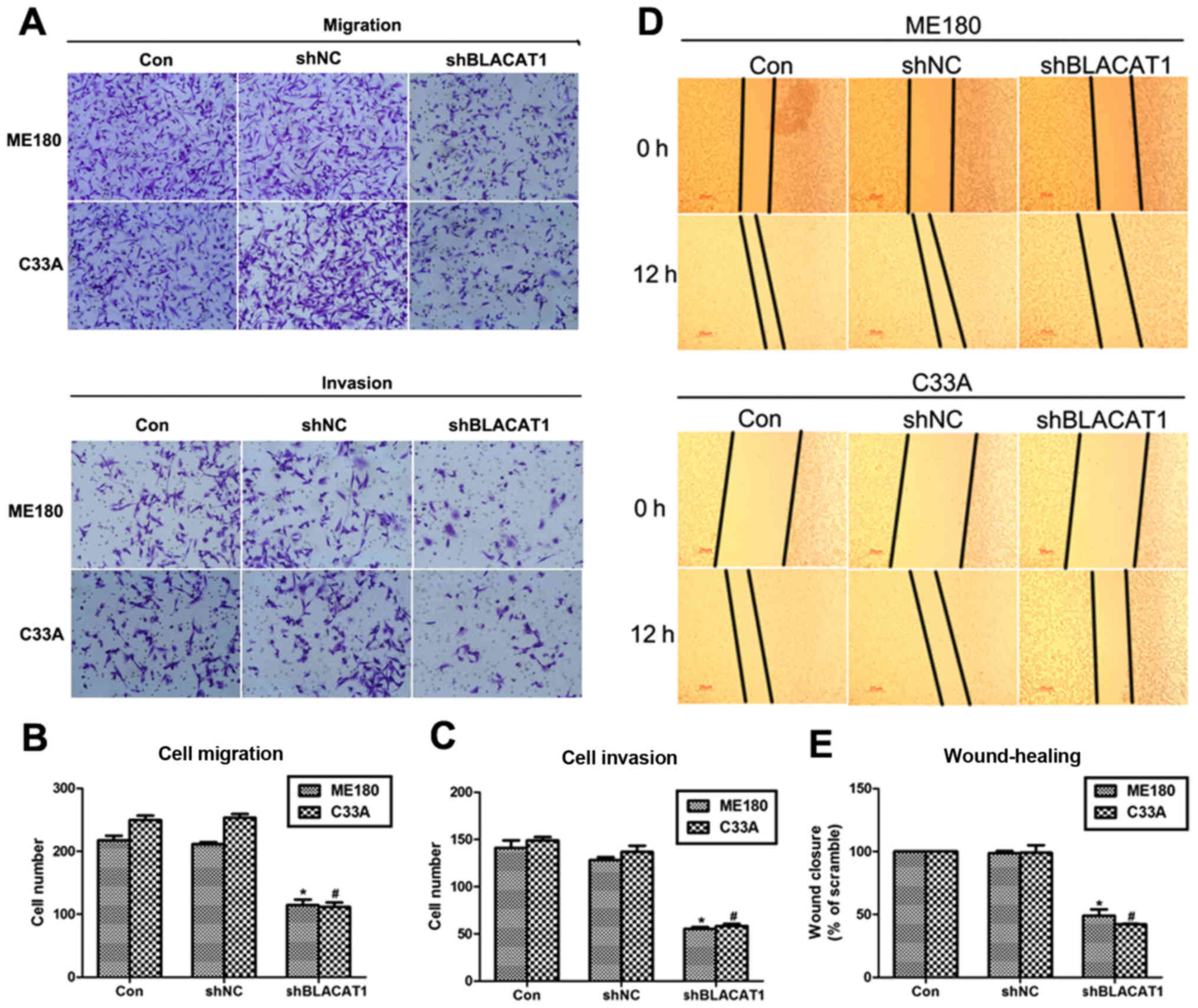
Depletion of BLACAT1 suppresses cell
metastasis in ME180 and C33A cells
Cell proliferation and cell metastasis are two main
manifestations of various malignancy. Thus, the role of BLACAT1 in
cell metastasis was also explored with Transwell assay and
wound-healing analysis. It was shown that more than 200 ME180 cells
and 240 C33A cells migrated through the membrane, and only 110
cells were observed after transfection of shBLACAT1 (Fig. 3A and B). Similarly, cell invasion was
also inhibited by knockdown of BLACAT1 in ME180 and C33A cells.
Approximate 100 ME180 and C33A cells were observed to be retarded
to invade through the membrane upon knockdown of BLACAT1 (Fig. 3A and C). Afterwards, wound-healing
assay further demonstrated this observation again. Cell capacity to
close the wound was inhibited by more than 50% after depletion of
BLACAT1 in both cell lines (Fig. 3D and
E). These results revealed that depletion of BLACAT1 inhibited
cell metastasis in human cervical cancer in vitro.
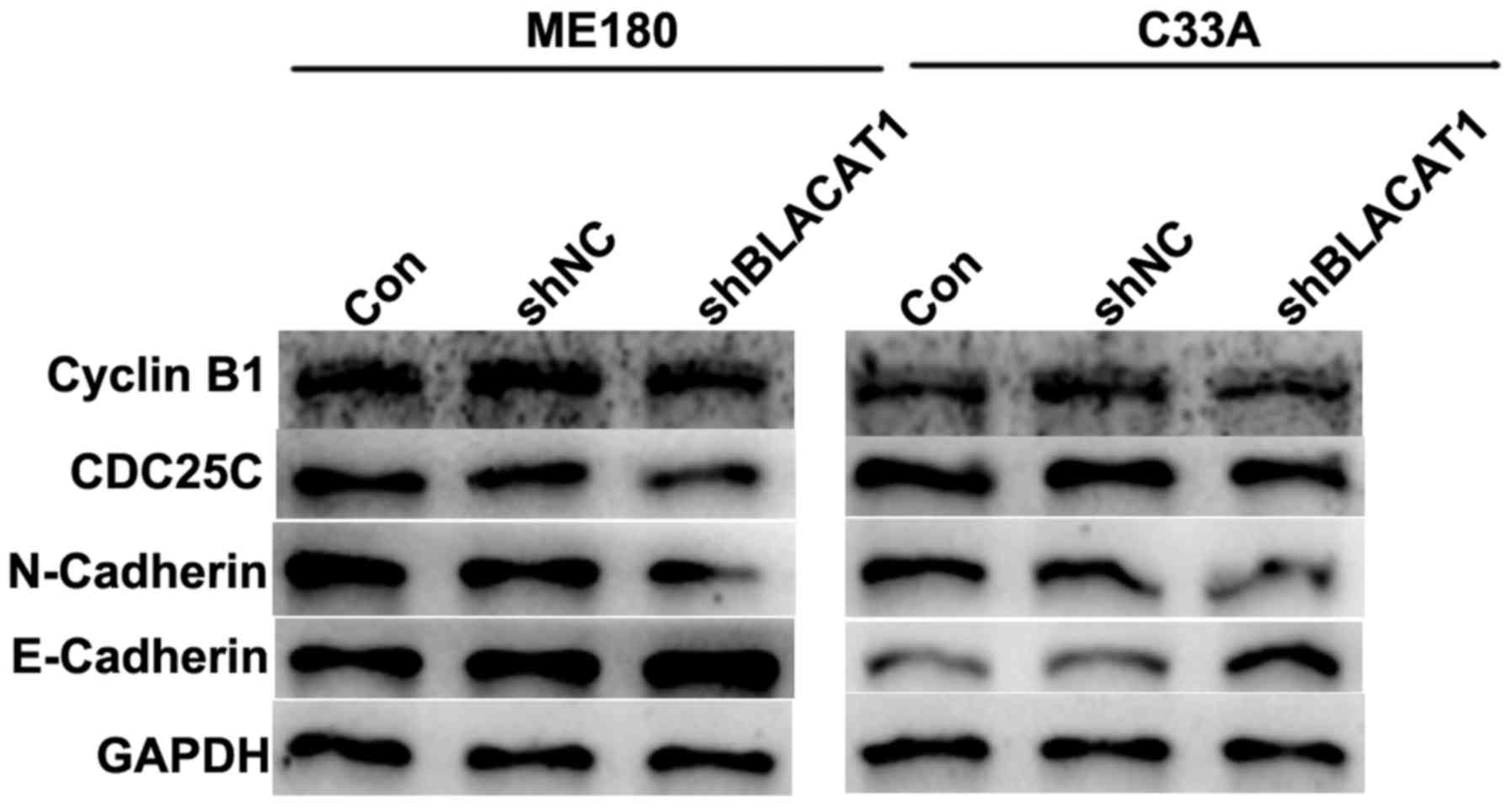
Depletion of BLACAT1 in ME180 and C33A
cells decreases cell cycle regulators and suppressed EMT
process
EMT is associated with tumorigenesis. Therefore,
cell cycle regulators cyclin B1 and CDC25C, and EMT markers
including mesenchymal N-cadherin and epithelial E-cadherin, were
detected in ME180 and C33A cells with shBLACAT1 transfection using
western blot analysis. As presented in Fig. 4, after BLACAT1 was knocked down by
specific shRNAs in cervical cancer ME180 and C33A cells, the
protein levels of cyclin B1, CDC25C and N-cadherin were decreased,
while the expression of E-cadherin was increased. The results of
the present study were consistent with the above functional
observations, reinforcing that knockdown of BLACAT1 inhibited cell
proliferation and metastasis.
Discussion
Cervical cancer remains one of the most common
gynecological malignancies (1). In
spite of traditional treatments consisting of surgery, chemotherapy
and radiotherapy the long-term prognosis for patients with cervical
cancer remains poor due to metastatic or recurrent property
(2). In view that uncontrolled cell
growth and metastasis are common features of cervical cancer
(4), identification of novel factors
associated with cervical cancer cell proliferation, migration and
invasion would aid in developing novel effective therapies.
Recent advances have implicated lncRNAs in the
development and progression of cervical cancer. BLACAT1 is a newly
identified lncRNA that was initially characterized in bladder
cancers (13), and later found to be
associated with prognosis of gastric cancer (14) and colorectal cancer (15). The present study was the first one to
uncover the expression and biological character of BLACAT1 in
cervical cancer.
Our data have shown that the relative expression of
BLACAT1 in cervical cancer tissues were significantly upregulated
as compared with the adjacent non-cancerous tissues. This
expression profile was consistent with previous studies (14,15).
Moreover, with the use of a specific shRNA against BLACAT1, it was
further found that depletion of BLACAT1 inhibited cell
proliferation in cervical cancer ME180 and C33A cells. Cell cycle
deregulation is a hallmark of tumorigenesis and associated closely
with aberrant cell growth (17). Cell
growth primarily depends on cell cycle progression in mitosis and
is basically regulated by cell cycle regulators, including cyclins
and cyclin-dependent kinases (CDKs) (18,19). Our
western blot analysis revealed that knockdown of BLACAT1 decreased
the protein levels of key cell cycle regulators Cyclin B1 and
CDC25C, supporting the cell proliferation inhibition by BLACAT1
depletion. The accumulation of cells in G0/G1 phase with less cells
in S and G2/M phases also supported that the proliferation was
inhibited after knockdown of BLACAT1 in ME180 and C33A cells.
Interestingly, knockdown of BLACAT1 also slowed down wound
recovery, migration and invasion capacities. Epithelial to
mesenchymal transition is a hallmark of tumor distant metastasis
(20). Decrease of epithelial markers
and increase of mesenchymal markers were always accompanied with
cancer malignancies. Our western blot analysis also revealed that
epithelial property (E-cadherin) was over mesenchymal property
(N-cadherin) after BLACAT1 knockdown, which significantly
reinforced the metastasis-inhibition effects by BLACAT1 depletion.
Therefore, our data have identified BLACAT1 as a critical mediator
of cell proliferation and metastasis in cervical cancer.
Mechanisms of how BLACAT1 functions in cervical
cancer remain mysterious to date. Available literature has shown
that BLACAT1 exerts a functional role in bladder cancer by
recruiting and binding to polycomb repressive complex 2 (PRC2)
(13). A more recent study also
reported that BLACAT1 regulated cell proliferation by
epigenetically silencing of p15 (15). The two available studies suggested
that regulation of proteins, either via physical interaction
or epigenetic regulation, might underlie the functional basis of
BLACAT1. However, more work needs to be done. We will further work
to reveal these mechanistic networks that contributes to the
biological character of BLACAT1 in cervical cancer and we will
begin with the key regulator of PI3K/AKT, ERK1/2 AND NF-κB
signaling pathway and examine the expression of p-AKT and
p-ERK.
In all, the present study have identified a novel
lncRNA, BLACAT1, as a critical mediator of cell proliferation and
metastasis in cervical cancer. Our results have shown that BLACAT1
was highly expressed in cervical cancer. Depletion of BLACAT1
negatively affected cell proliferation, migration and invasion.
Previous reports mainly focused on the clinical outcome of BLACAT1
in human cancers with its involvement in cancer cell aggressiveness
largely unknown. Our data may represent the first one to provide
experimental data supporting the pro-oncogenic property of BLACAT1
in cervical cancer. The conclusion drawn from the present study may
aid in developing novel therapeutic strategies against cervical
cancer in clinic.
References
|
1
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann. Oncol. 22:2675–2686. 2011.
|
|
2
|
Yeung TL, Leung CS, Yip KP, Yeung Au CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A Review in the Theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Gao H, Gao X, Huang S, Wu K, Yu
X, Yuan K and Zeng T: Elevated expression of Kin17 in cervical
cancer and its association with cancer cell proliferation and
invasion. Int J Gynecol Cancer. 27:628–633. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou N, Fei D, Zong S, Zhang M and Yue Y:
MicroRNA-138 inhibits proliferation, migration and invasion through
targeting hTERT in cervical cancer. Oncol Lett. 12:3633–3639. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Hoon M, Shin JW and Carninci P:
Paradigm shifts in genomics through the FANTOM projects. Mamm
Genome. 26:391–402. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pennisi E: Genomics. ENCODE project writes
eulogy for junk DNA. Science. 337:1159–1161. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo X and Hua Y: CCAT1: An oncogenic long
noncoding RNA in human cancers. J Cancer Res Clin Oncol.
143:555–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Martin L and Chang HY: Uncovering the role
of genomic ‘dark matter’ in human disease. J Clin Invest.
122:1589–1595. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han P, Li W, Lin CH, Yang J, Shang C,
Nuernberg ST, Jin KK, Xu W, Lin CY, Lin CJ, et al: A long noncoding
RNA protects the heart from pathological hypertrophy. Nature.
514:102–106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu Y, Sun X, Mao C, Guo G, Ye S, Xu J, Zou
R, Chen J, Wang L, Duan P and Xue X: Upregulation of long noncoding
RNA TUG1 promotes cervical cancer cell proliferation and migration.
Cancer Med. 6:471–482. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han J, Zhou W, Jia M, Wen J, Jiang J, Shi
J, Zhang K, Ma H, Liu J, Ren J, et al: Expression quantitative
trait loci in long non-coding RNA PAX8-AS1 are associated with
decreased risk of cervical cancer. Mol Genet Genomics.
291:1743–1748. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu L, Jin L, Zhang W and Zhang L: Roles of
long non-coding RNA CCAT2 in cervical cancer cell growth and
apoptosis. Med Sci Monit. 22:875–879. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He W, Cai Q, Sun F, Zhong G, Wang P, Liu
H, Luo J, Yu H, Huang J and Lin T: Linc-UBC1 physically associates
with polycomb repressive complex 2 (PRC2) and acts as a negative
prognostic factor for lymph node metastasis and survival in bladder
cancer. Biochim Biophys Acta. 1832:1528–1537. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu Y, Pan J, Wang Y, Li L and Huang Y:
Long noncoding RNA linc-UBC1 is negative prognostic factor and
exhibits tumor pro-oncogenic activity in gastric cancer. Int J Clin
Exp Pathol. 8:594–600. 2015.PubMed/NCBI
|
|
15
|
Su J, Zhang E, Han L, Yin D, Liu Z, He X,
Zhang Y, Lin F, Lin Q, Mao P, et al: Long noncoding RNA BLACAT1
indicates a poor prognosis of colorectal cancer and affects cell
proliferation by epigenetically silencing of p15. Cell Death Dis.
8:e26652017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benson C, Kaye S, Workman P, Garrett M,
Walton M and de Bono J: Clinical anticancer drug development:
Targeting the cyclin-dependent kinases. Br J Cancer. 92:7–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Coudreuse D and Nurse P: Driving the cell
cycle with a minimal CDK control network. Nature. 468:1074–1079.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|