Introduction
Ovarian cancer is the seventh most common type of
cancer and the eighth most common cause of cancer-associated
mortality among females until 2012 (1). During 2012, ~238,700 female ovarian
cancer cases were diagnosed, and globally 151,900 females succumbed
to this disease (1). The majority of
the female patients who developed ovarian cancer were not aware of
the condition, or received diagnoses until an advanced stage, which
were primary causes of recurrence and early mortality (2,3). Despite
advances in imaging diagnosis, preoperative and postoperative care,
and chemotherapy delivery, there has been little improvement in
5-year overall survival (4–6).
Gene expression assays have been introduced in daily
clinical treatments for the care of patients with numerous
conditions, for example, patients with newly diagnosed breast
cancer (7). The Oncotype DX assay
(Genomic Health, Inc., Redwood City, CA, USA) is a 21-gene assay
that is designed to quantify risk of distant recurrence at 10 years
for a group of women with early stage breast cancer. The assay
includes genes associated with cell proliferation (Ki-67, STK15,
survivin, cyclin B1, MYBL2), invasion (stromelysin 3, cathepsin
L2), HER2, estrogen (ER, PR, Bcl2, SCUBE2), in addition to GSTM1,
CD68, BAG1, and several reference genes (β-actin, GAPDH, RPLP0,
GUS, and TFRC) (7). Zhan et al
(8) identified a five-gene
(cytoskeleton associated protein 4, Solute carrier family 40 member
1, otoferlin, mannosidase-α class 2A member 2, isoprenoid synthase
domain containing) panel that was significantly associated with
patient survival in those with renal clear cell carcinoma from The
Cancer Genome Atlas (TCGA) database. Using a publicly available
microarray database, an inverse association between elevated
SHANK-associated RH domain interactor gene expression with reduced
patient survival in PR+ or ER+ breast cancer
was identified by De Melo and Tang (9).
In the study of ovarian cancer, gene expression
profiling has been utilized extensively. Previous studies have
focused on differential gene expression between the tissue of
normal and tumors (10),
characterizing between histologic subtypes (11,12) and
marking differences between invasive and tumors with low malignancy
potential (13,14). Several studies have attempted to
target the gene expression signatures that correlate with clinical
data, to identify genes that are determinative of survival, to
generate predictive biomarkers (15,16). The
present study aimed to identify genes that were associated with the
overall survival time of patients with ovarian cancer by analyzing
high-throughput RNA sequencing data downloaded from TCGA using the
random survival forests variable hunting (RSFVH) algorithm
(17). Multiple genes were selected
to predict the survival time of patients following fitting, and
then used to verify the expression of the predicted genes in fresh
ovarian cancer tissue by using polymerase chain reaction (PCR)
analysis, and evaluate the prognostic value, sensitivity and
specificity of the model.
Materials and methods
Materials and kits
A total of two ovarian serous cystadenocarcinoma
fresh tissues were obtained during surgery from patients undergoing
surgical treatment in Hubei Maternal and Child Health Hospital
(Wuhan, China). Patients provided written informed consent.
Reverse transcription PCR
(RT-PCR)
For RT-PCR experiments, tissue RNA was extracted
using TRIzol (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's protocol. cDNA was synthesized
using random hexamers (Takara Biotechnology Co., Ltd., Dalian,
China). Briefly, and synthesized according to the following
conditions: 96°C for 5 min; 96°C for 20 sec; 55°C for 30 sec and
72°C for 1 min for 30 cycles. RT-PCR was performed with the RT-PCR
kit KOD HOT Start polymerase chain Reaction (EMD Millipore,
Billerica, MA, USA). The PCR cycling conditions were as follows:
95°C, for 5 sec, 95°C for 15 sec, 50°C for 5 sec and 60°C for 90
sec, for 35 cycles. With the use of ethidium bromide, amplified
products were visualized on 1.5% agarose gels. Finally, a UV-IV UV
analyzer instrument (Beyotime Institute of Biotechnology, Haimen,
China) was used to capture images. The following primer pairs were
used: Forward primer, 5′-ACCTTGGTCTGCGTTTG-3′; and reverse
5′-GCACATCTGGGTCTTGG-3′ for clathrin heavy chain-like 1 (CLTCL1);
forward, 5′-ATCCTGGAGGCTGTGCT-3′; and reverse,
5′-CTGAACGCTGGAACTGG-3′ for calcium/calmodulin dependent protein
kinase 11α (CAMK2A); forward 5′-ACCAGGACCTCAAAGACAGA-3′ and
reverse, 5′-GGGCATATTTAGGCATCAGT-3′ for mesencephalic
astrocyte-derived neurotrophic factor (MANF); forward primer,
5′-GAGCAGGAAATGGAGGA-3′; and reverse, 5′-TGGTTGTGATGCGAGAC-3′ for
dedicator of cytokinesis 11 (DOCK11); forward,
5′-CAGCAGCCTCGGCAGTA-3′; and reverse, 5′-CCGCAGGGTTTCTTTCAT-3′ for
dehydrogenase/reductase 4-like 1 (DHRS4L1).
Ovarian cancer gene expression data
from TCGA
The mRNA level 3 expression data of 413 patients
with ovarian cancer were downloaded from the TCGA database via the
data portal (https://cancergenome.nih.gov; accessed 23rd July
2016), including 22,547 human genes and the corresponding clinical
data. A total of 3 patients with missing data were excluded. Next,
the 410 ovarian cancer samples were randomly divided into a
training set (n=204) and a testing set (n=206). The training set
was used to identify gene expression signature, and the testing set
was used for validation.
Statistical analysis
A univariate Cox regression analysis was used to
evaluate the association between the expression level of genes and
patient OS. Next, based on the corresponding result, a risk score
formula was built to calculate the risk score for each patient.
Risk score (RS)=∑Ni=1(explg × coef), where N
is the number of genes, explg is the expression value of genes and
‘coef’ is the estimated regression coefficient of genes in the
univariate Cox regression analysis. Considering that a model with a
smaller number of genes would be more practical, genes that were
significantly associated with patient survival were identified
using the RSFVH algorithm (P<0.001). Kaplan-Meier and Cox
proportional-hazard regression analyses were performed for two
genes, with the expectation of identifying an improved model for
predicting survival. The cut-off values for the two genes were
computed with X-tile (18). The
survival differences between the low- and high-risk groups were
evaluated, and the sensitivity and specificity of the model in the
survival prediction was also compared using receiver operating
characteristic (ROC) analysis. All analyses were performed using R
program (http://www.r=project.org) including
packages named survival ROC. Survival and random Forest SRC was
downloaded from Bio-conductor.
Results
Patient characteristics
All 410 patients involved in the present study were
clinically and pathologically diagnosed with ovarian serous
cystadenocarcinoma, and the data were downloaded from TCGA
database. The mean age of these 410 patients was 60 years (range,
30–87). Using the International Federation of Gynecology and
Obstetrics classification (19),
clinical stages of the tumor were classified into stages I–IV. In
the present study, there were 0 patients with stage I, 22 patients
with stage II, 326 patients with stage III, and 62 patients with
stage IV disease. The 3 patients lacking clinical staging data were
not included in any analysis. All other patient information is
summarized in Table I. A total of 2
patients whose tissues, obtained from surgical resection, were used
were clinically and were pathologically diagnosed with ovarian
serous cystadenocarcinoma. Their ages were 44 and 53 respectively,
the clinical stages of tumor were stage III, and they were labeled
Patients 1 and 2.
 | Table I.Summary of patient demographics and
clinical characteristics. |
Table I.
Summary of patient demographics and
clinical characteristics.
|
Characteristics | Training set | Testing set | Total |
|---|
| Age |
|
|
|
| Median,
years | 60 | 60 | 60 |
| Range,
years | 30–87 | 30–87 | 30–87 |
| Clinical stage |
|
|
|
| Stage
I, n | 0 | 0 | 0 |
| Stage
II, n | 11 | 11 | 22 |
| Stage
III, n | 163 | 163 | 326 |
| Stage
IV, n | 30 | 32 | 62 |
| Patient status |
|
|
|
| Alive,
n | 84 | 95 | 179 |
|
Deceased, n | 120 | 111 | 231 |
Detection of genes associated with
overall survival time of patients with ovarian cancer in the
training set by RT-PCR
To identify the genes potentially associated with
overall survival time in patients with ovarian serous
cystadenocarcinoma, a total of 22,547 genes were identified by
random survival forests analysis. The order of analyses to develop
the risk score model and validate the efficiency of the signature
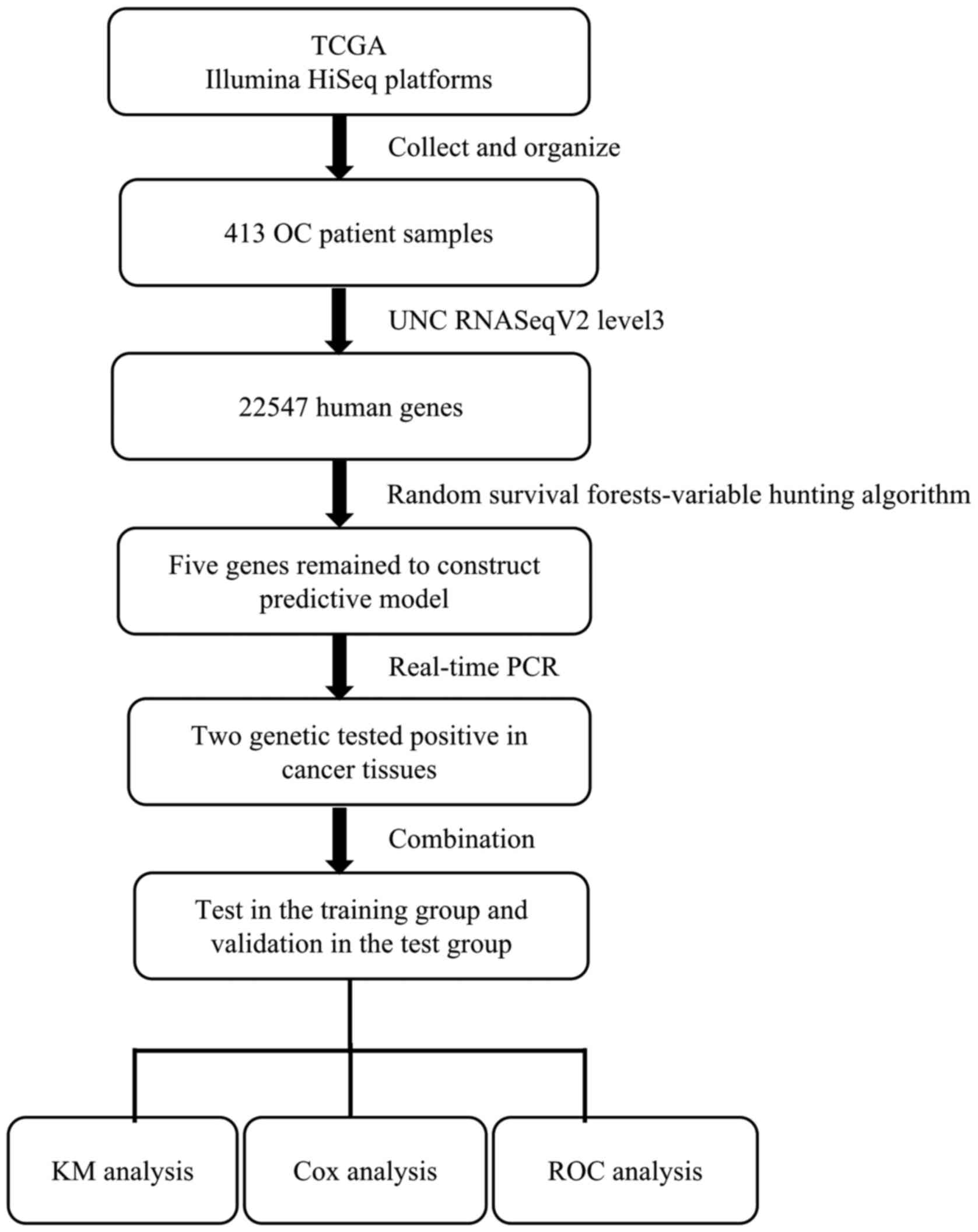
to predict prognostic outcomes is demonstrated in Fig. 1. A univariate Cox proportional hazards
regression analysis of the genes expression profiling data with
survival time, and survival status as the dependent variable was
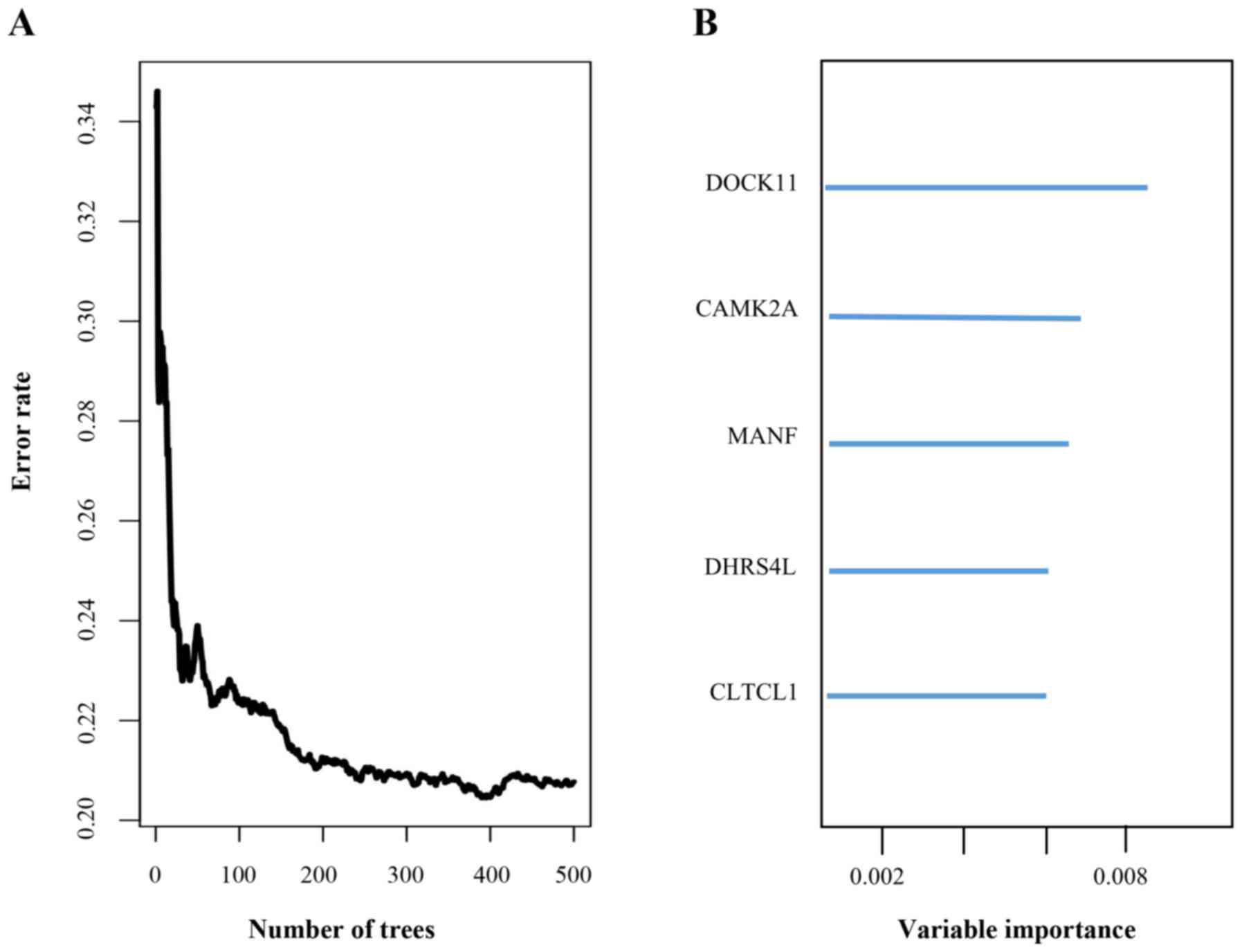
conducted. Using a random forest supervised classification
algorithm, a total of 5 genes (MANF, DOCK11, CLTCL1, CAMK2A,
DHRS4L1) with the highest association with the prognostic
classification were selected according to the permutation
importance scores for verification with PCR in 2 fresh ovarian
serous cystadenocarcinoma tissues (Fig.
2; Table II). Within the
selection of the five genes and subsequent RT-PCR analysis, despite
a number of changes in the amplification conditions and primers
referring to the optimization of the RT-PCR step, only two genes
exhibited positive expression (Fig.
3). The information concerning these two genes is summarized in
Table III. Following this
comparison, the optimum model including these two genes was
determined. The risk score formula for this model was (−0.53179 ×
expression value of MANF) + (0.324759 × expression value of
DOCK11). Using X-tile to determine the cut-off values, the values
of the training and testing sets was −2.60 and −2.86, respectively
(18). These values were included in
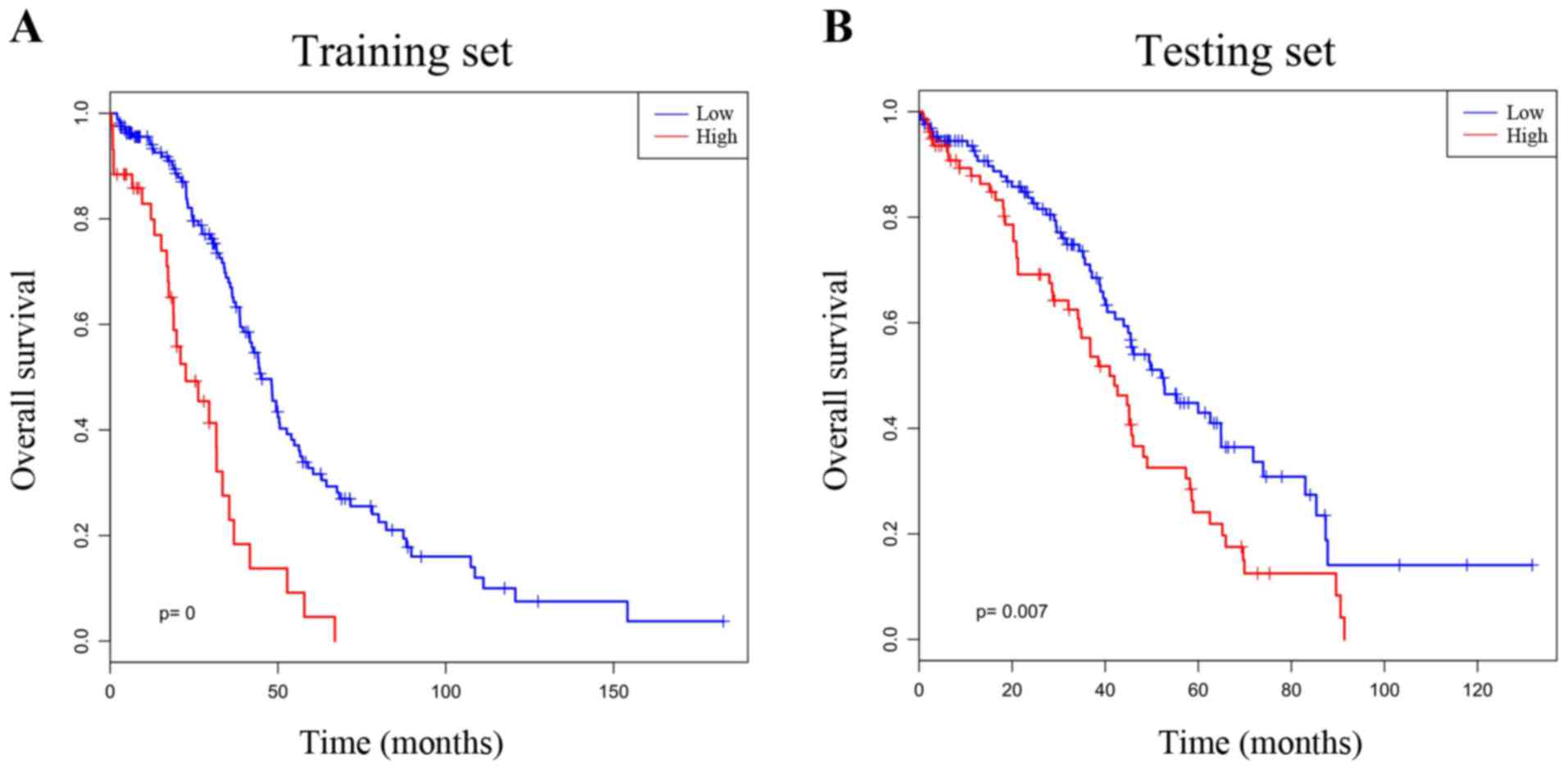
the low group. Survival analysis was performed by using the
Kaplan-Meier method with a log-rank statistical test between the
high-risk group and low-risk group. As demonstrated in Fig. 4A, Kaplan-Meier curves indicated that
patients in the high-risk group exhibited significantly
(P<0.001) poorer prognosis than those in the low-risk group.
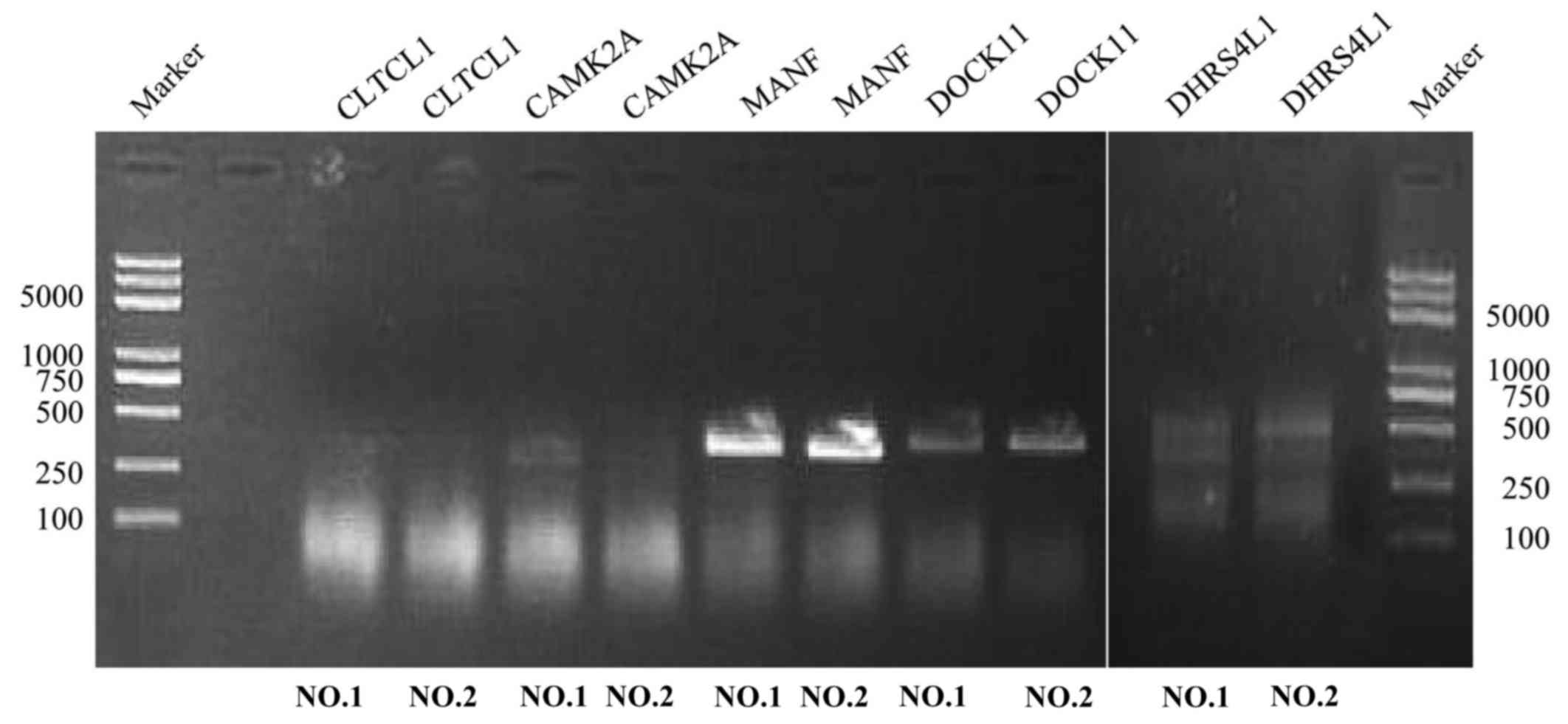 | Figure 3.Reverse transcription PCR. The
expression of five genes was assessed in two cases of ovarian
serous cystadenocarcinoma. The molecular length of the five genes
(CLTCL1, CAMK2A, MANF, DOCK11, DHRS4L1) was 1,146, 657, 396, 1,349
and 710 bp, respectively. Experiments were repeated in triplicate.
MANF, mesencephalic astrocyte-derived neurotrophic factor; DOCK11,
dedicator of cytokinesis 11; CLTCL1, clathrin heavy chain-like 1;
CAMK2A, calcium/calmodulin dependent protein kinase 11α; DHRS4L1,
dehydrogenase/reductase 4-like 1. |
 | Table II.Five genes significantly associated
with the survival time of patients in the training set. |
Table II.
Five genes significantly associated
with the survival time of patients in the training set.
| Gene name | Coefficient | HR | P-value |
|---|
| MANF | −0.53179 | 0.587553 | 0.002083 |
| DOCK11 | 0.324759 | 1.383697 | 0.022425 |
| CLTCL1 | 0.550443 | 1.734021 | 0.029124 |
| CAMK2A | 4.112609 | 61.10596 | 0.000217 |
| DHRS4L1 | −0.81199 | 0.443976 | 0.040793 |
 | Table III.Analysis of the function of the
two-gene model. |
Table III.
Analysis of the function of the
two-gene model.
| Gene name | Chromosomal
position | Start site | End site | Function |
|---|
| MANF | chr3 | 50674969 | 51536662 | Inhibits cell
proliferation and ER stress-induced cell death |
| DOCK11 | chrX | 118146063 | 118826973 | GEF that activates
CDC42 by exchanging bound GDP for free GTP |
Verification of survival-associated
genes in the testing set
To determine the prognostic potential of the
two-gene signature, Kaplan-Meier survival analysis was performed on
the testing set. There was a statistically significant difference
(P=0.007) between the high- and low-risk groups, which was in
agreement with that in the training test, revealing that this
two-gene signature may serve a role in predicting the survival of
ovarian cancer patients (Fig. 4B). To
confirm the clinical performance of the two-gene model as a
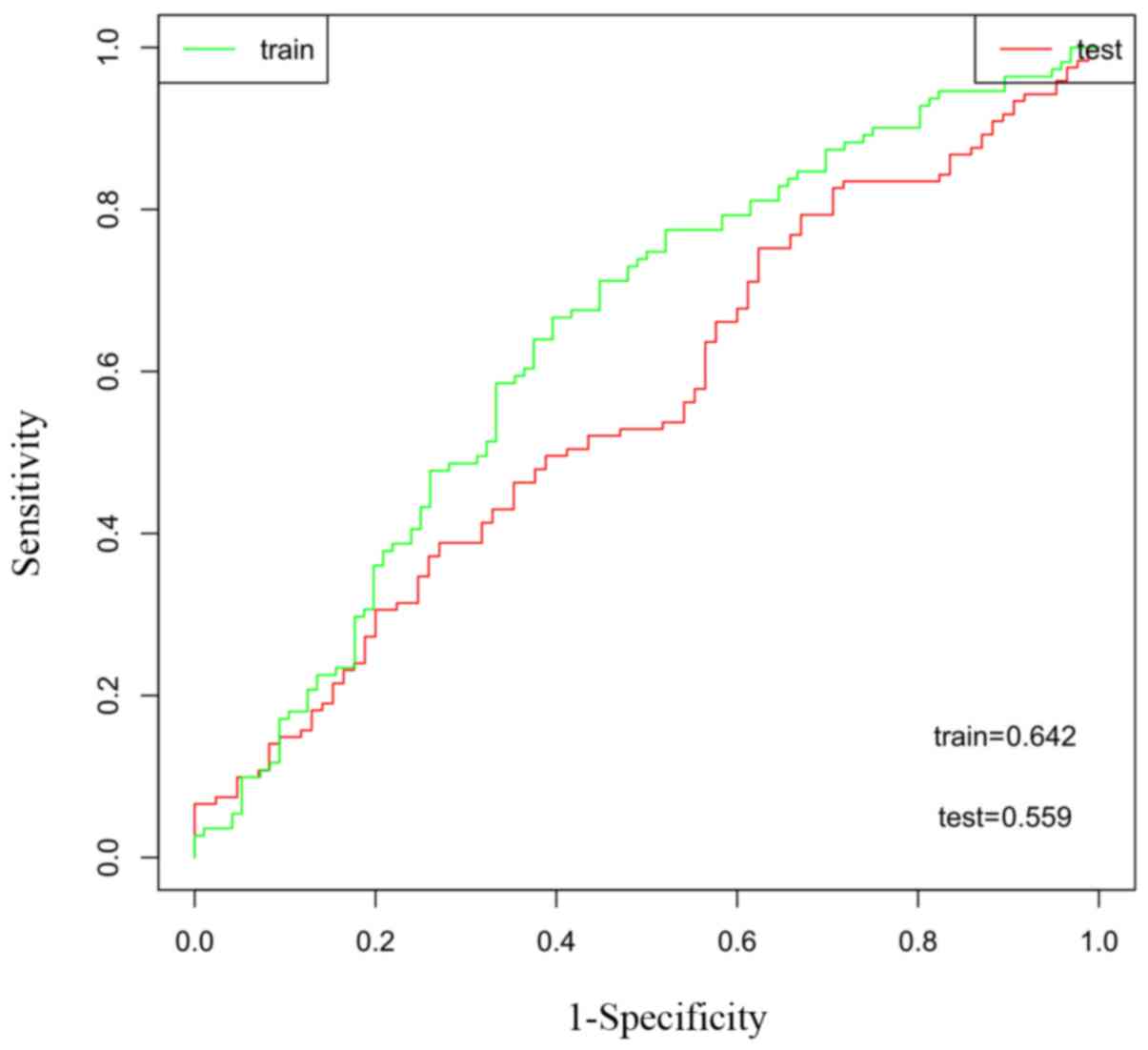
biomarker for predicting prognosis further, ROC analysis was used
to evaluate the validity of the gene signature on patient survival.
The area under the ROC curve (AUC) of the training and testing sets
were 0.642 and 0.559, respectively, demonstrating that the two-gene
model exhibited high sensitivity and specificity, and could be used
as a biomarker to predict the prognostic survival of patients
(Fig. 5).
To examine whether the two-gene model distinguished
between the high- and low-risk patients, Cox regression analyses
were performed in the training and testing sets. The results
confirmed that the two-gene model was an independent prognostic
factor for the prognosis of ovarian cancer in the training and the
test sets (Table IV).
 | Table IV.Two-gene model of Cox regression in
training and testing sets. |
Table IV.
Two-gene model of Cox regression in
training and testing sets.
| Sample sets | Parameter
estimate | Standard error | P-value | HR | 95% CI |
|---|
| Training set | 0.64009 | 0.21858 | 0.0034 | 1.897 | 1.236–2.911 |
| Testing set | 0.59023 | 0.25254 | 0.0194 | 1.804 | 1.100–2.960 |
Discussion
Ovarian cancer (OC) is one of the leading causes of
cancer mortality in gynecological oncology, exhibiting a 5-year
survival rate of 44% (20). The
serous ovarian cancer high-grade subtype is one of the most
aggressive and metastatic forms of ovarian cancer (21). In the present study, a five-gene
signature that was significantly associated with patient survival
in ovarian serous cystadenocarcinoma was predicted, based on
genome-wide RNA profiling of 413 ovarian cancer patients from the
TCGA database using the RSFVH algorithm and Cox analysis. Using
PCR, 2 genes (MANF and DOCK11) were verified to exhibit positive
expression in ovarian serous cystadenocarcinoma tissues.
Subsequently, it was confirmed that the two-gene model was an
independent prognostic predictor of survival using Cox regression
analysis on the training and testing sets.
MANF has been discussed in previous studies as a
survival-promoting factor for embryonic midbrain dopaminergic
neurons in vitro (22). In
HeLa cells, MANF is localized in the endoplasmic reticulum, which
is expressed at particularly high levels in secretory tissues with
extensive protein production (23).
Notably, prior studies have indicated that MANF is important for
protein homeostasis in the endoplasmic reticulum, as the knockdown
of MANF in cultured cells and the knockout of MANF in mice and
Drosophila resulted in the activation of the unfolded
protein response, a signaling pathway induced by endoplasmic
reticulum stress (23–25). Evidence indicates that the endoplasmic
reticulum is involved in apoptotic signaling pathways (26), and that it participates in the
occurrence and development of many types of cancer, including
cervical cancer (27), hepatocellular
carcinoma (28) and head and neck
squamous cell carcinoma (29). In
addition, MANF may reduce the inflammatory response and prevent
proliferation of inflamed cells by inhibiting DNA binding of the
transcription factor p65 subunit, consequently suppressing the
inflammatory pathways induced by nuclear factor-κ
light-chain-enhancer of activated B cells binding to its target
genes (30).
The other positive gene identified in the present
study, DOCK11, is a gene that belongs to the dedicator of
cytokinesis (Dock) protein family, a class of guanine nucleotide
exchange factors (GEFs) that activate the Rho GTPases, and one of
the three members of the Dock-D subfamily. Dock proteins are large
proteins, which constitute a major class, together with the
Dbl-homology proteins, of Rho GEFs (31,32).
Membrane receptors promote the reorganization of the actin
cytoskeleton downstream of the Rho GTPases by their GEFs to
regulate cell adhesion and migration (33). There are two classes of exchange
factors that are associated with GTPases: The classical
Dbl-associated exchange factors and, the more recently identified
atypical Dock-family exchange factors. The Dock family of exchange
factors was identified only 12 years ago as a novel class of Rho
GTPase activators, particularly Ras-related C3 botulinum toxin
substrates 1, 2 and 3, and Cdc42 (34,35). In
mammals, there are 11 Dock genes, which are grouped into 4
subfamilies: A, B, C and D (35). The
D subfamily, characterized by an N-terminal pleckstrin homology
domain, is made up of 3 members, Dock9, Dock10, and Dock11
(32,36,37).
Dock11 mediates a positive feedback activation of cell division
control protein Cdc42 homolog (Cdc42), as active Cdc42 may in turn
bind to Dock11 and enhance its GEF activity (37). Sakabe et al (38) revealed that Dock11 recruitment
downstream of Fc γ-receptor III and TLR4 activated Cdc42 to promote
cell migration. As TLR4 has been demonstrated to promote the
epithelial-mesenchymal transition and cancer cell migration
(39–41), we hypothesized that Dock11 activity is
associated with cancer-induced pathological cell migration. In
support of this hypothesis, Dock11 was detected among the top-20
highest expressed genes in testicular carcinoma (42).
Expression of MANF and DOCK11 in human tissues has
not been extensively studied, including in ovarian tissues. The
Human Protein Atlas (www.proteinatlas.org) database (43) was used to identify that MANF was not
expressed in the follicle cells and was expressed at low levels in
the stroma cells of normal ovarian tissues; DOCK11 was not detected
in the stromal cells and detected at low levels in the follicle
cells of the normal ovarian tissue. MANF exhibited high, medium and
low expression, and DOCK11 presented a medium, low, no expression
in different clinical staging tissue of ovarian serous
cystadenocarcinoma. In the present study, five genes associated
with ovarian cancer survival were predicted through analysis of
TCGA database, and the positive expression of two genes (MANF and
DOCK11) was validated in ovarian cancer. In subsequent experiments,
the expression of MANF and DOCK11 at the protein level in ovarian
serous cystadenocarcinoma and adjacent normal tissues should be
verified, and the clinical relevance should be identified to
determine the use of these genes as novel biomarkers to predict the
treatment outcomes of patients with ovarian cancer.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holschneider CH and Berek JS: Ovarian
cancer: Epidemiology, biology, and prognostic factors. Semin Surg
Oncol. 19:3–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klint A, Tryggvadóttir L, Bray F, Gislum
M, Hakulinen T, Storm HH and Engholm G: Trends in the survival of
patients diagnosed with cancer in female genital organs in the
Nordic countries 1964–2003 followed up to the end of 2006. Acta
Oncol. 49:632–643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markman M, Bundy BN, Alberts DS, Fowler
JM, Clark-Pearson DL, Carson LF, Wadler S and Sickel J: Phase III
trial of standard-dose intravenous cisplatin plus paclitaxel versus
moderately high-dose carboplatin followed by intravenous paclitaxel
and intraperitoneal cisplatin in small-volume stage III ovarian
carcinoma: An intergroup study of the Gynecologic Oncology Group,
Southwestern Oncology Group, and Eastern Cooperative Oncology
Group. J Clin Oncol. 19:1001–1007. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA:
Gynecologic Oncology Group: Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coleman MP, Forman D, Bryant H, Butler J,
Rachet B, Maringe C, Nur U, Tracey E, Coory M, Hatcher J, et al:
Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and
the UK, 1995–2007 (the International Cancer Benchmarking
Partnership): An analysis of population-based cancer registry data.
Lancet. 377:127–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baker J: Genomic Health, Inc.
Pharmacoqenomics. 8:397–399. 2007. View Article : Google Scholar
|
|
8
|
Zhan Y, Guo W, Zhang Y, Wang Q, Xu XJ and
Zhu L: A five-gene signature predicts prognosis in patients with
kidney renal clear cell carcinoma. Comput Math Methods Med.
2015:8427842015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Melo J and Tang D: Elevation of SIPL1
(SHARPIN) increases breast cancer risk. PLoS One. 10:e01275462015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Welsh JB, Zarrinkar PP, Sapinoso LM, Kern
SG, Behling CA, Monk BJ, Lockhart DJ, Burger RA and Hampton GM:
Analysis of gene expression profiles in normal and neoplastic
ovarian tissue samples identifies candidate molecular markers of
epithelial ovarian cancer. Proc Natl Acad Sci USA. 98:1176–1181.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schwartz DR, Kardia SL, Shedden KA, Kuick
R, Michailidis G, Taylor JM, Misek DE, Wu R, Zhai Y, Darrah DM, et
al: Gene expression in ovarian cancer reflects both morphology and
biological behavior, distinguishing clear cell from other
poor-prognosis ovarian carcinomas. Cancer Res. 62:4722–4729.
2002.PubMed/NCBI
|
|
12
|
Schaner ME, Ross DT, Ciaravino G, Sorlie
T, Troyanskaya O, Diehn M, Wang YC, Duran GE, Sikic TL, Caldeira S,
et al: Gene expression patterns in ovarian carcinomas. Mol Biol
Cell. 14:4376–4386. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bonome T, Lee JY, Park DC, Radonovich M,
Pise-Masison C, Brady J, Gardner GJ, Hao K, Wong WH, Barrett JC, et
al: Expression profiling of serous low malignant potential,
low-grade, and high-grade tumors of the ovary. Cancer Res.
65:10602–10612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gilks CB, Vanderhyden BC, Zhu S, van de
Rijn M and Longacre TA: Distinction between serous tumors of low
malignant potential and serous carcinomas based on global mRNA
expression profiling. Gynecol Oncol. 96:684–694. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mok SC, Chao J, Skates S, Wong K, Yiu GK,
Muto MG, Berkowitz RS and Cramer DW: Prostasin, a potential serum
marker for ovarian cancer: Indentification through microarray
technology. J Natl Cancer Inst. 93:1458–1464. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spentzos D, Levine DA, Kolia S, Otu H,
Boyd J, Libermann TA and Cannistra SA: Unique gene expression
profile based on pathologic response in epithelial ovarian cancer.
J Clin Oncol. 23:7911–7918. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Chen Z, Tian L, Zhou C, He MY, Gao
Y, Wang S, Zhou F, Shi S, Feng X, et al: LncRNA profile study
reveals a three-lncRNA signature associated with the survival of
patients with oesophageal squamous cell carcinoma. Gut.
63:1700–1710. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Camp RL, Dolled-Filhart M and Rimm DL:
X-tile: A new bio-informatics tool for biomarker assessment and
outcome-based cut-point optimization. Clin Cancer Res.
10:7252–7259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nguyen HN, Averette HE, Hoskins W, Sevin
BU, Penalver M and Steren A: National survey of ovarian carcinoma.
VI. Critical assessment of current International Federation of
Gynecology and Obstetrics staging system. Cancer. 72:3007–3011.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baldwin LA, Huang B, Miller RW, Tucker T,
Goodrich ST, Podzielinski I, DeSimone CP, Ueland FR, van Nagell JR
and Seamon LG: Ten-year relative survival for epithelial ovarian
cancer. Obstet Gynecol. 120:612–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCluggage WG: Morphological subtypes of
ovarian carcinoma: A review with emphasis on new developments and
pathogenesis. Pathology. 43:420–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petrova P, Raibekas A, Pevsner J, Vigo N,
Anafi M, Moore MK, Peaire AE, Shridhar V, Smith DI, Kelly J, et al:
MANF: A new mesencephalic, astrocyte-derived neurotrophic factor
with selectivity for dopaminergic neurons. J Mol Neurosci.
20:173–188. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Apostolou A, Shen Y, Liang Y, Luo J and
Fang S: Armet, a UPR-upregulated protein, inhibits cell
proliferation and ER stress-induced cell death. Exp Cell Res.
314:2454–2467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lindahl M, Danilova T, Palm E, Lindholm P,
Võikar V, Hakonen E, Ustinov J, Andressoo JO, Harvey BK, Otonkoski
T, et al: MANF is indispensable for the proliferation and survival
of pancreatic β cells. Cell Rep. 7:366–375. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Palgi M, Greco D, Lindström R, Auvinen P
and Heino TI: Gene expression analysis of Drosophilaa Manf mutants
reveals perturbations in membrane traffic and major metabolic
changes. BMC Genomics. 13:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang YM, Yang Y, Dai WW, Li XM, Ma JQ and
Tang LP: Genistein-induced apoptosis is mediated by endoplasmic
reticulum stress in cervical cancer cells. Eur Rev Med Pharmacol
Sci. 20:3292–3296. 2016.PubMed/NCBI
|
|
28
|
Yeh TC, Chiang PC, Li TK, Hsu JL, Lin CJ,
Wang SW, Peng CY and Guh JH: Genistein induces apoptosis in human
hepatocellular carcinomas via interaction of endoplasmic reticulum
stress and mitochondrial insult. Biochem Pharmacol. 73:782–792.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
El Jamal SM, Taylor EB, Elmageed Abd ZY,
Alamodi AA, Selimovic D, Alkhateeb A, Hannig M, Hassan SY,
Santourlidis S, Friedlander PL, et al: Interferon gamma-induced
apoptosis of head and neck squamous cell carcinoma is connected to
indoleamine-2,3-dioxygenase via mitochondrial and ER
stress-associated pathways. Cell Division. 11:112016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Feng L, Wang X, Du J, Chen Y, Yang
W, Zhou C, Cheng L, Shen Y, Fang S, et al: Mesencephalic
astrocyte-derived neurotrophic factor is involved in inflammation
by negatively regulating the NF-κB pathway. Sci Rep. 5:81332015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rossman KL, Der CJ and Sondek J: GEF means
go: Turning on RHO GTPases with guanine nucleotide-exchange
factors. Nat Rev Mol Cell Biol. 6:167–180. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meller N, Irani-Tehrani M, Kiosses WB, Del
Pozo MA and Schwartz MA: Zizimin1, a novel Cdc42 activator, reveals
a new GEF domain for Rho proteins. Nat Cell Biol. 4:639–647. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gadea G and Blangy A: Dock-family exchange
factors in cell migration and disease. Eur J Cell Biol. 93:466–477.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cook DR, Rossman KL and Der CJ: Rho
guanine nucleotide exchange factors: Regulators of Rho GTPase
activity in development and disease. Oncogene. 33:4021–4035. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Côté JF and Vuori K: Identification of an
evolutionarily conserved superfamily of DOCK180-related proteins
with guanine nucleotide exchange activity. J Cell Sci.
115:4901–4913. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishikimi A, Meller N, Uekawa N, Isobe K,
Schwartz MA and Maruyama M: Zizimin2: A novel, DOCK180-related
Cdc42 guanine nucleotide exchange factor expressed predominantly in
lymphocytes. FEBS Lett. 579:1039–1046. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin Q, Yang W, Baird D, Feng Q and Cerione
RA: Identification of a DOCK180-related guanine nucleotide exchange
factor that is capable of mediating a positive feedback activation
of Cdc42. J Biol Chem. 281:35253–35262. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakabe I, Asai A, Iijima J and Maruyama M:
Age-related guanine nucleotide exchange factor, mouse Zizimin2,
induces filopodia in bone marrow-derived dendritic cells. Immun
Ageing. 9:22012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jing YY, Han ZP, Sun K, Zhang SS, Hou J,
Liu Y, Li R, Gao L, Zhao X, Zhao QD, et al: Toll-like receptor 4
signaling promotes epithelial-mesenchymal transition in human
hepatocellular carcinoma induced by lipopolysaccharide. BMC Med.
10:982012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liao SJ, Zhou YH, Yuan Y, Li D, Wu FH,
Wang Q, Zhu JH, Yan B, Wei JJ, Zhang GM and Feng ZH: Triggering of
Toll-like receptor 4 on metastatic breast cancer cells promotes
αvβ3-mediated adhesion and invasive migration. Breast Cancer Res
Treat. 133:853–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rakhesh M, Cate M, Vijay R, Shrikant A and
Shanjana A: A TLR4-interacting peptide inhibits
lipopolysaccharide-stimulated inflammatory responses, migration and
invasion of colon cancer SW480 cells. Oncoimmunology. 1:1495–1506.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Almstrup K, Leffers H, Lothe RA,
Skakkebaek NE, Sonne SB, Nielsen JE, Rajpert-De Meyts E and
Skotheim RI: Improved gene expression signature of testicular
carcinoma in situ. Int J Androl. 30:292–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pontén F, Jirström K and Uhlen M: The
human protein atlas-a tool for pathology. J Pathol. 216:387–393.
2008. View Article : Google Scholar : PubMed/NCBI
|