Introduction
Esophageal cancer is a highly lethal disease with a
rapidly increasing incidence. In China, esophageal cancer is the
fourth most common cause of mortality, resulting in 16.77
mortalities per 100,000 of the population in 2009 (1). In China, esophageal squamous cell
carcinoma (ESCC) is a major histopathological subtype of esophageal
cancer. Locally advanced ESCC is conventionally treated through
radiotherapy; however, a large proportion of ESCC tumors develop
resistance to radiation. This phenomenon emphasizes the importance
of enhancing tumor radiosensitization in ESCC.
Hypoxia is a common phenomenon observed in solid
tumors. Hypoxia-inducible factor (HIF)-1 is an important regulator
of adaptive responses to hypoxia (2).
HIF-1 plays an important role in tumor growth, proliferation,
metastasis and therapeutic resistance. HIF-1 is a heterodimer
protein consisting of HIF-1α and HIF-1β. HIF-1α is primarily
involved in HIF-1 protein stabilization and transactivation
(3). Therefore, HIF-1α expression
should be suppressed to enhance the sensitivity of tumor cells to
radiotherapy.
Laboratory and clinical studies have demonstrated
that Brucea javanica oil emulsion (BJOE) provides an
antitumor potential. However, BJOE, in combination with
radiotherapy has yet to be used to treat esophageal cancer. The
present study was designed to explore the radiosensitizing effect
of BJOE on human esophageal cancer in vitro and in
vivo.
Materials and methods
Cell culture and treatment
Human esophageal carcinoma cell line ECA109 was
provided by the Central Experimental Laboratory, Nanjing Medical
University (Nanjing, China). The cells were cultured in RPMI-1640
medium (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China)
containing 10% Sijiqing newborn bovine serum (Zhejiang Tianhang
Biotech Co., Ltd., Huzhou, China) in a 5% CO2
thermostatic incubator (Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C. The cells were also incubated at 37°C in a sealed
tank filled with complex air consisting of 1% O2, 94%
N2, and 5% CO2 to stimulate growth under
hypoxic conditions for 24 h.
The cells were irradiated with a single dose at a
dose rate of 200 cGy/min using a 6 MV linear accelerator (Precise
Treatment System™; Elekta AB, Stockholm, Sweden). The
source-cell distance was 100 cm, and the radiation field was 20×20
cm.
Patients and treatment
A total of 20 patients with histological evidence of
invasive thoracic ESCC were enrolled between January 2013 and
December 2014. They underwent radiotherapy at the Center of
Radiation Oncology in the First Affiliated Hospital of Nanjing
Medical University (Nanjing, China). Patients with tumors in
clinical stage T1N1 or T2-3N0-1 and M0, in accordance with the
Union for International Cancer Control Tumor Node Metastasis
classification (Preoperative Chemoradiotherapy for Esophageal or
Junctional Cancer) (4), were chosen.
The enrolled patients (12 males, 8 females) were between 40–70
years old, and were characterized by an Eastern Cooperative
Oncology Group performance status score of ≤2. Adequate
hematologic, renal, hepatic and pulmonary functions, absence of
other cancer types, or previous radiotherapy or chemotherapy were
required. The patients signed an informed consent prior to
treatment administration. The present study was approved by the
Ethics Committee of Huai'an First People's Hospital in Huai'an
(Jiangsu, China).
The patients were randomly divided into two groups:
Radiation group and radiation + BJOE group. They received a total
radiation dose of 56–60 Gy in 28–30 fractions with 2 Gy/fraction, 5
days/week using three-dimensional conformal radiotherapy technique.
In the radiation + BJOE group, 30 ml of BJOE dissolved in 250 ml
normal saline was intravenously injected once daily.
Reagents and antibodies
BJOE was provided by Shenyang Yaoda Pharmaceutical
Co., Ltd. (Liaoning, China). Anti-HIF-1α (cat, no. sc13515) and
anti-β-actin monoclonal antibodies (cat. no. sc70319) were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
Rabbit antihuman phosphorylated H2A histone family member X (γH2AX)
polyclonal antibody (cat. no. 309-005-003), horseradish peroxidase
(HRP)-conjugated secondary antibody (cat. no. 111-035-003) and
fluorescein (FITC)-conjugated secondary antibody (cat. no.
111-005-003) were obtained from Jackson ImmunoResearch
Laboratories, Inc. (Westgrove, PA, USA).
MTT assay
ECA109 cells in the logarithmic growth phase were
inoculated in 96-well plates at a density of 5×103
cells/ml. The cells were cultured in an incubator containing 5%
CO2 at 37°C. The medium was removed and replaced with
fresh medium supplemented with different BJOE concentrations (1.25,
2.5, 5, 10, 20, 40, 80, and 100 mg/ml), when the monolayers had
reached confluency. An untreated cell culture medium was used as an
experimental control. After treatments were administered for 24 and
48 h, medium was removed, and cells were washed with PBS (pH 7.4),
once or twice. Following this, 20 µl MTT (5 mg/ml; Beyotime
Institute of Biotechnology, Haimen, China) was added to each well.
After the medium was inoculated for another 4 h, the supernatant
was removed and 150 µl dimethyl sulfoxide (Nanjing KeyGen Biotech
Co., Ltd.) was added to each well to dissolve formazan crystals.
Cell viability was determined through MTT cell proliferation and
cytotoxicity assay (Beyotime Institute of Biotechnology).
Absorbance was obtained at a wavelength of 490 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Clone formation assay
ESCC cells in the logarithmic growth phase were
trypsinized and diluted to single-cell suspensions. Following this,
200 cells/ml were seeded into 6-well plates and cultured overnight.
Adherent cells were randomly divided into two groups: Radiation
group and the BJOE (2.5 mg/ml) + radiation group. After the cells
were cultured under hypoxic conditions, both groups were irradiated
with different absorbance doses of 0, 2, 4, 6, and 8 Gy. The cells
were then cultured in an incubator with 5% CO2 at 37°C
for ~2 weeks until the colonies were visible. The colonies were
fixed with methanol for 10 min at room temperature and stained with
Giemsa for 15 min at room temperature. The number of colonies
(>50 cells/colony) were counted. A cell survival curve was
fitted on the basis of single-hit multiple-target model by using
GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA) to
determine the survival
fraction=1-(1-e−D/D0)n, where D0 is
the dose required when the curve index decreased by 63%. D37 is the
required dose when the curve index decreased by 37%. The
sensitization enhancement ratio (SER) was calculated as follows: D0
in the control group/D0 in the experimental group.
γH2AX immunofluorescent
measurement
ECA109 cells were cultured on 6-well plates at a
density of 5×104 cells/ml. The cells were treated with
or without BJOE (2.5 mg/ml) and exposed to 6 Gy radiation. The
control cells did not receive BJOE or radiation treatment. Each
group was incubated under hypoxic conditions for 24 h. Thereafter,
the cells were fixed with 4% paraformaldehyde for 15 min at 4°C,
permeabilized with 0.3% Triton X-100 for 15 min at 4°C, and blocked
with 1% bovine serum albumin for 2 h at room temperature (all from
Nanjing KeyGen Biotech Co., Ltd.). The cells were incubated with an
anti-γH2AX primary antibody (dilution, 1:250) at 4°C overnight and
the following day with secondary antibody conjugated with FITC
(dilution, 1:150) for 1 h at room temperature. The nuclei were
counterstained with DAPI (Nanjing KeyGen Biotech Co., Ltd.) for 3
min. Focal formation was verified by using a laser scanning
confocal microscope.
Flow cytometric analysis of
apoptosis
Single-cell suspension of ESCC cells lines were
seeded onto 6-well plates at a density of 5×106
cells/ml. The control group was cultured under hypoxic conditions
for 24 h without any treatment. The two groups were treated with 6
Gy irradiation and cultured for 24 h under hypoxic conditions. They
were further cultured for 48 h. The cells were routinely
trypsinized, rinsed with cold PBS, resuspended in 1X
Annexin-binding buffer and stained with the Annexin V-flourescein
isothiocyanate FITC apoptosis kit (Nanjing KeyGen Biotech Co.,
Ltd.), according to the manufacturer's protocol. The samples were
immediately analyzed by using a flow cytometer. Data was analyzed
using FlowJo 7.6.2 (FlowJo LLC, Asland, OR, USA).
Western blot analysis
ESCC cells were treated with BJOE under hypoxic
conditions for 24 h. The control cells were cultured without BJOE
under normoxic, and hypoxic conditions for 12, 24, and 48 h.
Subsequently, proteins were extracted from cells using RIPA lysis
buffer (Beyotime Institute of Biotechnology), and protein
concentration was determined through a BCA assay. Equal amounts of
protein (40 µg) were loaded into each lane and separated through
SDS-PAGE with 8% resolving gel and 5% stacking gel using an
electrophoresis instrument (Bio-Rad Laboratories, Inc.) at 60 V for
15 min and at 120 V for 30 min. Proteins were transferred onto
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA) using a wet electrophoretic transfer instrument (Bio-Rad
Laboratories, Inc.) at 250 mA for 180 min. The membranes were
blocked with 5% skim milk for 1 h at room temperature, and then
incubated with anti-HIF-1α monoclonal antibodies (diluted 1:1,000)
at 4°C overnight. The following day, membranes were incubated with
goat anti-rabbit IgG (H+L) HRP-conjugated secondary antibody
(diluted 1:5,000) for 1 h at room temperature. The immunostained
membranes were visualized using an enhanced chemiluminescence
detection kit (Nanjing KeyGen Biotech Co., Ltd.) with a Chemidoc
XRS imaging system (Bio-Rad, Laboratories, Inc.).
Enzyme-linked immunosorbent assay
(ELISA)
Venous blood samples were collected from the
enrolled patients before and after radiotherapy was completed.
Serum was isolated at (2,014 × g) and 4°C for 10 min. Serum HIF-1α
levels were assayed using a human HIF-1α ELISA kit (Shanghai Bogoo
Biological Technology Co., Ltd., Shanghai, China) in accordance
with the manufacturer's protocol. Absorbance was determined at a
wavelength of 450 nm on a microplate reader (Bio-Rad Laboratories,
Inc.).
Statistical analysis
Data were statistically analyzed using SPSS 17.0
(SPSS Inc., Chicago, IL, USA) and expressed as the mean ± standard
deviation. All experiments were performed at least in triplicate,
and differences between treatment groups were determined via
one-way analysis of variance with post hoc contrasts by
Student-Newman-Keuls test. P<0.05 was considered to indicate a
statistically significant difference.
Results
BJOE enhances the radiosensitivity of
hypoxic ESCC cell lines
The viability of ESCC cell lines was detected
following treatment with different BJOE concentrations for 24 and
48 h. MTT assay revealed that BJOE inhibited the growth of ECA109
in a concentration- and time-dependent manner (Fig. 1). At 24 h, IC20 of ECA109
was 4.02 mg/ml. The treatment with 2.5 mg/ml BJOE for 24 h did not
significantly affect the OD of ECA109 cells. Thus, 2.5 mg/ml BJOE
was chosen for the radiosensitization experiments to avoid BJOE
cytotoxicity.
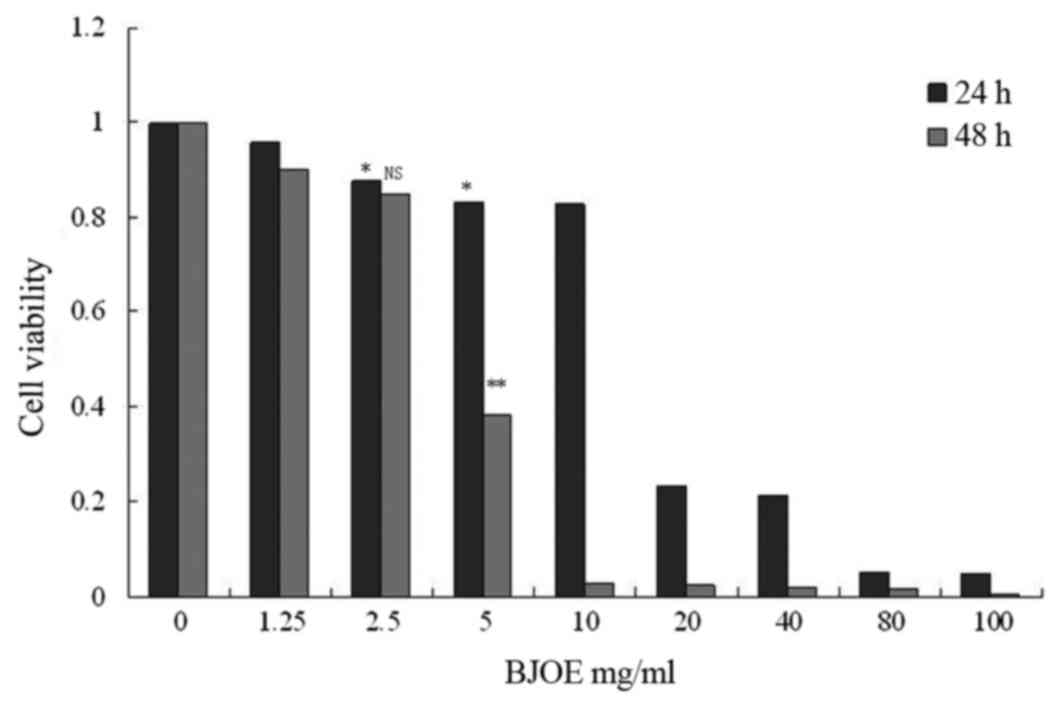 | Figure 1.Effects of different concentrations of
BJOE on cell viability of ECA109 cells determined by an MTT assay.
ECA109 cells were inoculated in 96-well plates at a density of
5×103 cells/ml. The cells were cultured in an incubator
containing 5% CO2 at 37°C. The medium was removed and
replaced with fresh medium supplemented with different BJOE
concentrations (1.25, 2.5, 5, 10, 20, 40, 80, and 100 mg/ml), when
the monolayers had reached confluency. An untreated cell culture
medium was used as an experimental control. After 24 and 48 h, cell
viability was determined. BJOE inhibited the growth of ECA109 in a
concentration- and time-dependent manner. The treatment with 2.5
mg/ml BJOE for 24 h did not significantly affect the optical
density of ECA109 cells. Data are shown as mean ± standard
deviation. *P<0.05 and **P<0.01, compared with the control;
NS, no significance, compared with the control; BJOE, Brucea
javanica oil emulsion. |
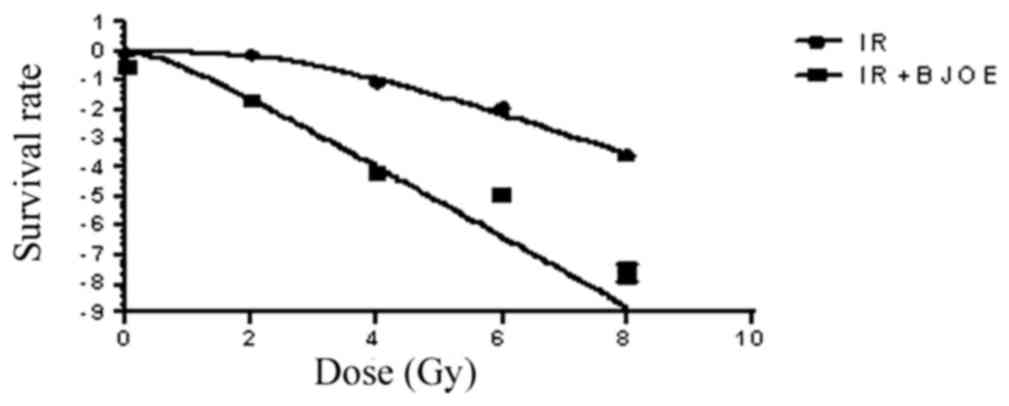
Effects of radiation and BJOE on
colony formation
ECA109 cells were exposed to different radiation
doses after they were pretreated with 2.5 mg/ml BJOE for 24 h under
hypoxic conditions to assess clone formation. The dose-survival
curves (Fig. 2) were generated by
using GraphPad Prism 6.0. The survival fraction data were fitted
into the aforementioned single-hit multiple-target model. For
ECA109 cells, the radiation group and the radiation + BJOE group
yielded D0 of 1.98±0.04, and 1.17±0.03 Gy, respectively. The SERs
of ECA109 was 1.66. The results from the present study revealed
that BJOE significantly sensitized hypoxia cancer cells to
radiation. ECA109 reached D37 of 5.21. Therefore, 6 Gy was the
administered radiation dose for the focal formation experiment and
apoptosis analysis.
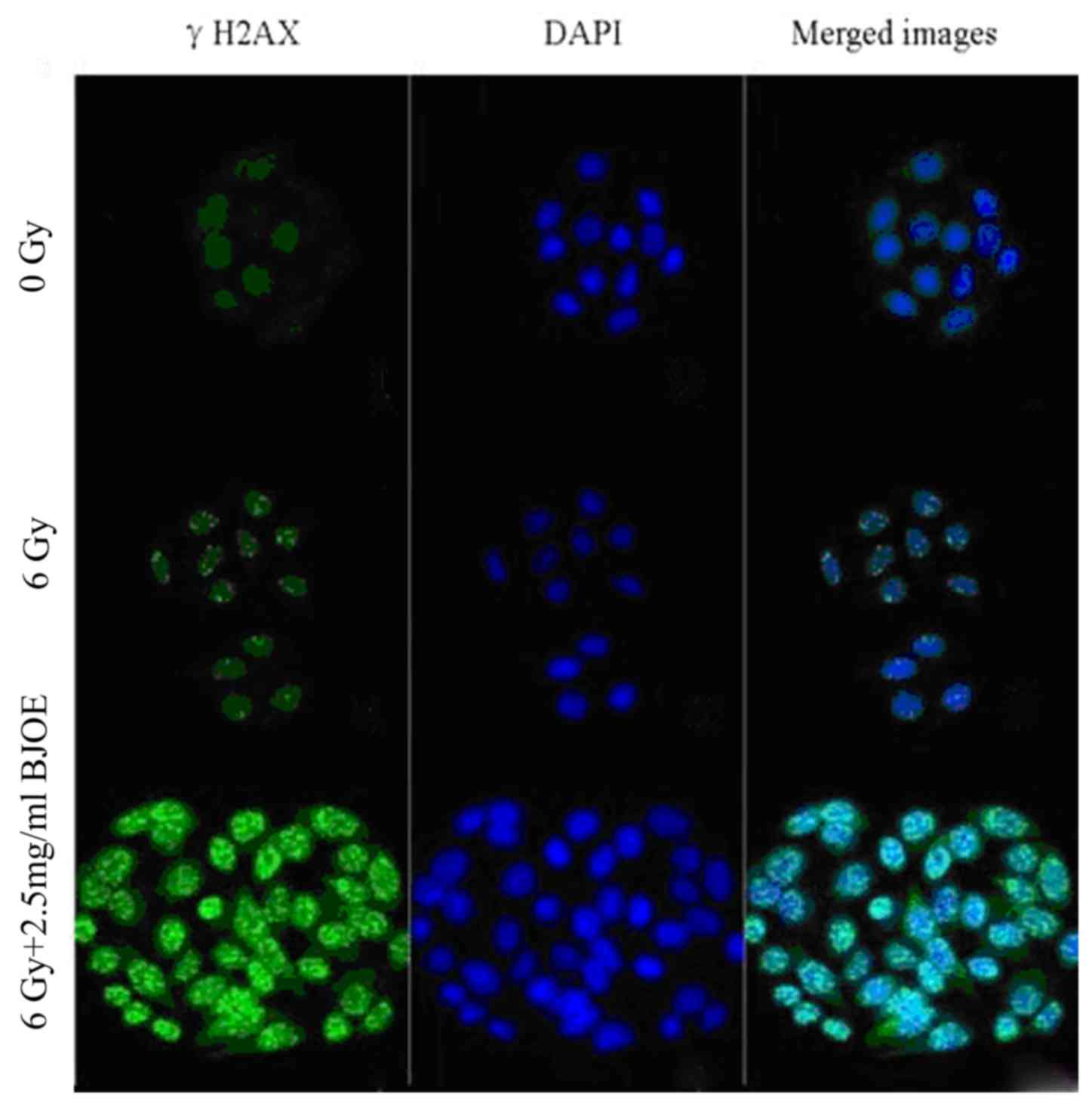
Immunofluorescence of γH2AX
The γH2AX focal formation levels were determined
through immunofluorescent measurement to investigate the
radiosensitization effect of BJOE on ESCC cells (Fig. 3). The control cells barely exhibited
focal formation. However, following exposure to radiation, focal
formation occurred primarily in the nuclei. BJOE + radiation
treatment markedly promoted focal formation. This observation
suggested that BJOE elicited a radiosensitizing effect.
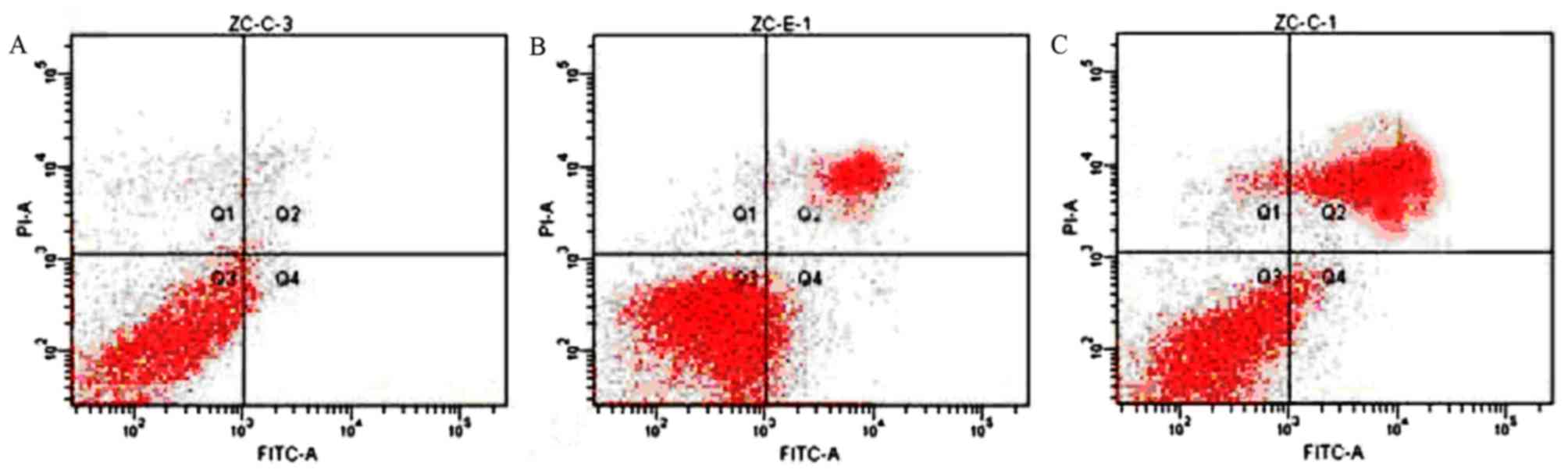
Radiation and BJOE renders ESCC cells
to increased apoptosis
The effects of BJOE on the apoptosis of hypoxic ESCC
cells treated with or without radiation were detected (Fig. 4). ECA109 in the control, radiation and
radiation + BJOE groups yielded apoptotic rates of 5.65±0.80,
39.09±4.57, and 67.38±3.69%, respectively. Irradiation exposure
significantly induced apoptotic events in ECA109 compared with the
control cells (P<0.001). Compared with radiation alone, BJOE +
radiation significantly increased apoptotic rates (P<0.001).
BJOE inhibits HIF-1α in ESCC cells
under hypoxic conditions
The HIF-1α expression in ESCC cells was analyzed by
western blotting at different time points under hypoxic conditions
(Fig. 5). Comparable to normal
protein levels were detected after 12 h under hypoxic conditions.
However, HIF-1α levels increased to the maximum in ECA109 cells
after 24 h under hypoxic conditions. In response to treatment with
5 mg/ml BJOE for 24 h under hypoxic conditions, the HIF-1α protein
levels in ECA109 cells were markedly inhibited by BJOE.
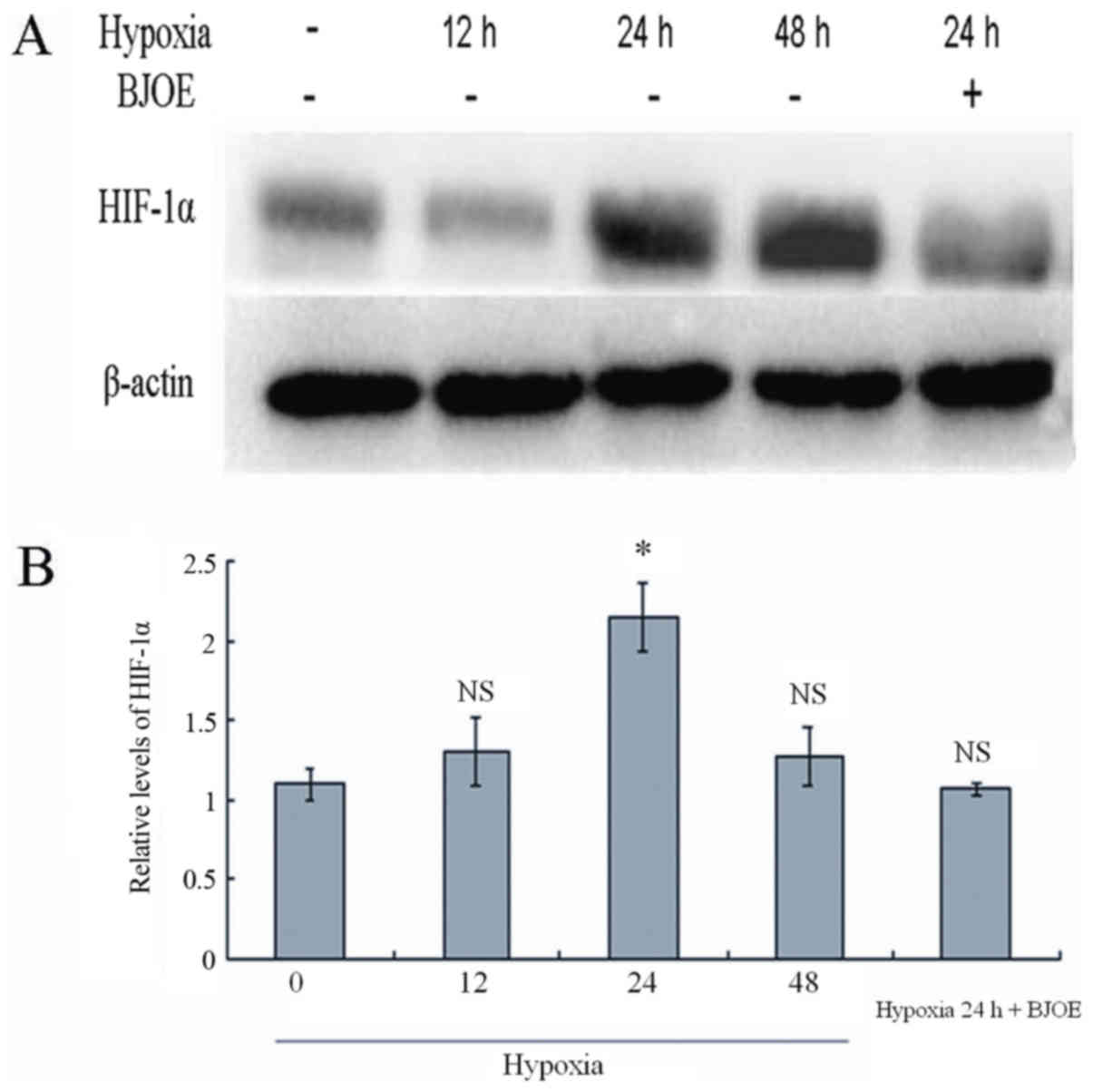 | Figure 5.(A) Western blot analysis for HIF-1α
in ECA109. (B) Semi-quantitative levels of HIF-1α in ECA109. ECA109
cells were treated with BJOE under hypoxic conditions for 24 h. The
control cells were cultured without BJOE under normoxic, and
hypoxic conditions for 12, 24, and 48 h. Subsequently, proteins
were extracted from cells. A total of 40 µg protein were loaded.
The experiments were performed according to the procedures
aforementioned in the materials and methods section. Comparable to
normal protein levels were detected after 12 h under hypoxic
conditions. However, HIF-1α levels increased to the maximum in
ECA109 cells after 24 h under hypoxic conditions. In response to
treatment with 5 mg/ml BJOE for 24 h under hypoxic conditions, the
HIF-1α protein levels in ECA109 cells were notably inhibited by
BJOE. Data are shown as mean ± standard deviation. *P<0.05,
compared with the control; NS, no significance, compared with the
control. HIF-1α, hypoxia-inducible factor 1α; BJOE, Brucea
javanica oil emulsion. |
BJOE increases the radiation
sensitivity of ESCC in vivo
HIF-1α levels did not significantly differ between
the radiation group and the radiation + BJOE group before the
treatment was administered. Conversely, the HIF-1α level in the
radiation + BJOE group was significantly higher compared with that
in the radiation group following treatment (Table I). In terms of demographic parameters,
including age and gender, HIF-1α levels did not significantly
differ between the two groups.
 | Table I.Comparison of HIF-1α levels between
pre-radiotherapy and post-radiotherapy. |
Table I.
Comparison of HIF-1α levels between
pre-radiotherapy and post-radiotherapy.
|
|
| HIF-1α (ng/ml) |
|---|
|
|
|
|
|---|
| Group | n | Pre-treatment | Post-treatment |
|---|
| Radiotherapy | 10 | 35.66±7.26 | 29.15±4.77 |
|
BJOE+Radiotherapy | 10 |
38.79±10.64 | 17.76±3.66 |
| P-valuea |
| 0.45 |
<0.001a |
Discussion
Tumor hypoxia is a well-recognized characteristic
associated with resistance to radiotherapy and chemotherapy. Tumor
cells adapt to hypoxic microenvironments by activating associated
signaling pathways (5,6). HIF-1α plays a crucial role in tumor
growth and metastasis. The following mechanisms are involved in
these phenomena: i) HIF-1α promotes tumor angiogenesis by
upregulating the expression of vascular endothelial growth factor
(VEGF), angiopoietin-1, angiopoietin-2 and other factors (7). The correlation between HIF-1α and VEGF
has been clarified (8). However,
blood and nutrient supply of tumors become insufficient as they
grow in mass. HIF-1α overexpression promotes VEGF expression as a
result of hypoxia, and VEGF further stimulates VEGF-R1 and VEGF-R2
expression. This phenomenon increases the number of tumor blood
vessels (8). ii) HIF-1α activates
target genes encoding glucose transporters, namely, glucose
transporter (GLUT)1 and GLUT3, and hexokinase, the first enzyme of
the glycolytic pathway. This mechanism produces a rapid and
effective pathway for tumor energy metabolism (9). iii) HIF-1α overexpression is able to
inhibit P53 gene expression. Nevertheless, P53 is an important
tumor suppressor. P53 induces apoptosis by upregulating Bcl-2
associated-X protein (Bax) and downregulating B-cell lymphoma-2
(Bcl-2) (10). iv) HIF-1α upregulates
the expression of c-Met and matrix metalloproteinases (MMPs), and
these proteins are associated with tumor progression and
metastasis. HIF-1α may also inhibit β-catenin and E-cadherin
expression. These associated catenins are the major constituents of
cell-cell adhesion. Therefore, the decreased expression of
β-catenin and E-cadherin is correlated with tumor progression
(11,12). HIF-1α increases the metastatic
potential of tumor cells by increasing VEGF expression, as
previously described.
BJOE is a traditional Chinese medicine, which is
extracted from B. javanica. In China, BJOE has been used to
clinically treat patients with lung cancer, brain metastases and
gastrointestinal cancer for numerous years. Clinical studies have
revealed that BJOE combined with radiotherapy or chemotherapy is
able to increase therapeutic efficacy, and reduce the side effects
of patients with lung cancer, brain metastases and gastrointestinal
cancer (13–16). Furthermore, BJOE elicits cytotoxic
effects on tumor cell lines, including A431 (human skin cancer cell
line), HT1080 (human fibrosarcoma cell line), LNCaP (human prostate
cancer cell line), MCF-7 (human breast carcinoma cell line),
HTB-126 (human breast carcinoma cell line), HeLa (HPV 18 positive
human cervical carcinoma cell line), Caski (HPV 16 positive human
cervical carcinoma cell line), SiHa (HPV 16 positive human cervical
carcinoma cell line), HCT116 (human colon carcinoma cell line), KB
(human oral cancer cell line), and ORL-48 (human oral cancer cell
line) (16–19). However, the potential effect of the
combination of BJOE with irradiation on ESCC has yet to be
investigated.
Therefore, in the present study, the effect and
mechanism of the combination of BJOE with irradiation on ESCC were
preliminarily investigated in vitro and in vivo. MTT
assay demonstrated that BJOE inhibited the proliferation of
esophageal cancer cells in a concentration- and time-dependent
manner, and this finding is consistent with that of Zhang et
al (17). The dose-survival
curves fitted the single-hit multiple-target model and the SERs of
ECA109 was 1.66. The shoulder region of the survival curve was also
shortened and the D0 value decreased. These findings demonstrate
that BJOE enhanced the radiosensitivity of hypoxic ECA109 cells.
γH2AX is closely associated with DNA double-strand breaks (DSB)
(20). DSB is also directly
associated with radiosensitivity. Immunofluorescent measurement
further demonstrated that BJOE efficiently increased the
radiosensitivity of hypoxic esophageal cancer cells. Majid et
al (18) demonstrated that BJOE
exhibits apoptosis-inducing activity on human oral cancer cell
lines KB and ORL-48. Zhang et al (17) also described the pro-apoptotic
function of BJOE on human acute myeloid leukemia cells U937 and
HL-60. In the present study, similar results were obtained in
ECA109 cells. BJOE + irradiation induced a markedly higher
apoptotic rate of ESCC cells compared with the effects of
irradiation alone. BJOE also increased the radiosensitivity of ESCC
cells by enhancing radiation-induced apoptosis. The signaling
mechanism by which BJOE increased the radiosensitivity of ESCC
cells was examined through western blot analysis. Hypoxia
stimulated the HIF-1α expression in ESCC cells. Thus, hypoxia
contributes to tumor radiotherapy resistance. However, BJOE
markedly downregulated HIF-1α expression. Taken together, these
data suggested that BJOE may increase the radiosensitivity of ESCC
cells by suppressing the activation of HIF-1α.
In vivo data also revealed that BJOE
potentially enhanced tumor radiosensitivity by decreasing HIF-1α
expression. In conclusion, the present study provided in
vitro and in vivo evidence supporting the hypothesis
that BJOE enhances the radiosensitivity of ESCC cells. This
phenomenon is associated with the downregulation of HIF-1α
expression. Therefore, BJOE is a potential radiotherapy
sensitization agent due to its anti-hypoxia activity.
Glossary
Abbreviation
Abbreviations:
|
BJOE
|
Brucea javanica oil
emulsion
|
References
|
1
|
Chen W, He Y, Zheng R, Zhang S, Zeng H,
Zou X and He J: Esophageal cancer incidence and mortality in China,
2009. J Thorac Dis. 5:19–26. 2013.PubMed/NCBI
|
|
2
|
Ajduković J: HIF-1: A big chapter in the
cancer tale. Exp Oncol. 38:9–12. 2016.PubMed/NCBI
|
|
3
|
Cavadas MA, Nguyen LK and Cheong A:
Hypoxia-inducible factor (HIF) network: Insights from mathematical
models. Cell Commun Signal. 11:422013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumors. 7th edition.
Wiley-Blackwell; Oxford: 2010
|
|
5
|
Tang CM and Yu J: Hypoxia-inducible
factor-1 as a therapeutic target in cancer. J Gastroenterol
Hepatol. 28:401–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dhani N, Fyles A, Hedley D and Milosevic
M: The clinical significance of hypoxia in human cancers. Semin
Nucl Med. 45:110–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pereira ER, Frudd K, Awad W and Hendershot
LM: Endoplasmic Reticulum (ER) stress and hypoxia response pathways
interact to potentiate hypoxia-inducible factor 1 (HIF-1)
transcriptional activity on targets like Vascular Endothelial
Growth Factor (VEGF). J Biol Chem. 289:3352–3364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Catalán V, Gómez-Ambrosi J, Rodríguez A,
Ramírez B, Silva C, Rotellar F, Hernández-Lizoain JL, Baixauli J,
Valentí V, Pardo F, et al: Up-regulation of the novel
proinflammatory adipokines lipocalin-2, chitinase-3 like-1 and
osteopontin as well as angiogenic-related factors in visceral
adipose tissue of patients with colon cancer. J Nutr Biochem.
22:634–641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iyer NV, Kotch LE, Agani F, Leung SW,
Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY
and Semenza GL: Cellular and developmental control of O2
homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev.
12:149–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang YR, Wei JL, Mo XF, Yuan ZW, Wang JL,
Zhang C, Xie YY, You QD and Sun HP: Discovery and optimization of
new benzofuran derivatives against p53-independent malignant cancer
cells through inhibition of HIF-1 pathway. Bioorg Med Chem Lett.
26:2713–2718. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng JC, Klausen C and Leung PC:
Hypoxia-inducible factor 1 alpha mediates epidermal growth
factor-induced down-regulation of E-cadherin expression and cell
invasion in human ovarian cancer cells. Cancer Lett. 329:197–206.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans AJ, Russell RC, Roche O, Burry TN,
Fish JE, Chow VW, Kim WY, Saravanan A, Maynard MA, Gervais ML, et
al: VHL promotes E2 box-dependent E-cadherin transcription by
HIF-mediated regulation of SIP1 and snail. Mol Cell Biol.
27:157–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu YY, Huang XE, Cao J, Xu X, Wu XY, Liu
J, Xiang J and Xu L: Phase II study on Javanica oil emulsion
injection (Yadanzi®) combined with chemotherapy in
treating patients with advanced lung adenocarcinoma. Asian Pac J
Cancer Prev. 14:4791–4794. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nie YL, Liu KX, Mao XY, Li YL, Li J and
Zhang MM: Effect of injection of Brucea javanica oil
emulsion plus chemoradiotherapy for lung cancer: A review of
clinical evidence. J Evid Based Med. 5:216–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Huang XE, Tian GY, Cao J, Lu YY, Wu
XY and Xiang J: Phase II study on safety and efficacy of
Yadanzi® (Javanica oil emulsion injection) combined with
chemotherapy for patients with gastric cancer. Asian Pac J Cancer
Prev. 14:2009–2012. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji ZQ, Huang XE, Wu XY, Liu J, Wang L and
Tang JH: Safety of Brucea javanica and cantharidin combined
with chemotherapy for treatment of NSCLC patients. Asian Pac J
Cancer Prev. 15:8603–8605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Yang JY, Zhou F, Wang LH, Zhang
W, Sha S and Wu CF: Seed oil of Brucea javanica induces
apoptotic death of acute myeloid leukemia cells via both the death
receptors and the mitochondrial-related pathways. Evid Based
Complement Alternat Med. 2011:9650162011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Majid MZ, Zaini ZM and Razak FA:
Apoptosis-inducing effect of three medicinal plants on oral cancer
cells KB and ORL-48. ScientificWorldJournal. 2014:1253532014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan Z, Zhang B, Huang Y, Qiu H, Chen P and
Guo GF: Involvement of autophagy inhibition in Brucea
javanica oil emulsion-induced colon cancer cell death. Oncol
Let. 9:1425–1431. 2015. View Article : Google Scholar
|
|
20
|
Valdiglesias V, Giunta S, Fenech M, Neri M
and Bonassi S: γH2AX as a marker of DNA double strand breaks and
genomic instability in human population studies. Mutat Res.
753:24–40. 2013. View Article : Google Scholar : PubMed/NCBI
|