Introduction
Elemene is a new drug first extracted in China using
the herbs that activates blood circulation such as Curcuma Wenyujin
in recent years. It has the broad-spectrum antineoplastic, immune
protection and other effects (1). The
main active component is β-elemene. The current research shows that
β-elemene has the effects to induce cell apoptosis and
differentiation, with reverse multiple drug resistance of tumor,
and enhance sensibilization of combined radiotherapy and
chemotherapy etc (2). Moreover, its
toxic and side effects are very few (2). At present, it has been widely applied in
the clinical treatment of liver cancer, lung cancer, breast cancer,
cervical cancer, gastrointestinal tumor, carcinoma of urinary
bladder, brain tumor and other superficial tumors, such as bladder
cancer and gland cancer (3). The
research shows that β-elemene can prevent NSCLC-H460 cell of human
lung cancer, Hep-2 cell of human laryngocarcinoma, A2780 cell of
human ovarian cancer, U251 cell of human cerebral glioma and HXO-RB
44 cell of human retinoblastoma entering phase G2/M from phase S
(4). Besides, it also can reduce
mitosis, suppress tumor growth and induce cancer cell apoptosis
(5). Meanwhile, the research also
finds that β-elemene can inhibit the hematogenous metastasis and
lymphatic metastasis of tumor. It plays the role of down-regulation
for the protein level of VEGF-C and VEGFR-3 in cell SPC-A-1 of
human pulmonary carcinoma, which shows that β-elemene also can
inhibit the hematogenous metastasis and lymphatic metastasis by
reducing vascular growth factor and its receptors (6). In the experimental research on nude
mouse model to find the cure of human laryngocarcinoma, it is found
that β-elemene can inhibit the expression of VEGF-C and VEGFR-3 to
block the growth of cell Hep-2 in laryngocarcinoma and its
hematogenous metastasis accordingly (7).
Ligusticum wallichii, also known as
Xiongqiong, is an artemisia plant in umbrelliferae family of
Chinese traditional medicine, which is mainly produced in Sichuan,
Yunnan, Guizhou, Guangxi, Hubei and other places in China.
Ligustrazine, one of the effective components of Ligusticum
wallichii, belongs to amides alkaloid, whose chemical structure
is tetramethylpyrazine (TMP) and contains many biological functions
as well, including promoting blood circulation, removing blood
stasis and dredging veins, etc (8).
Modern researches have further proven that it has various
pharmacological effects and can be used in the treatment for many
deadly diseases such as cardiovascular diseases, pulmonary
hypertension, chronic renal failure, liver cirrhosis, and with
radioactive treatment of pulmonary fibrosis. Latest research
suggests TMP has significant protective effects on spinal cord
injury. Meanwhile, we come to know that TMP also can inhibit the
apoptosis of spinal cord injury cells (9) as well as can help in the production of
endothelin, which can dilate vessels and increase the effect of
blood supply in the ischemic area (10). It can inhibit the aggregation of
platelet thus, reducing blood viscosity simultaneously improve
microcirculation and regulate the expression of interleukin in
order to inhibit inflammation (11).
Angiogenesis might be a condition for the growth of
primary and metastatic tumors. The recent research finds that the
increase of GPR124 expression is closely related to angiogenesis,
which plays an important part in in its significant role of
promoting tumor invasion, metastasis and further in poor prognosis
(12). At present, the limited
research related to GPR124 function is mostly focused on the
angiogenesis of central nervous system. The research related to its
specific mechanism of action is very few. A prior research,
suggests that GPR124 in endothelial cells may bind with PDZ region
of hDIg through PDZ binding motif at the terminal of its carboxyl,
by regulate as well as control cell proliferation, and adhesion
through regulating, controlling the anchorage of hDIg which further
affect the angiogenesis (13).
When chemotherapy is combined with elemene to treat
patient with gastric cancer, the result shows that chemotherapy
drug can remarkably increase the chemotherapy sensitivity to
gastric cancer, which has effectively reversed the patient
tolerance with advanced gastric cancer for chemotherapy drug
(14). The research also finds that
β-elemene can effectively inhibit the growth of gastric cancer cell
after get combined with fluorouracil drug. Its mechanism is closely
related to the induction of cell apoptosis. The development of bone
sarcoma is rapid (15). The prognosis
is poor and the death rate, high. It is a kind of malignant disease
severely life threatening. In order to reduce the toxic side
effects of drug and effectively kill the sarcoma cell, we choose to
combine the low-concentration of β-elemene and ligustrazine to
apply them into the cell of bone sarcoma to research the combined
effect which reduces the drug toxicity so as to realize the
sufficient anti-sarcoma effect of drug (16). In this research, we observe the
external cell and the experiment on animal body, illustrating the
mechanism through the experimental technique of molecular biology,
and making a sufficient research for the combined effect of
β-elemene and ligustrazine.
Materials and methods
Cell line
OS-732 human osteosarcoma cells was purchased from
ATCC (American Type Culture Collection, Manassas, VA, USA). The
cells were cultured using RPMI1640 medium (Biosera, Nuaille,
France) with 10% FBS at 37°C in 5% CO2 incubator
(MCO-20AIC; SANYO, Tokyo, Japan).
MTT assay
Culture solution was added to adjust the
concentration of OS-732 cells in logarithmic growth phase added to
2×104/dish, then transferred to the 96-well culture
plate with 50 µl per well, and placed in incubator for 5%
CO2 at 37°C for 24 h. β-elemene-ligustrazine (Zhengzhou
Cheuk-Fung Pharmaceutical Co., LTD) were added into 96-well plate
having 50 µl per well. In the meantime 50 µl culture solution was
added to the control group and cultured in CO2 incubator
for 48 h. Subsequently, the control group was added with MTT
solution after the removal of supernatant thereafter incubated for
4 h. In the blank control group 100 µl DMSO was added after removal
of the supernatant and shocked for 30 min, the enzyme standard
instrument were used to detect at 570 nm (680 Microplate reader,
Bio-Rad, CA, USA) (17).
Flow cytometry assay
Centrifugation of single cell suspension should be
done in order to remove stationary liquid and washed by 3 ml PBS
twice, followed by centrifugation of 5 min; added with 1 ml PI
staining solution and incubated in refrigerator at 4°C for 30 mins
while keeping it in a dark place as to prohibit sunlight exposure;
and then filtered by 500-hole copper mesh; flow cytometry detection
(Accuri C6, BD Biosciences, La Jolla, CA, USA) and argon ion laser
with 15 mA excitation light source with 488 nm wavelength was used
for testing, and 630 nm band-pass filter to receive the light.
Selection of 10,000 cells were taken place using FSC/SSC scattered
point diagram method, we also used gating technology to eliminate
adhesive cells and cell debris, to analyze the percentage of
apoptotic cells in PI fluorescence histogram (17).
Real-time quantitative PCR assay
Whole RNA of cancer cells and mice tumor tissues
were extracted using RNAzol, and DNase RNase-free was adopted to
digest total RNA at 37°C for 15 min, and then RNase kit to purify
RNA in order to adjust its concentration to 1 µg/µl. The 2 µg RNA
was used as the template to synthetize cDNA by reacting it with
reverse transcriptase at 37°C for 120 min, at 99°C for 4 min, and
at 4°C for 3 min. Followed by, reverse transcription-polymerase
chain reaction method was adopted to amplify the gene expression of
GPR124, TIMP-1, TIMP-2, MMP-2, MMP-9, VEGF, endostatin, NF-κB,
IL-8, CXCR4, uPA, caspase-3, caspase-8 and caspase-9 to determine
the transcription level of mRNA, and β-actin was used as the
housekeeping genes of internal control group (StepOne, Applied
Biosystems, Alameda, CA, USA) (18).
Mice experiment
7 weeks (n=60) old male BALB/c-nu/nu nude mice were
purchased from Beijing University; they were sustained in a
temperature-controlled facility at 23±1°C and relative humidity of
50±5% with a 12-h light/dark cycle. The experiment of this study
was performed following the protocols approved by the Animal Ethics
Committee of Beijing University (Beijing, China).
In vivo experiment
Cultivate OS-732 cells into 100 ml culture solution
of RPMI-1640. The cell grows rapidly and vigorously, one passage
grows within three to four days, in total there were four passages.
Collect OS-732 cells in logarithmic phase, and wash once with PBS.
Then adjust the cell concentration to make it as
5×107/ml cell suspension. Carry out a 0.2 ml
subcutaneous inoculation in the posterior axillary of the right
forelimb of nude mouse under aseptic condition, and subcutaneously
inoculating about 1×107 oncocyte for each mouse. After
the inoculation, we observe the general condition and local wound
status of nude mouse each day. From the first day, the β-elemene,
ligustrazine and β-elemene-ligustrazine combination group mice were
treated with 1.2 mg/kg β-elemene, 1.2 mg/kg ligustrazine and 0.8
mg/kg β-elemene + 0.4 mg/kg ligustrazine respectively by
intravenous injection. From the eighth day after the operation, we
weigh the nude mouse with electronic balance after every four days.
Measure the short diameter (a) and the long diameter (b) of tumor
with vernier caliper. According to the formula,
V=0.5xa2xb. calculation of relative tumor volume and
draw growth curve was done. According to the formula, RTVn=Vn/V 8,
we able calculate the relative growth rate of tumor at the 28th
days. At the 28th day after the inoculation, we sacrificed the nude
mouse by the method of cervical vertebra dislocation to get the
tumor tissue followed by weigh the tumor (19).
Statistical analysis
The experiments data were expressed as mean ±
standard deviation (SD). The significant difference (P<0.05) of
data of different groups were calculated using Duncan's multiple
range test using SPSS Statistics 22.0 (IBM, Armonk, NY, USA).
Results
The growth of β-elemene and
ligustrazine in OS-732 cells
Using MTT assay, after treated with different
concentrations of β-elemene (1, 5, 10, 50, 100, 500 µg/ml) for 24
and 48 h, the IC50 of OS-732 cells were 41.36±1.89 and
19.12±0.88 µg/ml respectively. Meanwhile, after the treatment of
different concentrations of ligustrazine (0.1, 0.5, 1, 5, 10, 50
µg/ml) for 24 and 48 h, the IC50 of OS-732 cells were
9.42±0.33 and 3.98±0.28 µg/ml respectively. The 20 µg/ml β-elemene
and 10 µg/ml ligustrazine were used as a combination treatment for
next part of the experiments. The 30 µg/ml β-elemene, 30 µg/ml
ligustrazine and β-elemene-ligustrazine combination (20 µg/ml
β-elemene and 10 µg/ml ligustrazine) showed the inhibitory effects
at 59.3, 72.6 and 87.8%, respectively (Table I).
 | Table I.Growth inhibitory effects of OS-732
human osteosarcoma cells by MTT assay. |
Table I.
Growth inhibitory effects of OS-732
human osteosarcoma cells by MTT assay.
| Treatment | OD570
value | Inhibitory rate
(%) |
|---|
| Control | 0.482±0.006 | / |
| β-elemene |
0.196±0.007a | 59.3±3.1a |
| Ligustrazine |
0.132±0.005a |
72.6±3.4a |
|
β-elemene-ligustrazine combination |
0.059±0.004a |
87.8±2.8a |
Sub-G1 content of OS-732 cells
Using the flow cytometry assay,
β-elemene-ligustrazine combination treatment for OS-732 cells had
the most apoptotic cells (41.3±2.6%, sub-G1 DNA content), β-elemene
and ligustrazine when treated OS-732 cells separately also had many
apoptotic cells (6.5±0.2 and 18.7±1.1%), these apoptotic cells were
more than the control group cells (2.4±0.2%).
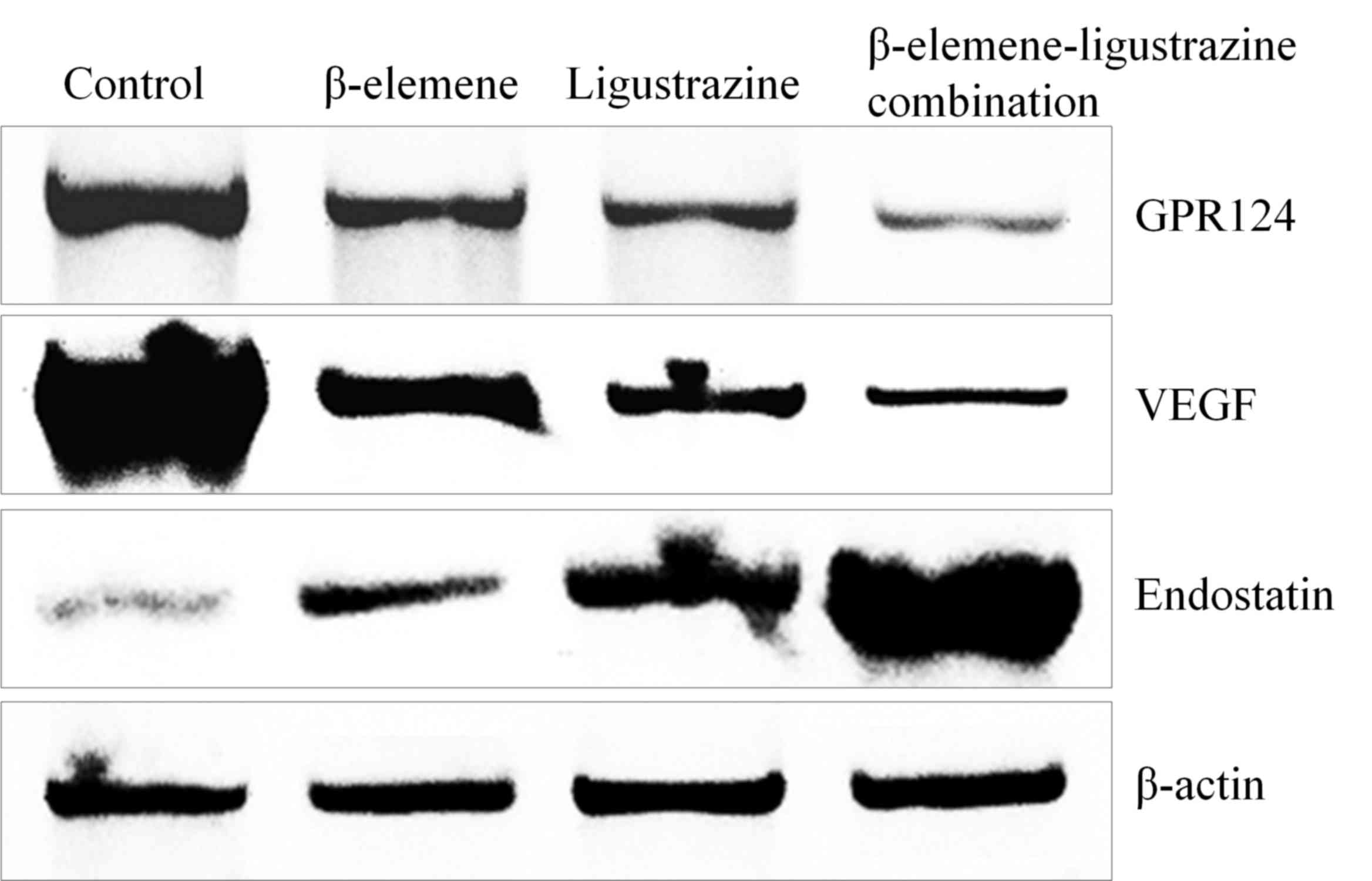
mRNA and protein expression of GPR124,
VEGF and endostatin in OS-732 cells
The control group cells had the highest GPR124, VEGF
mRNA expression and lowest expression for endostatin.
β-elemene-ligustrazine combination could reduce GPR124, VEGF
expression and raise endostatin expression significantly higher as
compared with the other group cells (Fig.
1, Table II).
 | Table II.Quantitative analysis of GPR124, VEGF
and endostatin mRNA expressions in OS-732 cells (Folds of
control). |
Table II.
Quantitative analysis of GPR124, VEGF
and endostatin mRNA expressions in OS-732 cells (Folds of
control).
| Treatment | GPR124 | VEGF | Endostatin |
|---|
| Control | 1.00±0.05 | 1.00±0.03 | 1.00±0.26 |
| β-elemene |
0.61±0.04a |
0.55±0.04a |
2.36±0.35a |
|
P-value | (0.005) | (0.005) | (0.008) |
| Ligustrazine |
0.43±0.05a |
0.35±0.03a |
4.57±0.39a |
|
P-value | (0.006) | (0.007) | (0.005) |
|
β-elemene-ligustrazine |
0.22±0.03a |
0.18±0.03a |
7.03±0.42b |
| combination | (0.005) | (0.007) | (0.006) |
mRNA and protein expression of TIMP-1,
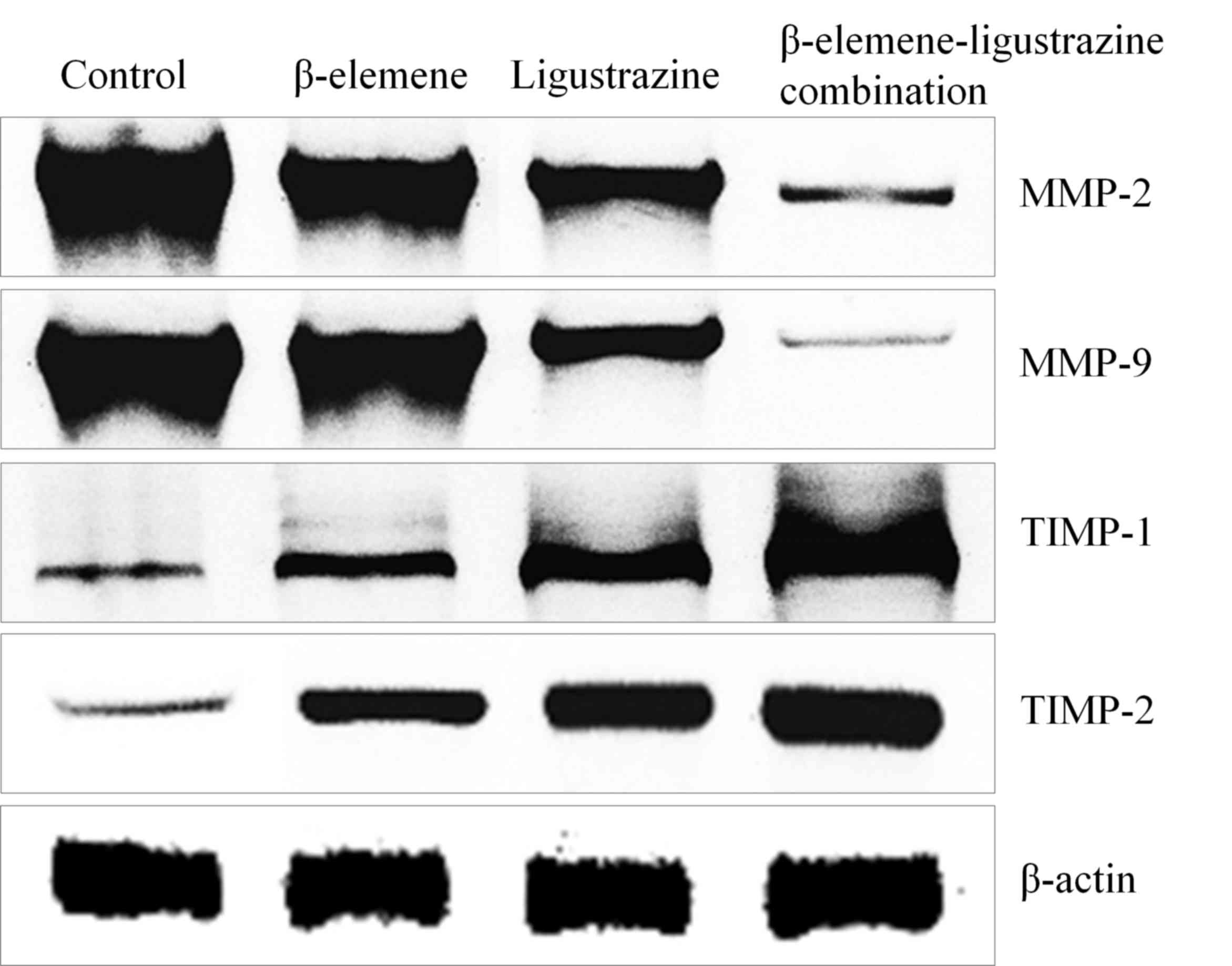
TIMP-2, MMP-2 and MMP-9 in OS-732 cells
The control cells showed the lowest TIMP-1, TIMP-2
expression and highest MMP-2, MMP-9 expression (Fig. 2, Table
III), and β-elemene, ligustrazine, β-elemene-ligustrazine
combination treated OS-732 cells showed the higher TIMP-1, TIMP-2
expression and lower MMP-2, MMP-9 expression than control cells,
β-elemene-ligustrazine combination showed the highest TIMP-1,
TIMP-2 expression and lowest MMP-2, MMP-9 expression.
 | Table III.Quantitative analysis of TIMP-1,
TIMP-2, MMP-2 and MMP-9 mRNA expressions in OS-732 cells (folds of
control). |
Table III.
Quantitative analysis of TIMP-1,
TIMP-2, MMP-2 and MMP-9 mRNA expressions in OS-732 cells (folds of
control).
| Treatment | MMP-2 | MMP-9 | TIMP-1 | TIMP-2 |
|---|
| Control | 1.00±0.04 | 1.00±0.04 | 1.00±0.26 | 1.00±0.15 |
| β-elemene |
0.68±0.04a |
0.77±0.06a |
3.02±0.35a |
3.45±0.25a |
|
P-value | (0.003) | (0.005) | (0.005) | (0.008) |
| Ligustrazine |
0.37±0.03a |
0.45±0.05a |
5.41±0.39a |
7.12±0.21a |
|
P-value | (0.006) | (0.005) | (0.003) | (0.005) |
|
β-elemene-ligustrazine combination |
0.15±0.02a |
0.32±0.04a |
6.89±0.42a |
8.59±0.51a |
|
P-value | (0.005) | (0.006) | (0.005) | (0.007) |
mRNA expression of NF-κB, IL-8, CXCR4
and uPA in OS-732 cells
β-elemene-ligustrazine combination could reduce the
NF-κB, IL-8, CXCR4 and uPA expression as compared to control cells
(Table IV), β-elemene and
ligustrazine also could reduce these expression, but higher than
β-elemene-ligustrazine combination treated cells.
 | Table IV.Quantitative analysis of NF-κB, IL-8,
CXCR4 and uPA mRNA expressions in OS-732 cells (folds of
control). |
Table IV.
Quantitative analysis of NF-κB, IL-8,
CXCR4 and uPA mRNA expressions in OS-732 cells (folds of
control).
| Treatment | NF-κB | IL-8 | CXCR4 | uPA |
|---|
| Control | 1.00±0.03 | 1.00±0.03 | 1.00±0.03 | 1.00±0.03 |
| β-elemene |
0.82±0.03a |
0.65±0.06a |
0.76±0.06a |
0.54±0.03a |
| P-value | (0.003) | (0.002) | (0.005) | (0.002) |
| Ligustrazine |
0.52±0.05a |
0.45±0.04a |
0.48±0.06a |
0.36±0.06a |
| P-value | (0.005) | (0.006) | (0.004) | (0.004) |
|
β-elemene-ligustrazine combination |
0.31±0.03a |
0.33±0.02a |
0.21±0.04a |
0.18±0.04a |
| P-value | (0.008) | (0.007) | (0.005) | (0.006) |
mRNA expression of caspases in OS-732
cells
As shown in Table V,
β-elemene-ligustrazine combination could increase the caspase-3,
caspase-8 and caspase-9 expressions higher as compared to control
OS-732 cells, and these expressions of β-elemene and ligustrazine
treated OS-732 cells were also higher than the control cells.
 | Table V.Quantitative analysis of caspase-3,
caspase-8 and caspase-9 mRNA expressions in OS-732 cells (Folds of
control). |
Table V.
Quantitative analysis of caspase-3,
caspase-8 and caspase-9 mRNA expressions in OS-732 cells (Folds of
control).
| Treatment | Caspase-3 | Caspase-8 | Caspase-9 |
|---|
| Control | 1.00±0.12 | 1.00±0.15 | 1.00±0.18 |
| β-elemene |
3.54±0.18a |
2.97±0.22a |
3.12±0.15A |
|
P-value | (0.008) | (0.005) | (0.008) |
| Ligustrazine |
6.12±0.45a |
4.58±0.35a |
4.77±0.29a |
|
P-value | (0.006) | (0.007) | (0.005) |
|
β-elemene-ligustrazine combination |
8.41±0.32a |
7.74±0.40a |
7.52±0.25a |
|
P-value | (0.007) | (0.007) | (0.006) |
Weight and bulk of tumor in mice
The untreated mice showed the heaviest weight and
bulk of tumor (Table VI), β-elemene,
ligustrazine and β-elemene-ligustrazine combination decreased the
weight and bulk of tumor in mice, and β-elemene-ligustrazine
combination showed the best effects.
 | Table VI.Weight and bulk of tumor in mice. |
Table VI.
Weight and bulk of tumor in mice.
| Treatment | Weight of tumor
(g) | Bulk of tumor
(mm3) |
|---|
| Control | 3.42±0.24 |
1,839.47±245.12 |
| β-elemene |
2.71±0.17a |
1,287.15±137.71a |
| Ligustrazine |
1.79±0.12b |
915.87±66.58b |
|
β-elemene-ligustrazine combination |
0.69±0.09a |
435.21±44.29b |
mRNA and protein expression of GPR124,
VEGF and endostatin in mice
The control mice showed the highest GPR124, VEGF
mRNA expression and lowest endostatin expression like the OS-732
cells (Fig. 3, Table VII). -elemene-ligustrazine
combination showed the weaker GPR124, VEGF expression and little
raised endostatin expression compared than other group mice.
 | Table VII.Quantitative analysis of GPR124, VEGF
and endostatin mRNA expressions of tumor tissue in mice (Folds of
control). |
Table VII.
Quantitative analysis of GPR124, VEGF
and endostatin mRNA expressions of tumor tissue in mice (Folds of
control).
| Treatment | GPR124 | VEGF | Endostatin |
|---|
| Control | 1.00±0.05 | 1.00±0.03 | 1.00±0.02 |
| β-elemene |
0.74±0.04a |
0.69±0.02a |
3.02±0.17a |
|
P-value | (0.005) | (0.005) | (0.003) |
| Ligustrazine |
0.46±0.06a |
0.48±0.03a |
6.71±0.29a |
|
P-value | (0.003) | (0.004) | (0.005) |
|
β-elemene-ligustrazine combination |
0.16±0.03a |
0.26±0.03a |
7.33±0.26a |
|
P-value | (0.005) | (0.002) | (0.004) |
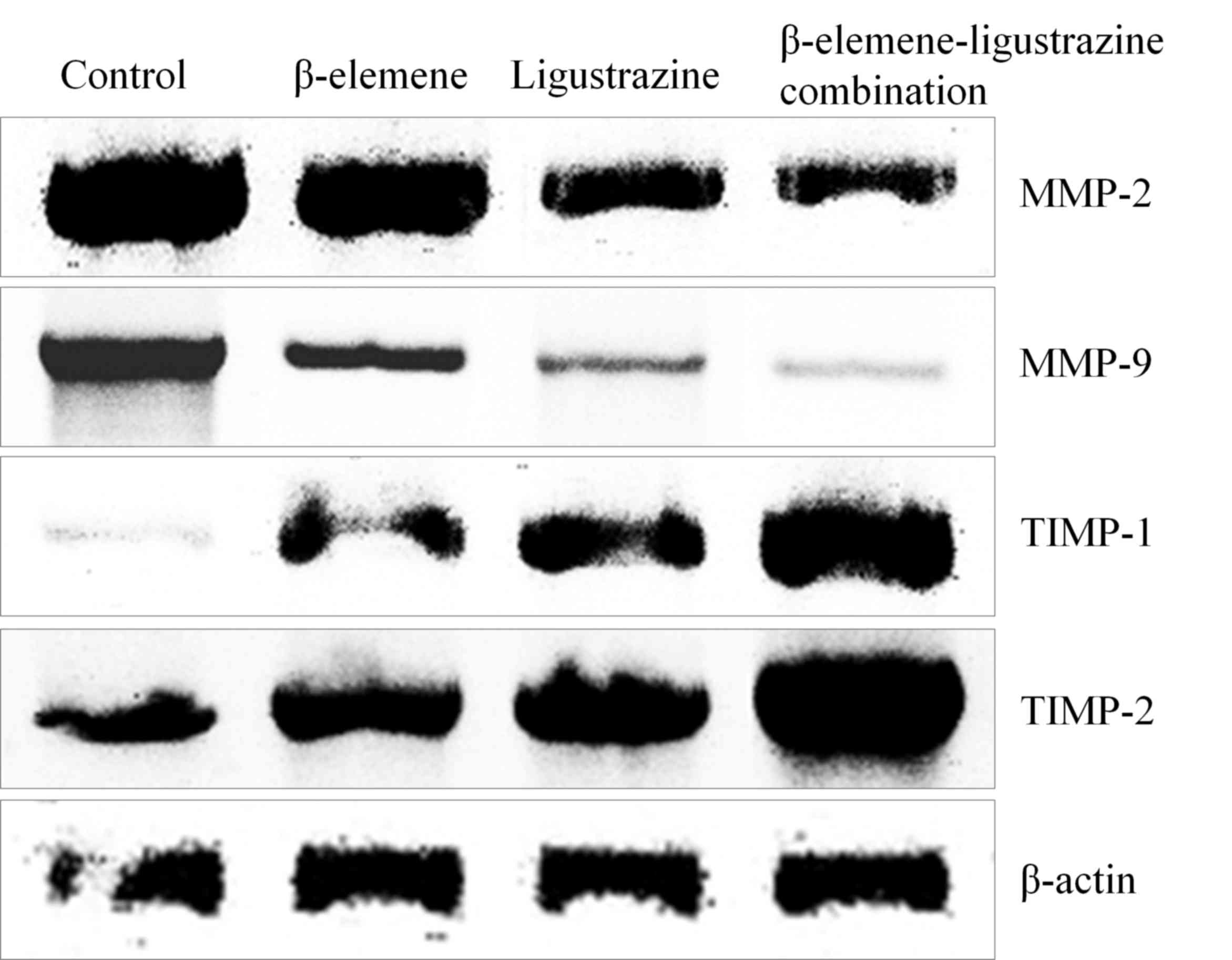
mRNA and protein expression of TIMP-1,
TIMP-2, MMP-2 and MMP-9 in mice
The control mice had the lowest TIMP-1, TIMP-2
expression and highest MMP-2, MMP-9 expression (Fig. 4, Table
VIII), and β-elemene-ligustrazine combination treated mice had
the strongest TIMP-1, TIMP-2 expression and weaker MMP-2, MMP-9
expression than control mice.
 | Table VIII.Quantitative analysis of TIMP-1,
TIMP-2, MMP-2 and MMP-9 mRNA expressions of tumor tissue in mice
(Folds of control). |
Table VIII.
Quantitative analysis of TIMP-1,
TIMP-2, MMP-2 and MMP-9 mRNA expressions of tumor tissue in mice
(Folds of control).
| Treatment | MMP-2 | MMP-9 | TIMP-1 | TIMP-2 |
|---|
| Control | 1.00±0.04 | 1.00±0.04 | 1.00±0.15 | 1.00±0.15 |
| β-elemene |
0.84±0.02a |
0.66±0.03a |
4.02±0.30a |
2.88±0.18a |
|
P-value | (0.005) | (0.005) | (0.002) | (0.003) |
| Ligustrazine |
0.65±0.03a |
0.32±0.03a |
6.12±0.25a |
5.12±0.21a |
|
P-value | (0.004) | (0.004) | (0.003) | (0.005) |
|
β-elemene-ligustrazine combination |
0.42±0.02a |
0.11±0.03a |
7.89±0.36a |
7.39±0.35a |
|
P-value | (0.005) | (0.003) | (0.007) | (0.004) |
mRNA expression of NF-κB, IL-8, CXCR4
and uPA in mice
β-elemene-ligustrazine combination decreased the
NF-κB, IL-8, CXCR4 and uPA expression even less then the control
mice (Table IX, and these expression
in β-elemene and ligustrazine treated mice were higher than
β-elemene-ligustrazine combination treated mice, but lower than
control mice.
 | Table IX.Quantitative analysis of NF-κB, IL-8,
CXCR4 and uPA mRNA expressions of tumor tissue in mice (Folds of
control). |
Table IX.
Quantitative analysis of NF-κB, IL-8,
CXCR4 and uPA mRNA expressions of tumor tissue in mice (Folds of
control).
| Treatment | NF-κB | IL-8 | CXCR4 | uPA |
|---|
| Control | 1.00±0.03 | 1.00±0.03 | 1.00±0.03 | 1.00±0.03 |
| β-elemene |
0.74±0.03a |
0.75±0.03a |
0.68±0.06a |
0.65±0.03a |
|
P-value | (0.004) | (0.004) | (0.005) | (0.008) |
| Ligustrazine |
0.36±0.04a |
0.65±0.04a |
0.55±0.02a |
0.35±0.03a |
|
P-value | (0.005) | (0.003) | (0.007) | (0.007) |
|
β-elemene-ligustrazine combination |
0.12±0.03a |
0.41±0.02a |
0.18±0.02a |
0.22±0.02a |
|
P-value | (0.003) | (0.005) | (0.005) | (0.004) |
mRNA expression of caspases in
mice
β-elemene-ligustrazine combination could increase
the caspase-3, caspase-8 and caspase-9 expression in tissues which
is greater as compared to the control group mice (Table X). β-elemene and ligustrazine treated
mice also had stronger caspase-3, caspase-8 and caspase-9
expression than control group mice, and these effects of
ligustrazine treated mice were stronger than β-elemene treated
mice.
 | Table X.Quantitative analysis of caspase-3,
caspase-8 and caspase-9 mRNA expressions of tumor tissue in mice
(Folds of control). |
Table X.
Quantitative analysis of caspase-3,
caspase-8 and caspase-9 mRNA expressions of tumor tissue in mice
(Folds of control).
| Treatment | Caspase-3 | Caspase-8 | Caspase-9 |
|---|
| Control | 1.00±0.12 | 1.00±0.12 | 1.00±0.18 |
| β-elemene |
3.87±0.20a |
4.52±0.16a |
2.84±0.15a |
|
P-value | (0.004) | (0.004) | (0.007) |
| Ligustrazine |
5.26±0.26a |
5.87±0.26a |
5.26±0.18a |
|
P-value | (0.004) | (0.005) | (0.009) |
|
β-elemene-ligustrazine combination |
7.54±0.36a |
8.26±0.26a |
8.03±0.31a |
|
P-value | (0.005) | (0.008) | (0.005) |
Discussion
A great deal of experimental researches showed that
the tumor cell with the volume, less than 2 mm3 mainly
depends on diffusion for supply of energy and oxygen, waste
excretion as well as to maintain its own survival till the growth
of any blood vessel in tumor (20–22). At
this stage, the apoptosis and the hyperplasia of tumor cells are in
the balance. The volume of tumor can remain unchanged for several
months or years, no metastasis will happen in this time period
(23). In addition, the infiltration
and metastasis occur easily after generation and growth process of
blood vessel, GPR124 plays a role in migration and differentiation
in endothelial cells. The growth and the metastasis of tumor need
the generation of new vessels. In addition, the expression of
GPR124 has been found in some tumors. Tumor recurrence and
metastasis are the main reason in the failure of treatment
(24). In the research for liver
cancer tissue and para-cancerous tissue which adopts
immunohistochemical, western blot and RT-PCR technologies, the
expression of GPR124 in liver cancer tissue is positive. But the
one in para-cancerous tissue mainly is negative. Based on the
analysis for clinical data, it is found that the expression of
GPR124 is positively related to tumor size, node quantity, TNM
staging and microvascular invasion. Meanwhile, it is also found
that the total survival rate of GPR124 high expression group is
somehow lower than the one of low expression group. This shows that
the expression of GPR124 can promote the development of cancer as
well as affect the prognosis (25).
The expressions of MMPs, TIMP-1 and TIMP-2 all occur
in various tumors. MMPs also have the important function in
promoting the tumor invasion and metastasis. As a natural
inhibitor, it does have the higher expression but also will result
in the poor prognosis. The over expression of TIMP-1 and TIMP-2 is
the reactive over expression after MMPs rise (26). When the balance between them is
broken, MMPs will pay a dominant role. However, recently, there are
many evidences by many different studies shows that TIMP-1 is a
kind of multifunctional protein. In addition of inhibiting MMP
activity, it also has the unique function to stimulate tumor,
participating in the process of cell apoptosis, cell proliferation
and tumor angiogenesis etc., which has explained the mechanism to
hint the poor prognosis in the breast cancer with its increased
content (27). In addition of
inhibiting MMP hydrolytic activity, it also has the unique
cytokine-like function, capable of participating in the
proliferation, apoptosis of tumor cell and tumor angiogenesis
through signal transduction pathway (28).
VEGF is the key regulator of tumor metastasis. The
research proves that VEGF can promote the permeation, activation,
survival, proliferation, infiltration and migration of endothelial
cells; it is closely related to tumor metastasis and
lymphoangiogenesis (29). Meanwhile,
it also can block the signal pathway of VEGF to inhibit the growth
and metastases of tumor. Endostatin can inhibit the endothelial
cell proliferation of tumor vessel, and in a dose-dependent manner.
Moreover, it also can inhibit the endothelial cell proliferation
through competing with fibroblast growth factor, stopping phase
G0/G1 transforming to phase S of cell cycle and in many other ways,
as a result there'll be change in cell signaling pathway and can
effectively inhibit the growth of multiple primary solid tumors as
well as tumors which were metastasis. The expression and the
function of multiple cytokines required by the growth of tumor cell
also depend on NF-κB (30). The
growth factor, EGF can activate NF-κB to cause the growth of solid
tumor. VEGF is the principal member of angiogenic factor family,
whose transcription is regulated and controlled by NF-κB (31).
When the activity of NF-κB is inhibited, it can
reduce the angiogenic factor, VEGF and IL-8 in both in vitro
and in vivo remarkably to reduce the tumor angiogenesis. In
promoting the metastasis of tumor, the activity of NF-κB can make
the factor expression of cell line of high-metastatic breast
cancer, MMPs and uPA as well as some cells high. The further
research finds that NF-κB can regulate the motility of breast
cancer cell line through directly increasing the expression of
CXCR4. Moreover, the expression of cell line, CXCR4 which has
higher pulmonary metastasis (32).
In the molecules of signaling pathways and pathways
of apoptosis, caspase family has an important role, caspase family
do the major work in cutting off cell signaling, recombination
cytoskeleton, closing dna replication as well as repair, destroying
DNA and nuclear structure throughout the downstream effect
(33). Caspase family also induces
apoptotic bodies in order to perform apoptosis by inducing
apoptosis in tumor cells, especially in chemotherapy resistant
tumor cells, the action of drugs can be enhanced by mediating the
activity of caspase-3 and caspase-8 (34). Caspase-9 can regulate chemically
induced dipolymer infiltration, other caspase activation and
apoptosis in mitotic as well as in non-dividing cells (35).
Through various parts of experiments, this research
finds that the combined effect of β-elemene and ligustrazine can
promote the apoptosis in tumor more precisely. Especially, it is
proved by the help of many molecular experiments that the combined
effect of β-elemene and ligustrazine can regulate and control the
expression of GPR124, and significantly affect the metastasis
expression of relevant tumor so as to realize the purpose to
control the tumor metastasis. The tumor can be effectively
inhibited by regulating the expression of GPR124 in bone cancer
cell and we can effectively control its migration.
References
|
1
|
Zou K, Liu C, Zhang Z and Zou L: The
effect of elemene on lung adenocarcinoma A549 cell radiosensitivity
and elucidation of its mechanism. Clinics (Sao Paulo). 70:556–562.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mu L, Wang T, Chen Y, Tang X, Yuan Y and
Zhao Y: β-elemene enhances the efficacy of gefitinib on
glioblastoma multiforme cells through the inhibition of the EGFR
signaling pathway. Int J Oncol. 49:1427–1436. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi F, Yang G, Ren J, Guo T, Du Y and Feng
N: Formulation design, preparation, and in vitro and in vivo
characterizations of β-elemene-loaded nanostructured lipid
carriers. Int J Nanomedicine. 8:2533–2541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang G, Li X, Huang F, Zhao J, Ding H,
Cunningham C, Coad JE, Flynn DC, Reed E and Li QQ: Antitumor effect
of β-elemene in non-small-cell lung cancer cells is mediated via
induction of cell cycle arrest and apoptotic cell death. Cell Mol
Life Sci. 62:881–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gan Z, Han K, Lin S, Hu H, Shen Z and Min
D: Knockdown of ubiquitin-specific peptidase 39 inhibited the
growth of osteosarcoma cells and induced apoptosis in vitro. Biol
Res. 50:152017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen W, Lu Y, Wu J, Gao M, Wang A and Xu
B: Beta-elemene inhibits melanoma growth and metastasis via
suppressing vascular endothelial growth factor-mediated
angiogenesis. Cancer Chemother Pharmacol. 67:799–808. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tao L, Zhou L, Zheng L and Yao M: Elemene
displays anti-cancer ability on laryngeal cancer cells in vitro and
in vivo. Cancer Chemother Pharmacol. 58:24–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zengyong Q, Jiangwei M and Huajin L:
Effect of Ligusticum wallichii aqueous extract on oxidative injury
and immunity activity in myocardial ischemic reperfusion rats. Int
J Mol Sci. 12:1991–2006. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Y, Liu Y and Chen K: Mechanisms and
clinical application of tetramethylpyrazine (an interesting natural
compound isolated from Ligusticum wallichii): Current status and
perspective. Oxid Med Cell Longev. 2016:21246382016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu J, Lang Y, Cao Y, Zhang T and Lu H: The
neuroprotective effect of tetramethylpyrazine against contusive
spinal cord injury by activating PGC-1α in rats. Neurochem Res.
40:1393–1401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu X, Liu H, Zeng Z, Zhou W, Liu J and He
Z: Pharmacokinetics of ligustrazine ethosome patch in rats and
anti-myocardial ischemia and anti-ischemic reperfusion injury
effect. Int J Nanomedicine. 6:1391–1398. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou Y and Nathans J: Gpr124 controls CNS
angiogenesis and blood-brain barrier integrity by promoting
ligand-specific canonical wnt signaling. Dev Cell. 31:248–256.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu XZ, Liu LF, Zhou XJ, Xiao S, Long JW
and Zhou X: GPR124 expression and its relationship with prognosis
in hepatocellular carcinoma. Cancer Res Prev Treat. 41:1082–1086.
2014.
|
|
14
|
Li P, Zhou X, Sun W, Sheng W, Tu Y, Yu Y,
Dong J, Ye B, Zheng Z and Lu M: Elemene induces apoptosis of human
gastric cancer cell line BGC-823 via extracellular signal-regulated
kinase (ERK) 1/2 signaling pathway. Med Sci Monit. 23:809–817.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Liu X, Qiu G, Zhang Z, Fan L, Zhao
W, He S, Chang S and Che X: Effects of β-elemene on proliferation
and apoptosis of SGC7901 gastric cancer cells in vitro and the
underlying mechanisms. Nan Fang Yi Ke Da Xue Xue Bao. 35:1234–1238.
2015.(In Chinese). PubMed/NCBI
|
|
16
|
Cai DY, Gao X, Wu XH and Hong TT:
Synergistic effect of beta-elemene injection combined paclitaxel
injection on human breast cancer MB-468 cells: An in vitro study.
Zhongguo Zhong Xi Yi Jie He Za Zhi. 33:978–982. 2013.(In Chinese).
PubMed/NCBI
|
|
17
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao X, Wang Q, Li GJ, Chen F, Qian Y and
Wang R: In vitro antioxidant, anti-mutagenic, anti-cancer and
anti-angiogenic effects of Chinese Bowl tea. J Funct Food.
7:590–598. 2014. View Article : Google Scholar
|
|
19
|
Liu WF, Guo W, Niu XH, Yuan RY, Zhang Q,
Xu HR and Chen DF: The establishment of a model of human
osteosarcoma OS-732 xenograft in nude mice. Chin J Bone Tumor Bone
Disease. 7:204–207. 2008.
|
|
20
|
Abdelraouf A, Hamdy H, El Erian AM,
Elsebae M, Taha S, Elshafey HE, Ismail S and Hassany M: Initial
experience of surgical microwave tissue precoagulation in liver
resection for hepatocellular carcinoma in cirrhotic liver. J Egypt
Soc Parasitol. 44:343–350. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Y, Qiu W, Liang J, Yu Z and Yue L:
Anti-tumor effects of pEgr-1-endostatin-TNF-α recombinant plasmid
expression induced by ionizing radiation. Asian Pac J Cancer Prev.
12:2933–2937. 2011.PubMed/NCBI
|
|
22
|
Kan Z, Phongkitkarun S, Kobayashi S, Tang
Y, Ellis LM, Lee TY and Charnsangavej C: Functional CT for
quantifying tumor perfusion in antiangiogenic therapy in a rat
model. Radiology. 237:151–158. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carlson J, Alobuia W and Mizell J: Rectal
gastrointestinal stromal tumor with metastasis to the penis: Case
report and review of literature. Int J Surg Case Rep. 29:172–175.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Y, Cho SG, Wu X, Siwko S and Liu M:
G-protein coupled receptor 124 (GPR124) in endothelial cells
regulates vascular endothelial growth factor (VEGF)-induced tumor
angiogenesis. Curr Mol Med. 14:543–554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Posokhova E, Shukla A, Seaman S, Volate S,
Hilton MB, Wu B, Morris H, Swing DA, Zhou M, Zudaire E, et al:
GPR124 functions as a WNT7-specific coactivator of canonical
β-catenin signaling. Cell Rep. 10:123–130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma R, Yuan B, Du J, Wang L, Ma L, Liu S,
Shu Q and Sun H: Electroacupuncture alleviates nerve injury after
cerebra ischemia in rats through inhibiting cell apoptosis and
changing the balance of MMP-9/TIMP-1 expression. Neurosci Lett.
633:158–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vafadari B, Salamian A and Kaczmarek L:
MMP-9 in translation: From molecule to brain physiology, pathology,
and therapy. J Neurochem. 139 Suppl 2:S91–S114. 2016. View Article : Google Scholar
|
|
28
|
Breynaert C, de Bruyn M, Arijs I, Cremer
J, Martens E, Van Lommel L, Geboes K, De Hertogh G, Schuit F,
Ferrante M, et al: Genetic deletion of tissue inhibitor of
metalloproteinase-1/TIMP-1 alters inflammation and attenuates
fibrosis in dextran sodium sulphate-induced murine models of
colitis. J Crohns Colitis. 10:1336–1350. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu D, Shen Z, Liu J, Chen J, Liu Y, Hu C,
Li Z and Li Y: The ROS-mediated activation of STAT-3/VEGF signaling
is involved in the 27-hydroxycholesterol-induced angiogenesis in
human breast cancer cells. Toxicol Lett. 264:79–86. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gong YF, Zhang XM, Liu F, Wang ZZ, Deng
XF, Jiao Y, Li XJ and Huang XY: Inhibitory effect of recombinant
human endostatin on the proliferation of hypertrophic scar
fibroblasts in a rabbit ear model. Eur J Pharmacol. 791:647–654.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Zhang J, Chen Z, Wang L, Wu X and
Ou M: Epidermal growth factor (EGF) triggers the malignancy of
hemangioma cells via activation of NF-κB signals. Biomed
Pharmacother. 82:133–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qiu X, Guo S, Wu H, Chen J and Zhou Q:
Identification of Wnt pathway, uPA, PAI-1, MT1-MMP, S100A4 and
CXCR4 associated with enhanced metastasis of human large cell lung
cancer by DNA microarray. Minerva Med. 103:151–164. 2012.PubMed/NCBI
|
|
33
|
Nicholson DW and Thorberry NA: Caspase:
Killer proteases. Thernds Bichem Sci. 22:299–306. 1997. View Article : Google Scholar
|
|
34
|
Evdokiou A, Bouralexis S, Atkins GJ, Chai
F, Hay S, Clayer M and Findlay DM: Chemotherapeutic agents
sensitize osteogenic sarcoma cells, but not normal human bone
cells, to Apo2L/TRAIL-induced apoptosis. Int J Cancer. 99:491–504.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie X, Zhao X, Liu Y, Zhang J, Matusik RJ,
Slawin KM and Spencer DM: Adenovirus-mediated tissue-targeted
expression of a caspase-9-based artificial death swith for the
treatment of prostate cancer. Cancer Res. 61:6795–6804.
2001.PubMed/NCBI
|