Introduction
Lung cancer remains the most common and lethal
malignant tumor type worldwide, despite extensive research and
numerous clinical trials (1,2). The first therapeutic choice for patients
with early-stage lung cancer is surgical excision. However, in the
majority of cases, tumors have developed to the unresectable stage
by the time of initial diagnosis, and surgical resection is no
longer a viable option (3). Thus,
systemic chemotherapy has become the principal treatment for lung
cancer. However, it possesses limitations with regard to its
efficacy, a prime example being that patients rarely survive for an
extended time period following treatment. In this regard,
identifying increasingly sensitive markers for predicting
chemotherapeutic efficacy, and therapeutic targets to promote the
development of individualized treatment has become a popular area
of research.
The mechanisms underlying resistance to anticancer
agents may be categorized into two types, comprising tumor cell
intrinsic factors and non-tumor cell intrinsic factors. The former
includes increased drug efflux by ATP-binding cassette superfamily
proteins, dose-associated toxicities, increased DNA repair
mechanisms, apoptosis deficiency and drug alteration (4–7). However,
these insights into drug resistance have not thus far led to major
improvements in the survival rates of patients with lung cancer,
who usually exhibit immune suppression and cancer-associated
inflammation (8). For instance,
inflammatory leukocytes, together with proteolytic enzymes and
dysregulated vessels form the tumor microenvironment. High levels
of-tumor-associated macrophages (TAM), one of the most important
types of inflammatory leukocytes (9),
exhibit a crucial role in the chemotherapy-resistance of patients
with lung cancer (10). Furthermore,
cancer-associated fibroblasts have been demonstrated to contribute
to the doxorubicin-resistance of breast cancer cells (11). Gene polymorphisms in DNA repair
pathways and multi-drug resistance gene 1 (MDR1) could also
contribute to the response of non-small cell lung cancer (NSCLC) to
chemotherapy (12).
In the 19th century, Rudolf Virchow (13) identified the association between
inflammation and cancer. Thereafter, studies confirmed an extensive
association between inflammation and cancer (14–16).
Cancer may induce an inflammatory state, resulting in multiple
inflammatory cells being recruited to the cancer microenvironment,
and contributing to the hallmarks of cancer (16,17).
Notably, indexes of inflammatory cells [including the pretreatment
neutrophil count (PNC), macrophage, neutrophil-lymphocyte ratio
(NLR) and platelet-lymphocyte ratio (PLR)] in the bloodstream can
reflect the scope and extent of inflammation (18,19).
Previous studies have reported that elevations in the PNC, NLR or
PLR indicate a poor prognosis in various human cancer types, most
notably in colorectal cancer (19),
hepatocellular carcinoma (20), and
in gastric (21), esophageal
(22), breast (23,24),
ovarian (25), cervical (26) and lung cancer (27,28).
Increasingly, studies have suggested that inflammation serves an
important role in the regulation of chemoresistance (8,29–32). This is consistent, with the notion
that certain inflammatory indexes correlate with chemotherapeutic
responses; for example, NLR correlates with chemotherapeutic
responses in breast cancer (33). Van
Glabbeke et al (34)
demonstrated that an elevated baseline neutrophil count correlated
with initial, as well as late, resistance to imatinib treatment in
gastrointestinal stromal tumors (GIST). However, studies regarding
the association between chemotherapeutic sensitivity and
inflammatory indexes are scarce, particularly in lung cancer.
Despite this, we hypothesized that common inflammatory indexes
could serve as important predictors for chemotherapeutic response.
The available literature leaves certain questions unanswered, such
as which indexes may be used for predicting the response to
chemotherapy in lung cancer, and which index is superior.
The primary aim of this study was to investigate and
compare the predictive value of commonly used inflammatory indexes
on chemotherapeutic efficacy in advanced lung cancer.
Materials and methods
Ethical approval
This protocol of this retrospective study was
reviewed and approved by the Ethics Committee of Shandong
Provincial Hospital Affiliated to Shandong University, and written
informed consent was obtained from all participants for their
clinical records to be used in this study.
Patient and data collection
All participating patients with stage III and IV
unresectable lung cancer (NSCLC and SCLC) received chemotherapy as
the initial treatment between January 2007 and December 2011 at the
Department of Oncology, Shandong Provincial Hospital Affiliated to
Shandong University (Jinan, China) and Jinling Hospital (Nanjing,
China) were retrospectively identified from the hospital's original
electronic databases. The primary inclusion criteria were as
follows: i) Definitive diagnosis of primary lung cancer; ii)
explicit pathological pattern before treatment; and iii)
chemotherapy as a first-line therapy without prior treatment. The
exclusion criteria were as follows: i) Patients with a history of
other types of cancer; ii) patients whose data were incomplete;
iii) patients for whom chemotherapeutic efficacy was not evaluated;
and iv) patients who received concurrent radiochemotherapy prior to
response evaluation.
Progression-free survival (PFS) was defined as the
time period from the start of treatment to the date that tumor
progression was detected. The patients’ clinicopathological data
were collected using electronic medical records. This,
clinicopathological information included sex, age, inflammatory
manifestation, smoking status, obstructive pneumonia, central tumor
location, clinical stage, chemotherapeutic response, overall
response and histopathological pattern. PNC, NLR and PLR were
calculated from the medical records at the time of the first
explicit diagnosis prior to chemotherapy.
Response assessment
Chemotherapeutic response was assessed after 2 or 3
chemotherapeutic cycles using the revised Response Evaluation
Criteria in Solid Tumors (version 1.1) (34). The criteria classified the responses
into four categories: Complete response (CR), partial response
(PR), stable disease (SD) and progressive disease (PD). CR was
defined as the complete disappearance of all measurable lesions
sustained for ≥4 weeks. PR was defined as a minimum 30% reduction
in measurable lesions sustained for ≥4 weeks. SD was defined as a
<30% decrease or <20% increase in the size of measurable
lesions. PD was assigned to patients when measurable lesions
increased by >20%, or when new lesions were identified (35). Overall response included CR and PR.
The overall response rate was the ratio of overall response
patients to the total patients.
Statistical analysis
All statistical analyses were performed using SPS
version 20.0 (IBM Corp., Armonk, NY, USA). Analysis of inflammatory
indexes was performed according to the overall response by receiver
operating characteristic (ROC) curves, which were used to detect
the value of each index for predicting the response to
chemotherapy. Associations between inflammatory indexes and
clinicopathological parameters were investigated using the
χ2 test. A nonparametric test of numerical variables was
used for testing differences in the distribution of inflammatory
indexes among different groups. PFS was estimated using the
Kaplan-Meier method and the log-rank test was used for comparison
of outcomes. The Cox proportional hazards regression model was used
to confirm independent predictors of PFS, and multivariate Cox
analyses were performed with a step-forward logistic regression
approach. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
All the main clinicopathological characteristics of
samples are detailed in Table I. A
total of 390 patients, based on the original electronic files, were
retrospectively enrolled in the study. All samples were from
patients with stage III or IV unresectable lung cancer who received
platinum-based chemotherapy as a first-line treatment. There were
277 (71.0%) males and 113 (29.0%) females, with 121 (31.0%)
patients >65 years of age and the other 269 ≤65 (69.0%; range,
18–84 years). There were 58 (14.9%) patients with inflammatory
manifestation (fever, yellow sputum, purulent sputum, etc.) while
this was not present in the other 332 (85.1%). In terms of clinical
tumor stage, 200 (51.3%) patients had stage III and 190 (48.7%) had
stage IV disease. Regarding chemotherapeutic response, no patients
achieved CR, 261 achieved PR, 66 had SD and 63 had PD. In total 261
patients (66.9%) achieved an overall response, and the other 129
patients (33.1%) had no marked chemotherapeutic response. PNC and
NLR were divided into two levels by the critical values, and PLR
did not indicate significance according to the ROC curve.
 | Table I.Association between inflammatory
indexes and clinicopathological data. |
Table I.
Association between inflammatory
indexes and clinicopathological data.
|
|
| PNC |
| NLR |
|
|---|
|
|
|
|
|
|
|
|---|
|
Characteristics | Total patients, n
(%) | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
≤65 | 269 (69.0) | 152 (56.5) | 117 (43.5) | 0.955 | 127 (47.2) | 142 (52.8) | 0.353 |
|
>65 | 121 (31.0) | 68 (56.2) | 53 (43.8) |
| 51 (42.1) | 70 (57.9) |
|
| Sex |
|
|
|
|
|
|
|
|
Male | 277 (71.0) | 152 (54.9) | 125 (45.1) | 0.338 | 129 (46.6) | 148 (53.4) | 0.564 |
|
Female | 113 (29.0) | 68 (60.2) | 45 (39.8) |
| 49 (43.4) | 64 (56.6) |
|
| Inflammatory
manifestation |
|
|
|
|
|
|
|
|
Positive | 58 (14.9) | 25 (43.1) | 33 (56.9) | 0.027 | 17 (29.3) | 41 (70.7) | 0.007a |
|
Negative | 332 (85.1) | 195 (58.7) | 137 (41.3) |
| 161 (48.5) | 171 (51.5) |
|
| Smoking status |
|
|
|
|
|
|
|
| Current
smokers | 210 (53.8) | 120 (57.1) | 90 (42.9) | 0.074 | 102 (48.6) | 108 (51.4) | 0.450 |
|
Non-smokers | 134 (34.4) | 81 (60.4) | 53 (39.6) |
| 57 (42.5) | 77 (57.5) |
|
|
Ex-smokers | 46 (11.8) | 19 (41.3) | 27 (58.7) |
| 19 (41.3) | 27 (58.7) |
|
| Obstructive
pneumonia |
|
|
|
|
|
|
|
|
Positive | 137 (35.1) | 78 (56.9) | 59 (43.1) | 0.878 | 56 (40.9) | 81 (59.1) | 0.164 |
|
Negative | 253 (64.9) | 142 (56.1) | 111 (43.9) |
| 122 (48.2) | 131 (51.8) |
|
| Central tumor
location |
|
|
|
|
|
|
|
|
Positive | 176 (45.1) | 99 (56.2) | 77 (43.8) | 0.954 | 79 (44.9) | 97 (55.1) | 0.786 |
|
Negative | 214 (54.9) | 121 (56.5) | 93 (43.5) |
| 99 (46.3) | 115 (53.7) |
|
| Clinical stage |
|
|
|
|
|
|
|
| Stage
III | 200 (51.3) | 113 (56.5) | 87 (43.5) | 0.971 | 99 (49.5) | 101 (50.5) | 0.116 |
| Stage
IV | 190 (48.7) | 107 (56.3) | 83 (43.7) |
| 79 (41.6) | 111 (58.4) |
|
| Chemotherapeutic
response |
|
|
|
|
|
|
|
| PR | 261 (66.9) | 172 (65.9) | 89 (34.1) | <0.001 | 129 (49.4) | 132 (50.6) | 0.103 |
| SD | 66 (16.9) | 31 (47.0) | 35 (53.0) |
| 25 (37.9) | 41 (62.1) |
|
| PD | 63 (16.2) | 17 (27.0) | 46 (73.0) |
| 24 (38.1) | 39 (61.9) |
|
| Overall
response |
|
|
|
|
|
|
|
|
CR+PR | 261 (66.9) | 172 (65.9) | 89 (34.1) | <0.001 | 129 (49.4) | 132 (50.6) | 0.033a |
|
SD+PD | 129 (33.1) | 48 (37.2) | 81 (62.8) |
| 49 (38.0) | 80 (62.0) |
|
| Histopathological
types |
|
|
|
|
|
|
|
|
SCLC | 104 (26.7) | 68 (65.4) | 36 (34.6) | 0.031 | 55 (52.9) | 49 (47.1) | 0.083 |
|
NSCLC | 286 (73.3) | 152 (53.1) | 134 (46.9) |
| 123 (43.0) | 163 (57.0) |
|
The association between inflammatory
indexes and overall response rate
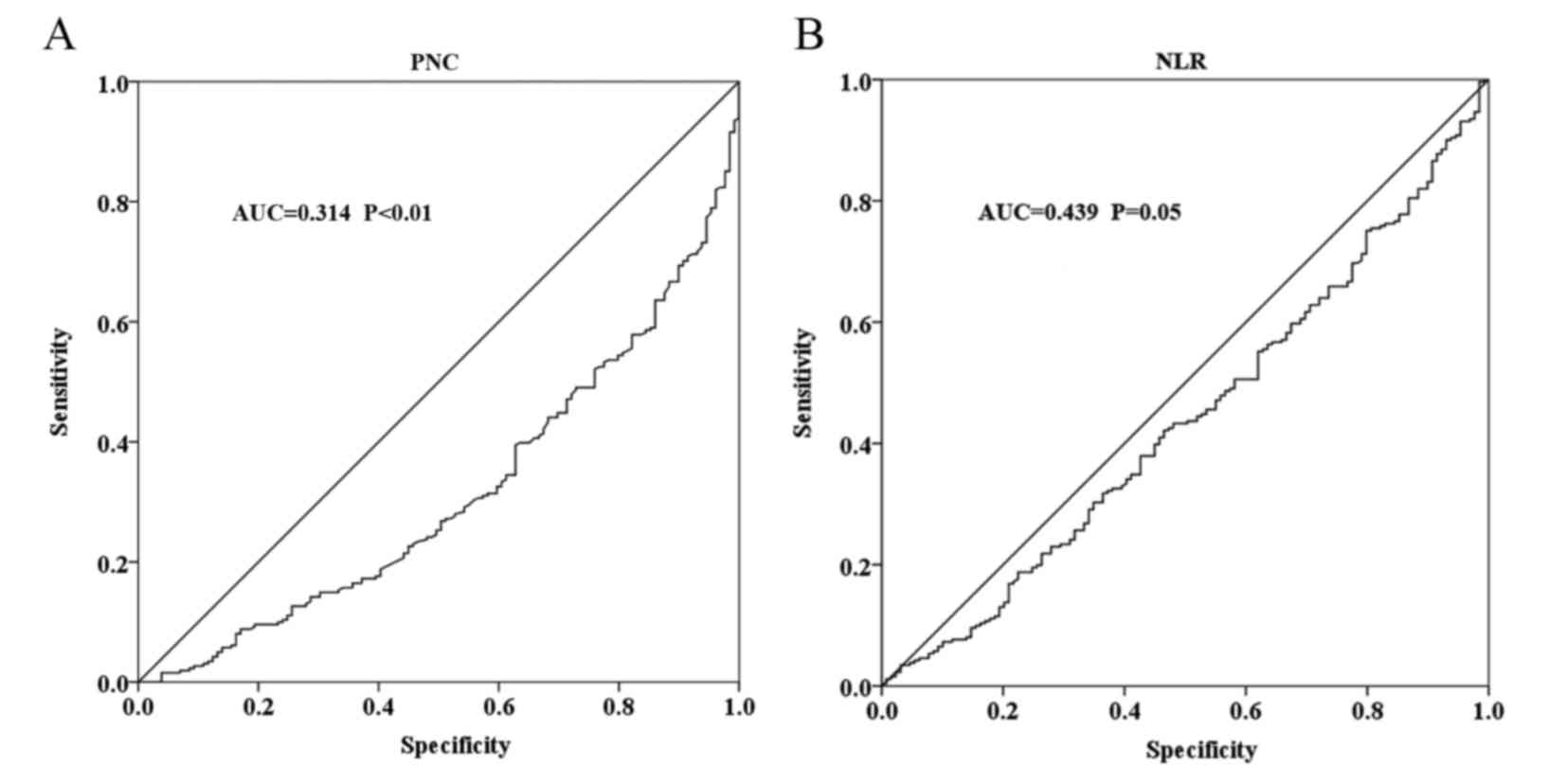
The cut-off values based on the ROC curve were
calculated as 4.635×109/l for PNC and 2.443 for NLR.
However, the PLR failed to reach diagnostic accuracy for the
overall response (Fig. 1). According
to the cut-off values, PNC and NLR were each divided into low and
high groups. The ROC curve indicated that PNC [area under curve
(AUC), 0.314; P<0.001)] and NLR (AUC, 0.439; P=0.05) were
significant predictors of overall response. The overall response
rate was 78.2% (172/220) in the low PNC group, and only 52.4%
(89/170) in the high PNC group (odds ratio, 3.261; 95% CI,
2.102–5.060; P<0.001). The overall response rate was 72.5%
(129/178) in the low NLR group, and was reduced to 62.3% (132/212)
in high group (odds ratio, 1.596; 95% CI, 1.037–2.454;
P=0.033).
Associations between inflammatory
indexes and clinicopathological characteristics
The associations between the clinicopathological
parameters and inflammatory indexes are presented in Table I. High levels of PNC were found to be
significantly associated with inflammatory manifestation (P=0.027),
non overall response (SD+PD; P<0.001), chemotherapeutic response
(P<0.001) and histopathological types (P=0.031). Conversely,
there was no significant association between PNC level and other
clinical parameters, such as age (P=0.955), sex (P=0.338), smoking
status (P=0.074), obstructive pneumonia (P=0.878), central tumor
location (P=0.954) or clinical tumor stage (P=0.971). NLR was
significantly associated with inflammatory manifestation (P=0.007)
and overall response (P=0.033); however, there was no significant
association with other parameters.
Distribution of inflammatory indexes
according to chemotherapeutic response and overall response
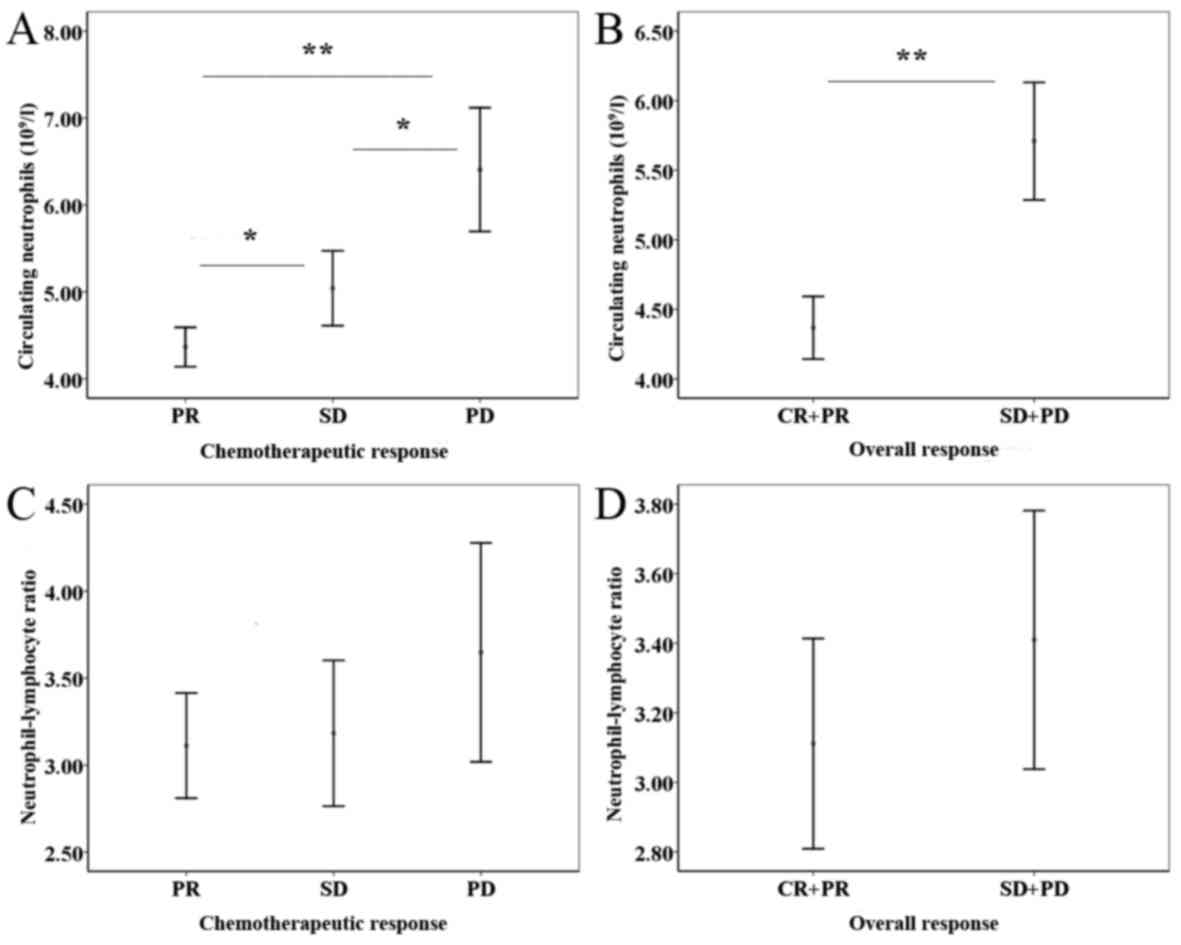
To analyze differences in the distribution of PNC
and NLR between patients who exhibited a chemotherapeutic response
and overall response, a Mann-Whitney U test was used. Comparison of
the PNC and NLR values among the three chemotherapeutic response
groups revealed that the differences were statistically significant
(PR<SD, P=0.008; SD<PD, P=0.001; PR<PD, P<0.001)
(Fig. 2A). Consistently, patients
with low PNC were more likely to exhibit an overall response to
chemotherapy (P<0.001; Fig. 2B).
However, the distribution of NLR demonstrated no significant
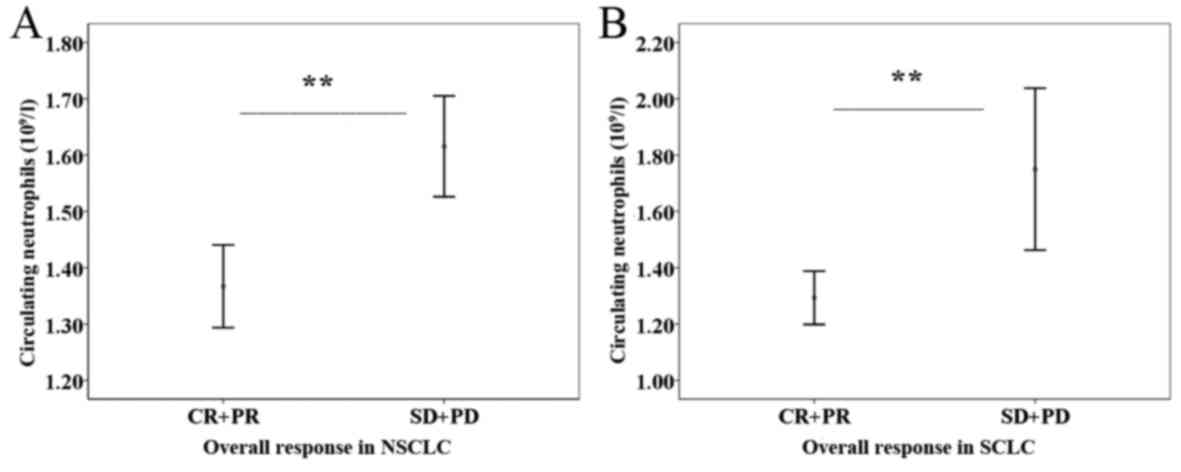
difference (Fig. 2C and D). Further
analyses, revealed the predictive efficiency of PNC for overall
response existed in the NSCLC (P<0.001) and SCLC (P<0.001)
subgroups (Fig. 3).
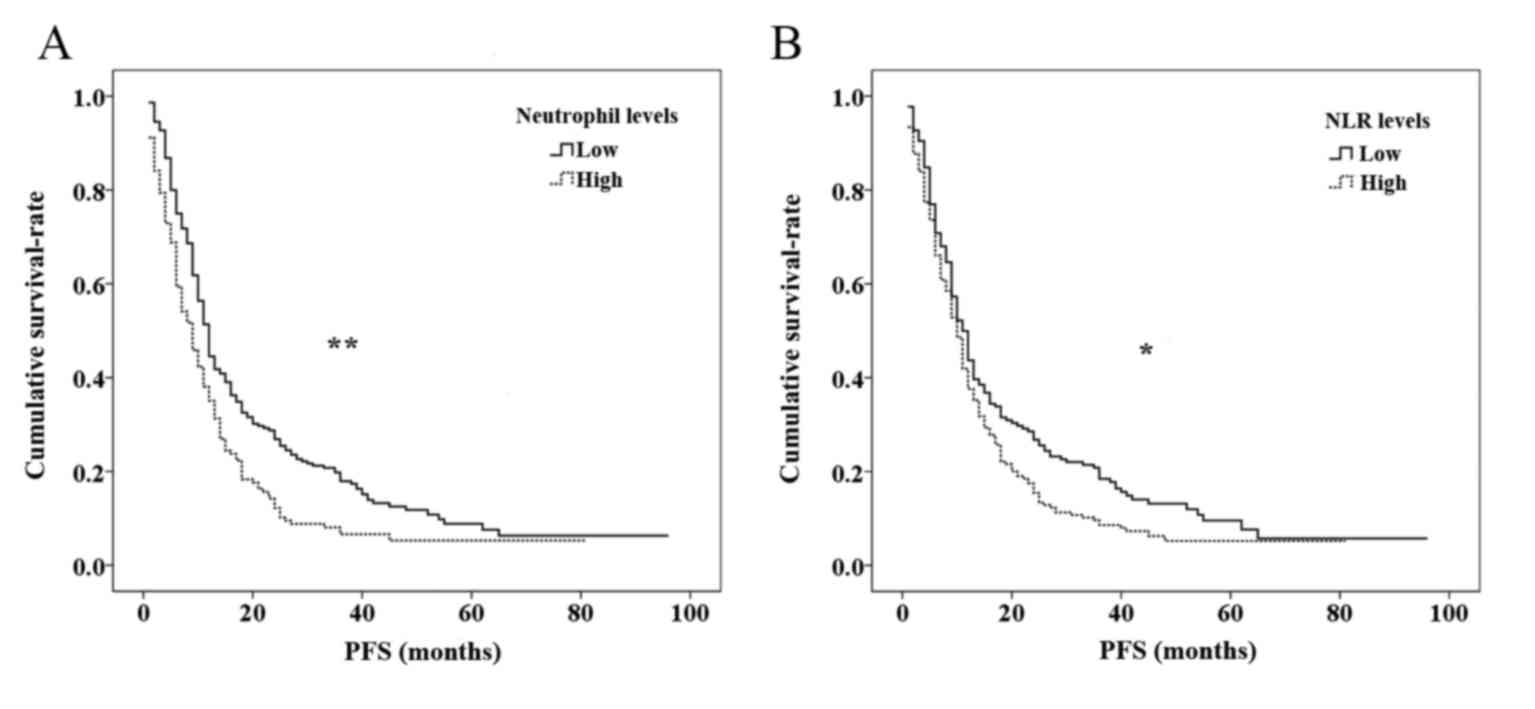
Survival analyses
The last follow-up was performed in December 2014,
and 24 (6.2%) patients were lost to follow up. In the present
study, PFS was the endpoint for the entire cohort. Kaplan-Meier
survival curves based on PNC and NLR levels are shown in Fig. 4. Statistically significant differences
in survival were identified between the different levels of PNC
(P<0.001) and NLR (P=0.016); high levels of PNC and NLR were
significantly associated with recurrence risk and poor prognosis.
The Cox proportional hazards regression model was used for
confirming the independent predictors of PFS (Table II). According to univariate Cox
regression analysis, PFS was correlated with NLR levels (HR, 1.288;
95% CI, 1.041–1.594; P=0.020), PNC levels (HR, 1.487, 95% CI,
1.200–1.841; P<0.001), overall response (HR, 2.349; 95% CI,
1.878–2.939; P<0.001) and clinical tumor stage (HR, 1.537, 95%
CI=1.241–1.904; P<0.001). No significant associations were
identified between survival and sex, age or smoking status.
Multivariate Cox analysis confirmed that PNC levels, overall
response and clinical stage were independent predictors of PFS
(P=0.011, P<0.001 and P=0.001, respectively).
 | Table II.Cox proportional hazard's
analyses. |
Table II.
Cox proportional hazard's
analyses.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
|
Characteristics | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
|
Female | 1 (ref.) | 0.253 | – | – |
|
Male | 1.145
(0.908–1.444) |
| – |
|
| Age (years) |
|
|
|
|
|
>65 | 1 (ref.) | 0.837 | – | – |
|
≤65 | 1.024
(0.817–1.283) |
| – |
|
| Smoking status |
|
|
|
|
| Current
smokers | 1 (ref.) | 0.783 | – | – |
|
Non-smokers | 0.941
(0.748–1.183) |
| – |
|
|
Ex-smokers | 1.056
(0.750–1.487) |
| – |
|
| NLR levels |
|
|
|
|
|
Low | 1 (ref.) | 0.020a | – | – |
|
High | 1.288
(1.041–1.594) |
| – |
|
| Circulating
neutrophil levels |
|
|
|
|
|
Low | 1 (ref.) |
<0.001a | 1 | 0.011a |
|
High | 1.487
(1.200–1.841) |
| 1.326
(1.066–1.650) |
|
| Overall
response |
|
|
|
|
|
CR+PR | 1 (ref.) |
<0.001a | 1 |
<0.001a |
|
SD+PD | 2.349
(1.878–2.939) |
| 2.146
(1.707–2.698) |
|
| Clinical stage |
|
|
|
|
| Stage
III | 1 (ref.) |
<0.001a | 1 | 0.001a |
| Stage
IV | 1.537
(1.241–1.904) |
| 1.454
(1.172–1.803) |
|
Discussion
Millions of individuals worldwide receive a lung
cancer diagnosis each year, and the majority of the cases are
detected, whereas the disease has developed into unresectable tumor
stage (1,2,36).
Chemotherapy serves crucial role in the treatment of unresectable
lung cancer (3); however,
chemoresistance has become a vital factor that negatively
influences curative effects. Therefore, there is an urgent
requirement to identify powerful biomarkers associated with the
chemotherapeutic response that can contribute to selecting optimal
therapies for individuals. Therefore, the present study attempted
to establish the relevance of certain inflammatory indexes to
chemotherapeutic efficacy in 390 patients with stage III or IV
unresectable lung cancer. To the best of our knowledge, this is the
first study to identify and compare chemotherapeutic efficacy among
PNC, NLR and PLR in advanced lung cancer.
Links between systemic inflammation and cancer have
garnered academic interest and have been the focus of numerous
studies (14–16). Emerging evidence suggests that
systemic inflammation and the tumor-associated inflammatory
microenvironment serve an important additional role in modulating
chemotherapeutic responsiveness and chemoresistance; however, the
underlying mechanisms remain largely unclear (8,29).
Hematological markers of systemic inflammation, including
C-reactive protein, PNC, NLR, PLR, albumin-neutrophil prognostic
grade etc., are well established as useful in the prediction of
outcomes in a number of cancer types (19–23,25–28,37–40).
Nevertheless, there is a paucity of studies regarding the
associations between inflammatory indexes and chemotherapeutic
response. Furthermore, the conclusions of these studies have been
inconsistent. For example, Van Glabbeke et al (34) identified that elevated PNC was
correlated with chemoresistance in GIST. An additional study
demonstrated that a normal neutrophil ratio was independently
associated with the response to chemotherapy in SCLC (41). Previous research also demonstrated
that NLR was correlated with chemotherapeutic response (33,42).
Conversely, Eryilmaz et al (43) demonstrated no significant association
between CR and NLR levels. In the present study, it was confirmed
that PNC and NLR were significant predictors of chemotherapeutic
response. Notably, PNC presented an increased sensitivity value,
compared with NLR and PLR, for predicting chemoresistance.
Therefore, PNC has the potential to function as a novel and
powerful factor for predicting chemotherapeutic efficacy in lung
cancer.
Hematological inflammatory cells are indispensable
components of the tumor microenvironment, which is analogized to
‘soil supporting the growth of plants’, to support tumor
progression and chemoresistance (8,15).
Firstly, tumor-infiltrating neutrophils (TINs), the most important
‘fertilizers’ in the ‘soil’ (8,15), lose
their conventional antitumor characteristics and acquire a
pro-tumor phenotype in the presence of transforming growth factor-β
(TGF-β) (44). In our previous study,
TIN count was revealed to serve as a prognostic factor and to
promote epithelial-mesenchymal transition (EMT) in esophageal
cancer (45), which may be a key
process involved in regulating chemoresistance in malignant tumors
(46–48). However, TIN is not a conventional
indicator for detection due to difficulty in its measurement.
Furthermore, PNC is largely recruited via chemoattractant
mediators, including chemokines, lipids, complement anaphylotoxins
and N-formylated peptides into tumor microenvironment, and then is
converted into TIN (49). PNC is
correlated with TIN in quantitative terms (27); therefore, PNC can indirectly reflect
and influence the chemotherapeutic response. Elevated PNC can
stimulate upregulation of cytokines and chemokines (13), and this confers cancer cells with
acquired resistance to chemotherapeutic drugs (8,50,51). These possible mechanisms are
consistent with the present results: Elevated PNC is significantly
associated with a poor chemotherapeutic response and poor prognosis
in patients with advanced lung cancer. NLR is the ratio of the PNC
to the pretreatment lymphocyte count, and therefore the association
between high NLR and poor chemotherapeutic response, as revealed in
the present study, may indicate that chemoresistance is associated
with neutrophilia. However, lymphocytes destroy not only invading
pathogens but also malignancies, via the induction of cytotoxic
death. A consequential decrease in the lymphocyte count may lead to
a weaker immune reaction against cancer cells (15,52).
Additionally, neutrophilia suppresses lymphocyte activity by
releasing reactive oxygen species (ROS), nitric oxide (NO) and
arginase (23), therefore hindering
the antitumor immune response (21).
As established, systemic inflammation associated immune suppression
is the predominant non-tumor-cell-intrinsic mechanism of
chemoresistance (8). The present
study demonstrated that individuals in the high NLR group have an
increased risk of experiencing a poor response to chemotherapy and
a poor PFS time. Finally, PLR (the ratio of platelets to
lymphocytes,) was not significantly associated with
chemotherapeutic response in the present study, despite two
previous studies obtaining the opposite results (28,53).
In the study, the associations between common
inflammatory indexes and the chemotherapeutic response and
clinicopathological parameters, in addition the outcome of patients
with stage III or IV unresectable lung cancer, were investigated.
PLR failed to indicate a statistically significant result.
According to previous studies, high PNC and NLR were associated
with poor PFS and a lower rate of response to chemotherapy. The
novel and notable finding from this data that may have practical
implications is that high PNC exhibits a higher overall response
and HR than NLR. Furthermore, significant distributional
differences in PNC were identified in the different chemotherapy
response groups and overall response groups, whereas the NLR
distribution did not significantly differ. This indicates that PNC
may be more powerful and sensitive in predicting chemotherapeutic
response. This may be due to the superior sensitivity of
neutrophils for indicating inflammatory states, and their direct
participation in cancer-associated inflammatory microenvironments.
An additional reason may be that different ages, tumor stages and
histopathological phenotypes correspond with different immune
responses, and therefore hematological data varies.
Additionally, the PNC distribution according to
overall response was also examined in different histopathological
subtypes. The results revealed that the significant differences in
distribution were universal in NSCLC and SCLC, despite the
differences between the two in terms of biological properties and
therapeutic measures.
The major limitations of the present study are as
follows: First, numerous individuals were excluded due to
incomplete data or the unsuccessful completion of follow-up, which
may have led to selective bias; second, patients with different
types of lung cancer exhibited different immune responses and
different inflammatory states, and a stratified analysis of each
subtype was not conducted; third, this study failed to investigate
more inflammatory indexes, such as the Glasgow prognostic score
(GPS); furthermore, conclusions were solely drawn from the
objective clinical data, as it was beyond the scope of our study to
elucidate the mechanism of association between inflammation and
chemoresistance. The combined aforementioned limitations suggest
that the results require validation in additional independent
cohorts of patients with specific lung cancer types, ideally
through large-scale prospective clinical studies.
In summary, the present study indicated that PNC and
NLR were clinically important predictors of chemotherapeutic
response in patients with stage III and IV unresectable lung cancer
who received chemotherapy as first-line treatment. Additionally,
PNC represents a more robust indicator for chemoresistance compared
with other inflammatory indexes. This study has the potential to
provide a highly reproducible, easily obtainable, inexpensive,
reliable and practical index for predicting chemotherapeutic
efficacy, and to facilitate the administration of therapy in
patients with a high PNC in order to reach an improved
chemotherapeutic response, thereby enhancing the long-term outcomes
for patients with unresectable lung cancer. However, the potential
underlying mechanisms and the performance of PNC in clinical
practice should be validated in further prospective studies.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. Ca
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu L, Chang W, Zhao J, Yu Y, Tan X, Su T,
Zhao L, Huang S, Liu S and Cao G: Development of autoantibody
signatures as novel diagnostic biomarkers of non-small cell lung
cancer. Clin Cancer Res. 16:3760–3768. 2010.PubMed/NCBI
|
|
4
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH: Eastern
Cooperative Oncology Group: Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chang A: Chemotherapy, chemoresistance and
the changing treatment landscape for NSCLC. Lung Cancer. 71:3–10.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Raymond E, Chaney SG, Taamma A and
Cvitkovic E: Oxaliplatin: A review of preclinical and clinical
studies. Ann Oncol. 9:1053–1071. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Borst P, Jonkers J and Rottenberg S: What
makes tumors multidrug resistant? Cell Cycle. 6:2782–2787. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
de Visser KE and Jonkers J: Towards
understanding the role of cancer-associated inflammation in
chemoresistance. Current Pharm Des. 15:1844–1853. 2009. View Article : Google Scholar
|
|
9
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Palma M and Lewis CE: Macrophage
regulation of tumor responses to anticancer therapies. Cancer Cell.
23:277–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amornsupuk K, Insawang T, Thuwajit P,
O-Charoenrat P, Eccles SA and Thuwajit C: Cancer-associated
fibroblasts induce high mobility group box 1 and contribute to
resistance to doxorubicin in breast cancer cells. BMC cancer.
14:9552014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du Y, Su T, Zhao L, Tan X, Chang W, Zhang
H and Cao G: Associations of polymorphisms in DNA repair genes and
MDR1 gene with chemotherapy response and survival of non-small cell
lung cancer. PLoS One. 9:e998432014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carus A, Gurney H, Gebski V, Harnett P,
Hui R, Kefford R, Wilcken N, Ladekarl M, von der Maase H and
Donskov F: Impact of baseline and nadir neutrophil index in
non-small cell lung cancer and ovarian cancer patients: Assessment
of chemotherapy for resolution of unfavourable neutrophilia. J
Transl Med. 11:1892013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zou ZY, Liu HL, Ning N, Li SY, Du XH and
Li R: Clinical significance of pre-operative neutrophil lymphocyte
ratio and platelet lymphocyte ratio as prognostic factors for
patients with colorectal cancer. Oncol Lett. 11:2241–2248. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lai Q, Santa Castro E, Juri Rico JM,
Pinheiro RS and Lerut J: Neutrophil and platelet-to-lymphocyte
ratio as new predictors of dropout and recurrence after liver
transplantation for hepatocellular cancer. Transpl Int. 27:32–41.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee S, Oh SY, Kim SH, Lee JH, Kim MC, Kim
KH and Kim HJ: Prognostic significance of neutrophil lymphocyte
ratio and platelet lymphocyte ratio in advanced gastric cancer
patients treated with FOLFOX chemotherapy. BMC Cancer. 13:3502013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng JF, Huang Y and Chen QX: Preoperative
platelet lymphocyte ratio (PLR) is superior to neutrophil
lymphocyte ratio (NLR) as a predictive factor in patients with
esophageal squamous cell carcinoma. World J Surg Oncol. 12:582014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Azab B, Shah N, Radbel J, Tan P, Bhatt V,
Vonfrolio S, Habeshy A, Picon A and Bloom S: Pretreatment
neutrophil/lymphocyte ratio is superior to platelet/lymphocyte
ratio as a predictor of long-term mortality in breast cancer
patients. Med Oncol. 30:4322013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krenn-Pilko S, Langsenlehner U, Thurner
EM, Stojakovic T, Pichler M, Gerger A, Kapp KS and Langsenlehner T:
The elevated preoperative platelet-to-lymphocyte ratio predicts
poor prognosis in breast cancer patients. Br J Cancer.
110:2524–2530. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asher V, Lee J, Innamaa A and Bali A:
Preoperative platelet lymphocyte ratio as an independent prognostic
marker in ovarian cancer. Clin Transl Oncol. 13:499–503. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carus A, Ladekarl M, Hager H, Nedergaard
BS and Donskov F: Tumour-associated CD66b+ neutrophil count is an
independent prognostic factor for recurrence in localised cervical
cancer. Br J Cancer. 108:2116–2122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carus A, Ladekarl M, Hager H, Pilegaard H,
Nielsen PS and Donskov F: Tumor-associated neutrophils and
macrophages in non-small cell lung cancer: No immediate impact on
patient outcome. Lung Cancer. 81:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Wu Y, Wang Z, Yao Y, Chen F, Zhang
H, Wang Y and Song Y: Pretreatment platelet-to-lymphocyte ratio
(PLR) as a predictor of response to first-line platinum-based
chemotherapy and prognosis for patients with non-small cell lung
cancer. J Thorac Dis. 5:783–789. 2013.PubMed/NCBI
|
|
29
|
DeNardo DG, Brennan DJ, Rexhepaj E,
Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD
and Junaid SA: Leukocyte complexity predicts breast cancer survival
and functionally regulates response to chemotherapy. Cancer Discov.
1:54–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muccioli M and Benencia F: Toll-like
receptors in ovarian cancer as targets for immunotherapies. Front
Immunol. 5:3412014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jinushi M and Komohara Y: Tumor-associated
macrophages as an emerging target against tumors: Creating a new
path from bench to bedside. Biochim Biophys Acta. 1855:123–130.
2015.PubMed/NCBI
|
|
32
|
Delitto D, Black BS, Sorenson HL, Knowlton
AE, Thomas RM, Sarosi GA, Moldawer LL, Behrns KE, Liu C, George TJ,
et al: The inflammatory milieu within the pancreatic cancer
microenvironment correlates with clinicopathologic parameters,
chemoresistance and survival. BMC Cancer. 15:7832015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Chen K, Xiao X, Nie Y, Qu S, Gong
C, Su F and Song E: Pretreatment neutrophil-to-lymphocyte ratio is
correlated with response to neoadjuvant chemotherapy as an
independent prognostic indicator in breast cancer patients: A
retrospective study. BMC Cancer. 16:3202016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Van Glabbeke M, Verweij J, Casali PG, Le
Cesne A, Hohenberger P, Ray-Coquard I, Schlemmer M, van Oosterom
AT, Goldstein D, Sciot R, et al: Initial and late resistance to
imatinib in advanced gastrointestinal stromal tumors are predicted
by different prognostic factors: A european organisation for
research and treatment of cancer-italian sarcoma group-australasian
gastrointestinal trials group study. J Clin Oncol. 23:5795–5804.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Brower V: Biomarker studies abound for
early detection of lung cancer. J Natl Cancer Inst. 101:11–13.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sun H, Hu P, Shen H, Dong W, Zhang T, Liu
Q and Du J: Albumin and neutrophil combined prognostic grade as a
new prognostic factor in non-small cell lung cancer: Results from a
large consecutive cohort. PLoS One. 10:e01446632015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kishida Y, Kawahara M, Teramukai S, Kubota
K, Komuta K, Minato K, Mio T, Fujita Y, Yonei T, Nakano K, et al:
Chemotherapy-induced neutropenia as a prognostic factor in advanced
non-small-cell lung cancer: Results from japan multinational trial
organization LC00-03. Br J Cancer. 101:1537–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schmidt H, Bastholt L, Geertsen P,
Christensen IJ, Larsen S, Gehl J and von der Maase H: Elevated
neutrophil and monocyte counts in peripheral blood are associated
with poor survival in patients with metastatic melanoma: A
prognostic model. Br J Cancer. 93:273–278. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Negrier S, Mejean A, Oudard S and Escudier
B: (Metastatic kidney cancer: New therapeutic approaches). Prog
Urol. 12:703–708. 2002.PubMed/NCBI
|
|
41
|
Paesmans M, Sculier JP, Lecomte J,
Thiriaux J, Libert P, Sergysels R, Bureau G, Dabouis G, Van Cutsem
O, Mommen P, et al: Prognostic factors for patients with small cell
lung carcinoma: Analysis of a series of 763 patients included in 4
consecutive prospective trials with a minimum follow-up of 5 years.
Cancer. 89:523–533. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sato H, Tsubosa Y and Kawano T:
Correlation between the pretherapeutic neutrophil to lymphocyte
ratio and the pathologic response to neoadjuvant chemotherapy in
patients with advanced esophageal cancer. World J Surg. 36:617–622.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eryilmaz MK, Mutlu H, Salim DK, Musri FY,
Tural D and Coskun HS: The neutrophil to lymphocyte ratio has a
high negative predictive value for pathologic complete response in
locally advanced breast cancer patients receiving neoadjuvant
chemotherapy. Asian Pac J Cancer Prev. 15:7737–7740. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hu P, Wang G, Shen M, Zhang P, Zhang J, Du
J and Liu Q: Intratumoral polymorphonuclear granulocyte is
associated with poor prognosis in squamous esophageal cancer by
promoting epithelial-mesenchymal transition. Future Oncol.
11:771–783. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hoshino H, Miyoshi N, Nagai K, Tomimaru Y,
Nagano H, Sekimoto M, Doki Y, Mori M and Ishii H:
Epithelial-mesenchymal transition with expression of SNAI1-induced
chemoresistance in colorectal cancer. Biochem Biophys Res Commun.
390:1061–1065. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kurrey NK, Jalgaonkar SP, Joglekar AV,
Ghanate AD, Chaskar PD, Doiphode RY and Bapat SA: Snail and slug
mediate radioresistance and chemoresistance by antagonizing
p53-mediated apoptosis and acquiring a stem-like phenotype in
ovarian cancer cells. Stem Cells. 27:2059–2068. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Canadas I, Rojo F, Taus Á, Arpí O,
Arumí-Uría M, Pijuan L, Menéndez S, Zazo S, Dómine M and Salido M:
Targeting epithelial-to-mesenchymal transition with Met inhibitors
reverts chemoresistance in small cell lung cancer. Clin Cancer Res.
20:938–950. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dumitru CA, Lang S and Brandau S:
Modulation of neutrophil granulocytes in the tumor
microenvironment: Mechanisms and consequences for tumor
progression. Semin Cancer Biol. 23:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kelly MG, Alvero AB, Chen R, Silasi DA,
Abrahams VM, Chan S, Visintin I, Rutherford T and Mor G: TLR-4
signaling promotes tumor growth and paclitaxel chemoresistance in
ovarian cancer. Cancer Res. 66:3859–3868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Nakanishi C and Toi M: Nuclear
factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Miao Y, Yan Q, Li S, Li B and Feng Y:
Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio are
predictive of chemotherapeutic response and prognosis in epithelial
ovarian cancer patients treated with platinum-based chemotherapy.
Cancer Biomark. 17:33–40. 2016. View Article : Google Scholar : PubMed/NCBI
|