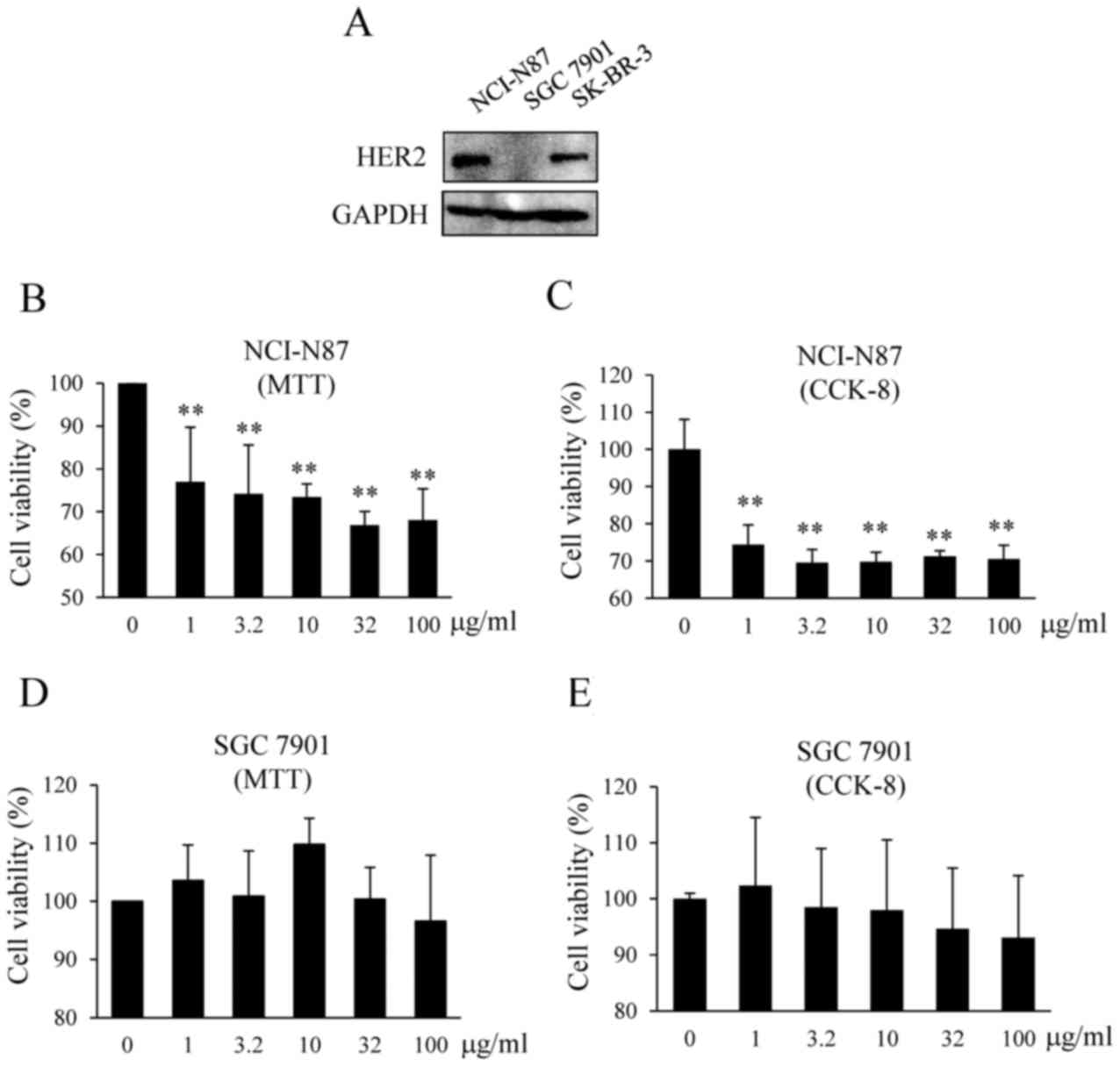
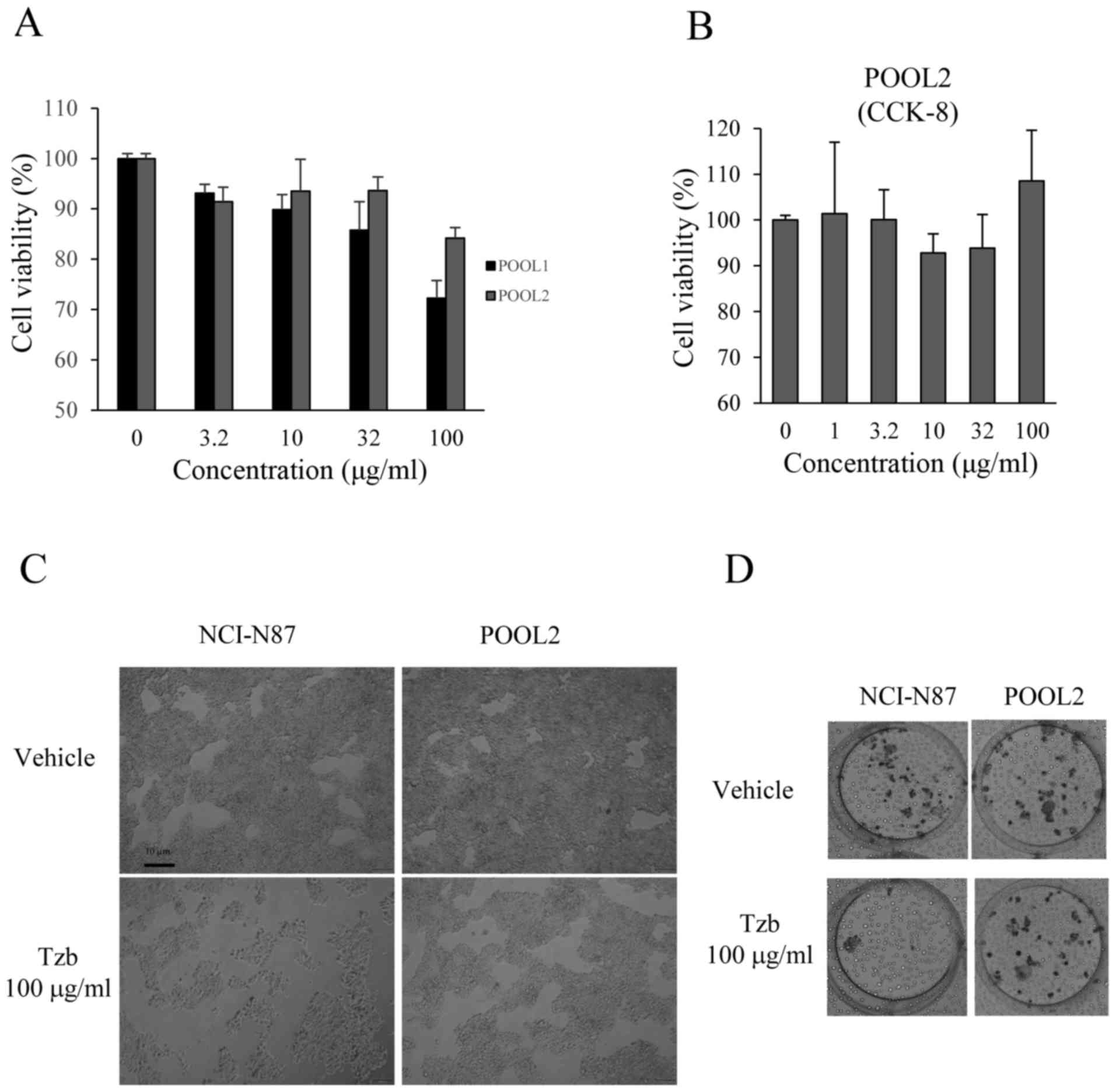
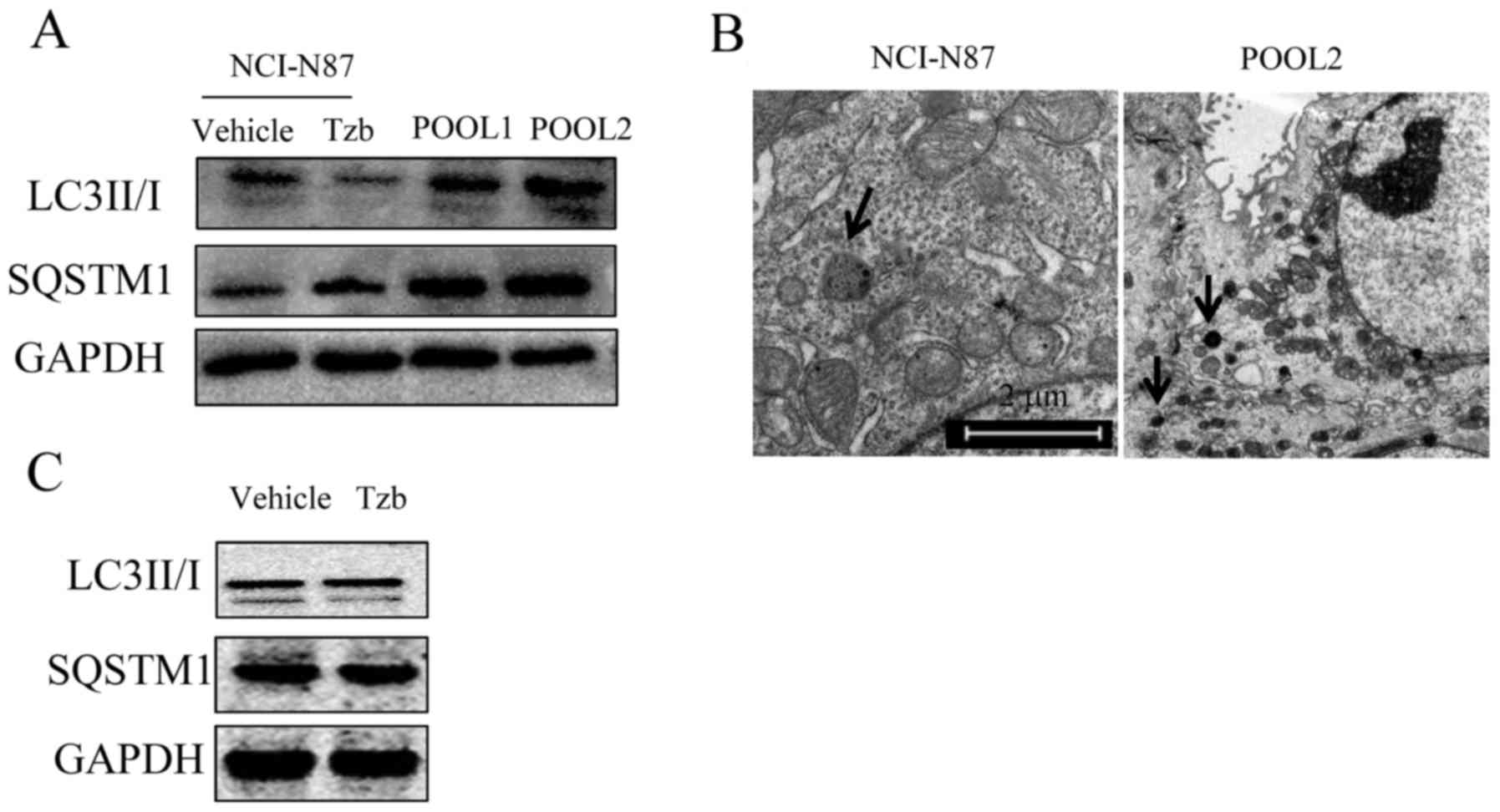
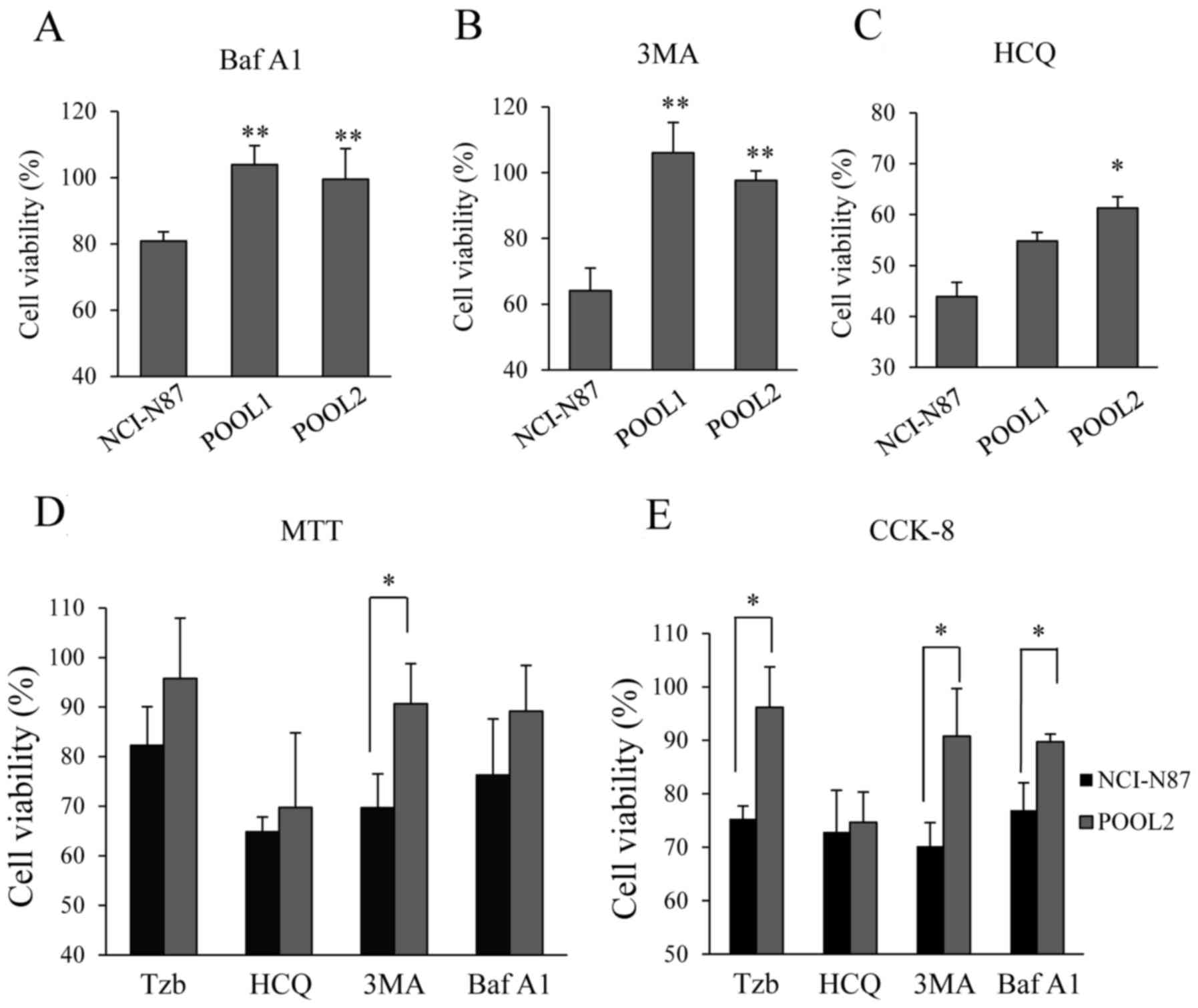
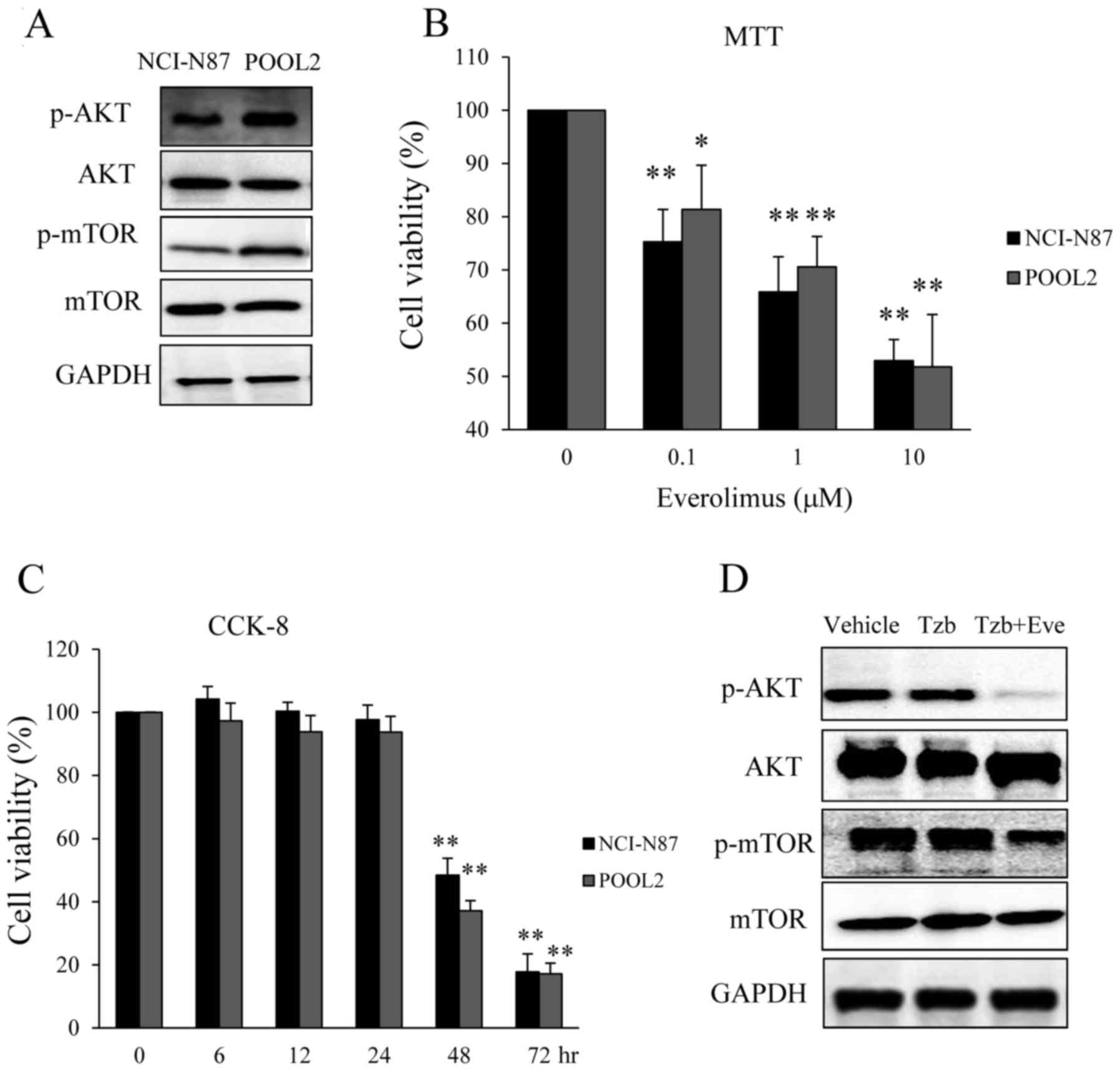
|
1
|
Wang Q, Zhang X, Shen E, Gao J, Cao F,
Wang X, Li Y, Tian T, Wang J, Chen Z, et al: The anti-HER3 antibody
in combination with trastuzumab exerts synergistic antitumor
activity in HER2-positive gastric cancer. Cancer Lett. 380:20–30.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arienti C, Zanoni M, Pignatta S, Del Rio
A, Carloni S, Tebaldi M, Tedaldi G and Tesei A: Preclinical
evidence of multiple mechanisms underlying trastuzumab resistance
in gastric cancer. Oncotarget. 7:18424–18439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abrahao-Machado LF and Scapulatempo-Neto
C: HER2 testing in gastric cancer: An update. World J
Gastroenterol. 22:4619–4625. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhi X and Zhong Q: Autophagy in cancer.
F1000prime Rep. 7:182015. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao Z, Han F, Yang S, Wu J and Zhan W:
Oxamate-mediated inhibition of lactate dehydrogenase induces
protective autophagy in gastric cancer cells: Involvement of the
Akt-mTOR signaling pathway. Cancer Lett. 358:17–26. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan WR, Chen YL, Hsu HC and Chen WJ:
Antimicrobial peptide GW-H1-induced apoptosis of human gastric
cancer AGS cell line is enhanced by suppression of autophagy. Mol
Cell Biochem. 400:77–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu M, Lao Y, Xu N, Wang X, Tan H, Fu W,
Lin Z and Xu H: Guttiferone K induces autophagy and sensitizes
cancer cells to nutrient stress-induced cell death. Phytomedicine.
22:902–910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qian HR and Yang Y: Functional role of
autophagy in gastric cancer. Oncotarget. 7:17641–17651. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cufi S, Vazquez-Martin A,
Oliveras-Ferraros C, Corominas-Faja B, Urruticoechea A,
Martin-Castillo B and Menendez JA: Autophagy-related gene 12
(ATG12) is a novel determinant of primary resistance to
HER2-targeted therapies: Utility of transcriptome analysis of the
autophagy interactome to guide breast cancer treatment. Oncotarget.
3:1600–1614. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodriguez CE, Reidel SI, Bal de Kier Joffé
ED, Jasnis MA and Fiszman GL: Autophagy protects from
trastuzumab-induced cytotoxicity in HER2 overexpressing breast
tumor spheroids. PLoS One. 10:e01379202015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cufi S, Vazquez-Martin A,
Oliveras-Ferraros C, Corominas-Faja B, Cuyàs E, López-Bonet E,
Martin-Castillo B, Joven J and Menendez JA: The anti-malarial
chloroquine overcomes primary resistance and restores sensitivity
to trastuzumab in HER2-positive breast cancer. Sci Rep. 3:24692013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vazquez-Martin A, Oliveras-Ferraros C and
Menendez JA: Autophagy facilitates the development of breast cancer
resistance to the anti-HER2 monoclonal antibody trastuzumab. PLoS
One. 4:e62512009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartlett BJ, Isakson P, Lewerenz J,
Sanchez H, Kotzebue RW, Cumming RC, Harris GL, Nezis IP, Schubert
DR, Simonsen A and Finley KD: p62, Ref(2)P and ubiquitinated
proteins are conserved markers of neuronal aging, aggregate
formation and progressive autophagic defects. Autophagy. 7:572–583.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pamarthy S, Jaiswal MK, Kulshreshtha A,
Katara GK, Gilman-Sachs A and Beaman KD: The vacuolar ATPase
a2-subunit regulates Notch signaling in triple-negative breast
cancer cells. Oncotarget. 6:34206–34220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Manic G, Obrist F, Kroemer G, Vitale I and
Galluzzi L: Chloroquine and hydroxychloroquine for cancer therapy.
Mol Cell Oncol. 1:e299112014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lui A, New J, Ogony J, Thomas S and
Lewis-Wambi J: Everolimus downregulates estrogen receptor and
induces autophagy in aromatase inhibitor-resistant breast cancer
cells. BMC Cancer. 16:4872016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CI, Whang EE, Donner DB, Du J, Lorch
J, He F, Jiang X, Price BD, Moore FD Jr and Ruan DT: Autophagy
induction with RAD001 enhances chemosensitivity and
radiosensitivity through Met inhibition in papillary thyroid
cancer. Mol Cancer Res. 8:1217–1226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Said A, Bock S, Lajqi T, Muller G and
Weindl G: Chloroquine promotes IL-17 production by CD4+
T cells via p38-dependent IL-23 release by monocyte-derived
Langerhans-like cells. J Immunol. 193:6135–6143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Spears LD, Tran AV, Qin CY, Hobbs SB,
Burns CA, Royer NK, Zhang Z, Ralston L and Fisher JS: Chloroquine
increases phosphorylation of AMPK and Akt in myotubes. Heliyon.
2:e000832016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
O'Reilly KE, Rojo F, She QB, Solit D,
Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al:
mTOR inhibition induces upstream receptor tyrosine kinase signaling
and activates Akt. Cancer Res. 66:1500–1508. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang XL, Lin FJ, Guo YJ, Shao ZM and Ou
ZL: Gemcitabine resistance in breast cancer cells regulated by
PI3K/AKT-mediated cellular proliferation exerts negative feedback
via the MEK/MAPK and mTOR pathways. OncoTargets Ther. 7:1033–1042.
2014.
|
|
25
|
Zhang L, Wang H, Xu J, Zhu J and Ding K:
Inhibition of cathepsin S induces autophagy and apoptosis in human
glioblastoma cell lines through ROS-mediated PI3K/AKT/mTOR/p70S6K
and JNK signaling pathways. Toxicol Lett. 228:248–259. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saran U, Foti M and Dufour JF: Cellular
and molecular effects of the mTOR inhibitor everolimus. Clin Sci
(Lond). 129:895–914. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fuereder T, Wanek T, Pflegerl P,
Jaeger-Lansky A, Hoeflmayer D, Strommer S, Kuntner C, Wrba F,
Werzowa J, Hejna M, et al: Gastric cancer growth control by BEZ235
in vivo does not correlate with PI3K/mTOR target inhibition but
with [18F]FLT uptake. Clin Cancer Res. 17:5322–5332. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Y, Tian T, Zou J, Wang Q, Li Z, Li Y,
Liu X, Dong B, Li N, Gao J and Shen L: Dual PI3K/mTOR inhibitor
BEZ235 exerts extensive antitumor activity in HER2-positive gastric
cancer. BMC Cancer. 15:8942015. View Article : Google Scholar : PubMed/NCBI
|