Introduction
Gastric cancer is the second leading cause of
cancer-associated mortalities globally and is a major cause of
mortality in Asia (1–3). According to a survey in 2015, the
mortality rate from gastric cancer ranked second in overall cancer
mortality in China (4). Accordingly,
therapeutic strategies for treating gastric cancer are in
demand.
Surgery and radiotherapy form the mainstay of
current therapeutic strategies for localized tumors; however, these
treatments are not completely successful and subsequence tumor
recurrence and metastases may occur (5). Furthermore, their high cost may be
prohibitive and postoperative complications may be severe (6).
Fluorouracil (5-FU) is a classic drug used for
gastric cancer treatment (7). It is
generally known to exert its antitumor effects by stopping the
production of DNA (8). However,
numerous cases of drug resistance have been previously reported
(7,8).
Therefore, alternative treatment options with improved efficacy and
fewer side effects are required. For this reason, 5-FU was selected
as a positive control for the present study.
In recent years, there has been interest in the
application of traditional Chinese medicine for the treatment of
tumors (9–11). Proponents claim that ‘Lei-Wan’, the
scientific term for Omphalia lapidescens, possesses
medicinal activity against a variety of parasites, including
roundworms, spirometra and ancylostomes (12). Numerous studies have sought to
elucidate the anti-tumor effects of ‘Lei-Wan’ and its underlying
molecular mechanisms (13,14). Purified Omphalia lapidescens
protein (pPeOp) is a protein extracted from the sclerotium
of Omphalia lapidescens by polyvinyl pyrrolidone (PVP)
extraction buffer. In a previous study, it was demonstrated that
pPeOp promotes apoptosis and cell cycle arrest of tumor
cells (MC-4), but did not cause toxicity to normal gastric cells
(MC-1) (15). Those wishing to study
the underlying biology of pPeOp would have to study its
influence on the intricate network of numerous regulatory molecules
that govern tumor genesis.
In the present study, the ability of pPeOp to
inhibit the migration of SGC-7901 gastric cancer cells was
investigated. SGC-7901 cells were treated with different dosages of
pPeOp, following which, the extent of apoptosis and cell
cycle arrest was determined. Furthermore, the potential underlying
mechanism of action of pPeOp was investigated by studying
the Janus kinase (JAK)-signal transducer and activator of
transcription (STAT) signaling pathway and a number of key
regulatory molecules [matrix metallopeptidase (MMP)2, MMP9, cyclin
D1, cyclin A2, cyclin B1, cyclin dependent kinase (CDK)1, CDK2,
CDK4, B-cell lymphoma (Bcl)-2, p53 and caspase-3].
Materials and methods
pPeOp extraction and purification
The fruiting body of Omphalia lapidescens was
provided by Fang Hui Chun Tang (Hangzhou, China). A total of 200 mg
of the fruiting body of Omphalia lapidescens was washed
three times with distilled water at 4°C and placed into 0.875 ml of
cold PVP extraction buffer (15% 1.0 M Tris-HCl, pH 8.0; 2% PVP; 25%
glycerol) on ice for 4 h. The samples were then centrifuged (12,000
× g, 20 min) at 4°C, and the supernatant was collected and retained
for purification.
A SephadexG-50 column (GE Healthcare Life Sciences,
Little Chalfont, UK), pre-equilibrated with 50 mM Tris-HCl buffer
(pH 8.5), was employed to purify the sample (1 ml sample for each
experiment). The absorbance was measured at 280 nm, and the flow
rate was 0.2 ml/min, producing three peaks. In accordance with a
previously described protocol (15),
the second A280 nm peak fraction was ultrafiltrated
using a Millipore ultrafiltration tube (EDM Millipore, Billerica,
MA, USA). Finally, the protein was sterilized by filtration using a
0.22 µm filter, and then stored at −20°C for later use.
Cell culture
The human gastric cancer cell line SGC-7901 was
provided from Zhejiang Provincial Center for Disease Control and
Prevention (Zhejiang, China). SGC-7901 cells were cultured in
RPMI-1640 medium (cat no. GNM31800; Hangzhou Genom Biomedical
Technology Co., Ltd., Hangzhou, China; http://www.genom.com.cn/) supplemented with 5% (v/v)
fetal bovine serum (cat no. 22011-8612; Zhejiang Tianhang
Biotechnology Co., Ltd., Zhejiang, China), 100 U/ml penicillin and
100 µg/ml streptomycin at 37°C, 5% CO2.
Cell viability assay
Cultured SGC-7901 cells were detached with 0.25%
trypsin during the logarithmic growth phase. The suspension of
SGC-7901 cells containing 1×106/ml cells was seeded into
a 96-well plate and incubated at 37°C with 5% CO2 for 24
h in preparation for an MTS assay. Subsequently, pPeOp (30,
60 and 90 µg/ml), 100 µg/ml5-FU (cat no. 51-21-8; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) or PVP (90 µg/ml) diluted with
RPMI-1640, which had been sterilized through a 0.22 µm filter, were
added to the cells and incubated for 24 h at 37°C. A CellTiter
96®AQueous One Solution Assay kit (cat no. G3580;
Promega Corporation, Madison, WI, USA) was used for the MTS assay,
according to the manufacture protocol. According to the
manufacturer's protocol, 20 µl MTS solution (Promega Corporation)
was added to each well, briefly agitated for 30 sec and incubated
at 37°C for 60 min. Absorbance at 490 nm was measured using an
automatic microwell plate reader (Thermo Labsystems, Helsinki,
Finland). The light microscope used to visualize cells was the
Nikon eclipse ti (Nikon Corporation, Tokyo, Japan) at a
magnification of ×100.
Cell migration assay
Cellular migration was investigated using a
wound-healing assay. SGC-7901 cells (1×106/ml) were
seeded into 6-well plates and cultured under standard
aforementioned conditions for ~24 h. When cells were ~80%
confluent, a wound was scratched into the cell layer with a 200 µl
pipette tip. Cells were then treated with 30, 60 or 90 µg/ml
pPeOp, 90 µg/ml PVP or 100 µg/ml 5-FU; for 24 h at 37°C.
Cells were then washed with pre-warmed PBS (37°C) to remove cell
debris and allowed to migrate for 24 h prior to image capture.
Flow cytometry detection of cell
cycle
SGC-7901 cells were located in 6-well plates and
incubated under standard aforementioned conditions for 24 h in
preparation for a cell cycle assay. Then, cells were treated with
pPeOp (30, 60 or 90 µg/ml), PVP (100 µg/ml) or 5-FU (100
µg/ml) under standard aforementioned conditions for 24 h. Cultured
cells were harvested with EDTA-free trypsin (0.25%) and washed
twice with ice-cold PBS. Subsequently, cells (1×106 /ml)
were incubated with 0.5 ml propidium iodide (PI)/RNase staining
buffer (cat no. 550825; BD Biosciences, San Jose, CA, USA) for 15
min at room temperature (25°C) in the dark prior to being fixed
with 1% paraformaldehyde (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) at 25°C for 30 min, and stored in 70%
ethanol at −20°C for 15 min. Cell cycle progression analyses were
performed using a Beckman FC500 (Beckman Coulter, Inc., Brea, CA,
USA) flow cytometer with red fluorescent light at a wavelength of
488 nm. Win Cycle software (version 32; Beckman Coulter, Inc.) were
used for analyses.
Flow cytometry detection of
apoptosis
FITC Annexin V Apoptosis Detection kit I (cat no.
556547; BD Biosciences) was used to assay cell apoptosis. Cultured
cells, subsequent to treatment with pPeOp (30, 60 or 90
µg/ml), PVP (100 µg/ml) or 5-FU (100 µg/ml) under standard
aforementioned conditions for 24 h, were collected with EDTA-free
trypsin (0.25%) and washed twice with ice-cold PBS. Subsequently,
cells (1×106/ml) were resuspended in 100 µl 1Xbinding
buffer solution (BD Biosciences), to which 5 µl of fluorescein
isothiocyanate/Annexin V (BD Biosciences) and 5 µl PI (BD
Biosciences) were added. Cells were then gently vortexed and
incubated for 15 min at room temperature (25°C) in the dark.
Incubated cells were washed again with 1X binding buffer and
resuspended in 200 µl of 1X binding buffer. PI (5 µl) and 400 µl of
1X binding buffer were added to each sample, and samples were
analyzed using a Beckman FC500 flow cytometer (Beckman Coulter,
Inc.). The software used to analyze apoptosis was CXP analysis
(version 32; Beckman Coulter, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
MMP2, MMP9, cyclin D1, cyclin A2, cyclin B1, CDK1,
CDK2, CDK4, Bcl-2, p53, caspase-3, JAK1, JAK2 and STAT3) were
analyzed by RT-qPCR. In brief, cells (1×106 /ml),
subsequent to treatment with pPeOp (30, 60 or 90 µg/ml), PVP
(100 µg/ml) or 5-FU (100 µg/ml) under standard aforementioned
conditions for 24 h, were trypsinized and total RNA was isolated
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). cDNA was synthesized using a Maxima First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol, which was used as a template for qPCR.
Taq®PCR Master Mix (cat no. A6001; Promega Corporation)
for qPCR, and performed using a Step One Plus Real-Time PCR System
(Applied Biosystems; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. Data were normalized to the expression
level of a GAPDH reference gene. Fold-changes in the amplified cDNA
were calculated by the (2−ΔΔCq) method (16). Primer sequences used are presented in
Table I. All primers were purchased
from Sangon Biotech Co., Ltd. (Shanghai, China). RT-qPCR was
performed using an Eppendorf Realplex-4 Real Time PCR Machine
(Eppendorf, Hamburg, Germany). The PCR amplification conditions
were as follows: One cycle at 95°C pre-denaturing for 2 min,
followed by 40 cycles at 95°C denaturing for 15 sec, and annealing
temperature of 62.9°C (GAPDH, JAK1, JAK2, STAT3, caspase-3, p53,
Bcl-2), 57.8°C (MMP2, MMP9, cyclin A2, cyclin B1, cyclin D1) or
61°C (CDK1, CDK2, CDK4) for 1 min. At the end of the PCR cycle, a
dissociation curve was performed with StepOne™ Software (version
2.2.2; Applied Biosystems; Thermo Fisher Scientific, Inc.) to
confirm amplification of a single product. The results are
represented as the fold-change in gene expression relative to
GAPDH.
 | Table I.Specific primers used for polymerase
chain reaction analysis. |
Table I.
Specific primers used for polymerase
chain reaction analysis.
| Gene | Forward primer,
5′-3′ | Reverse primer,
5′-3′ | Ampl |
|---|
| GAPDH |
TGACTTCAACAGCGACACCCA |
CACCCTGTTGCTGTAGCCAAA | 121 |
| CDK1 |
TGCTGGGGTCAGCTCGTTACTCA |
TGGGATGCTAGGCTTCCTGGTT | 326 |
| CDK2 |
GTGGGCCCGGCAAGATTTTAG |
GCCGAAATCCGCTTGTTAGGG | 378 |
| CDK4 |
CTTGATCTGAGAATGGCTACCTCT |
CATGAAGGAAATCTAGGCCTCTTA | 682 |
| Cyclin D1 |
CATCTCTGTACTTTGCTTGCTCAT |
CGCTATTTCCTACACCTATTGGAC | 499 |
| Cyclin B1 |
TCGAGCAACATACTTTGG |
GCAAAAAGCTCCTGCTGC | 101 |
| Cyclin A2 |
AGACCCTGCATTTGGCTGTG |
ACAAACTCTGCTACTTCTGG | 1,110 |
| MMP2 |
TGATGGTGTCTGCTGGAAAG |
GACACGTGAAAAGTGCCTTG | 280 |
| MMP9 |
GGAGACCTGAGAACCAATCTC |
TCCAATAGGTGATGTTGTGG | 1,078 |
| Caspase-3 |
CATGGAAGCGAATCAATGGACT |
CTGTACCAGACCGAGATGTCA | 3,036 |
| p53 |
TAGTGTGGTGGTGCCCTATG |
CCAGTGTGATGATGGTGAGG | 699 |
| JAK1 |
CCTGCTGGTGGCTACTAAGA |
AGATGTGTGTTCTCGTGCCT | 3,262 |
| JAK2 |
GCCTTCTTTCAGAGCCATCA |
CCAGGGCACCTATCCTCATA | 1,148 |
| STAT3 |
GACATGGAGTTGACCTCGGAGTG |
GGTGGCAGAATGCAGGTAGGC | 103 |
| Bcl-2 |
GGATTGTGGCCTTCTTTGAG |
CCAAACTGAGCAGAGTCTTC | 234 |
Western blotting assay
MMP2, MMP9, cyclin D1, cyclin A2, cyclin B1, CDK1,
CDK2, CDK4, Bcl-2, p53, caspase-3, JAK1, JAK2 and STAT3 were
analyzed by western blot analysis. Cells (1×106),
subsequent to treatment with pPeOp (30, 60 or 90 µg/ml), PVP
(100 µg/ml) or 5-FU (100 µg/ml) under standard aforementioned
conditions for 24 h, were collected following treatment with PBS (1
ml, 30 min); centrifuged for 10 min at 200 × g and the supernatant
was discarded. The pellet was then incubated with 200 µl lysis
buffer (cat no. C0201; Beyotime Institute of Biotechnology,
Shanghai, China) for 30 min on ice, and agitated every 10 sec.
Lysates were separated using centrifugation at 4°C for 15 min at
10,000 × g, the supernatant was collected, and protein determined
by BCA Protein Assay kit (cat no. KGP902; Nanjing KeyGen Biotech
Co., Ltd., Nanjing, China). Protein (40 µg per lane) was loaded
onto a 12% standard polyacrylamide gel and resolved by SDS-PAGE.
Resolved proteins were subsequently transferred onto an
Immobilon®-P Transfer Membrane (cat no. IPVH00010; Merck
KGaA), saturated with 5% milk in Tris-buffered saline and 0.5%
Tween-20 (TBST) at 25°C for 2 h. Antibodies against JAK1 (cat no.
ab138005; 1:1,000 dilution), JAK2 (cat no. ab32101; 1:1,000
dilutions) and STAT3 (cat no. ab76315; 1:200,000 dilutions) were
purchased from Abcam (Cambridge, UK). Antibodies against MMP2 (cat
no. 13132; 1:1,000 dilutions), MMP9 (cat no. 3852; 1:1,000
dilutions), cyclin A2 (cat no. 4656; 1:2,000 dilutions), cyclin B1
(cat no. 4138; 1:1,000 dilutions), cyclin D1 (cat no. 2978; 1:1,000
dilutions), CDK1 (cat no. ab32384; 1:1,000 dilutions), CDK2 (cat
no. 2546; 1:1,000 dilutions), CDK4 (cat no. 12790; 1:1,000
dilutions), Bcl-2 (cat no. 2870; 1:1,000 dilutions), p53 (cat no.
2527; 1:1,000 dilutions) and caspase-3 (cat no. 14220; 1:1,000
dilutions) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-GAPDH (cat no. YM3029; 1:5,000 dilutions),
used as a control, was purchased from Immuno Way Biotechnology
Company (TX, USA). Horseradish peroxidase (HRP)-conjugated goat
anti-rabbit immunoglobulin G (cat no. A0208; 1:1,000 dilutions) or
goat anti-mouse immunoglobulin G (cat no. A0216;1: 1,000 dilutions)
were used as secondary antibodies. All secondary antibodies were
purchased from the Beyotime Institute of Biotechnology. The
membranes were incubated overnight with primary antibodies
(described above) at 4°C. Membranes were washed three times with
TBST. Membranes that were incubated with GAPDH antibodies were then
incubated with goat anti-mouse IgG secondary antibody (described
above), while membranes which were incubated with MMP2, MMP9,
cyclin D1, cyclin A2, cyclin B1, CDK1, CDK2, CDK4, Bcl-2, p53,
caspase-3, JAK1, JAK2 and STAT3 antibodies were then incubated with
goat anti-rabbit IgG HRP secondary antibodies (described above) for
2 h at 4°C, and washed three times with TBST. Membranes were
visualized on film in a dark room using ECL substrate solution (cat
no. P0018A; Beyotime Institute of Biotechnology) and results
quantified using ImageJ software (version 1.8.0; National
Institutes of Health, Bethesda, MA, USA).
Statistical analyses
All experiments were conducted in triplicate. Data
are presented as the mean ± standard deviation. SPSS software
(version 20.0; IBM Corp., Armonk, NY, USA) was used for statistical
analyses. The MTS cell viability data were subjected to one-way
analysis of variance (ANOVA), followed by Fisher's least
significant difference (LSD) post-hoc test. Flow cytometry-based
analyses were also analyzed using one-way ANOVA, followed by
Fisher's LSD. Western blot results were quantitated using Image J
software and analyzed by one-way ANOVA, followed by Fisher's LSD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of pPeOp on the viability of
SGC-7901 cells
Initially, the effect of pPeOp on the
proliferation of SGC-7901 gastric cancer cells was investigated.
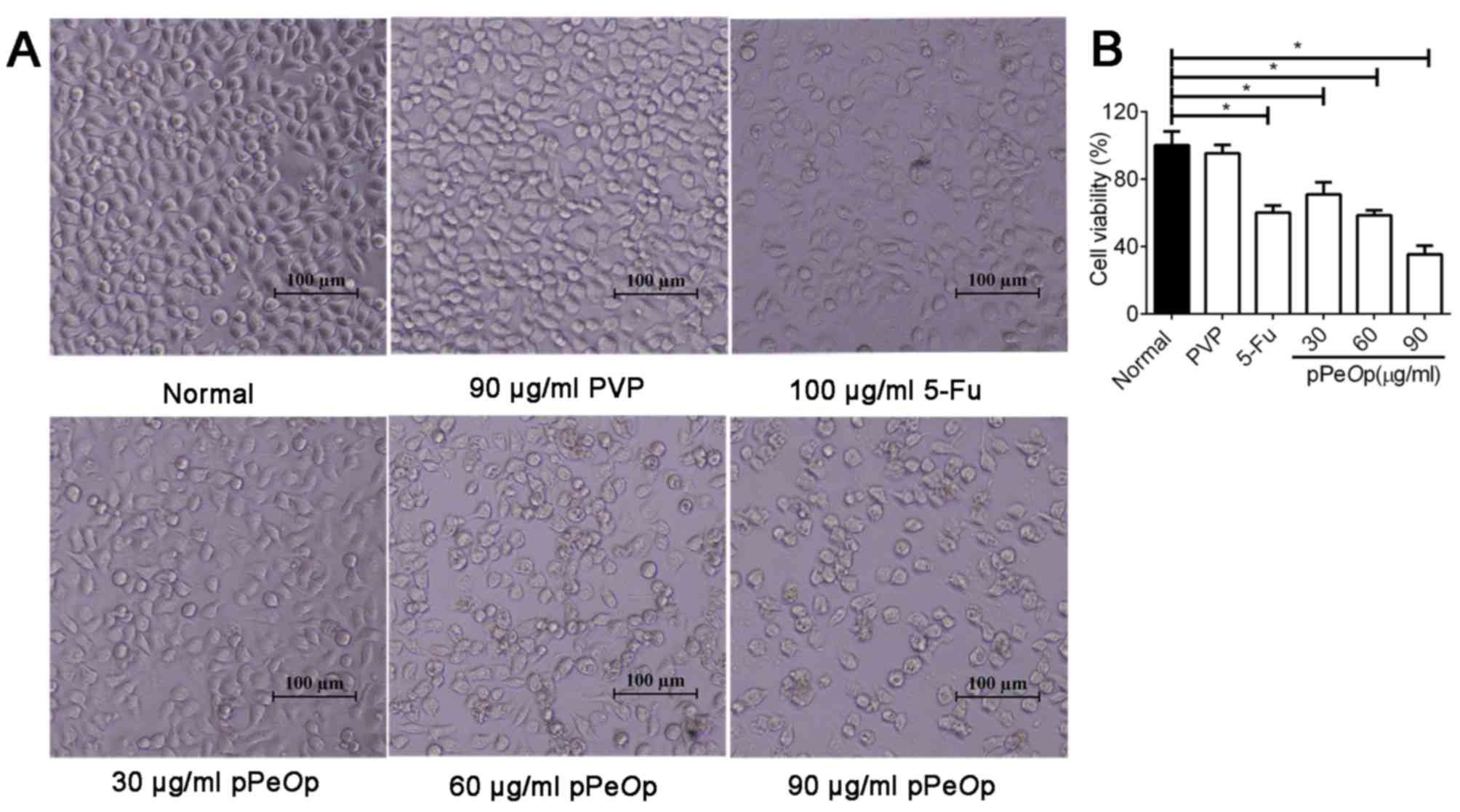
Cells treated with PVP (90 µg/ml, 24 h) maintained a normal
morphology comparable to that of untreated cells (Fig. 1). However, when SGC-7901 cells were
treated for 24 h with graded concentrations of pPeOp (30, 60
and 90 µg/ml), cells decreased in size and presented a spherical
appearance (Fig. 1A). In addition to
the morphological changes, the viability was also affected. The
survival rates of cells after 24 h of treatment were as follows:
PVP (100 µg/ml), 95.26±5.21%; 5-FU (100 µg/ml), 60.13±3.13%;
pPeOp (30 µg/ml), 70.97±6.18%; pPeOp (60 µg/ml),
58.44±5.74%; pPeOp (90 µg/ml) 35.22±2.33%. The half maximal
inhibitory concentration was 64.326 µg/ml. An increase in the
concentration of pPeOp resulted in a proportional and
significant increase in the rate of inhibition (P<0.05; Fig. 1B). These results suggested that
pPeOp inhibits the viability of SGC-7901 cells.
Effect of pPeOp on the migration of
SGC-7901 cells
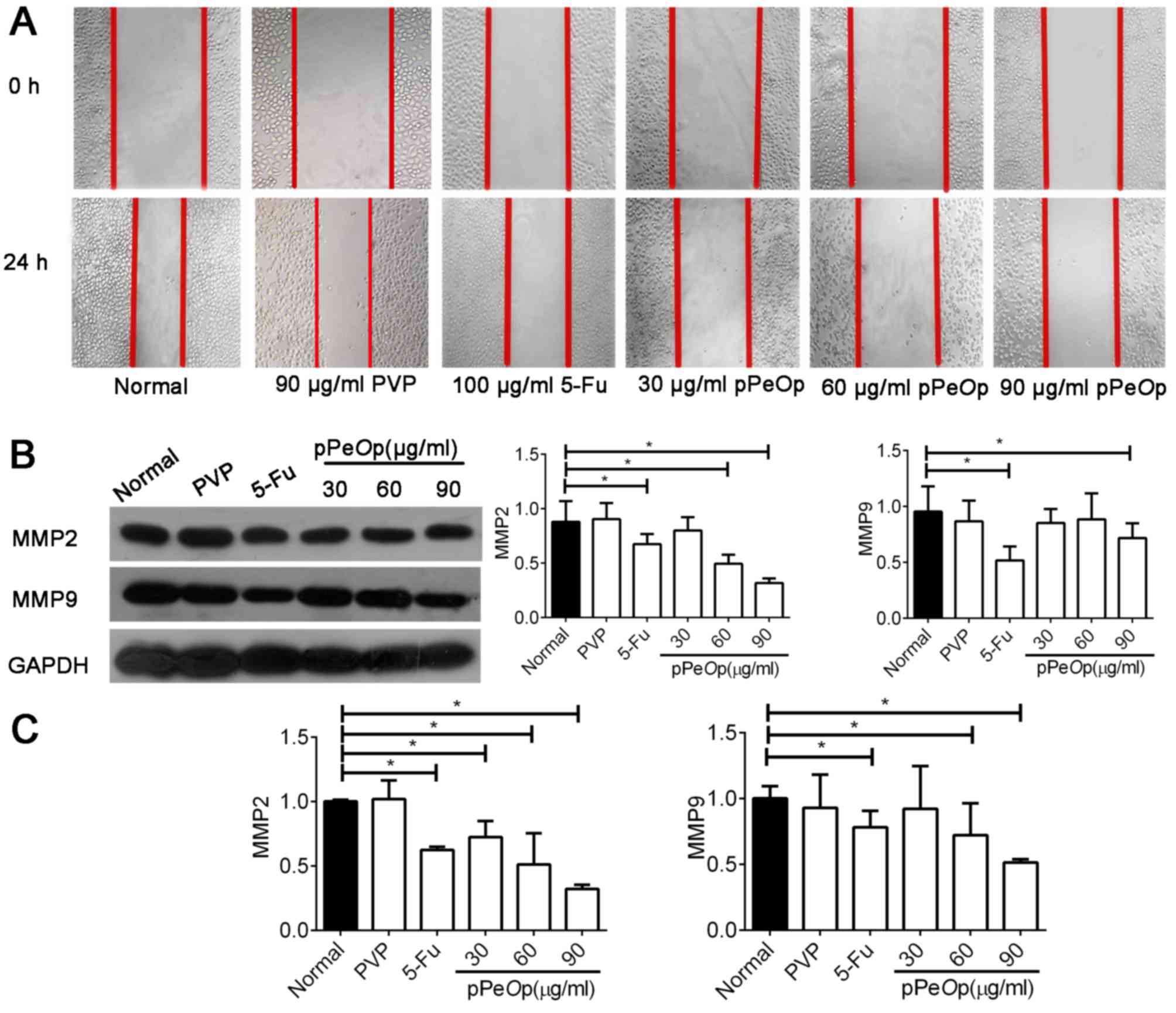
A wound healing assay was used to assess the
migration ability of SGC-7901 cells following treatment with
pPeOp. As presented in Fig.
2A, compared with the normal and negative normal treatments,
the migration ability of SGC-7901 cells was markedly decreased
following pPeOp treatment.
Invasion and metastasis of carcinomas are closely
associated with extracellular matrix (ECM) degradation (17,18). MMPs
are well characterized and demonstrate proteolytic activity in the
ECM, and have recently emerged as key molecules involved in
mediating tumor invasion and metastasis (19). Therefore, MMP2 and MMP9 were selected
for further studies. MMP2 and MMP9 levels were evaluated by RT-qPCR
and western blot analysis. The results demonstrated that MMP2 and
MMP9 were significantly downregulated at the mRNA and protein
levels compared with the normal control, in proportion to the
pPeOp dosage (P<0.05; Fig. 2B
and C). However, compared with the normal group, cells treated
with PVP did not show any significant difference in gene or protein
expression profiles (P>0.05; Fig. 2B
and C). Thus, the results suggest that pPeOp inhibits
the ability of SGC-7901 cells to migrate.
Effect of pPeOp on the cell cycle
progression of SGC-7901 cells
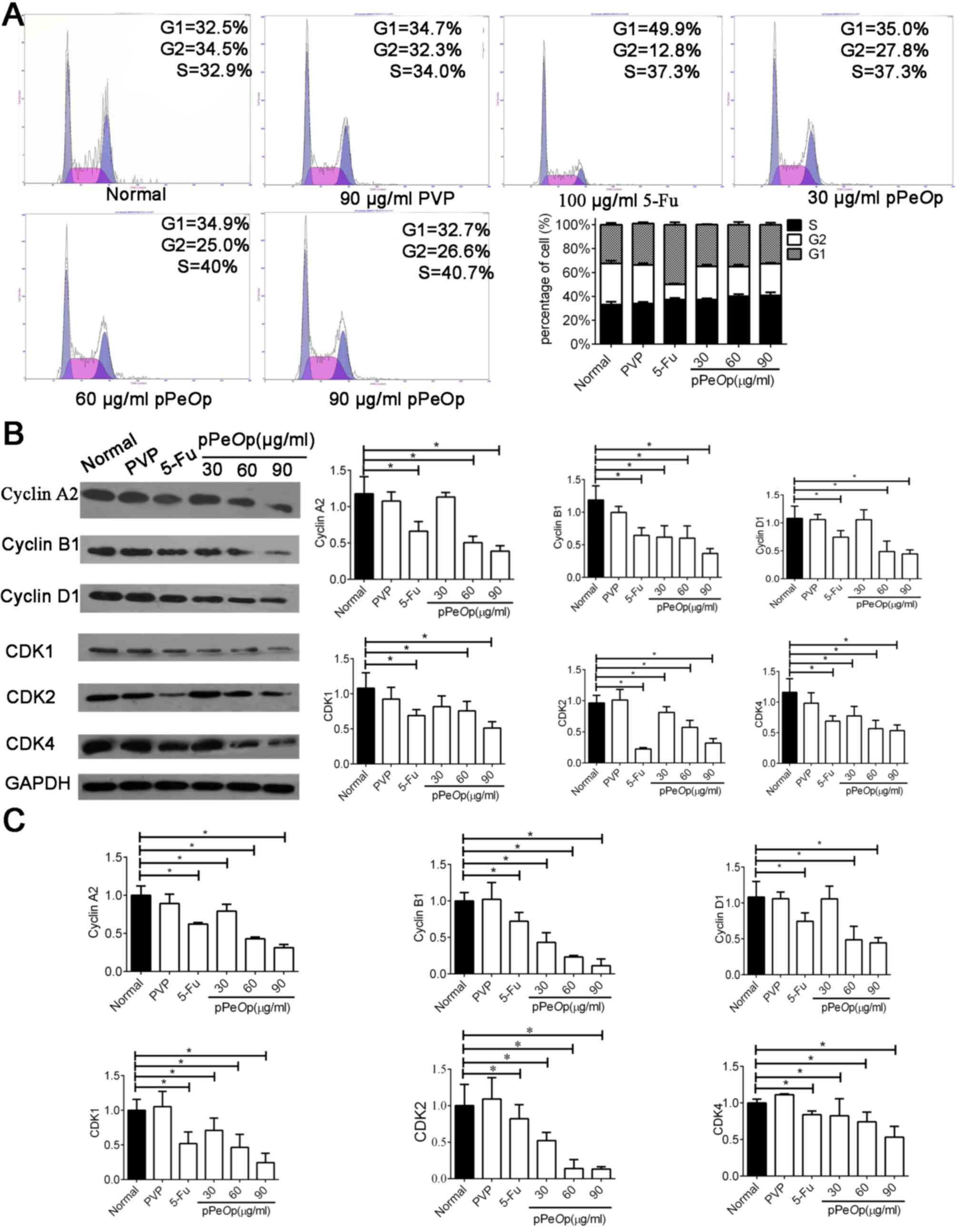
The influence pPeOp has on the progression of
SGC-7901 cells through the cell cycle was investigated using flow
cytometry of cells post-treatment with either normal/5-FU/PVP or
graded concentrations of pPeOp (Fig. 3A). The percentage of cells in G2/M
phases decreased, whereas the proportion of cells in G0/G1, S
phases increased in the pPeOp-treated group compared with
the control (P<0.05, Fig. 3A).
This suggests that pPeOp blocks DNA replication, thereby
arresting cells in the S phase.
To verify the data obtained by flow cytometry, how
pPeOp treatment influenced the expression of several cell
cycle regulators, including cyclin A2, cyclin B1, cyclin D1, CDK1,
CDK2, and CDK4 was investigated using RT-qPCR and western blot
analysis. The results indicated that protein and mRNA expression
levels of all six genes were significantly decreased post-treatment
with pPeOp compared with the normal control (P<0.05;
Fig. 3B and C). Furthermore, no
significant differences were observed between untreated cells and
cells treated with PVP (P>0.05; Fig.
3B and C). Collectively, these results suggest that
pPeOp arrests cell cycle progression of SGC-7901 cells.
Effect of pPeOp on the apoptosis of
SGC-7901 cells
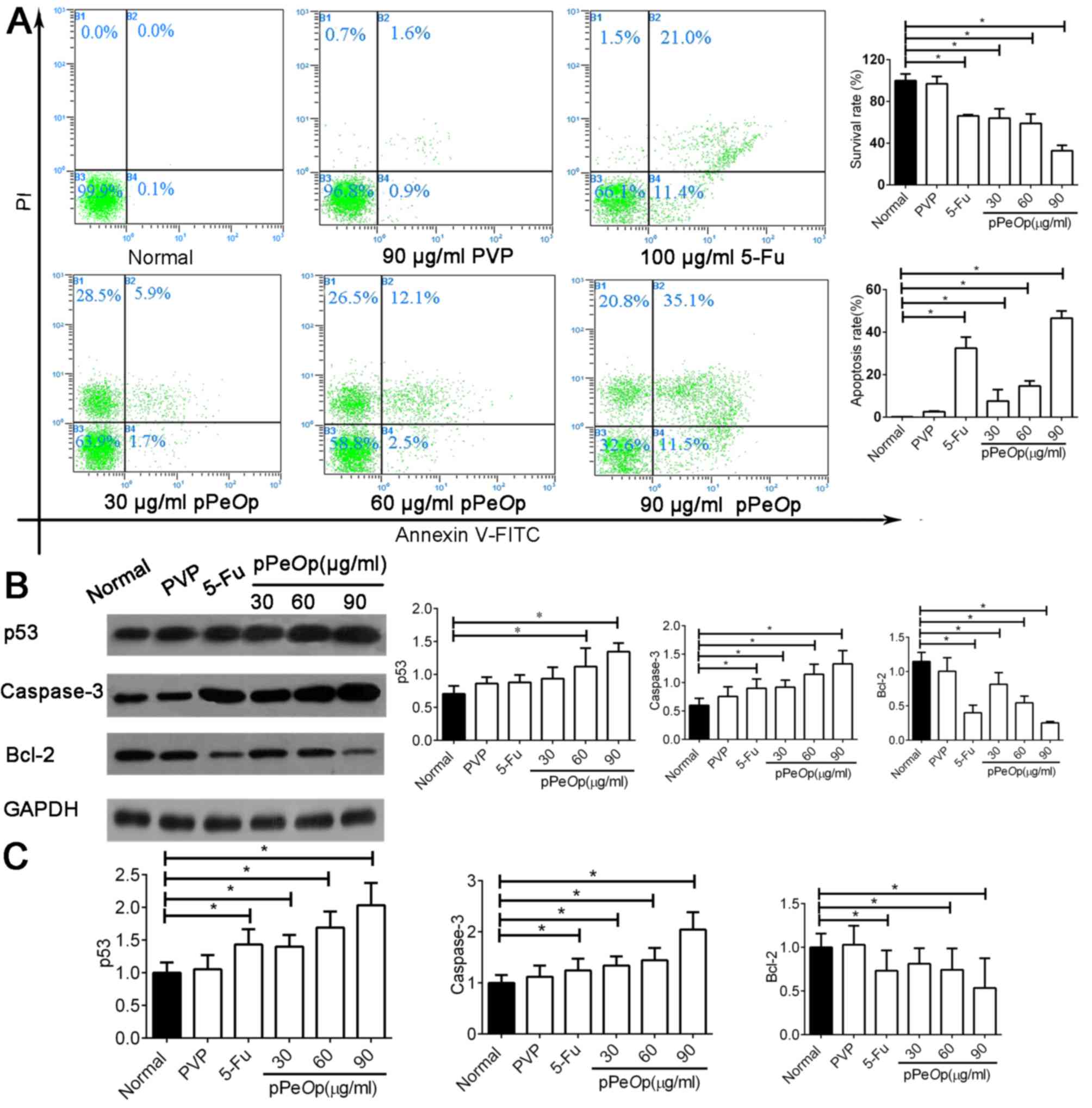
Flow cytometry was used to investigate how treatment
with normal/5-FU/PVP or varying dosages of pPeOp affects the
proportions of SGC-7901 cells undergoing apoptosis (Fig. 4A). The results demonstrated that
pPeOp treatment induced SGC-7901 cancer cells to undergo
apoptosis in a dose-dependent manner. Cells treated with 5-FU (100
µg/ml for 24 h) demonstrated a high rate of apoptosis (21%;
Fig. 4A). In addition, cells treated
with pPeOp (90 µg/ml for 24 h) experienced a markedly
increased rate of apoptosis (35.1%; Fig.
4A), surpassing that of 5-FU treatment.
Subsequently, the molecular events underlying this
modulation in apoptosis were investigated by determining the
expression of apoptosis-associated factors, namely caspase-3, p53
and Bcl-2, at the mRNA and protein levels. The results indicated
that pPeOp significantly upregulated the expression of
caspase-3 and p53, and markedly downregulated the expression of
Bcl-2 (P<0.05; Fig. 4B and C).
There was no significant difference in the expression of these
regulators between normal and PVP-treated cells (P>0.05;
Fig. 4B and C). These results
collectively demonstrate that pPeOp is effective for
inducing SGC-7901 cells to apoptosis, superseding even a
conventional chemotherapeutic agent.
Effect of pPeOp on the JAK-STAT
pathway
The aforementioned experiments highlight the effects
that pPeOp exhibits on a variety of important biological
programs, including cell proliferation, migration, apoptosis and
cell cycle progression. However, the mechanism underlying these
effects remains poorly understood. According to the results of a
microarray assay, numerous differentially expressed genes were
detected (data not shown). Following Kyoto Encyclopedia of Genes
and Genomes analysis, the JAK-STAT signaling pathway was the most
notable regulated signaling pathway influenced by pPeOp
proteins (data not shown). Therefore, it was hypothesized that
pPeOp may exert its anti-tumor effects through its
inhibition of the JAK-STAT signaling pathway in SGC-7901 human
gastric cells.
In order to investigate this hypothesis, SGC-7901
cells were treated with different concentrations of pPeOp
for 24 h and the expression of JAK1, JAK2 and STAT3 mRNA and
proteins were explored using RT-qPCR and western blotting. The
results demonstrated that the expression of JAK1, JAK2 and STAT3
mRNAs and proteins were significantly decreased post-treatment with
60 and 90 µg/ml pPeOp, compared with the control (P<0.05;
Fig. 5A and B). These results provide
evidence for the anti-tumor effects of pPeOp being
attributed to the inhibition of JAK-STAT signaling.
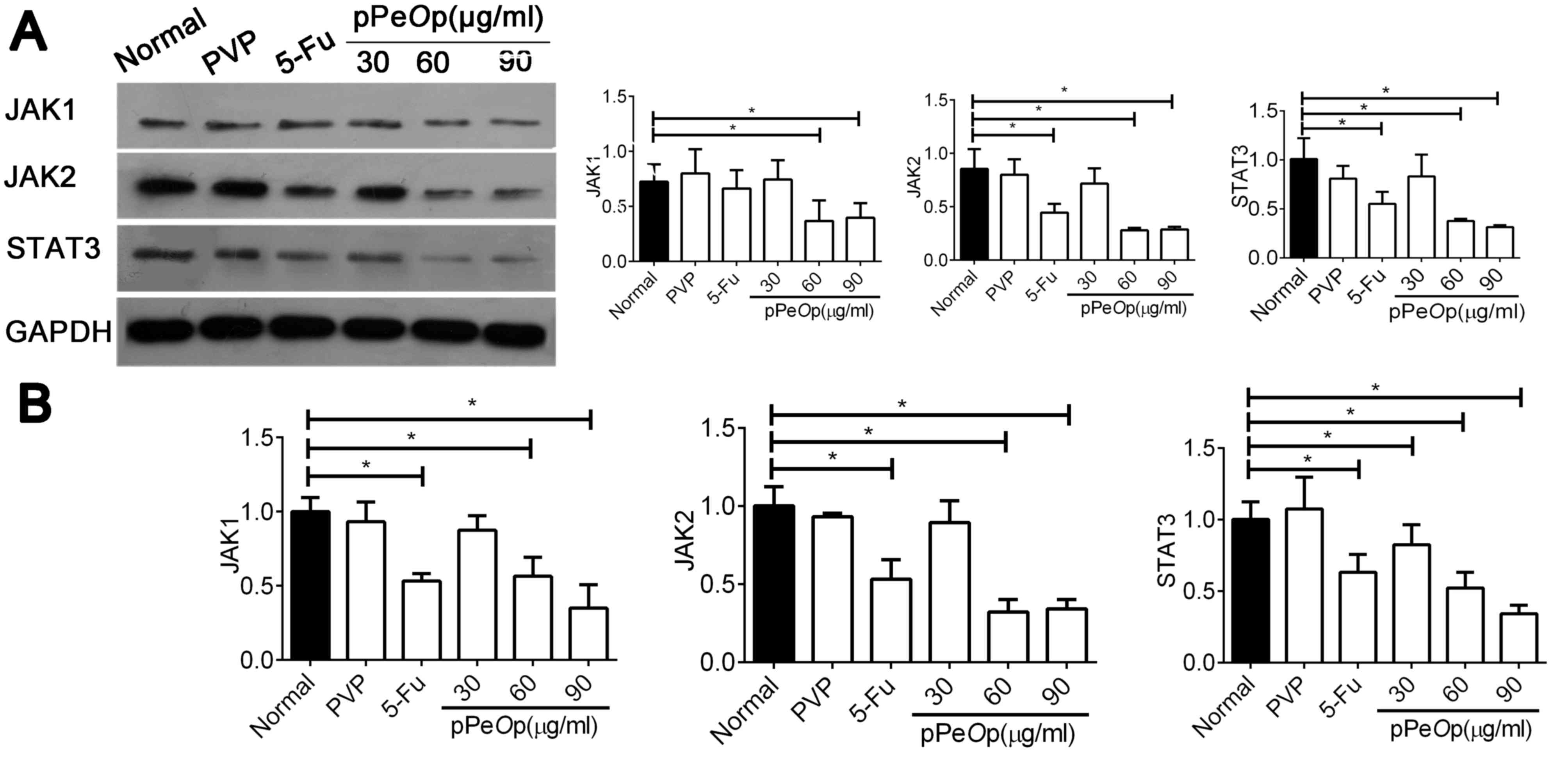 | Figure 5.pPeOp inhibited the JAK-STAT3
signaling pathway in SGC-7901 cells. (A) SGC-7901 cells treated
with 30, 60 and 90 µg/ml pPeOp, 90 µg/ml PVP or 100 µg/ml
5-FU for 24 h, data is presented as the mean ± standard deviation
for at least three independent experiments. Western blot analysis
was used to assess JAK1, JAK2 and STAT3 expression levels in
SGC-7901 cells. (B) Reverse transcription-quantitative polymerase
chain reaction was performed to evaluate JAK1, JAK2 and STAT3
expression levels in SGC-7901 cells. Data were normalized to the
Normal group. Quantification of mRNA levels was normalized to
GAPDH. *P<0.05 vs. Normal control. pPeOp, purified
Omphalia lapidescens protein; PVP, polyvinyl pyrrolidone;
5-FU, fluorouracil; JAK, Janus kinase; STAT, signal transducer and
activator of transcription. |
Discussion
Metastasis, the dissemination of cancer cells from
the primary site to distant sites, is the primary contributor to
the mortality of patients with cancer (20,21). The
ECM, which serves as a barrier for cell invasion, is breached by
MMPs in the evolution process of tumor metastasis (22). MMP9 and MMP2 have been reported to be
involved in cancer metastasis due to their ability to degrade type
IV collagen, a major component of the basement membrane (23). In the present study, the migration
ability of SGC-7901 cells was significantly decreased, whereas MMP2
and MMP9 were significantly downregulated at the mRNA and protein
levels in relation to the pPeOp dosage, compared with the
control group.
Dysregulation of cell cycle control is a major
hallmark underlying tumor growth and metastasis (24,25).
Cyclins are a family of regulators that control the progression of
the cell cycle through the activation of different CDKs. Cyclin D1,
cyclin A2 and cyclin B1 facilitate the cell's progression through
G0/G1, S and G2/M phases, respectively (26–28). In
the present study, pPeOp was demonstrated to arrest the cell
cycle in the S phase, and expression levels of cyclin A2, cyclin
B1, cyclin D1, CDK1, CDK2 and CDK4 were all decreased in SGC-7901
cells, compared with control group.
Apoptosis is an adenosine 5′triphosphate-dependent
and strictly regulated program, in which cells commit themselves to
physiological suicide (29).
Accordingly, apoptosis is essential for the elimination of excess,
redundant and otherwise unhealthy cells in the maintenance of
tissue homeostasis and stabilization (30,31). A
number of studies have reported that p53, which is described as a
‘tumor suppressor’, regulates a variety of apoptotic signals in
SGC-7901 cell (32,33). Bcl-2, an anti-apoptosis molecule,
serves a critical function in controlling apoptosis (34). Caspase-3, classified as an
executioner, mediates the final process of cell death (35). In the present study, pPeOp
treatment induced SGC-7901 cancer cells to undergo apoptosis in a
dose-dependent manner. Furthermore, the expression level of p53 and
caspase-3 were significantly upregulated, and Bcl-2 were
significantly downregulated, in SGC-7901 cells. In addition, the
apoptosis rate of SGC-7901 cells that were treated with 90 µg/ml
pPeOp group was increased compared with cells that were
treated with 100 µg/ml of the conventional chemotherapeutic agent
5-FU.
The JAK-STAT signaling pathway is abnormally
activated in gastric carcinoma, in which regulated gene expression
is involved in cell proliferation, cell cycle, invasion, metastasis
and survival (36–38). A constitutively active JAK-STAT
signaling pathway has been revealed to prevent apoptosis by
upregulating the expression of anti-apoptosis proteins, including
Bcl-2, Bcl-extra-large, myeloid cell leukemia 1 and surviving
(39–41). In addition, JAK-STAT has been
demonstrated to accelerate cell cycle progression by enhancing the
activity of cyclins (42,43). Masuda et al (44) and Sinibaldi et al (45) reported that inappropriate activation
of STAT3 may increase expression of cyclin D1 and subsequently
affect cell cycle progression. A number of studies have
demonstrated that the expression of activated STAT3 modulated
metastasis of tumor cells by promoting gene transcription of MMP2
and MMP9 (46,47). Therefore, inhibition of JAK-STAT is a
promising strategy for cancer therapy. In the present study, levels
of JAK1, JAK2, and STAT3 were significantly decreased at mRNA and
protein levels.
The results of the present study suggest that
pPeOp suppresses metastasis, arrests the cell cycle, induces
apoptosis and inhibits JAK-STAT signaling in SGC-7901 cells.
Therefore, pPeOp may be a promising agent for the treatment
of gastric cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation Project (grant no. 81374023),
the Zhejiang Provincial Natural Science Foundation (grant no.
Y207765) and the Zhejiang Provincial Medical and Health Science and
Technology Project (grant nos. 2015KYA038 and 2016KYA033).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han P, Wang QL and Zhang X: Expression of
TRAP1 in gastric cancer tissue and its correlation with malignant
biology. Asian Pac J Trop Med. 9:67–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choi HJ, Ki CS, Suh SP and Kim JW:
Presymptomatic identification of CDH1 germline mutation in a
healthy korean individual with family history of gastric cancer.
Ann Lab Med. 34:386–389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma B, Xu Q, Song Y, Gao P and Wang Z:
Current issues of preoperative radio(chemo)therapy and its future
evolution in locally advanced rectal cancer. Future Oncol.
13:2489–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robertson-Tessi M, Gillies RJ, Gatenby RA
and Anderson AR: Impact of metabolic heterogeneity on tumor growth,
invasion, and treatment outcomes. Cancer Res. 75:1567–1579. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ben Nasr S, Ayadi M, Bahloul R, Guesmi S,
Allani B, Chrait N, Rifi H, Rais H and Mezlini A: Perioperative
chemotherapy in locally advanced gastric cancer. A retrospective
study about 25 cases. Tunis Med. 93:228–230. 2015.PubMed/NCBI
|
|
8
|
Peters GJ, Backus HH, Freemantle S, van
Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J,
Calvert AH, Marsh S, et al: Induction of thymidylate synthase as a
5-fluorouracil resistance mechanism. Biochim Biophys Acta.
1587:194–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng H, Peng N, Yu M, Sun X, Ma Y, Yang G
and Wang X: Treatment of triple-negative breast cancer with Chinese
herbal medicine: A prospective cohort study protocol. Medicine
(Baltimore). 96:e84082017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai YT, Lai JN, Lo PC, Chen CN and Lin
JG: Prescription of Chinese herbal products is associated with a
decreased risk of invasive breast cancer. Medicine (Baltimore).
96:e79182017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Liang Y and He C: Anticancer
activities and mechanisms of heat-clearing and detoxicating
traditional Chinese herbal medicine. Chin Med. 12:202017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rashid N, Koh HA, Baca HC, Lin KJ, Malecha
SE and Masaquel A: Economic burden related to chemotherapy-related
adverse events in patients with metastatic breast cancer in an
integrated health care system. Breast Cancer (Dove Med Press).
8:173–181. 2016.PubMed/NCBI
|
|
13
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohno N, Miura T, Saito K, Nishijima M,
Miyazaki T and Yadomae T: Physicochemical characteristics and
antitumor activities of a highly branched fungal
(1–3)-beta-D-glucan, OL-2, isolated from Omphalia lapidescens. Chem
Pharm Bull (Tokyo). 40:2215–2218. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen YT, Lu QY, Lin MA, Cheng DQ, Ding ZS
and Shan LT: A PVP-extract fungal protein of Omphalia lapideacens
and its antitumor activity on human gastric tumors and normal
cells. Oncol Rep. 26:1519–1526. 2011.PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouyang J, Pan X, Lin H, Hu Z, Xiao P and
Hu H: GKN2 increases apoptosis, reduces the proliferation and
invasion ability of gastric cancer cells through down-regulating
the JAK/STAT signaling pathway. Am J Transl Res. 9:803–811.
2017.PubMed/NCBI
|
|
18
|
Zhang Y, Pan T, Zhong X and Cheng C:
Androgen receptor promotes esophageal cancer cell migration and
proliferation via matrix metalloproteinase 2. Tumour Biol.
36:5859–5864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dou CY, Cao CJ, Wang Z, Zhang RH, Huang
LL, Lian JY, Xie WL and Wang LT: EFEMP1 inhibits migration of
hepatocellular carcinoma by regulating MMP2 and MMP9 via ERK1/2
activity. Oncol Rep. 35:3489–3495. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tomizawa M, Shinozaki F, Motoyoshi Y,
Sugiyama T, Yamamoto S and Ishige N: 2-Deoxyglucose and sorafenib
synergistically suppress the proliferation and motility of
hepatocellular carcinoma cells. Oncol Lett. 13:800–804. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma J, Fu G, Wu J, Han S, Zhang L, Yang M,
Yu Y, Zhang M, Lin Y and Wang Y: 4-cholesten-3-one suppresses lung
adenocarcinoma metastasis by regulating translocation of HMGB1,
HIF1α and caveolin-1. Cell Death Dis. 7:e23722016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edatt L, Haritha K, Sruthi TV, Aswini P
and Sameer Kumar VB: 2-Deoxy glucose regulate MMP-9 in a SIRT-1
dependent and NFkB independent mechanism. Mol Cell Biochem.
423:197–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Radunovic M, Nikolic N, Milenkovic S,
Tomanovic N, Boricic I, Dimitrijevic M, Novakovic I and
Basta-Jovanovic G: The MMP-2 and MMP-9 promoter polymorphisms and
susceptibility to salivary gland cancer. J BUON. 21:597–602.
2016.PubMed/NCBI
|
|
24
|
Xu W and McArthur G: Cell cycle regulation
and melanoma. Curr Oncol Rep. 18:342016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hartwell LH and Kastan MB: Cell cycle
control and cancer. Science. 266:1821–1828. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen X, Wu Z, Chen S, Chen Y, Xia J, Lv Y
and Zhou Y: Induction of G2/M phase arrest and apoptosis by ZGDHU-1
in A549 and RERF-LC-MA lung cancer cells. Oncol Lett. 12:989–994.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shimura T, Fukumoto M and Kunugita N: The
role of cyclin D1 in response to long-term exposure to ionizing
radiation. Cell Cycle. 12:2738–2743. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loukil A, Cheung CT, Bendris N, Lemmers B,
Peter M and Blanchard JM: Cyclin A2: At the crossroads of cell
cycle and cell invasion. World J Biol Chem. 6:346–350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li YL, Zhang J, Min D, Hongyan Z, Lin N
and Li QS: Anticancer effects of
1,3-dihydroxy-2-methylanthraquinone and the ethyl acetate fraction
of hedyotis diffusa willd against HepG2 carcinoma cells mediated
via apoptosis. PLoS One. 11:e01515022016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamm I, Schriever F and Dörken B:
Apoptosis: Implications of basic research for clinical oncology.
Lancet Oncol. 2:33–42. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Azadmehr A, Hajiaghaee R, Baradaran B and
Haghdoost-Yazdi H: Apoptosis cell death effect of scrophularia
variegata on breast cancer cells via mitochondrial intrinsic
pathway. Adv Pharm Bull. 5:443–446. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhu BS, Zhao K, Jia X, Wu YY and Xing CG:
Effects of damage-regulated autophagy regulator gene on the SGC7901
human gastric cancer cell line. Oncol Lett. 8:657–662. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao K, Zhu BS, Gong W, Zhu ML, Gao ZT, Wu
YY, Chen Q, Yang XD and Xing CG: SN50 enhances the effects of
LY294002 on cell death induction in gastric cancer cell line
SGC7901. Arch Med Sci. 9:990–998. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu G, Xiang T, Wu QF and Wang WX:
Curcumin suppresses the proliferation of gastric cancer cells by
downregulating H19. Oncol Lett. 12:5156–5162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Xu J, Wang X and Yuan G: Protective
effect of ginsenoside Rg1 on lidocaine-induced apoptosis. Mol Med
Rep. 9:395–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu B, Lu Y, Li J, Liu Y, Liu J and Wang
W: Leukemia inhibitory factor promotes tumor growth and metastasis
in human osteosarcoma via activating STAT3. APMIS. 123:837–846.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yeh JE and Frank DA: STAT3-interacting
proteins as modulators of transcription factor function:
Implications to targeted cancer therapy. ChemMedChem. 11:795–801.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brambilla L, Genini D, Laurini E, Merulla
J, Perez L, Fermeglia M, Carbone GM, Pricl S and Catapano CV:
Hitting the right spot: Mechanism of action of OPB-31121, a novel
and potent inhibitor of the signal transducer and activator of
transcription 3 (STAT3). Mol Oncol. 9:1194–1206. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Khanna P, Chua PJ, Bay BH and Baeg GH: The
JAK/STAT signaling cascade in gastric carcinoma (Review). Int J
Oncol. 47:1617–1626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mukherjee A and Khuda-Bukhsh AR: Quercetin
down-regulates IL-6/STAT-3 signals to induce mitochondrial-mediated
apoptosis in a nonsmall-cell lung-cancer cell line, A549. J
Pharmacopuncture. 18:19–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yu RX, Hu XM, Xu SQ, Jiang ZJ and Yang W:
Effects of fucoxanthin on proliferation and apoptosis in human
gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway.
Eur J Pharmacol. 657:10–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Huang C and Xie K: Crosstalk of Sp1 and
Stat3 signaling in pancreatic cancer pathogenesis. Cytokine Growth
Factor Rev. 23:25–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kijima T, Niwa H, Steinman RA, Drenning
SD, Gooding WE, Wentzel AL, Xi S and Grandis JR: STAT3 activation
abrogates growth factor dependence and contributes to head and neck
squamous cell carcinoma tumor growth in vivo. Cell Growth Differ.
13:355–362. 2002.PubMed/NCBI
|
|
44
|
Masuda M, Suzui M, Yasumatu R, Nakashima
T, Kuratomi Y, Azuma K, Tomita K, Komiyama S and Weinstein IB:
Constitutive activation of signal transducers and activators of
transcription 3 correlates with cyclin D1 overexpression and may
provide a novel prognostic marker in head and neck squamous cell
carcinoma. Cancer Res. 62:3351–3355. 2002.PubMed/NCBI
|
|
45
|
Sinibaldi D, Wharton W, Turkson J, Bowman
T, Pledger WJ and Jove R: Induction of p21WAF1/CIP1and cyclin D1
expression by the Src oncoprotein in mouse fibroblasts: Role of
activated STAT3 signaling. Oncogene. 19:5419–5427. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ghosh A, Pechota A, Coleman D, Upchurch GR
Jr and Eliason JL: Cigarette smoke-induced MMP2 and MMP9 secretion
from aortic vascular smooth cells is mediated via the Jak/Stat
pathway. Hum Pathol. 46:284–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang F, Wang Z, Fan Y, Xu Q, Ji W, Tian R
and Niu R: Elevated STAT3 signaling-mediated upregulation of
MMP-2/9 confers enhanced invasion ability in multidrug-resistant
breast cancer cells. Int J Mol Sci. 16:24772–24790. 2015.
View Article : Google Scholar : PubMed/NCBI
|