Introduction
In order to improve the prognosis of patients with
head and neck cancer (HNC), it is essential to accurately diagnose
cervical lymph node metastases prior to the initiation of treatment
(1,2).
Although computed tomography (CT), magnetic resonance imaging (MRI)
and positron emission tomography (PET) may be used to identify
metastatic lymph nodes, the nodes can be difficult to detect,
particularly those that measure <10 mm in diameter (2). Ultrasonography (US) may be used to
evaluate lymph nodes that are <10 mm in diameter. The clinical
criteria currently being used to diagnose metastatic cervical lymph
nodes by ultrasonography are an increase in nodal size and cortical
thickness, a change in nodal shape, infiltration of surrounding
structures, the presence of inhomogeneous internal echo patterns
(including necrosis), the absence of echo-rich hilar structures and
extracapsular spread (3,4).
It has previously been demonstrated that lymph nodes
undergoing metastatic change experience an increase in blood vessel
volume and density prior to experiencing a change in nodal size
(5). Ultrasonic color Doppler imaging
is able to detect blood flow in and around lymph nodes but is
unable to delineate the blood vessels themselves (6).
Sonazoid™, a lipid-stabilized suspension of
perfluorocarbon microbubbles, was used in the present study as an
ultrasound contrast agent. In Japan, contrast-enhanced
ultrasonography (CEUS) with Sonazoid™ is used for the evaluation of
liver and mammary gland tumors (7,8). It was
hypothesized that it would also be possible to evaluate changes in
lymph node blood vessel volume and density using CEUS with
Sonazoid™. The objective of the present study was to evaluate the
usefulness of CEUS with Sonazoid™ for identifying metastatic
cervical lymph nodes in patients with HNC. Since enhancement of
lymph nodes occurs so rapidly and the time to peak is very short,
software capable of analyzing CEUS images of lymph nodes did not
previously exist. Thus, novel analysis software was developed by
the authors of the present study (9),
and its usefulness was evaluated in the present study. Another
problem faced was that adjustment of the scanning plane during
evaluation of the head and neck region was difficult. This also
occurred during evaluation of the sectioned surfaces of the
surgical specimens, although to a lesser degree. A novel technique
will be required to improve visualization of these areas. In the
present study, intra-operative US was performed to assist in the
acquisition of matched samples used for CEUS and histopathological
examination. The association between images obtained via CEUS and
histopathology performed on surgical specimens taken from
metastatic cervical lymph nodes was evaluated.
Materials and methods
Patients
Patients with histologically proven head and neck
squamous cell carcinoma who were treated at Iwate Medical
University Hospital (Morioka, Japan) between February and June 2016
were enrolled in the present study. Patients with untreated lymph
node metastasis diagnosed by a combination of physical examination,
CT scan, MRI and fluorodeoxyglucose (FDG)-PET scan met the
inclusion criterion. A total of five patients underwent surgery
including neck dissection.
The present study was approved by the review board
of Iwate Medical University Hospital and written informed consent
was obtained from each patient. All procedures followed were in
accordance with the ethical standards of The Responsible Committee
on Human Experimentation (institutional and national) and with The
Declaration of Helsinki (1975), as revised in 2013 (10).
Among the five patients, primary tumors were
detected in the oral cavity in two patients. Primary tumors were
also detected in the hypopharynx for two patients and in the larynx
for one patient (Table I). The
N-classification was N1 for one patient and N2b for four of the
patients, according to the TNM staging system (11). The ages of the patients ranged from 65
to 75 years (mean, 71.0 years, median, 72 years), and all patients
were male (Table I).
 | Table I.Profiles of the patients and lymph
nodes of interest. |
Table I.
Profiles of the patients and lymph
nodes of interest.
| Patient | Age | Gender | Primary tumor | Stagea | Lymph node of
interest | Major axis, mm | Minor axis, mm | Thickness, mm |
|---|
| 1 | 71 | M | Larynx | T2N2bM0 | Left II | 15 | 11 | 11 |
| 2 | 75 | M | Hypopharynx | T3N2bM0 | Left III | 24 | 21 | 19 |
| 3 | 72 | M | Hypopharynx | T4aN2bM0 | Right III | 20 | 10 | 10 |
| 4 | 65 | M | Tongue | rN1 | Left II | 31 | 31 | 19 |
| 5 | 72 | M | Floor of the
mouth | TN2bM0 | Left I | 31 | 15 | 10 |
Ultrasound imaging
Conventional US and CEUS were performed using a
LOGIQ E9 general imaging system (GE Healthcare, Chicago, IL, USA)
and a high-frequency probe (6–15 MHz) for the evaluation of the
cervical lymph nodes. In each patient, one cervical lymph node
suspected of having metastatic disease was selected as the lymph
node of interest prior to surgery. During neck dissection, the
probe was placed directly on the lymph node of interest and B-mode
data were obtained. Sonazoid™ was then injected into a peripheral
vein, and the probe was replaced. Video was obtained from the hard
disc drive of LOGIQ E9 for ~1 min following administration of the
contrast agent. During the ultrasonographic examination, the
mechanical index was set at 0.21, and the frame rate at 19 frames
per sec. The focus was on the lymph node of interest. The imaging
conditions were kept constant. The examinations using B-mode
ultrasound and CEUS were performed by the same investigator, and
all data were digitally recorded. The lymph nodes of interest were
analyzed using CEUS intraoperatively, and surgical specimens
obtained from these nodes were examined histopathologically. The
lymph nodes were carefully removed to allow an accurate match
between the cut surface of the surgical specimen and the section
examined via ultrasound.
Contrast agent for
ultrasonography
Sonazoid™ (Daiichi-Sankyo, Co., Ltd., Tokyo, Japan)
is a lyophilized preparation containing 16 µl perfluorobutane
microbubbles per vial that are reconstituted for injection. The
contents of each vial were resuspended in 2 ml of sterile water for
injection. For patients with liver or mammary gland tumors, a
single injection of 0.12 µMB/kg of the reconstituted suspension was
administered in a cubital vein. Previous research has identified
that similar enhancement effects can be obtained in cases of
metastatic HNC involving the cervical lymph nodes using a dose of
0.012 µMB/kg (data not shown). In the present study, an initial
dose of 0.012 µMB/kg was administered via a cubital vein
intraoperatively from an 18G needle followed by 10 ml saline
flush.
Analysis software
A novel image-analysis software (IwmUltrasonic;
version 2.0; Ditect Co., Ltd., Tokyo, Japan) was developed based on
an algorithm previously reported (9).
This software analyzes the video files obtained with CEUS. Visible
particles were used to draw accumulated color points revealing the
distribution of capillary vessels. This software is able to
simultaneously calculate the density of particles in the drawn
figures, which is hypothesized to represent the capillary density
of the lymph nodes.
Histopathological examination
Tissues were fixed in 10% formalin at room
temperature and embedded in paraffin wax. Sections were cut from
the paraffin blocks to a 3 µm thickness and mounted on
poly-Llysine-coated glass slides (Matsunami Glass Ind., Ltd.,
Tokyo, Japan). Paraffin sections (3-µm) were routinely dewaxed at
37°C for 7 h and rehydrated using 70–100% alcohol solution (70; 90;
95; 100; 100; 100%), 90 min for each interval according to routine
procedures (12). The first sections
were stained at room temperature for 40 min using hematoxylin and
eosin (100% Carrazi's hematoxylin and 0.5% eosin solution). The
second sections were stained with mouse anti-CD34 monoclonal
antibody (cat. no., IR632; ready-to-use; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) in order to make a
comparison between morphology and CD34 immunoreactivity, then
subjected to heat-induced epitope retrieval in High pH Target
Retrieval solution (Dako; Agilent Technologies, Inc.). The slides
were placed in 0.03% peroxidase-blocking reagent (Dako; Agilent
Technologies, Inc.) at room temperature for 5 min to inhibit
non-specific binding. Following primary antibody incubation at 97°C
for 20 min (mouse anti-CD34 monoclonal antibody; dilution,
ready-to-use; cat. no. IR632; Dako; Agilent Technologies, Inc.),
sections were analyzed using the EnVision™ HRP detection system
(cat. no. K8000, Dako; Agilent Technologies, Inc.) according to the
protocol of the manufacturer. The antigen-antibody complex was
visualized with DAB+ liquid chromogen (Dako; Agilent Technologies,
Inc.) and counterstained at room temperature for 2 min with 100%
Carrazi's hematoxylin prior to mounting.
Results
A newly developed image-analysis software was
utilized to construct images from a microbubble contrast agent,
allowing visualization of vessel distribution and density in
metastatic lymph nodes from patients with HNC. A typical result is
presented in Fig. 1.
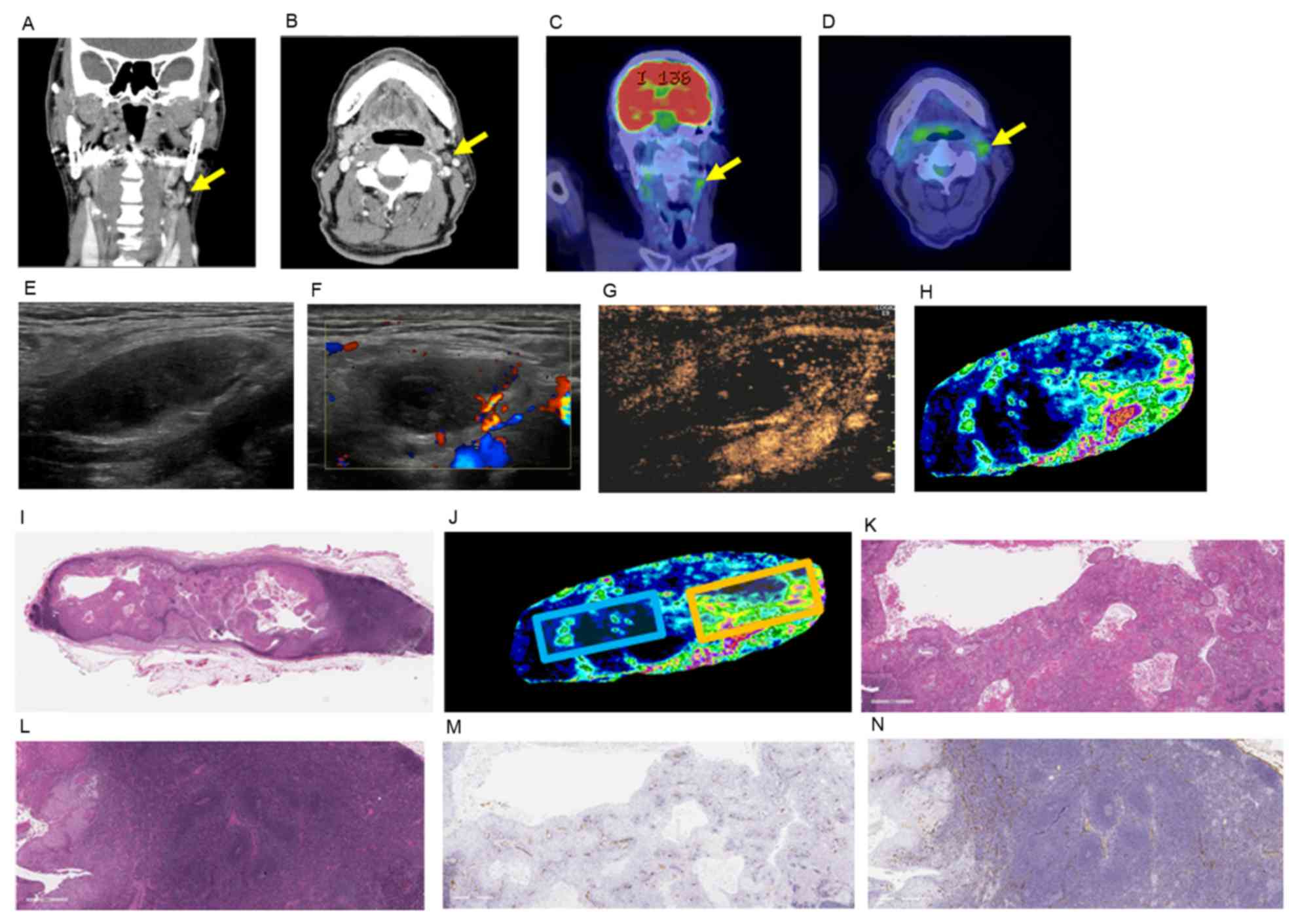 | Figure 1.Histopathological findings, clinical
and ultrasound images of case 5. Enhanced CT images in the (A)
coronal view and (B) axial view of the lymph node of interest.
Fluorodeoxyglucose-positron emission tomography/CT images in the
(C) coronal view and (D) axial view of the lymph node of interest.
The yellow arrows indicate the lymph node of interest. (E)
Ultrasound B-mode image of US including the major axis of the lymph
node of interest. (F) Color Doppler image of the lymph node of
interest. (G) CEUS and (H) analyzed CEUS images of the lymph node
of interest. (I) H&E staining of the lymph node of interest
including the major axis. Scale bar=4 mm. (J) The same image as H
indicating the selected area of interest. Blue square, area of low
contrast enhancement; orange square, area of high contrast
enhancement. H&E staining of (K) area of low contrast
enhancement and (L) area of high contrast enhancement. CD34
immunohistochemical staining of (M) area of low contrast
enhancement and (N) area of high contrast enhancement. Original
magnification, ×25 and scale bars=800 mm (K, L, M, and N). CT,
computed tomography; CEUS, contrast enhanced ultrasonography; CD34,
cluster of differentiation 34; H&E, hematoxylin and eosin. |
First, the internal structures of the metastatic
cervical lymph nodes of the patients with HNC were compared. A
total of 5 patients who underwent tumor resection with concomitant
neck dissection as an initial treatment and whose lymph nodes were
diagnosed as metastatic using CT, MRI or PET were enrolled in the
present study. The profiles of the patients are presented in
Table I. During neck dissection,
video files of the metastatic lymph nodes were obtained using CEUS,
and these images were subjected to analysis.
Typical results are presented in Fig. 1. Contrast-enhanced CT images (Fig. 1A and B) and FDG-PET images (Fig. 1C and D) identified a candidate lymph
node in case 5. Ultrasound images obtained from the same lymph node
using B-mode ultrasound, color Doppler, CEUS and CEUS with analysis
software can be compared in Fig.
1E-H. In the B-mode image (Fig.
1E), the fatty hilum and internal structure of the lymph node
are not clear. The CEUS mode image obtained using a microbubble
contrast agent is presented in Fig.
1G, and the CEUS mode image analyzed by the software is shown
in Fig. 1H. A comparison between
Fig. 1F and H identifies that color
Doppler provides only a rough sketch of the capillary vessels
present in lymph nodes, whereas the analyzed CEUS images depicts
micro-vascular patterns in the lymph node in greater detail.
Fig. 1I demonstrates the H&E
pattern of the plane including the major axis of the lymph node of
interest. In Fig. 1J, an area of high
contrast enhancement (orange square) and an area of low contrast
enhancement (blue square) were selected, and these areas (Fig. 1K-N) were examined by H&E (Fig. 1K and L) and CD34 staining (Fig. 1M and N). As indicated in Fig. 1L and N, the normal lymph follicles
were preserved, and around these lymph follicles thick capillary
vessels were present. Thin capillary vessels were prevalent in the
border of the tumor and normal lymph follicles. There were several
thin capillary vessels including a few with no lumen in the area of
low contrast enhancement (Fig. 1K and
M).
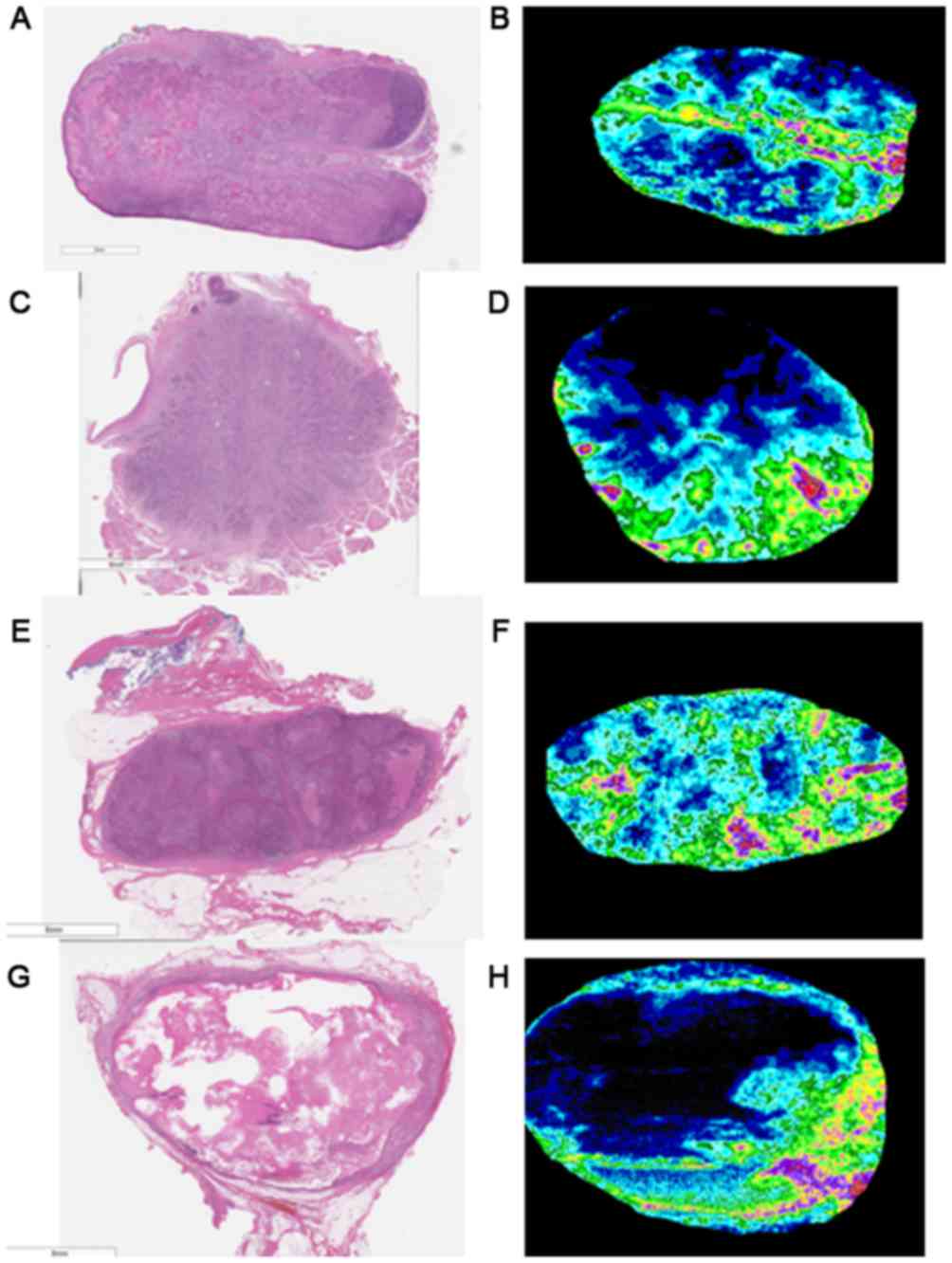
Histopathological images of lymph nodes and analyzed
CEUS images from cases 1–4 are presented in Fig. 2, and the characteristic features of
these metastatic lymph nodes are presented in Table II. Micronecroses were observed in the
lymph nodes from cases 3 and 5, and major necrosis was observed in
the lymph node from case 4. Non-metastatic normal lymph follicles
were observed in cases 1 and 5. Although histopathological
examination confirmed the presence of capillary vessels in lymph
nodes, color Doppler imaging was not able to detect them in two
cases. The analyzed CEUS image was successful in detecting those
same capillary vessels. However, a number of perfusion deficits
were observed in analyzed CEUS images of lymph nodes, suggesting
the presence of perfusion blocking factors in metastatic lymph node
tissue.
 | Table II.Histopathological and ultrasonography
image data of the lymph nodes. |
Table II.
Histopathological and ultrasonography
image data of the lymph nodes.
| Patient | Necrosis | Normal lymph
follicles | Color Doppler
imaging-matched capillary vessels | CEUS-matched
capillary vessels | Perfusion defect |
|---|
| 1 | − | + | + | ++ | ++ |
| 2 | − | − | − | ++ | + |
| 3 | + | − | + | ++ | + |
| 4 | +++ | − | − | ++ | − |
| 5 | + | + | + | ++ | ++ |
Discussion
Metastasis to cervical lymph nodes has previously
been identified using imaging modalities including CT, MRI and
FDG-PET. However, the diagnostic power of these imaging modalities
is limited when the lymph node is small, particularly when the
diameter is <10 mm and when characteristic features including
necrosis and calcification are missing (13). Ultrasound is readily available and its
power of resolution is greater compared with both CT and MRI
(3).
Ultrasound examination used to identify cervical
lymph node metastasis in patients with HNC is able to detect a
shift in or disappearance of the hilum of lymph nodes, an increase
in size of the nodes and central necrosis (3,4). However,
no specific diagnostic criteria have been established. Color
Doppler imaging is used to detect vessel flow in and around lymph
nodes, but its accuracy has not been investigated. In the present
study, the accuracy of color Doppler imaging was identified to be
inferior compared with CEUS imaging (Fig.
1).
In mouse models, it has been reported that an
increase in blood vessel volume and density precedes an increase in
lymph node size in the early stages of lymph node metastasis
(5). It has been hypothesized that
detection of these changes by CEUS may allow early diagnosis of
lymph node metastasis. This information may be applied to early
detection of cervical lymph node metastasis in patients with HNC.
Metastatic lymph nodes were examined in patients with advanced
stages of the disease (Table I and
Fig. 1). To allow accurate comparison
of the cut surface of the surgical specimen with the section
examined via ultrasound, intra-operative CEUS examination of the
lymph node of interest was performed. Although a number of studies
have evaluated intraoperative ultrasonography of the head and neck,
the majority of them involved transoral surgery (14–16). A
number of studies have investigated intraoperative evaluation of
the cervical lymph nodes (17,18). One
of these studies (18) reported the
usefulness of intraoperative US for patients with thyroid cancer,
and the surgical method used was similar to the method used in the
present study. To the best of our knowledge, the present study is
the first to describe intraoperative US and CEUS during neck
dissection in patients with HNC.
Regarding the accuracy of matching analyzed CEUS
images with histopathological images using HE staining, matching
was successful in the majority of cases, and the almost matched
patterns are presented in Figs. 1 and
2. These data indicate that
intraoperative US and CEUS are useful techniques for the
acquisition of accurate images for US and histopathological
examination. However, there was a defect on the upper side of the
analyzed CEUS image in case 2. Perhaps this phenomenon was due to
the direct touch of the ultrasound probe to the surface of the
lymph node. To avoid these image defects, an acoustic coupler could
be used for acquisition of the ultrasound data.
Although there have been several reports that
describe the usefulness of CEUS in the detection of metastatic
axillary lymph nodes in patients with breast cancer (19,20), there
have been a limited number of reports concerning the use of CEUS
for the identification of metastatic cervical lymph nodes (21,22). The
use of CEUS combined with the analysis software reveals the
distribution of blood vessels in metastatic cervical lymph nodes in
patients with HNC. A comparison of CEUS images and
histopathological patterns identifies that the use of CEUS combined
with software reveals a more detailed pattern of capillary vessels
in metastatic lymph nodes compared with color Doppler. Color
Doppler imaging provides only a rough sketch of the capillary
vessels present in lymph nodes. However, although CEUS revealed
vascular images in lymph nodes that were almost proportional to the
histopathological vascular patterns, there were a number of
perfusion deficits at CD34 stain positive areas. This indicated the
presence of immature capillary vessels accompanying tumor
infiltration of the cervical lymph nodes. Although there were
regular capillary vessels in non-affected regions of the lymph node
around the lymph follicles, capillary vessels were rich in the
border of the tumor and lymph follicles (Fig. 1L and N). Additionally, when CEUS was
employed for normal lymph nodes, capillary vessels were detected
mainly in the hilum of the lymph nodes, and peripheral flow from
the hilum was detected (data not shown). This was consistent with
the histopathological findings of the distribution of capillary
vessels in normal lymph nodes.
These results suggest that CEUS combined with the
usage of the image analysis software was useful in the
visualization of capillary distribution and density in the cervical
lymph nodes of patients with HNC. Histopathological examination
suggested that this may be used as a novel tool to analyze and
classify the internal structure of metastatic lymph nodes prior to
surgery or chemoradiotherapy. However, due to the limited number of
patients in the present study, the association between the internal
structure of the metastatic lymph nodes and the clinical
characteristics and prognoses of patients with HNC remains unclear.
Also as this study included only squamous cell carcinoma, other
types of head and neck malignancies including adenocarcinoma of the
salivary gland origin remain uninvestigated.
To conclude, intraoperative CEUS combined with novel
analysis software, intraoperative US was able to reveal the
distribution of blood vessels in the metastatic lymph nodes of
patients with HNC. Although this is a preliminary study, the
results indicated that the use of CEUS combined with software was
able to reveal a more detailed pattern of capillary vessels in
metastatic lymph nodes than color Doppler when these images were
compared to histopathological examination. Further investigation
with a larger number of samples from patients with HNC is required
to clarify the association between CEUS images and
histopathological patterns, as a number of perfusion defects were
noted in CD34 stain positive areas.
Acknowledgements
The present study was supported by a KAKENHI
grant-in-aid from the Japan Society for the Promotion of Science
(grant no. 26462619) and Health Labour Sciences Research Grant.
References
|
1
|
Schuller D, McGuirt WF, McCabe BF and
Young D: The prognostic significance of metastatic cervical lymph
nodes. Laryngoscope. 90:557–570. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spiro RH, Alfonso AE, Farr HW and Strong
EW: Cervical node metastasis from epidermoid carcinoma of the oral
cavity and oropharynx. A critical assessment of current staging. Am
J Surg. 128:562–567. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sumi M, Ohki M and Nakamura T: Comparison
of sonography and CT for differentiating benign from malignant
cervical lymph nodes in patients with squamous cell carcinoma of
the head and neck. AJR Am J Roentgenol. 176:1019–1024. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Furukawa MK and Furukawa MK: Diagnosis of
lymph node metastasis of head and neck cancer and evaluation of
effects of chemoradiotherapy using ultrasonography. J Clin Oncol.
15:23–32. 2010.
|
|
5
|
Li L, Mori S, Kodama M, Sakamoto M,
Takahashi S and Kodama T: Enhanced sonographic imaging to diagnose
lymph node metastasis: Importance of blood vessel volume and
density. Cancer Res. 73:2082–2092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giovagnorio F, Galluzzo M, Andreoli C, De
CM and David V: Color Doppler sonography in the evaluation of
superficial lymphomatous lymph nodes. J Ultrasound Med. 21:403–408.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Watanabe R, Matsumura M, Munemasa T,
Fujimaki M and Suematsu M: Mechanism of hepatic parenchyma-specific
contrast of microbubble-based contrast agent for ultrasonography:
Microscopic studies in rat liver. Invest Radiol. 42:643–651. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masumoto N, Kadoya T, Amioka A, Kajitani
K, Shigematsu H, Emi A, Matsuura K, Arihiro K and Okada M:
Evaluation of malignancy grade of breast cancer using
perflubutane-enhanced ultrasonography. Ultrasound Med Biol.
42:1049–1057. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ito K, Noro K, Yanagisawa Y, Sakamoto M,
Mori S, Shiga K, Kodama T and Aoki T: High-accuracy ultrasound
contrast agent detection method for diagnostic ultrasound imaging
systems. Ultrasound Med Biol. 41:3120–3130. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
World Medical Association, . World Medical
Association Declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumors. seventh. Blackwell
Publishing Ltd; 2009
|
|
12
|
Sugai T, Inomata M, Uesugi N, Jiao YF,
Endoh M, Orii S and Nakamura S: Analysis of mucin, p53 protein and
Ki-67 expressions in gastric differentiated-type intramucosal
neoplastic lesions obtained from endoscopic mucosal resection
samples: A proposal for a new classification of intramucosal
neoplastic lesions based on nuclear atypia. Pathol Int. 54:425–435.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Curtin HD, Ishwaran H, Mancuso AA, Dalley
RW, Caudry DJ and McNeil BJ: Comparison of CT and MR imaging in
staging of neck metastases. Radiology. 207:123–130. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Andrews GA, Kwon M, Clayman G, Edeiken B
and Kupferman ME: Technical refinement of ultrasound-guided
transoral resection of pharyngeal/retropharyngeal thyroid carcinoma
metastases. Head Neck. 33:166–170. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goepfert RP, Liu C and Ryan WR: Trans-oral
robotic surgery and surgeon-performed trans-oral ultrasound for
intraoperative location and excision of an isolated retropharyngeal
lymph node metastasis of papillary thyroid carcinoma. Am J
Otolaryngo. 36:710–714. 2015. View Article : Google Scholar
|
|
16
|
Clayburgh DR, Byrd JK, Bonfili J and
Duvvuri U: Intraoperative ultrasonography during transoral robotic
surgery. Ann Otol Rhinol Laryngol. 125:37–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-lami A, Riffat F, Alamgir F, Dwivedi R,
Berman L, Fish B and Jani O: Utility of an intraoperative
ultrasound in lateral approach mini-parathyroidectomy with
discordant pre-operative imaging. Eur Arch Otorhinolaryngo.
270:1903–1908. 2013. View Article : Google Scholar
|
|
18
|
Ertas B, Kaya H, Kurtulumus N, Yakupoglu
A, Giray S, Unal OF and Duren M: Intraoperative ultrasonography is
useful in surgical management of neck metastases in differentiated
thyroid cancers. Endocrine. 48:248–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsuzawa F, Einawa T, Abe H, Suzuki T,
Hamaguchi J, Kaga T, Sato M, Oomura M, Takata Y, Fujibe A, et al:
Accurate diagnosis of axillary lymph node metastasis using
contrast-enhanced ultrasonography with Sonazoid. Mol Clin Oncol.
3:299–302. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsuzawa F, Omoto K, Einawa T, Suzuki T,
Hamaguchi J, Kaga T, Sato M, Oomura M, Takata Y, Fujibe A, et al:
Accurate evaluation of axillary sentinel lymph node metastasis
using contrast-enhanced ultrasonography with Sonazoid in breast
cancer: A preliminary clinical trial. Springerplus. 4:5092015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu M, Liu Q, Song HP, Han ZH, Su HL, He GB
and Zhou XD: Clinical application of contrast-enhanced
ultrasonography in diagnosis of superficial lymphadenopathy. J
Ultrasound Med. 29:735–740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poanta L, Serban O, Pascu I, Pop S,
Cosgarea M and Fodor D: The place of CEUS in distinguishing benign
from malignant cervical lymph nodes: A prospective study. Med
Ultrason. 16:7–14. 2014. View Article : Google Scholar : PubMed/NCBI
|