Introduction
Prostate cancer (PCa) is the most prevalent type of
cancer among men in the United States (1) and currently accounts for ~30% of all
diagnosed cancers (2). PCa is the
second most common cause of cancer-associated mortality in men in
the United States (2). A recent study
estimated that 180,890 men in the United States were diagnosed with
PCa and 26,120 patients in the United States succumbed to PCa in
2016 (2). In China, the incidence of
PCa is also increasing (3,4). No standard methods for PCa prevention,
early diagnosis, treatment or prognosis are currently available
(5). Therefore, the majority of
patients with PCa are diagnosed at the late stage, leading to high
mortality rates (3). Identifying
novel diagnostic and prognostic markers that benefit the early
diagnosis and treatment of PCa are required to improve the
treatment of the disease.
MicroRNAs (miRNAs/miRs) are endogenous, small
non-coding RNAs that are ~22 nucleotides in length (6) and were initially discovered as a small
temporal RNA in Caenorhabditis elegans in 1993 (7). The functions of miRNA have been revealed
to serve a notable role in various biological processes, including
cell differentiation (8),
proliferation (8,9), apoptosis (10) and development (11). To date, >2,500 potential human
miRNAs are identified and recorded in the miRBase, and >30% of
all genes are estimated to be regulated by miRNAs (12). In recent years, mounting evidence has
demonstrated that miR-211 can impact cell proliferation and
migration in numerous human cancers (13–18).
However, the role of miR-211 in tumor progression remains
uncertain. In non-small lung cancer, Ye et al (18) revealed the miR-211 can directly
downregulate the expression of SRC kinase signaling inhibitor 1
(SRCIN1), and promote non-small cell lung cancer proliferation. As
a comparison, miR-211 expression was downregulated in
hepatocellular carcinoma (15,16),
gastric cancer (14) and epithelial
ovarian cancer (17), and was
regarded as a tumor suppressor by targeting the expression of its
downstream targets. However, the expression and role of miR-211 in
PCa remains unclear.
Secreted protein acidic and rich in cysteine
(SPARC), also known as osteonectin, is a matricellular glycoprotein
that serves instrumental roles during cell proliferation, migration
and cell differentiation (19,20). SPARC
was identified to be upregulated in various tumors, including PCa
(21–23); high SPARC expression was also revealed
to be associated with aggressive stages of melanoma (24). Additionally, the expression of SPARC
could be regulated by miR-211 in hepatocellular carcinoma (16). Meanwhile, a recent study demonstrated
that SPARC could mediate metastatic dormancy of PCa in the bone
(25), which highlighted the
significant role of SPARC in PCa. However, whether or not miR-211
could regulate the expression of SPARC in PCa remains unreported.
The present study aimed to determine the expression and function of
miR-211 in PCa and investigate the molecular mechanism of miR-211
in the progression of PCa.
Materials and methods
Participants
The study was approved by the Ethics Committee of
the First Affiliated Hospital of Jiamusi University (Jiamusi,
China), and was performed in accordance with the Declaration of
Helsinki. Written informed consent was obtained from all patients.
Matched PCa and normal prostate tissues 36 pairs, 44–71 years old
(mean age, 62) were obtained from patients who underwent radical
prostatectomy between October 2010 and February 2012 at the First
Affiliated Hospital of Jiamusi University (Jiamusi, China). None of
the patients had received radiotherapy or chemotherapy before
surgical resection. PCa stage was classified according to the
seventh American Joint Committee on Cancer (AJCC) classification
system (26). All samples were
snap-frozen in liquid nitrogen immediately and stored at −80°C
following surgery until further use.
Cell lines and cell culture
Two human PCa cell lines, DU145 and PC-3, were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and cultured in RPMI-1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 µg/ml penicillin
and 100 µg/ml streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Normal human prostate epithelial cells
(NHPE) were purchased from Lonza, Inc. (Allendale, NJ, USA) and
cultured in prostate epithelial cell growth medium containing
growth medium and supplements (cat no. CC-3166; Lonza, Inc.). All
the cell lines were incubated in a humidified atmosphere of 5%
CO2 at 37°C.
Cell transfection
The miR-211 mimic (5′-UUCCCUUUGUCAUCCUUCGCCU-3′),
inhibitor (5′-AGGCGAAGGAUGACAAAGGGAA-3′) and miRNA negative control
(5′-CAGUACUUUUGUGUAGUACAA-3′) molecules were purchased from
Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The small
interfering RNA (siRNA) against SPARC (5′-GCAGAGGUGACUGAGGUAUCU-3′)
(2.5 nmol) and negative control (5′-AGUCGAGAUCGGUGUUAGCAG-3′) (2.5
nmol) were designed and synthesized by Shanghai GenePharma Co.,
Ltd. (Shanghai, China). These nucleotides (miRNAs or siRNAs) were
transfected into the cell lines (2×105 cells/well, DU145
and PC-3) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) until a final concentration of 50 nM and
according to the manufacturer's protocol. At 48 h after
transfection, cells were collected for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses.
Total RNA isolation and RT-qPCR
Total RNA was extracted from cultured cells and from
surgically resected fresh PCa tissues using TRIzol reagent
(Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's protocol. The RNA concentration was quantified
using NanoDrop 2000 (Thermo Fisher Scientific, Inc.). To quantify
the level of miR-211 expression, total RNA was polyadenylated and
reverse transcribed using the TaqMan MicroRNA Reverse Transcription
kit and TaqMan miRNA Assay kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. To
quantify the level of SPARC expression, the SYBR Premix Ex TaqTM
kit (Takara Biotechnology Co., Ltd., Dalian, China) was used. PCR
conditions included an initial holding period at 95°C for 10 min
for 1 cycle, then 95°C for 15 sec and 60°C for 30 sec for 40
cycles. Primers used in the present study were as follows: SPARC
forward, 5′-AGCACCCCATTGACGGGTA-3′ and reverse,
5′-GGTCACAGGTCTCGAAAAAGC-3′; GAPDH forward,
5′-AAGGGAAGGTTGCTGGATAGG-3′ and reverse,
5′-CACATCCACCTCCTCCACATC-3′. Each sample was repeated in
triplicate. The relative expression of genes was calculated using
the 2−ΔΔCq method (27).
Western blot analysis
Total proteins were extracted from surgically
resected fresh PCa tissues using Radioimmunoprecipitation Assay
lysis buffer (Beyotime Institute of Biotechnology). The protein
concentration was quantified using a BCA protein concentration
determination kit (Beyotime Institute of Biotechnology). An equal
amount of protein samples (50 µg) was separated using 10% SDS-PAGE.
The separated protein was then transferred onto a polyvinylidene
fluoride membrane (Beyotime Institute of Biotechnology), blocked in
5% fat-free milk at room temperature for 1 h and incubated with
primary antibodies anti-SPARC (1:1,000; cat. no. ab207743; Abcam,
Cambridge, MA, USA) and anti-GAPDH (1:1,000; cat. no. ab37168;
Abcam) at 4°C overnight followed by incubation with a horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody
(1:10,000; ab97080, Abcam) at room temperature for 1 h. GAPDH was
selected as an internal control. Results were detected using the
BeyoECL Plus kit (Beyotime Institute of Biotechnology). The
densitometry was analyzed using ImageJ 1.48 software (National
Institutes of Health, Bethesda, MD, USA). Each experiment was
repeated independently three times.
Cell proliferation assay
The cell proliferation rate assay was conducted
using Cell Counting kit-8 (CCK-8; Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. In brief,
the cell lines were seeded into a 96-well plate at a density of
2×103 cells/well and cultured in 100 µl of the
aforementioned medium. In total, 10 µl CCK-8 reagent was added to
each well at the indicated time points (0, 24, 48 and 72 h) after
seeding and further incubated at the aforementioned conditions
(37°C) for 2 h. The absorbance was measured at 450 nm using a
microplate reader (Lifecare Medical Equipments Co., Ltd., Ningbo,
Zhejiang, China). Cell medium without cells was treated with 10 µl
CCK-8 reagent at 0, 24, 48 and 72 h and cultured for 2 h at 37°C
then used as control to eliminate the background. Each experiment
was performed in triplicate.
Bioinformatic analysis
The online algorithm TargetScan (http://www.targetscan.org/) (28) was used to predict the targets of
miR-211. SPARC, whose expression was upregulated in PCa, was
selected to further investigate whether it is regulated by
miR-211.
Statistical analysis
The data are expressed as the mean ± standard
deviation of triplicate experiments. Data analysis was performed
using the SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA).
Statistical analysis of differences between two groups was
performed using Student's t-test; multiple comparisons were
performed using one way analysis of variance with post hoc Tukey's
test. Pearson's χ2 test was used for analyzing the
association between the expression level of miR-211 and the
clinicopathological features of patients. P<0.05 was considered
to indicate a statistically significant difference in all
tests.
Results
miR-211 is downregulated in PCa
tissues and cell lines
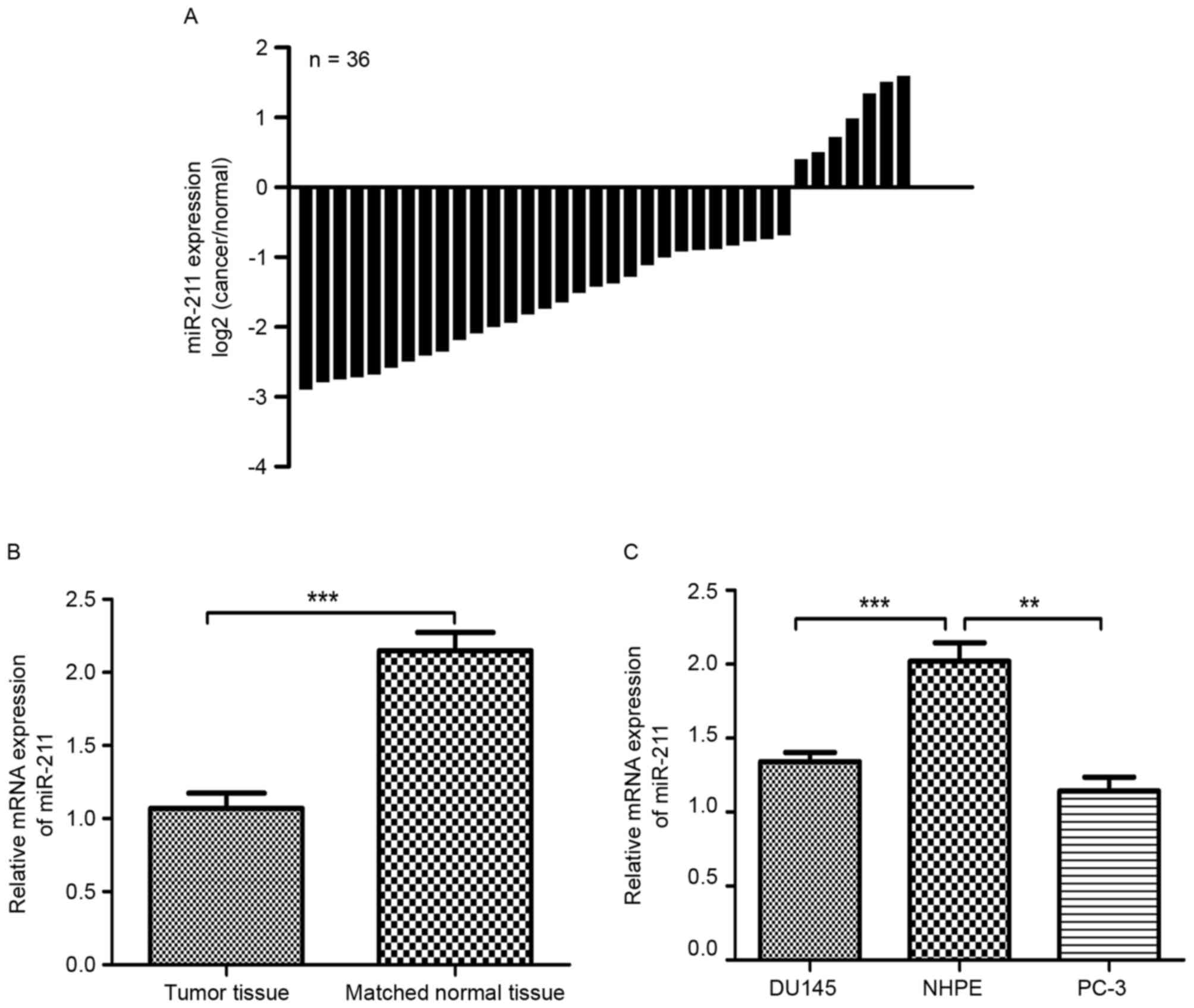
A decrease in miR-211 expression was identified in
29 of 36 PCa tissues compared with the matched non-tumor tissues
(Fig. 1A). As revealed in Fig. 1B, the expression of miR-211 in PCa
tissues was significantly lower than that in the matched normal
prostate tissues (P<0.001). The expression of miR-211 was also
assessed in two PCa cell lines. As depicted in Fig. 1C, the expression of miR-211 in the PCa
DU145 and PC-3 cell lines was also evidently lower than in the NHPE
cells (P<0.01).
miR-211 downregulation is associated
with clinicopathological features in patients with PCa
To assess whether miR-211 has any prognostic
significance, the association between miR-211 expression and
clinicopathological features of patients with PCa was analyzed. As
demonstrated in Table I, no
statistical difference was revealed between miR-211 expression and
age, nodal status and prostate specific antigen (PSA) levels (all
P>0.05). However, the expression of miR-211 was revealed to be
closely associated with the tumor stage (P=0.035) and higher
Gleason scores (29) (P=0.013).
 | Table I.Distribution of miR-211 expression
status in human prostate cancer according to clinicopathological
characteristics. |
Table I.
Distribution of miR-211 expression
status in human prostate cancer according to clinicopathological
characteristics.
|
|
| miR-211 expression,
n |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Patients, n | Low | High | P-value |
|---|
| Age, years |
|
|
| NS |
| ≥60 | 26 | 21 | 5 |
|
|
<60 | 10 | 8 | 2 |
|
| Tumor stage |
|
|
| 0.035 |
|
pT2a-c | 18 | 12 | 6 |
|
|
pT3a-4 | 18 | 17 | 1 |
|
| Gleason score |
|
|
| 0.013 |
| ≥8 | 28 | 25 | 3 |
|
|
<8 | 8 | 4 | 4 |
|
| Nodal status |
|
|
| NS |
|
pN0 | 27 | 23 | 4 |
|
|
pN1 | 9 | 6 | 3 |
|
| PSA levels,
ng/ml |
|
|
| NS |
|
≥10 | 24 | 19 | 5 |
|
|
<10 | 12 | 10 | 2 |
|
Upregulation of miR-211 inhibits cell
proliferation in vitro
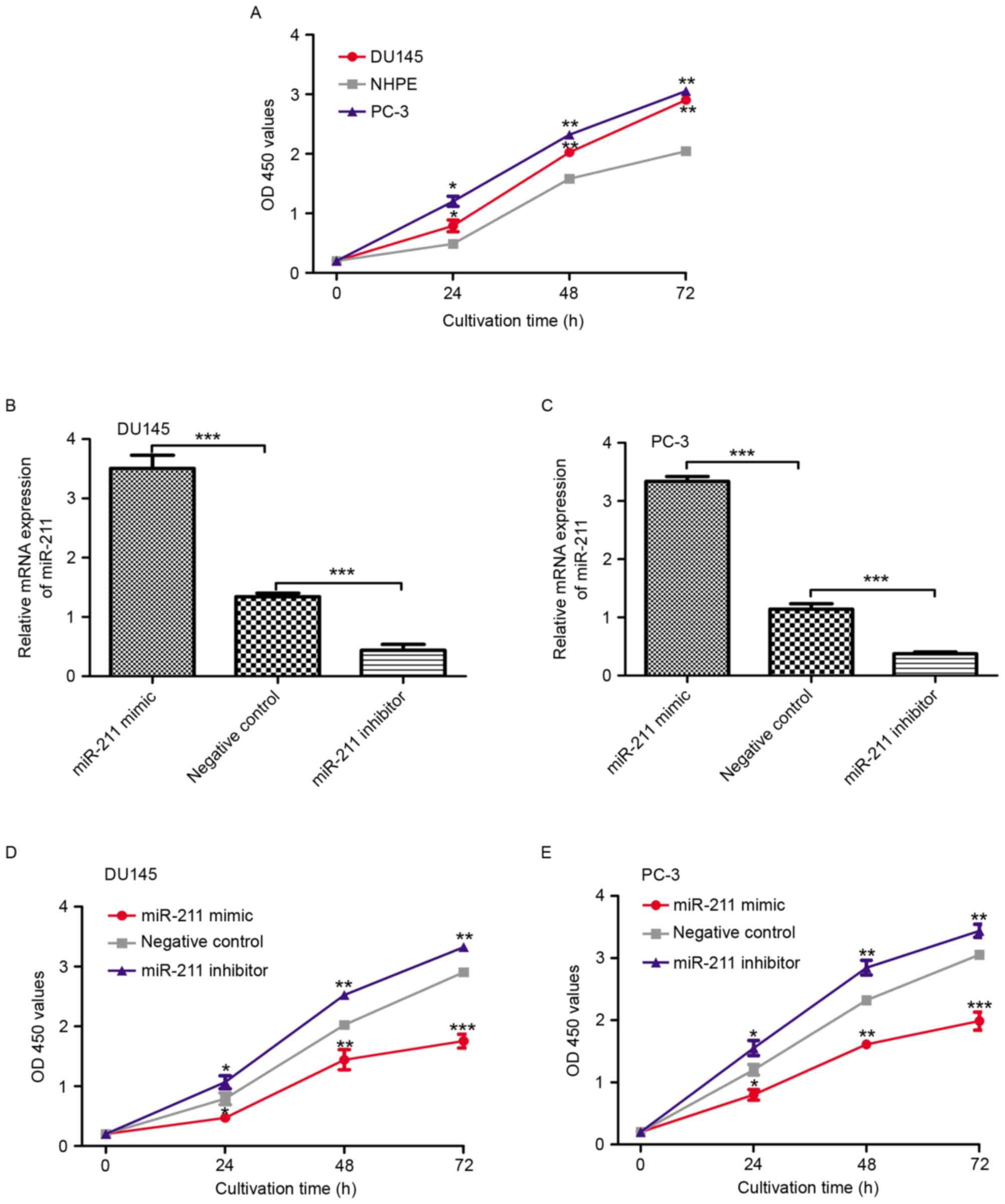
To examine the functional significance of miR-211 in
PCa, the rate of cell proliferation in PCa cell lines and NHPE
cells was measured and compared. As depicted in Fig. 2A, the cell proliferation rate was
evidently higher in the PCa cell lines than in NHPE cell line
(P<0.05). The miR-211 mimic, inhibitor and corresponding
negative control were selected to regulate the expression level of
miR-211 in the PCa cell lines. As depicted in Fig. 2B and C, the expression level of
miR-211 in the PCa cell lines transfected with miR-211 mimic was
significantly higher than those transfected with the negative
control miRNA (P<0.001). Conversely, the miR-211 expression
level in the PCa cell lines transfected with the miR-211 inhibitor
was significantly lower than those transfected with the negative
control miRNA (P<0.001). The cell proliferation rate in these
miRNA-transfected cell lines was also examined. As revealed in
Fig. 2D and E, the cell proliferation
rate in the PCa cell lines transfected with miRNAs was as follows:
PCa cell lines transfected with miR-211 inhibitor, PCa cell lines
transfected with negative control miRNA and PCa cell lines
transfected with miR-211 mimic.
Expression of SPARC is inversely
associated with miR-211
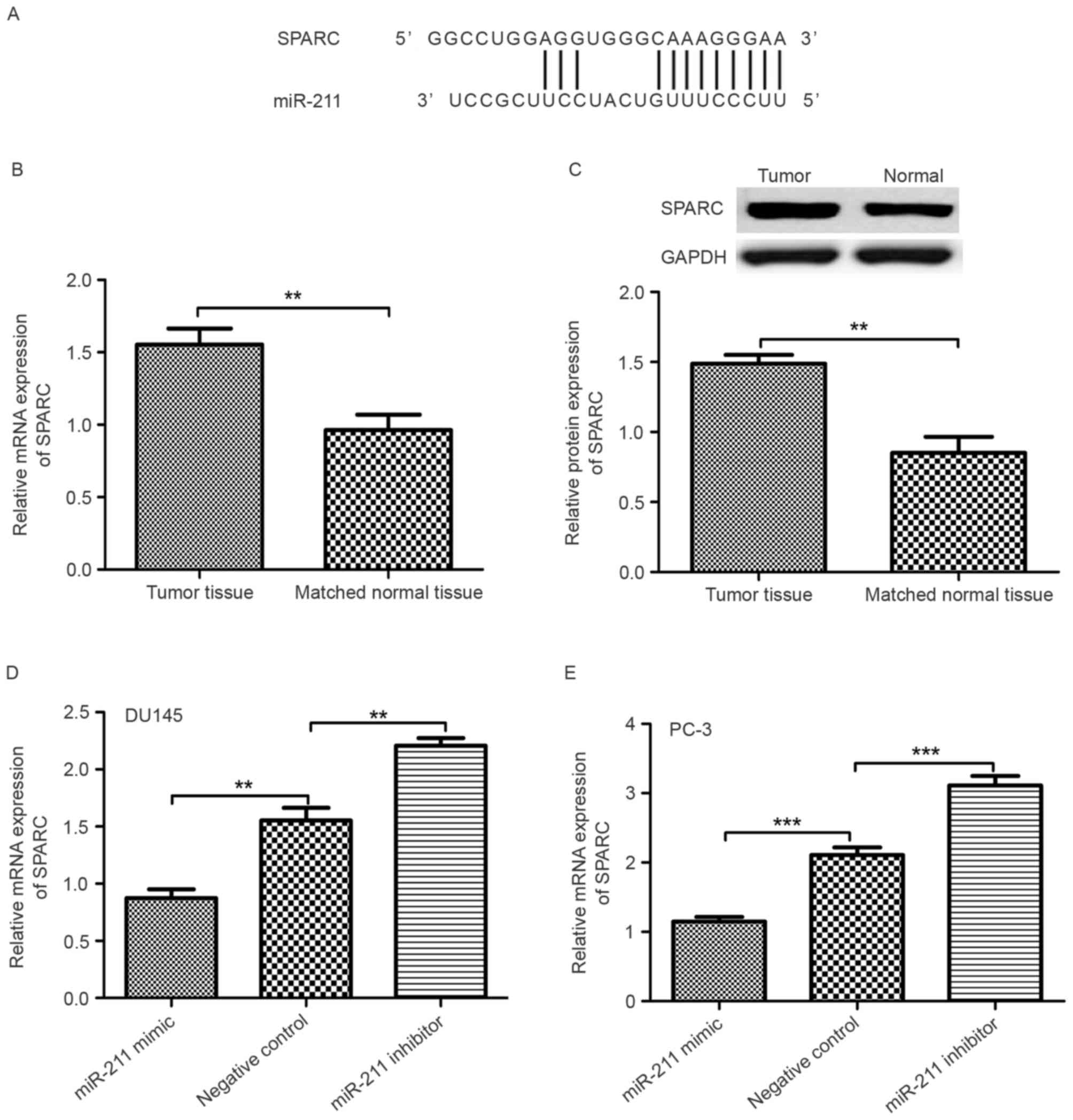
It was revealed that the 3′-untranslated region
(3′-UTR) of SPARC contained a conserved putative target site for
miR-211 using bioinformatics analysis (Fig. 3A). Therefore, the expression level of
SPARC in PCa tumor tissues and matched normal prostate tissue was
examined. As revealed in Fig. 3B and
C, RT-qPCR and western blot analyses demonstrated that the
expression of SPARC in PCa tumor tissues was significantly higher
than in matched normal prostate tissue (P<0.01). The expression
level of SPARC in the miR-211 mimic, miR-211 inhibitor and negative
control miRNA transfected PCa cell lines was also examined. The
SPARC mRNA expression level in the miR-211-mimic-transfected PCa
cell lines was significantly lower than in the
miR-211-inhibitor-transfected PCa cell lines (Fig. 3D and E; P<0.01).
Effect of SPARC on cell
proliferation
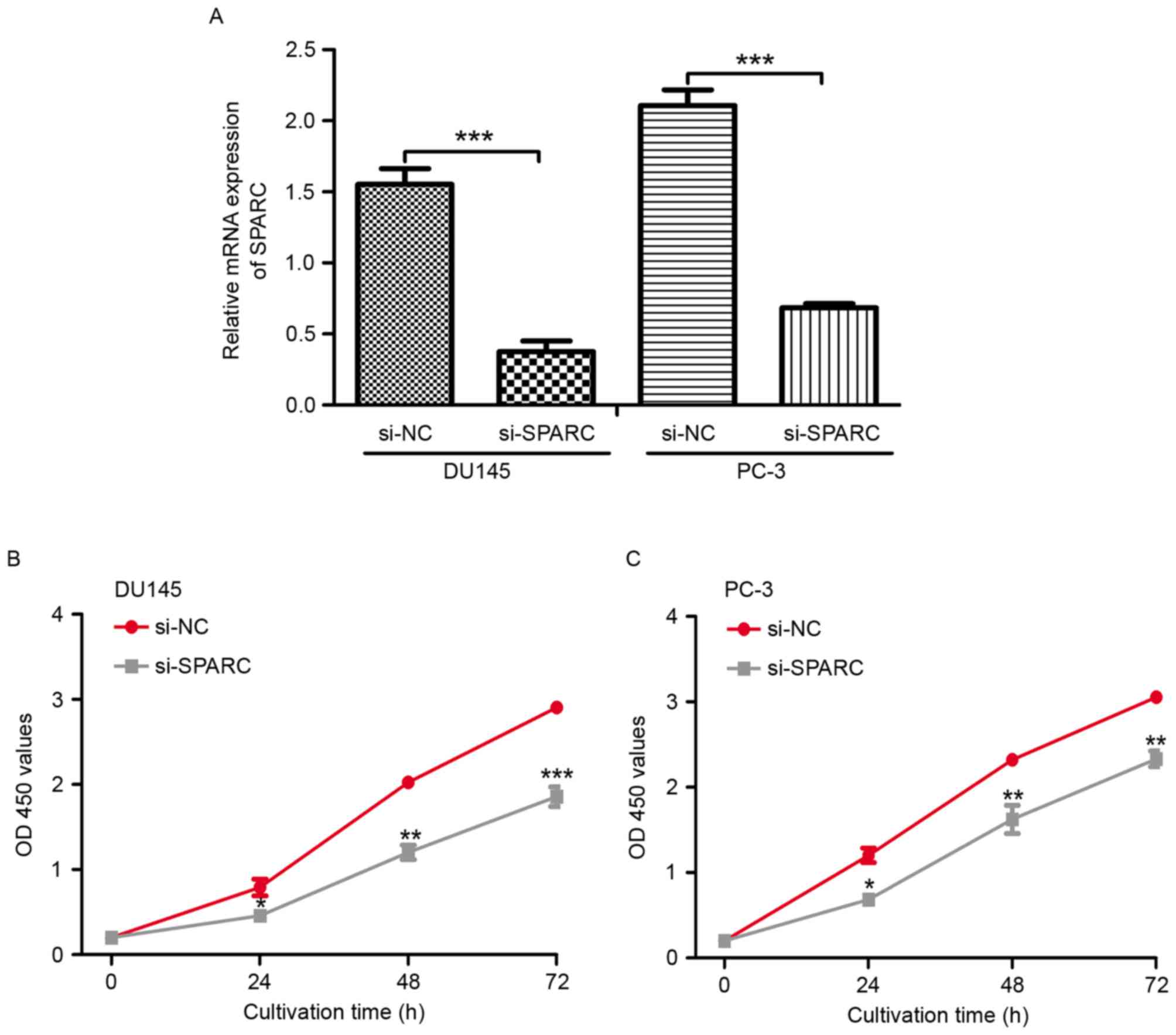
To determine the role of SPARC on cell
proliferation, knockdown of SPARC expression was performed using a
specific siRNA in PCa cell lines (DU145 and PC-3). The expression
of SPARC transfected with SPARC-siRNA was significantly decreased
compared with the negative control (Fig.
4A; P<0.001). As expected, knockdown of SPARC inhibited the
cell proliferation in PCa cell lines compared with the cells
transfected with the negative control (Fig. 4B and C; P<0.05).
Discussion
The established pre-treatment prognostic parameters
for patients with PCa currently include Gleason grade, PSA and
clinical stage (30–32). Although these parameters are
statistically powerful, they are not always sufficient for
individual treatment decision optimization (29). It can be hoped that further
understanding of PCa biology will lead to improvements in clinical
molecular tests that enable the reliable prediction of PCa
aggressiveness.
The role of miRNA dysregulation in cancer has been
demonstrated by numerous studies (33,34).
Previous studies have revealed that miR-211 is aberrantly expressed
in number of tumor types and serves an important role in tumor
progression (13–18). In the present study, the results of
RT-qPCR analysis indicated that the expression of miR-211 was
reduced in PCa tissues compared with the matched normal prostate
tissues. These results indicated that the dysregulation of miR-211
may be involved in the tumorigenesis of PCa. Furthermore, the
association between miR-211 expression and the clinicopathological
features was analyzed. It was revealed that the miR-211 expression
was significantly associated with tumor stage (P=0.035) and higher
Gleason scores (P=0.013), indicating that miR-211 expression was
associated with the PCa malignancy. It was also revealed that
miR-211 expression was reduced in PCa cell lines compared with NHPE
cells. Overexpression of miR-211 in PCa cell lines induced by
miR-211 mimics significantly inhibited the proliferation of PCa
cells; conversely, downregulation of miR-211 using a miR-211
inhibitor promoted the proliferation of PCa cell lines. These
results indicated that miR-211 may be a tumor suppressor in the
development and progression of PCa.
Previous studies have identified the target genes of
miR-211 in a variety of human types of cancer (13–18). To
investigate the molecular mechanism whereby miR-211 inhibits the
proliferation rate of selected cell lines further, bioinformatics
analysis was used to predict the potential downstream target in the
PCa cell lines. It was revealed that the 3′-UTR of SPARC mRNA
contained a complementary sequence for miR-211. The expression of
SPARC was increased in PCa tissues compared with matched normal
prostate tissues. In the PCa cell lines, it was revealed that the
enforced expression of miR-211 could reduce the expression of
SPARC. However, the downregulation of miR-211 could increase the
expression of SPARC. Therefore, it was assumed that SPARC was a
direct target of miR-211 in PCa cell lines.
A previous study has demonstrated that SPARC serves
an important role in the development of human hepatocellular
carcinoma (16). To investigate the
role of SPARC in PCa progression, the expression of SPARC in PCa
cell lines was knocked down by siRNA. It was revealed that the
proliferation of the PCa cell lines was altered through the
downregulation of the expression of SPARC. These results indicated
that the knockdown of SPARC elicited similar effects to miR-211
overexpression, and that SPARC is a functionally important target
of miR-211.
In conclusion, miR-211 was identified to be
frequently downregulated in PCa, which was associated with PCa
progression. Additionally, miR-211 overexpression inhibited cell
proliferation in vitro via direct targeting of SPARC.
Downregulation of SPARC in a miR-211-mediated manner could shed
further light on the molecular mechanisms behind malignancy.
References
|
1
|
Hashimoto Y, Shiina M, Kato T, Yamamura S,
Tanaka Y, Majid S, Saini S, Shahryari V, Kulkarni P, Desgupta P, et
al: The role of miR-24 as a race related genetic factor in prostate
cancer. Oncotarget. 8:16581–16593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hu J, He J, Kuang Y, Wang Z, Sun Z, Zhu H
and Liu X: Expression and significance of 90K/Mac-2BP in prostate
cancer. Exp Ther Med. 5:181–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade P, Zhang S, Zeng H,
Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China, 2015.
CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Etzioni R, Urban N, Ramsey S, McIntosh M,
Schwartz S, Reid B, Radich J, Anderson G and Hartwell L: The case
for early detection. Nat Rev Cancer. 3:243–252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee RC, Feinbaum RL and Ambros V: The C.
Elegans heterochronic gene lin-4 encodes small RNAs with antisense
complementarity to lin-14. Cell. 75:843–854. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen JF, Mandel EM, Thomson JM, Wu Q,
Callis TE, Hammond SM, Conlon FL and Wang DZ: The role of
microRNA-1 and microRNA-133 in skeletal muscle proliferation and
differentiation. Nat Genet. 38:228–233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ambros V: MicroRNA pathways in flies and
worms: Growth, death, fat, stress, and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu YC, Chang JT, Chan EC, Chao YK, Yeh TS,
Chen JS and Cheng AJ: miR-196, an emerging cancer biomarker for
digestive tract cancers. J Cancer. 7:650–655. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee H, Lee S, Bae H, Kang HS and Kim SJ:
Genome-wide identification of target genes for miR-204 and miR-211
identifies their proliferation stimulatory role in breast cancer
cells. Sci Rep. 6:252872016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang CY, Hua L, Sun J, Yao KH, Chen JT,
Zhang JJ and Hu JH: MiR-211 inhibits cell proliferation and
invasion of gastric cancer by down-regulating SOX4. Int J Clin Exp
Pathol. 8:14013–14020. 2015.PubMed/NCBI
|
|
15
|
Jiang G, Cui Y, Yu X, Wu Z, Ding G and Cao
L: miR-211 suppresses hepatocellular carcinoma by downregulating
SATB2. Oncotarget. 6:9457–9466. 2015.PubMed/NCBI
|
|
16
|
Deng B, Qu L, Li J, Fang J, Yang S, Cao Z,
Mei Z and Sun X: MiRNA-211 suppresses cell proliferation, migration
and invasion by targeting SPARC in human hepatocellular carcinoma.
Sci Rep. 6:266792016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia B, Yang S, Liu T and Lou G: miR-211
suppresses epithelial ovarian cancer proliferation and cell-cycle
progression by targeting Cyclin D1 and CDK6. Mol Cancer. 14:572015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye L, Wang H and Liu B: miR-211 promotes
non-small cell lung cancer proliferation by targeting SRCIN1. Tumor
Biol. 37:1151–1157. 2016. View Article : Google Scholar
|
|
19
|
Motamed K: SPARC (osteonectin/BM-40). Int
J Biochem Cell Biol. 31:1363–1366. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin M, Mizokami A, Kim J, Ofude M, Konaka
H, Kadono Y, Kitagawa Y, Miwa S, Kumaki M, Keller ET and Namiki M:
Exogenous SPARC suppresses proliferation and migration of prostate
cancer by interacting with integrin β1. Prostate. 73:1159–1170.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi Q, Bao S, Maxwell JA, Reese ED,
Friedman HS, Bigner DD, Wang XF and Rich JN: Secreted protein
acidic, rich in cysteine (SPARC), mediates cellular survival of
gliomas through AKT activation. J Biol Chem. 279:52200–52209. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomas R, True LD, Bassuk JA, Lange PH and
Vessella RL: Differential expression of osteonectin/SPARC during
human prostate cancer progression. Clin Cancer Res. 6:1140–1149.
2000.PubMed/NCBI
|
|
23
|
Zhao ZS, Wang YY, Chu YQ, Ye ZY and Tao
HQ: SPARC is associated with gastric cancer progression and poor
survival of patients. Clin Cancer Res. 16:260–268. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Massi D, Franchi A, Borgognoni L, Reali UM
and Santucci M: Osteonectin expression correlates with clinical
outcome in thin cutaneous malignant melanomas. Hum Pathol.
30:339–344. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma S, Xing F, Liu Y, Wu K, Said N,
Pochampally R, Shizawa Y, Lin HK, Balaji KC and Watabe K: Secreted
Protein Acidic and Rich in Cysteine (SPARC) mediates metastatic
dormancy of prostate cancer in bone. J Biol Chem. 291:19351–19363.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adensines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rusthoven CG, Carlson JA, Waxweiler TV,
Yeh N, Raben D, Flaig TW and Kavanagh BD: The prognostic
significance of Gleason scores in metastatic prostate cancer. Urol
Oncol. 32:707–713. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cuzick J, Yang ZH, Fisher G, Tikishvili E,
Stone S, Lanchbury JS, Camacho N, Merson S, Brewer D, Cooper CS, et
al: Prognostic value of PTEN loss in men with conservatively
managed localized prostate cancer. Br J Cancer. 108:2582–2589.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Noh BJ, Sung JY, Kim YW, Chang SG and Park
YK: Prognostic value of ERG, PTEN, CRISPR3 and SPINK1 in predicting
biochemical recurrence in prostate cancer. Oncol Lett.
11:3621–3630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duan K, Ge YC, Zhang XP, Wu SY, Feng JS,
Chen SL, Zhang LI, Yuan ZH and Fu CH: miR-34a inhibits cell
proliferation in prostate cancer by downregulating of SIRT1
expression. Oncol Lett. 10:3223–3227. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bier A, Giladi N, Kronfeld N, Lee HK,
Cazacu S, Finniss S, Xiang C, Poisson L, deCarvalho AC, Slavin S,
et al: MicroRNA-137 is downregulated in glioblastoma and inhibits
the stemness of glioma stem cells by targeting RTVP-1. Oncotarget.
4:665–676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu C, Liao Z, Cai M and Zhang G:
MicroRNA-320a downregulation mediates human liver cancer cell
proliferation through the Wnt/β-catenin signaling pathway. Oncol
Lett. 13:573–578. 2017. View Article : Google Scholar : PubMed/NCBI
|