Introduction
Intestinal cancer is a malignant lesion of the
mucous epithelium caused by multiple environmental or heritable
carcinogenic factors and was the third most common gastroenteric
tumor, and a common malignant tumor in 2012 (1). In the United States, 160,000 new cases
and ~57,000 colorectal cancer-associated mortalities were reported
in 2012. Globally, 900,000 additional cases of and 500,000
mortalities due to colorectal cancer are reported annually in 2012
(2). Globally, the incidence rate of
colorectal cancer ranks second among lethal tumors, and threatens
human health (3). Epidemiological
studies have revealed that colon cancer-associated morbidity
exhibits regional differences. Areas of increased incidence include
Western Europe, North America, New Zealand and Australia. Areas of
moderate incidence include Latin America, and southern and eastern
Europe (4). Areas of decreased
incidence include South America, Africa and Asia. The morbidity and
mortality of colorectal cancer differs between areas of increased
and decreased incidence (5).
The study of human microRNA (miRNA/miR) has advanced
in previous years. The number of submitted human mature miRNAs
increased from 870 to 1921 between March 2009 and November 2011
(6). A given miRNA may regulate
>100 genes (6). It is predicted
that >5,300 genes, 30% of all human genes, are regulated by
miRNAs (7).
Alterations to miRNA expression in early stage
colorectal cancer have been reported (8). MiRNA clone technology has detected 28
miRNAs in colorectal cancer and surrounding normal mucosal tissues,
including miR320, miR321, miR200c, miR143 and miR145 (9). The expression of miR143 and miR145 was
decreased compared with the surrounding normal mucosal tissues.
Bandres et al (10) detected
the expression of 156 types of miRNA in 15 colorectal cancer cell
lines and compared this result with miRNA expression in the normal
human colorectal cell line CCD-18C0. The results of that comparison
revealed that the expression of 13 miRNAs differed significantly
between the colorectal cancer and normal human cell lines.
Expression has been demonstrated to differ most markedly between
colorectal cancer and normal human cell lines for the following
miRNAs: miR31, miR96, miR133b, miR135b, miR145 and miR183 (8).
The Wnt signal pathway serves a key function in
multiple biological events, including development, cell transfer
and apoptosis (11). A previous study
demonstrated that the Wnt signaling pathway possesses ≥3 branches:
The classical Wnt and the the Wnt/catenin β1 (CTNNB1 signaling
pathways (12). In the Wnt/CTNNB1
signaling pathway, Wnt and frizzled proteins bind to induce the
phosphorylation of the downstream dishevelled segment polarity
proteins (DVL). Phosphorylated DVL and axins destroy, including
axins, APC and glycogen synthase kinase 3 (GSK3), resulting in
disassembly (13). Phosphorylated
GSK3 may not degrade CTNNB1, which is released, concentrated in the
cytoplasm, and enters the cell nucleus to function as a
transcription factor (TCF)/lymphoid enhancer binding factor (LEF),
and thereby activate the transcription of numerous downstream
immediate early genes, including MYC binding protein, cyclin D1
(CCND1) and matrix metallopeptidase 7, and help regulate cell
proliferation, apoptosis, movement and differentiation. Under
normal conditions, the Wnt/CTNNB1 signaling pathway regulates cell
growth and development effectively (14). When activated excessively, the pathway
may result in tumor development, or dysplasia (15). Numerous clinical and experimental
studies have demonstrated that abnormal activation of the
Wnt/CTNNB1 signaling pathway is associated with the occurrence and
development of rectal carcinoma, and breast, lung, skin, ovarian,
prostate, endometrial cancer, primary hepatic carcinoma, thyroid
cancer, and multiple other tumors (11,15). By
assessing the underlying upstream regulatory mechanism, the present
study demonstrated that Wnt/CTNNB1/miR183 predicted the recurrence
and prognosis of colorectal cancer.
Materials and methods
Patients
Patients (42 male, 56.81±12.21 years old) with
colorectal cancer were enrolled in the present study at Tianjin
Third Central Hospital (Tianjin, China) and provided written
informed consent. Colorectal cancer and paracarcinoma tissue
samples were obtained from these patients between October 2015 and
March 2016. The present study was approved by the Ethics Committee
at Tianjin Third Central Hospital (Tianjin, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from colorectal cancer and
paracarcinoma tissue samples using TRIzol reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's protocol. Total RNA (1 µg) was reverse transcribed
using a miDETECT A Track™ miRNA RT-qPCR kit (Guangzhou RiboBio Co.,
Ltd., Guangzhou, China) to cDNA. A SYBR Green-based detection
method (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used for qPCR analysis using the LightCycler®480 System
(Applied Biosystems Inc.; Thermo Fisher Scientific, Inc.). The
primers used were as follows: U6 forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′; and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′; and miR-183 forward,
5′-CGCTATGGCACTGGTAGAA-3′; and reverse, 5′-GCACTGGATACGACAGTGAA-3′;
Wnt5a forward, 5′-TGTGGTTTAATGGTGCCTGA-3′ and reverse,
5′-TTCGTCGTGCTCAAGGTATG-3′; β-catenin forward,
5′-CCAACGACTACCACCAACTTT-3′ and reverse,
5′-GCCAGGTCTGGTTCATTGCT-3′; GAPDH forward,
5′-CCATGTTCGTCATGGTGTG-3′ and reverse, 5′-GGTGCTAAGCAGTTGGTGGTG-3′.
The PCR steps were as follows: 5 min at 95°C, followed by 40 cycles
of 45 sec at 95°C, 30 sec at 60°C, and extension for 30 sec at
72°C. The relative expression levels were calculated using the
2−ΔΔCq method (repeats, N=3) (16). Relative expression levels <2 times
para-carcinoma tissue group were defined as low miR183 expression;
relative expression levels ≥2 times para-carcinoma tissue group
were defined as high expression of miR183.
Cell culture
The human colorectal cancer cell line HCT-116
(Invitrogen; Thermo Fisher Scientific, Inc.) was cultured in
RPMI-1640 medium (Invitrogen; Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc.), 1.5 g/l sodium bicarbonate and 1%
penicillin/streptomycin at 37°C in a humidified atmosphere
containing 5% CO2.
Cell viability assay
The HCT-116 cells (70–80% confluent) were treated
with 5 µM IWR-2 (MedchemExpress, Monmouth Junction, NJ, USA) for
12, 24 and 48 h, and with MTT (Invitrogen; Thermo Fisher
Scientific, Inc.) for 4 h, at 37°C in a humidified atmosphere
containing 5% CO2. Subsequently, the RPMI-1640 medium
was removed, and 150 µl dimethyl sulfoxide was added to the cells
for 10 min. Optical density was measured at a wavelength of 570 nm
relative to a 630 nm basal absorbance using a Victor3™ multilabel
counter (PerkinElmer, Inc., Waltham, MA, USA).
Cell apoptosis assay
The HCT-116 cells were treated with 5 µM IWR-2 for
24 h, and subsequently washed with PBS on ice. The cells were then
collected and resuspended in 100 µl cell buffer (Annexin
V-fluorescein isothiocyanate KITS; BD Biosciences San Jose, CA,
USA) and stained with 5 µl Annexin V-fluorescein isothiocyanate (BD
Biosciences) for 30 min at room temperature, and stained with 5 µl
of propidium iodide (BD Biosciences) for a further 10 min at room
temperature, in darkness. Cell apoptosis was subsequently detected
using a flow cytometer (C6, BD Biosciences) and analyzed using
FlowJo 7.6.1 (FlowJo LLC, Ashland, OR, USA).
Western blot analysis and caspase-3
activity
The HCT-116 cells were treated with 5 µM IWR for 48
h, and subsequently washed with PBS, on ice. The cells were lysed
using RIPA lysis buffer (BD Biosciences) and protein concentration
was measured using the Bradford method (BD Biosciences). Equal
amounts of protein (40 µg/lane) were separated using SDS-PAGE with
a 10% gel, and were subsequently transferred onto nitrocellulose
filter membranes. Subsequently, the separated proteins were
incubated with anti-Wnt (sc-30224; 1:300), anti-CTNNB1 (cat. no.
sc-7199; 1:500), anti-caspase (CASP) 3 (cat. no. sc-98785; 1:500),
anti-BCL2 associated X (Bax; cat. no. sc-6236; 1:300), anti-CCND1
(cat. no. sc-717; 1:300), all purchased from Santa Cruz
Biotechnology, Inc., Dallas, TX, USA, and anti-GAPDH (cat. no.
AG019; 1:2,000; Beyotime Institute of Biotechnology, Haimen, China)
primary antibodies at 4°C overnight. Membranes were blocked using
5% skimmed milk for 1 h at 37°C and subsequently incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG (cat. no.
A0208; 1:5,000; Beyotime Institute of Biotechnology) for 1 h at
37°C. Membranes were then visualized using an enhanced
chemiluminescence detection kit (GE Healthcare, Chicago, IL, USA)
and analyzed using ImageLab 3.0 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Subsequently, 10 µg total protein was used to
analyze the levels of caspase-3 activity using caspase-3 activity
kits (cat. no. C1116; Beyotime Institute of Biotechnology),
according to the manufacturer's protocol. Optical density was
measured using a Victor3™ multilabel counter (PerkinElmer, Inc.) at
405 nm.
Statistical analysis
All the data were expressed as the mean ± standard
deviation (n=3) using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
Statistical differences were determined using one-way analysis of
variance with Tukey's post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
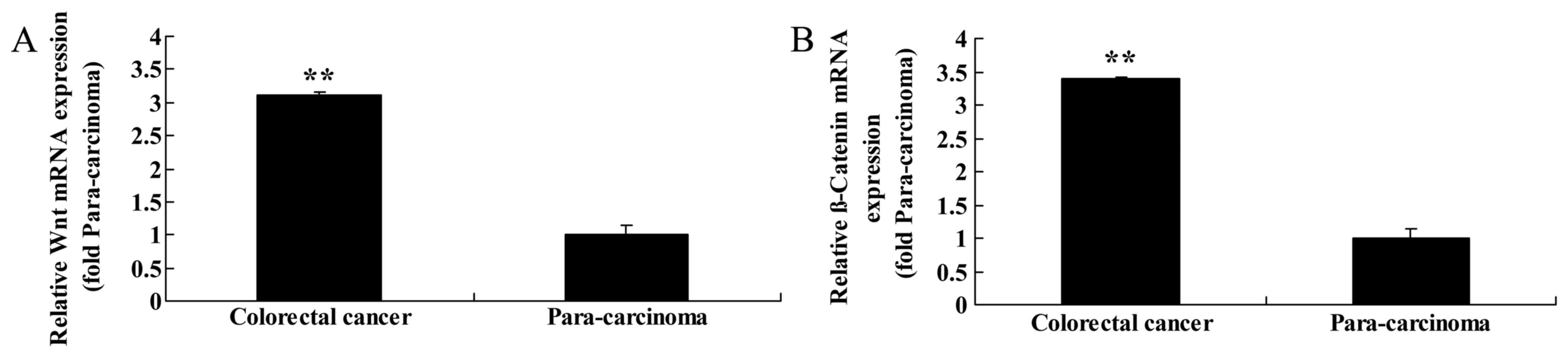
Expression of Wnt and CTNNB1 in
primary colorectal cancer tissue
To assess the function of Wnt and CTNNB1 in primary
colorectal cancer tissue, RT-qPCR analysis of Wnt and CTNNB1
expression was performed on colorectal cancer and paracarcinoma
tissue samples. Of the cases studied, colorectal cancer tissue
revealed concordant upregulation of Wnt and CTNNB1 expression
compared with the matched paracarcinoma tissue samples (Fig. 1).
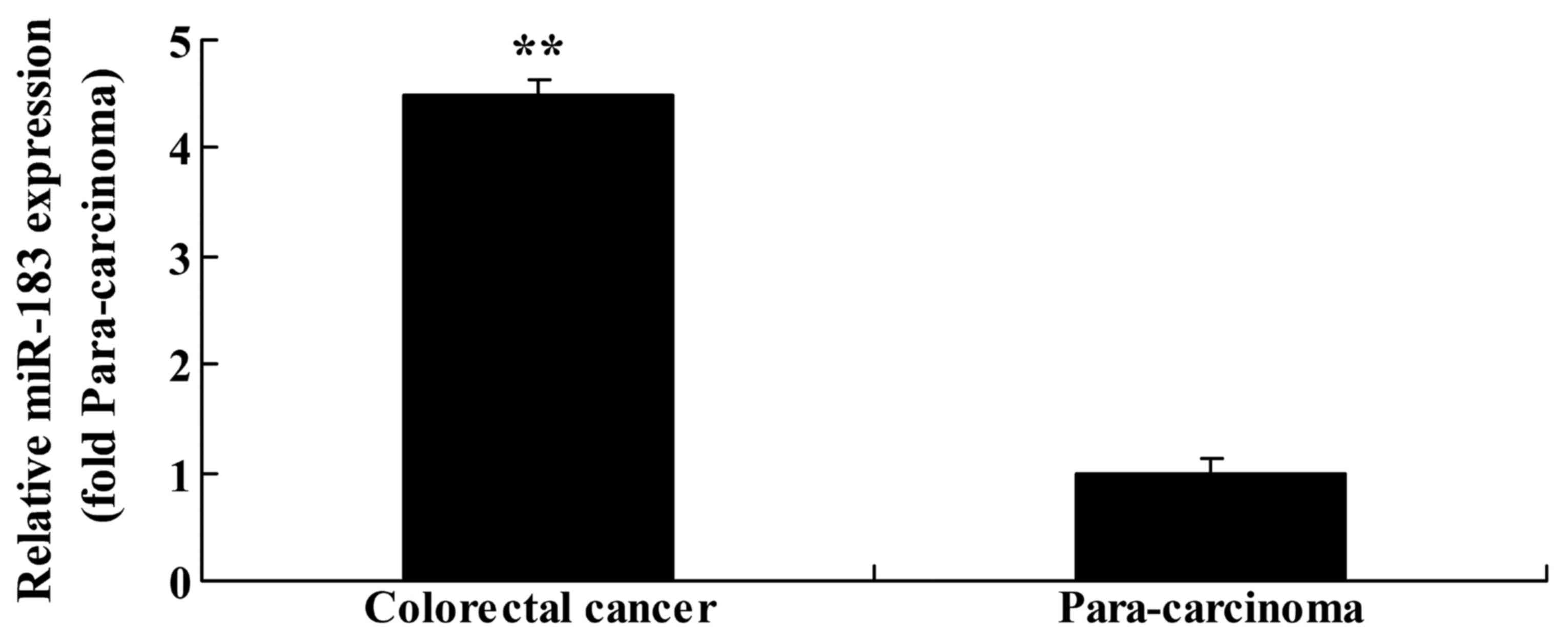
Expression of miR183 in primary
colorectal cancer tissue
To evaluate the function of miR183 in primary
colorectal cancer tissue, RT-qPCR analysis was performed to assess
miR183 expression in colorectal cancer and paracarcinoma tissue
samples. There was an upregulation of miR183 expression in
colorectal cancer tissue compared with the matched paracarcinoma
tissue samples (Fig. 2).
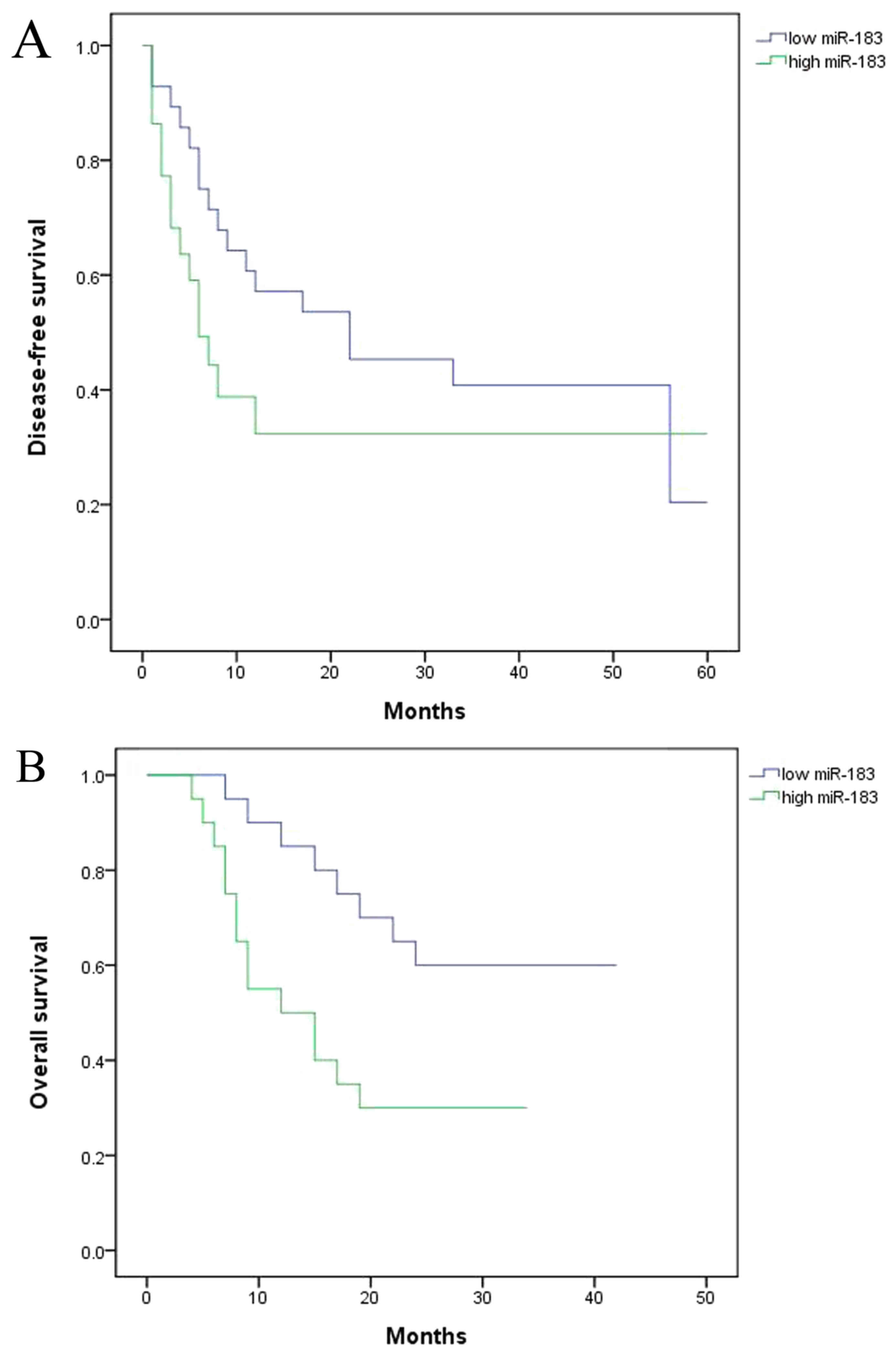
Survival analysis of patients with
colorectal cancer
To identify the functional effect of miR183/Wnt and
CTNNB1 on the survival of the patients with colorectal cancer, the
present study assessed disease-free survival (DFS) and overall
survival (OS). The results demonstrated that DFS and OS were
decreased in the high expression miR group compared with the low
miRNA expression group (Fig. 3).
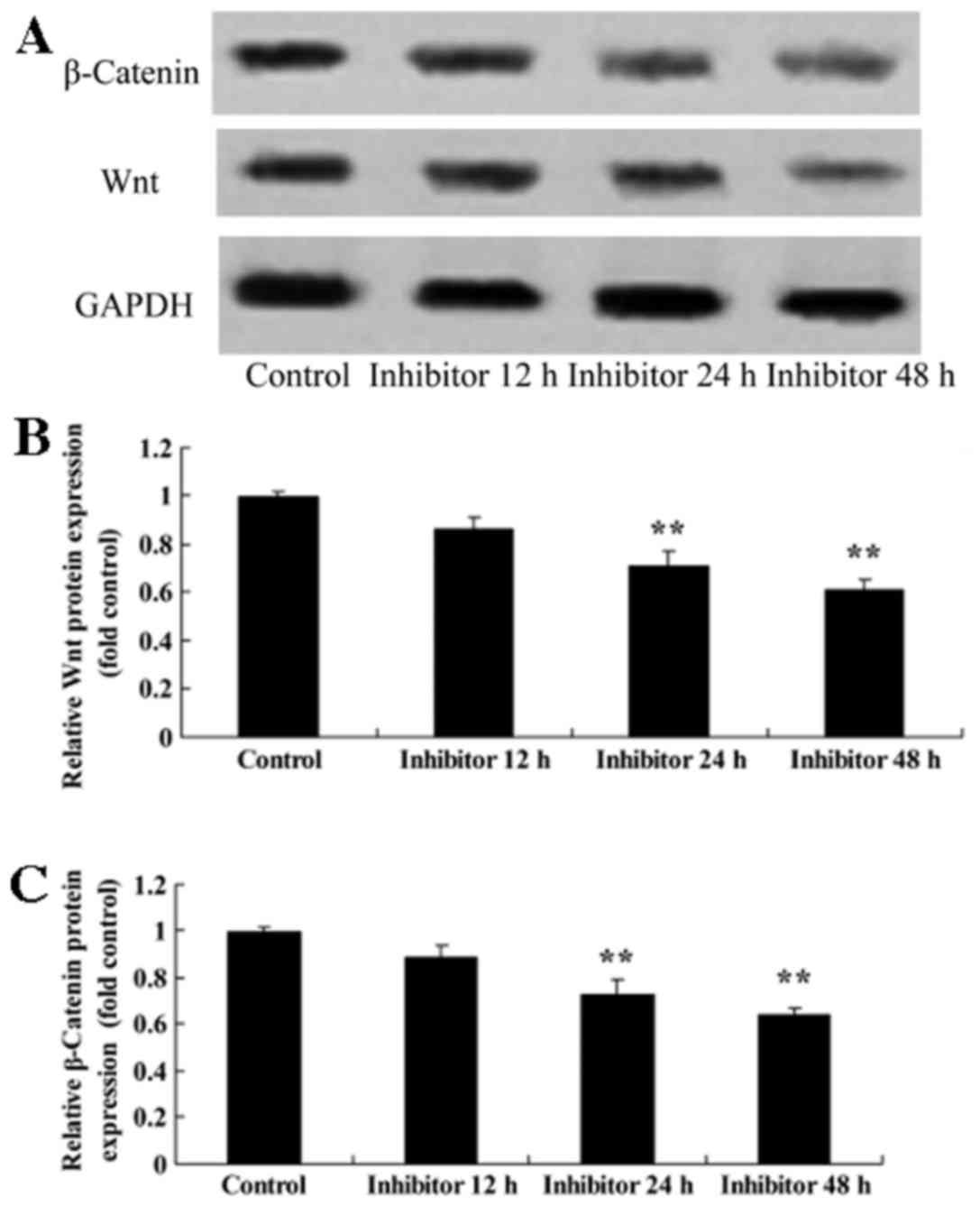
Effect of IWR treatment on Wnt and
CTNNB1 protein expression in HCT-116 cells
HCT-116 cells were treated with 5 µM IWR for 24 h,
which resulted in the inhibition of Wnt and CTNNB1 protein
expression compared with the untreated group (Fig. 4).
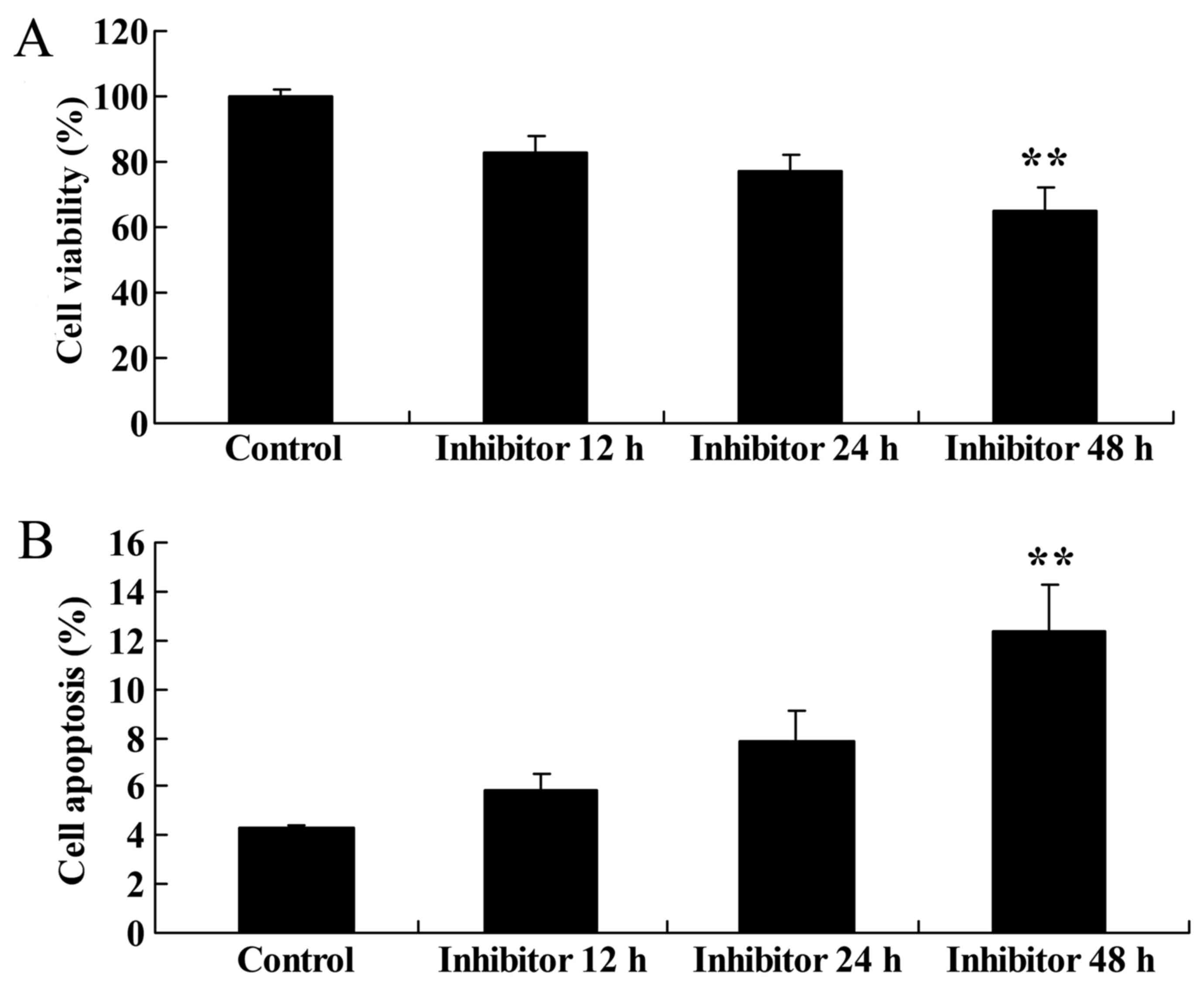
Effect of downregulating Wnt and
CTNNB1 expression on HCT-116 cell viability and apoptosis
The present study evaluated the effect of
downregulating Wnt and CTNNB1 expression on HCT-116 cell viability
and apoptosis. The apoptosis of HCT-116 cells was measured
following treatment with 5 µM IWR. HCT-116 cells were treated with
5 µM IWR for 12, 24 and 48 h, which resulted in the significant
inhibition of HCT-116 cell viability at 48 h compared with the
untreated group (Fig. 5A). Following
IWR treatment for 48 h, there was an increase in HCT-116 cell
apoptosis compared with the untreated group (Fig. 5B).
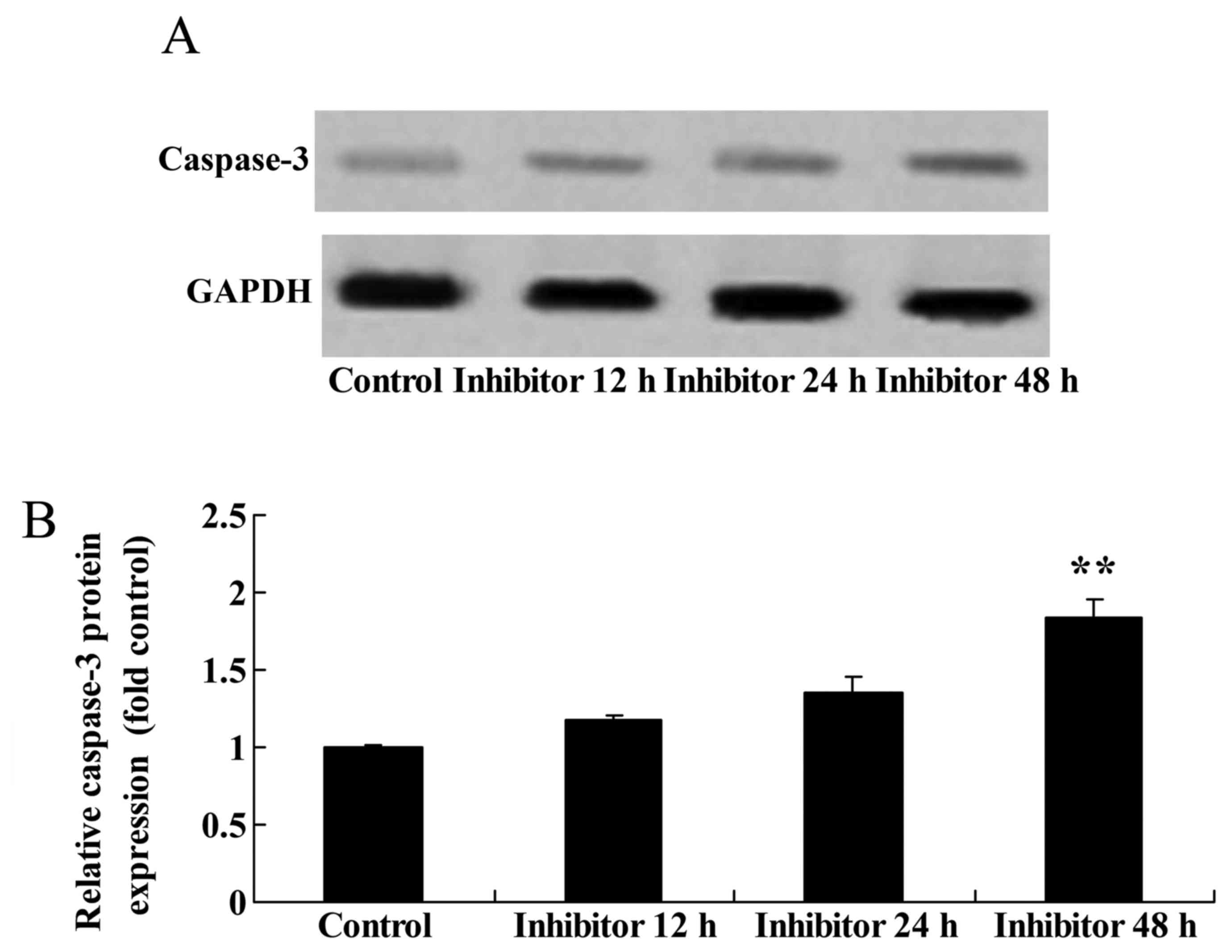
Effect of downregulating Wnt and
CTNNB1 expression on CASP3 function and protein expression in
HCT-116 cells
The present study assessed whether downregulating
Wnt and CTNNB1 affected CASP3 expression and function in HCT-116
cells using western blot analysis and a caspase-3 kit. The
downregulation of Wnt and CTNNB1, as induced by 48 h IWR treatment,
increased CASP3 function and protein expression in HCT-116 cells
compared with the untreated group (Fig.
6).
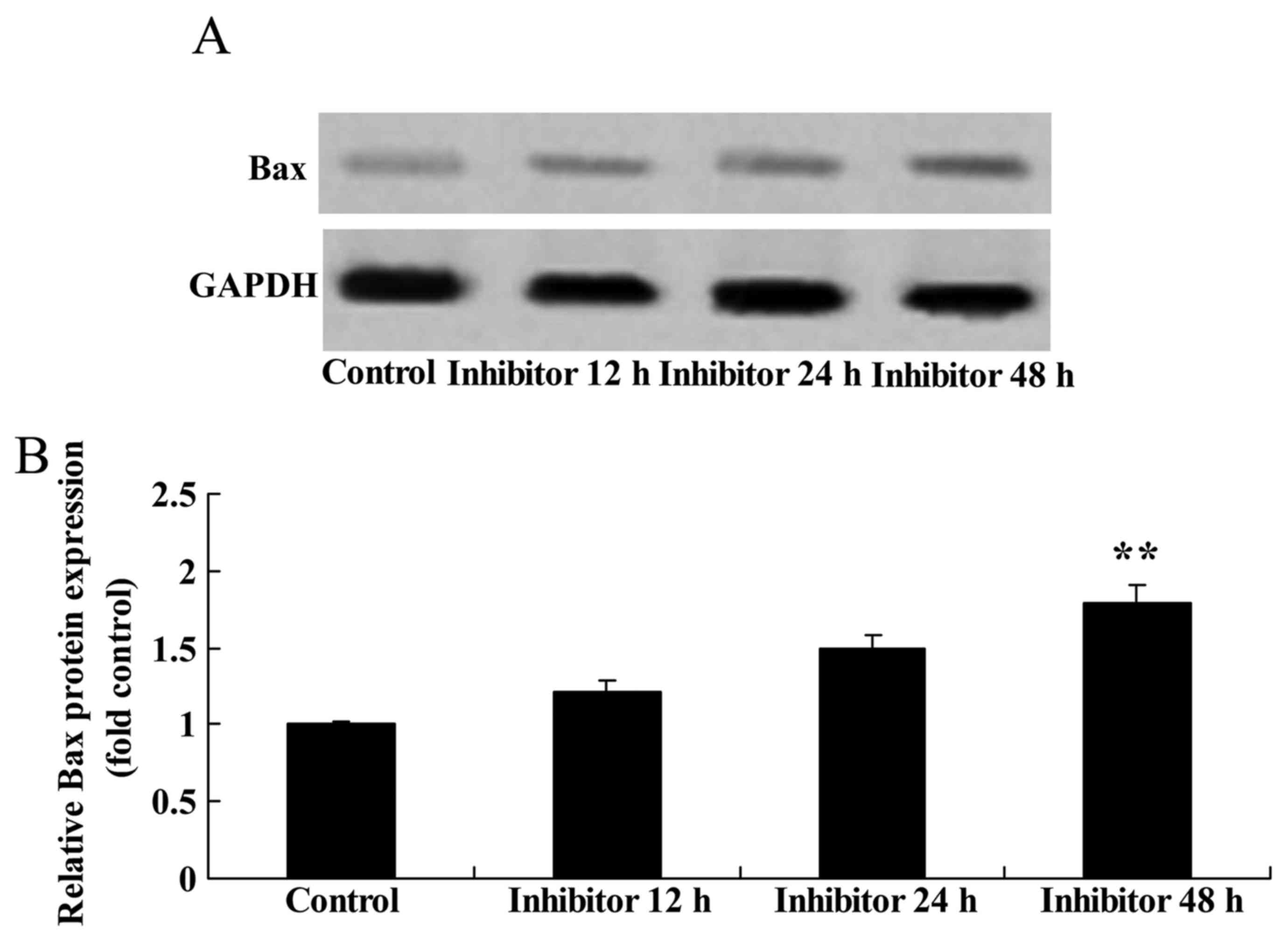
Effect of downregulating Wnt and
CTNNB1 on Bax protein expression in HCT-116 cells
Bax protein expression was measured following the
downregulation of Wnt and CTNNB1, as induced by IWR treatment. Bax
protein expression was significantly increased by the
downregulation of Wnt and CTNNB1 by 48 h post-IWR treatment in
HCT-116 cells compared with the untreated group (Fig. 7).
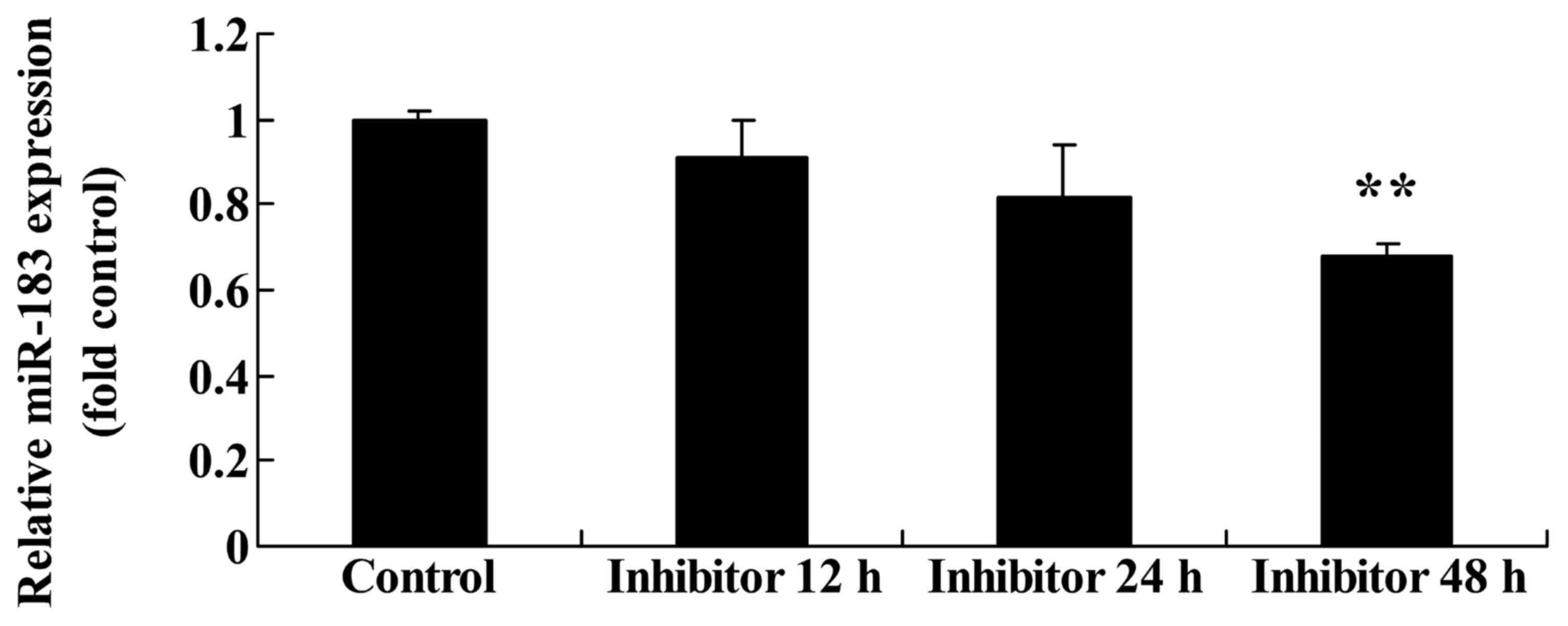
Effect of downregulating Wnt and
CTNNB1 on miR183 expression in HCT-116 cells
miR183 expression was evaluated following the
downregulation of Wnt and CTNNB1. The downregulation of Wnt and
CTNNB1, as induced by 48 h IWR treatment, significantly suppressed
miR183 expression in HCT-116 cells compared with the untreated
group (Fig. 8).
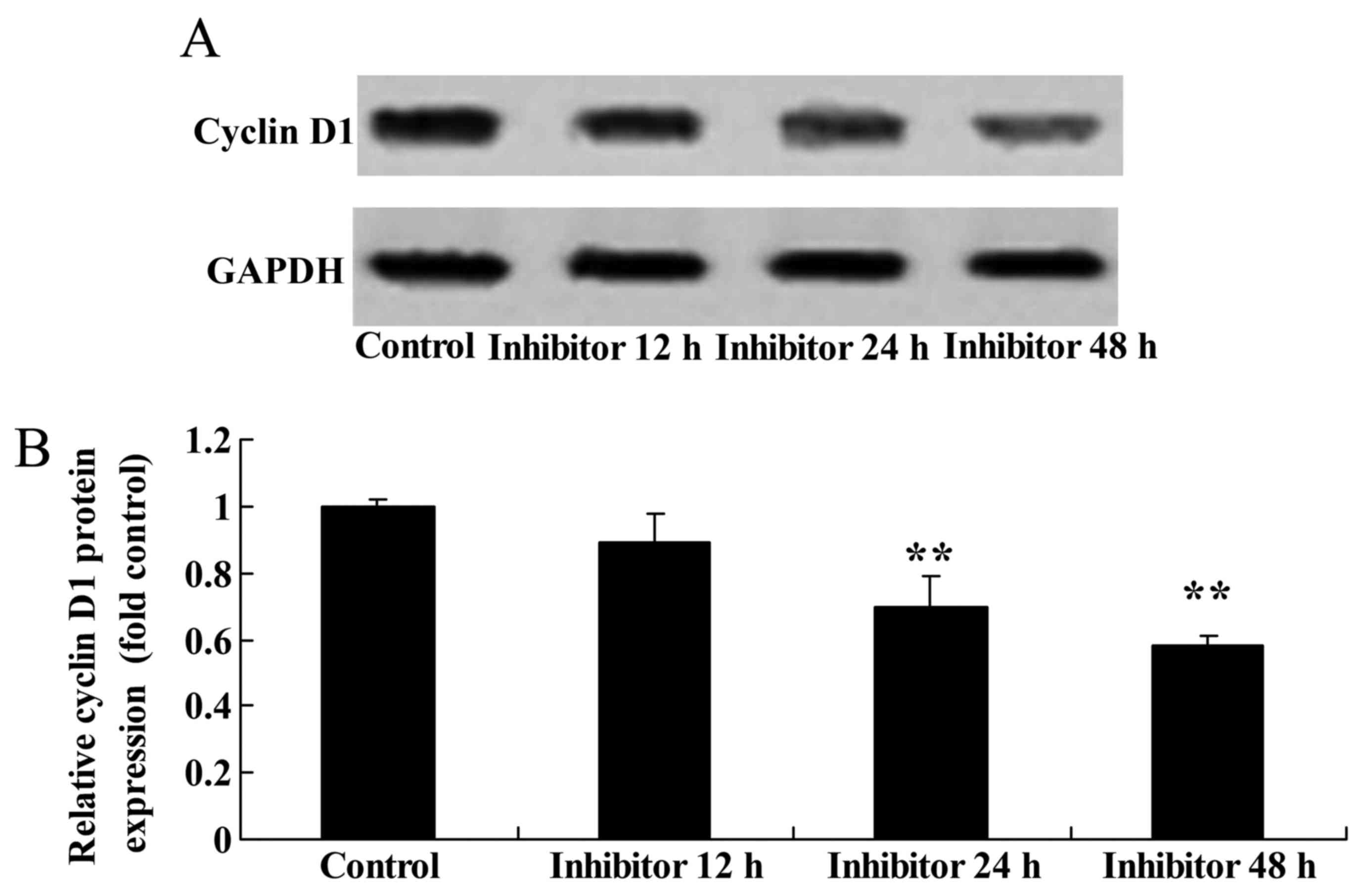
Effect of downregulating Wnt and
CTNNB1 on CCND1 protein expression in HCT-116 cells
Western blot analysis was performed to detect CCND1
protein expression in HCT-116 cells following the IWR-induced
downregulation of Wnt and CTNNB1. A significant decrease in CCND1
protein expression in 24 and 48 h IWR-treated HCT-116 cells was
detected compared with the untreated group (Fig. 9).
Discussion
Colorectal cancer is a common malignant tumor.
Globally, >1.2 million additional cases were estimated annually
in 2012 (17). Colorectal
cancer-associated morbidity ranks third and second among male and
female patients with malignant tumors, respectively (17). According to a 2008 report by the
International Agency for Research on Cancer, during the past 20–30
years, colorectal cancer has demonstrated the following
characteristics: i) Morbidity in the majority of countries and
areas has increased; ii) the increase in countries with previously
decreased morbidity is more marked; iii) the increase in developed
countries has decelerated and has reached a relatively stable
level; and iv) the morbidity in certain areas has decreased
(2). The present study revealed that
Wnt, CTNNB1 and miR183 expression in primary colorectal cancer
tissue was increased compared with that in paracarcinoma
tissue.
As the understanding of the human genome has
increased, it has been demonstrated that the expression of certain
genomic sequences may stabilize genetic ‘regulatory passwords’
under invariable conditions, a phenomenon that has attracted
increasing attention (8). These
epigenetic codes constitute a key information interface between
genes and phenotypes. Alteration of these codes is an important
means of regulating gene expression. MiRNAs are included among
these regulatory passwords (18). As
important factors regulated by gene transcription, miRNAs
participate widely in important life processes, including cell
proliferation, differentiation, apoptosis and development (18). In the present study, the DFS and OS
for patients with colorectal cancer with increased miR183
expression were decreased compared with that of the DFS and OS for
patients with colorectal cancer with decreased miR183
expression.
However, in different tumors, abnormal miR183
expression may serve different functions. MiR183 expression may be
upregulated in breast cancer cells; however, the overexpression of
miR183 may reduce the migration of breast cancer cells (19). A previous study revealed that miR183
expression is associated with the transfer potential of lung
carcinoma cells (20). The
overexpression of miR183 may reduce the transfer ability of lung
cancer cells, indicating that miR183 may potentially function as a
transfer-inhibiting factor of lung cancer cells (21). The results of another previous study
suggested that miR183 served a similar function to that of an
oncogene; by suppressing the expression of the transcription factor
early growth response 1, miR183 may promote the migration of
rhabdomyosarcoma tumor cells (21).
The present study revealed that downregulating Wnt and CTNNB1
expression suppressed viability, induced apoptosis and promoted
CASP3 activity and protein expression in HCT-116 cells.
The Wnt/CTNNB1 signaling pathway is conserved among
different species and controls multiple cell events including human
embryo growth, cell destiny, tissue and organ formation, and
tumorigenesis (15). The Wnt/CTNNB1
signaling pathway participates in the human central nervous sytem,
and in reproductive duct, breast, kidney, limb, placenta, hair and
bone development. Furthermore, dysregulated expression of the
components of the Wnt/CTNNB1 signaling pathway may induce embryo
mortality or abnormal embryonic development (22). In addition, the Wnt/CTNNB1 signaling
pathway is also associated with the renewal and differentiation of
embryonic and multiple tissue stem cells. Mucous epithelial cells
require regular renewal and, therefore, intestinal tract
stem/ancestral cells in the intestinal crypt regularly proliferate
and differentiate (22). Following
damage to, and the external stimulation of, mucous epithelial
cells, stem/ancestral cells may proliferate and differentiate into
numerous mucosal cells to maintain mucosal structural integrity
(23). In the gathering areas of the
stem/ancestral cells, in the bottom of the normal intestinal crypt,
the gathering of CTNNB1 in the cell nucleus may be observed. The
present study revealed that downregulating Wnt and CTNNB1
expression increased Bax protein expression in HCT-116 cells. Wu
et al (24) reported that the
Wnt/CTNNB1 signaling pathway promoted apoptosis, and enhanced CASP3
function and Bax protein expression in Bacillus Calmette-Guerin
vaccine-infected RAW264.7 macrophages.
A previous study indicated that the Wnt/CTNNB1
signaling pathway may promote the proliferation and maintain the
undifferentiated state in stem/ancestral cells of the bottom of the
intestinal crypt (25). TCF/LEF gene
knockout in animal intestinal tracts resulted in no crypt formation
in the hair. Furthermore, hair cells are seldom kept in the fission
(25). Mortality generally occurs in
the knockout animal following birth (26). The Wnt/CTNNB1 signaling pathway is
associated with the occurrence and development of tumors (26). In multiple malignant tumors, including
breast, gastric, thyroid, lung, prostate and skin cancer, the
abnormal activation of the Wnt/CTNNB1 signaling pathway and of
pathway-inhibiting proteins, including downregulated expression or
inactivation of dickkopf like acrosomal protein, secreted
frizzled-associated proteins or WNT inhibitory factor may be
demonstrated, and has been studied in the occurrence and
development of colorectal cancer (26). The abnormal activation of the
Wnt/CTNNB1 signaling pathway not only represents an early event of
colorectal cancer development, but is also important for its
progression (23). The present study
demonstrated that downregulating Wnt and CTNNB1 expression
suppressed miR183 expression and CCND1 protein expression in
HCT-116 cells. Leung et al (27) revealed that the Wnt/CTNNB1 signaling
pathway activated miR183 and promoted invasion in hepatocellular
carcinoma cells.
To conclude, the present study demonstrated that
downregulating Wnt and CTNNB1 expression suppressed miR183
expression and viability, promoted apoptosis and enhanced CASP3
activity and Bax protein expression in HCT-116 cells. The present
study also revealed that Wnt/CTNNB1/miR183 predicted the recurrence
and prognosis of colorectal cancer.
References
|
1
|
Feng Q, Wei Y, Ren L, Zheng P, Yu Y, Ye Q,
Ding J, Chen J, Chang W, Zhong Y, et al: Efficacy of continued
cetuximab for unresectable metastatic colorectal cancer after
disease progression during first-line cetuximab-based chemotherapy:
A retrospective cohort study. Oncotarget. 7:11380–11396.
2016.PubMed/NCBI
|
|
2
|
Sali L, Mascalchi M, Falchini M, Ventura
L, Carozzi F, Castiglione G, Delsanto S, Mallardi B, Mantellini P,
Milani S, et al: Reduced and full-preparation CT colonography,
fecal immunochemical test, and colonoscopy for population screening
of colorectal cancer: A randomized trial. J Natl Cancer Inst.
108:pii: djv3192015. View Article : Google Scholar
|
|
3
|
Lok SW, Wong HL, Kosmider S, Field K, Tie
J, Desai J, Bae S, Tacey M, Skinner I, Jones I and Gibbs P:
Translation of clinical trial outcomes to metastatic colorectal
cancer patients in community practice. Asia Pac J Clin Oncol.
10:361–367. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maxwell AE, Danao LL and Bastani R:
Dissemination of colorectal cancer screening by Filipino American
community health advisors: A feasibility study. Health Promot
Pract. 14:498–505. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu XL, Lin KJ, Bai AP, Wang WX, Meng XK,
Su XL, Hou MX, Dong PD, Zhang JJ, Wang ZY and Shi L: Osteopontin
knockdown suppresses the growth and angiogenesis of colon cancer
cells. World J Gastroenterol. 20:10440–10448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas J, Ohtsuka M, Pichler M and Ling H:
MicroRNAs: Clinical relevance in colorectal cancer. Int J Mol Sci.
16:28063–28076. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang JT, Wang JL, Du W, Hong J, Zhao SL,
Wang YC, Xiong H, Chen HM and Fang JY: MicroRNA 345, a
methylation-sensitive microRNA is involved in cell proliferation
and invasion in human colorectal cancer. Carcinogenesis.
32:1207–1215. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ma Y, Li W and Wang H: Roles of miRNA in
the initiation and development of colorectal carcinoma. Curr Pharm
Des. 19:1253–1261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong Y, Yu J and Ng SS: MicroRNA
dysregulation as a prognostic biomarker in colorectal cancer.
Cancer Manag Res. 6:405–422. 2014.PubMed/NCBI
|
|
10
|
Bandres E, Agirre X, Bitarte N, Ramirez N,
Zarate R, Roman-Gomez J, Prosper F and Garcia-Foncillas J:
Epigenetic regulation of microRNA expression in colorectal cancer.
Int J Cancer. 125:2737–2743. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Lu W, Saini SK, Moukha-Chafiq O,
Pathak V and Ananthan S: Identification of quinazoline compounds as
novel potent inhibitors of Wnt/β-catenin signaling in colorectal
cancer cells. Oncotarget. 7:11263–11270. 2016.PubMed/NCBI
|
|
12
|
Tarapore RS, Siddiqui IA, Adhami VM,
Spiegelman VS and Mukhtar H: The dietary terpene lupeol targets
colorectal cancer cells with constitutively active Wnt/β-catenin
signaling. Mol Nutr Food Res. 57:1950–1958. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Munding J, Ziebarth W, Pox CP, Ladigan S,
Reiser M, Hüppe D, Brand L, Schmiegel W, Tannapfel A and
Reinacher-Schick AC: The influence of 5-aminosalicylic acid on the
progression of colorectal adenomas via the β-catenin signaling
pathway. Carcinogenesis. 33:637–643. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fonseca BF, Predes D, Cerqueira DM, Reis
AH, Amado NG, Cayres MC, Kuster RM, Oliveira FL, Mendes FA and
Abreu JG: Derricin and derricidin inhibit Wnt/β-catenin signaling
and suppress colon cancer cell growth in vitro. PLoS One.
10:e01209192015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng
ZJ, Yuan SX, Wang DX, Luo JY, Zuo GW, et al: The PTEN/PI3K/Akt and
Wnt/β-catenin signaling pathways are involved in the inhibitory
effect of resveratrol on human colon cancer cell proliferation. Int
J Oncol. 45:104–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim K, Chandrasekar E and Lam H:
Colorectal cancer screening among Chinese, cambodian, and
vietnamese immigrants in Chicago. J Racial Ethn Health Disparities.
2:473–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang QH, Sun HM, Zheng RZ, Li YC, Zhang
Q, Cheng P, Tang ZH and Huang F: Meta-analysis of microRNA-183
family expression in human cancer studies comparing cancer tissues
with noncancerous tissues. Gene. 527:26–32. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huangfu L, Liang H, Wang G, Su X, Li L, Du
Z, Hu M, Dong Y, Bai X, Liu T, et al: miR-183 regulates autophagy
and apoptosis in colorectal cancer through targeting of UVRAG.
Oncotarget. 7:4735–4745. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Q, Ren W, Huang B, Yi L and Zhu H:
MicroRNA-183/182/96 cooperatively regulates the proliferation of
colon cancer cells. Mol Med Rep. 12:668–674. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou T, Zhang GJ, Zhou H, Xiao HX and Li
Y: Overexpression of microRNA-183 in human colorectal cancer and
its clinical significance. Eur J Gastroenterol Hepatol. 26:229–233.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bush BM, Brock AT, Deng JA, Nelson RA and
Sumter TF: The Wnt/β-catenin/T-cell factor 4 pathway up-regulates
high-mobility group A1 expression in colon cancer. Cell Biochem
Funct. 31:228–236. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong GZ, Shim AR, Hyeon JS, Lee HJ and Ryu
JH: Inhibition of Wnt/β-catenin pathway by dehydrocostus lactone
and costunolide in colon cancer cells. Phytother Res. 29:680–686.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu X, Deng G, Hao X, Li Y, Zeng J, Ma C,
He Y, Liu X and Wang Y: A caspase-dependent pathway is involved in
Wnt/β-catenin signaling promoted apoptosis in Bacillus
Calmette-Guerin infected RAW264.7 macrophages. Int J Mol Sci.
15:5045–5062. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi K, Hosono M, Sato I, Hata K,
Wada T, Yamaguchi K, Nitta K, Shima H and Miyagi T: Sialidase NEU3
contributes neoplastic potential on colon cancer cells as a key
modulator of gangliosides by regulating Wnt signaling. Int J
Cancer. 137:1560–1573. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang P and Li Z, Wang Y, Zhang L, Wu H and
Li Z: Secreted pyruvate kinase M2 facilitates cell migration via
PI3K/Akt and Wnt/β-catenin pathway in colon cancer cells. Biochem
Biophys Res Commun. 459:327–332. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Leung WK, He M, Chan AW, Law PT and Wong
N: Wnt/β-Catenin activates MiR-183/96/182 expression in
hepatocellular carcinoma that promotes cell invasion. Cancer Lett.
362:97–105. 2015. View Article : Google Scholar : PubMed/NCBI
|