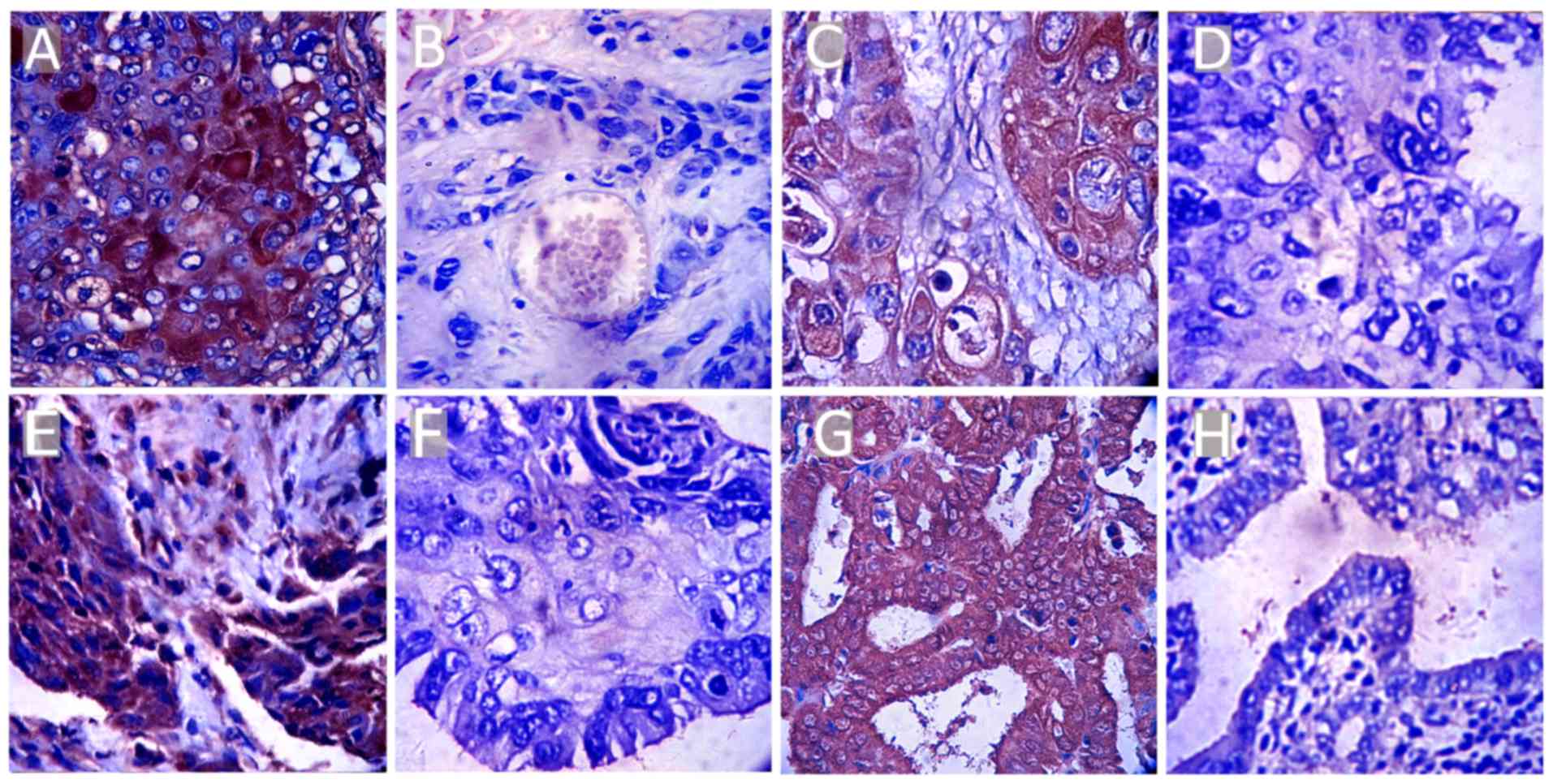
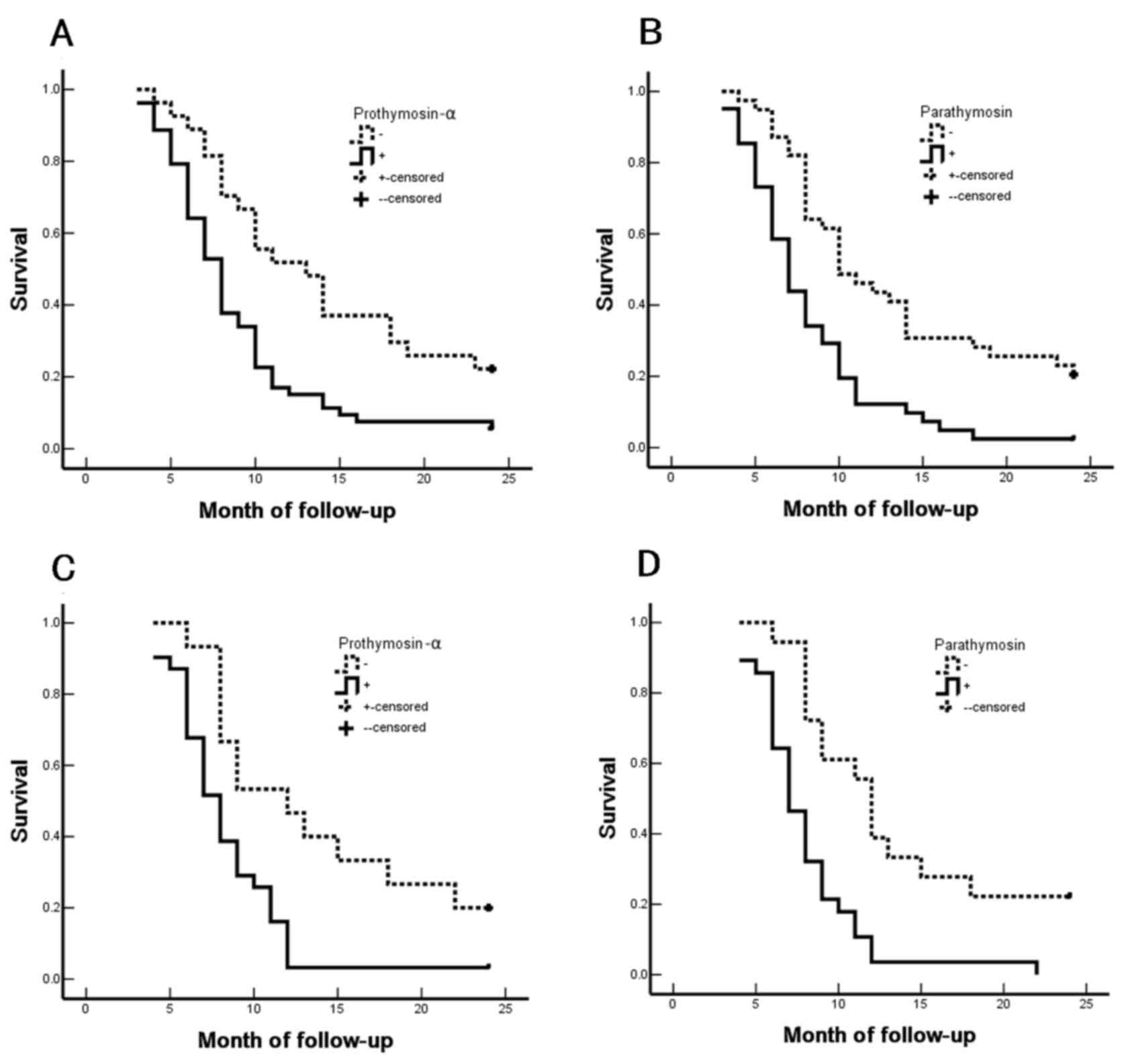
|
1
|
Roa JC, Tapia O, Cakir A, Basturk O,
Dursun N, Akdemir D, Saka B, Losada H, Bagci P and Adsay NV:
Squamous cell and adenosquamous carcinomas of the gallbladder:
Clinicopathological analysis of 34 cases identified in 606
carcinomas. Mod Pathol. 24:1069–1078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim WS, Jang KT, Choi DW, Choi SH, Heo JS,
You DD and Lee HG: Clinicopathologic analysis of
adenosquamous/squamous cell carcinoma of the gallbladder. J Surg
Oncol. 103:239–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kondo M, Dono K, Sakon M, Shimizu J,
Nagano H, Nakamori S, Umeshita K, Wakasa K and Monden M:
Adenosquamous carcinoma of the gallbladder. Hepatogastroenterology.
49:1230–1234. 2002.PubMed/NCBI
|
|
4
|
Nishihara K, Nagai E, Izumi Y, Yamaguchi K
and Tsuneyoshi M: Adenosquamous carcinoma of the gallbladder: A
clinicopathological, immunohistochemical and flow-cytometric study
of twenty cases. Jpn J Cancer Res. 85:389–399. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mingoli A, Brachini G, Petroni R,
Antoniozzi A, Cavaliere F, Simonelli L, Chirletti P and Modini C:
Squamous and adenosquamous cell carcinomas of the gallbladder. J
Exp Clin Cancer Res. 24:143–150. 2005.PubMed/NCBI
|
|
6
|
Chan KM, Yu MC, Lee WC, Jan YY and Chen
MF: Adenosquamous/squamous cell carcinoma of the gallbladder. J
Surg Oncol. 95:129–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rekik W, Ben Fadhel C, Boufaroua AL,
Mestiri H, Khalfallah MT, Bouraoui S and Mzabi-Rgaya S: Case
report: Primary pure squamous cell carcinoma of the gallbladder. J
Visc Surg. 148:e149–e151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oohashi Y, Shirai Y, Wakai T, Nagakura S,
Watanabe H and Hatakeyama K: Adenosquamous carcinoma of the
gallbladder warrants resection only if curative resection is
feasible. Cancer. 94:3000–3005. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haritos AA, Goodall GJ and Horecker BL:
Prothymosin alpha: Isolation and properties of the major
immunoreactive form of thymosin alpha 1 in rat thymus. Proc Natl
Acad Sci USA. 81:pp. 1008–1011. 1984; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Letsas KP and Frangou-Lazaridis M: Surfing
on prothymosin alpha proliferation and anti-apoptotic properties.
Neoplasma. 53:92–96. 2006.PubMed/NCBI
|
|
11
|
Jiang X, Kim HE, Shu H, Zhao Y, Zhang H,
Kofron J, Donnelly J, Burns D, Ng SC, Rosenberg S and Wang X:
Distinctive roles of PHAP proteins and prothymosin-alpha in a death
regulatory pathway. Science. 299:223–226. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jou YC, Tung CL, Tsai YS, Shen CH, Syue-Yi
C, Shiau AL, Tsai HT, Wu CL and Tzai TS: Prognostic relevance of
prothymosin-alpha expression in human upper urinary tract
transitional cell carcinoma. Urology. 74:951–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Letsas KP, Vartholomatos G, Tsepi C,
Tsatsoulis A and Frangou-Lazaridis M: Fine-needle aspiration
biopsy-RT-PCR expression analysis of prothymosin alpha and
parathymosin in thyroid: Novel proliferation markers? Neoplasma.
54:57–62. 2007.PubMed/NCBI
|
|
14
|
Wang M, Pan JY, Song GR, Chen HB, An LJ
and Qu SX: Altered expression of estrogen receptor alpha and beta
in advanced gastric adenocarcinoma: Correlation with prothymosin
alpha and clinicopathological parameters. Eur J Surg Oncol.
33:195–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang M, Cui F, Lu S, Lu H, Jiang T, Chen
J, Zhang X, Jin Y, Peng Z and Tang H: Increased expression of
prothymosin-α, independently or combined with TP53, correlates with
poor prognosis in colorectal cancer. Int J Clin Exp Pathol.
7:4867–4876. 2014.PubMed/NCBI
|
|
16
|
Frangou-Lazaridis M, Clinton M, Goodall GJ
and Horecker BL: Prothymosin alpha and parathymosin: Amino acid
sequences deduced from the cloned rat spleen cDNAs. Arch Biochem
Biophys. 263:305–310. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clinton M, Frangou-Lazaridis M,
Panneerselvam C and Horecker BL: The sequence of human parathymosin
deduced from a cloned human kidney cDNA. Biochem Biophys Res
Commun. 158:855–862. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haritos AA, Salvin SB, Blacher R, Stein S
and Horecker BL: Parathymosin alpha: A peptide from rat tissues
with structural homology to prothymosin alpha. Proc Natl Acad Sci
USA. 82:pp. 1050–1053. 1985; View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hannappel E and Huff T: The thymosins.
Prothymosin alpha, parathymosin, and beta-thymosins: Structure and
function. Vitam Horm. 66:257–296. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vareli K, Frangou-Lazaridis M, van der
Kraan I, Tsolas O and van Driel R: Nuclear distribution of
prothymosin alpha and parathymosin: Evidence that prothymosin alpha
is associated with RNA synthesis processing and parathymosin with
early DNA replication. Exp Cell Res. 257:152–161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lazcano-Ponce EC, Miquel JF, Muñoz N,
Herrero R, Ferrecio C, Wistuba II, Alonso de Ruiz P, Aristi Urista
G and Nervi F: Epidemiology and molecular pathology of gallbladder
cancer. CA Cancer J Clin. 51:349–364. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
Manual and the Future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hamilton SR and Aaltonen LA: Patholigy and
Genetics of Tumours of the Digestive System. IARC Press; Lyon:
2000
|
|
24
|
Okamoto K and Isohashi F: Purification and
primary structure of a macromolecular-translocation inhibitor II of
glucocorticoid-receptor binding to nuclei from rat liver. Inhibitor
II is the 11.5-kDa Zn2+-binding protein (parathymosin). Eur J
Biochem. 267:155–162. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoch K and Volk DE: Structures of thymosin
proteins. Vitam Horm. 102:1–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mosoian A, Teixeira A, High AA, Christian
RE, Hunt DF, Shabanowitz J, Liu X and Klotman M: Novel function of
prothymosin alpha as a potent inhibitor of human immunodeficiency
virus type 1 gene expression in primary macrophages. J Virol.
80:9200–9206. 2006. View Article : Google Scholar : PubMed/NCBI
|