Introduction
The high mobility group (HMG) proteins were
originally isolated from the nuclei of mammalian cells and named
according to their electrophoretic mobility (1). HMGs were later subdivided into three
families: HMG-AT-hook family (HMGA), the HMG-box family (HMGB) and
the HMG-nucleosome binding family (HMGN), each comprising several
protein members (2). The HMGN family
includes five chromatin architectural proteins, which are present
in higher vertebrates (3). A number
of previous studies have demonstrated that HMGNs are
transcriptional co-regulators that serve important functions in DNA
repair and cancer progression (3–6). HMGN2 is
the most conserved member of the HMGN family, involved in unfolding
higher-order chromatin structures and facilitating the
transcriptional activation of various mammalian genes (7,8). However,
the biological and pathological functions of HMGN2 are not well
understood. In the present study, HMGN2 expression was evaluated in
tumor cells and human oral squamous cell carcinoma (OSCC) tissues.
The results demonstrated that the expression of HMGN2 was
significantly increased in the majority of tumor cell lines
compared with periodontal ligament cells (PDLCs). Furthermore,
HMGN2 expression was increased in human OSCC tissues compared with
normal oral tissues. In metastatic OSCC tissues, HMGN2 expression
was increased, compared with non-metastatic OSCC tissues.
Materials and methods
Ethical approval
The Committee for Ethical Approval and the State Key
Laboratory of Oral Diseases of the West China School of
Stomatology, Sichuan University (Chengdu, China) approved the
present study (reference no. WCHSIRB-ST-2015-147). Protocols
regarding the use and manipulation of PDL tissues were approved by
the Institutional Review Board of West China Hospital of
Stomatology, Sichuan University (Institutional Review Board
reference number: WCHSIRB-D-2013-039). All PDLCs and OSSC tissues
donors have provided written informed consent.
Isolation and culture of periodontal
ligament cells (PDLCs)
PDL tissues were isolated from the extracted
premolar teeth of three healthy donors (two females and one male
aged 14±3 years old) who received orthodontic treatment at the West
China Hospital of Stomatology of Sichuan University from July to
September in 2016. Teeth with caries, periodontitis and periapical
periodontitis were excluded. The extracted teeth were rinsed with
Dulbecco's Modified Eagle Medium (HyClone; GE Healthcare, Chicago,
IL, USA) supplemented with 100 U/ml penicillin and 100 µg/ml
streptomycin. The remaining procedures for PDLC extraction were
performed as previously described by Arnold and Baram (9).
Western blotting and densitometry
The following human cell lines were kindly donated
by Dr Qianming Chen (West China Hospital of Stomatology of Sichuan
University), who purchased them from The American Type Culture
Collection (Manassas, VA, USA) and cultured them at the State Key
Laboratory of Oral Diseases; these included the lung adenocarcinoma
cell line, H1975; oral squamous carcinoma cell line, HSC-4; triple
negative breast cancer cell line, MDA-MB-468; tripe negative breast
cancer cell line, MDA-MB-231; oral squamous carcinoma cell line,
SCC-25, and leukemia cell line, THP-1. The H1975, HSC-4,
MDA-MB-2×3, MDA-MB-468 and THP-1 cell lines were cultured in RPMI
1640, the SCC-25 cell line was cultured in DEM/F-12 (Hyclone; GE
Healthcare) and the PDLC cell line was cultured in α-MEM medium
(all media were purchased from Hyclone; GE Healthcare). All cell
lines were supplemented with 10% fetal calf serum (Hyclone; GE
Healthcare), 100 mg/ml streptomycin and 100 IU/ml penicillin (both
Gibco; Thermo Fisher Scientific Inc.). All tumor cell lines and
PDLCs were cultured in a humidified incubator at 37°C with 5%
CO2, and all experiments were conducted while cells were
in the logarithmic growth phase. Total protein was extracted with a
total protein extraction kit (Jiangsu, Keygen Biotech Co., Ltd.,
Jiang Su, China), according to the manufacturer's protocol. Total
protein concentration was determined by BCA assay (Thermo Fisher
Scientific Inc., Waltham, MA, USA), and equal amounts of protein
(20 µg) from each sample were separated by SDS-PAGE (12% gel)
according to the molecular weights of the tested proteins. Proteins
were then transferred onto Immun-Blot polyvinylidenedifluoride
membranes (Invitrogen; Thermo Fisher Scientific, Inc.) and blocked
with 5% skimmed milk at room temperature for 1 h. Anti-HMGN2 and
anti-β-actin primary antibodies (both diluted 1:1,000; catalog nos.
ab199679 and ab8226; Abcam, Cambridge, UK) were incubated with the
membranes at 4°C overnight. Bound antibodies were detected by
incubation with corresponding horseradish peroxidase-conjugated
secondary antibodies (dilution, 1:2,000; catalog no. ZB-2301;
OriGene Technologies, Inc., Beijing, China) at room temperature for
2 h. Following three washes in tris-buffered saline with Tween 20,
immunoreactive bands were detected using the Easy ECL Western Blot
kit (TransGen Biotech, Inc., Beijing, China) and captured using the
Chemidoc XRS system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Quality One software version 4.62 (Bio-Rad Laboratories,
Inc.) was used to quantify relative protein level in different cell
lines; these levels were normalized to the concentration of
β-actin. Experiments for each cell line were performed in
triplicate.
Immunohistochemical analysis
Sections of human OSCC and normal oral tissues,
including 2 cases of tongue, 2 of palate, 1 of gingiva
non-metastatic OSCC and 1 of gingival, oropharynx, tongue, cheek
and floor of mouth metastatic OSCC respectively, in addition to 1
case of normal tissue serving as a control. These tissues were
evaluated for HMGN2 expression by immunohistochemical analysis. A
total of 11 specimens were selected from the Department of
Pathology of West China Hospital of Stomatology (Chengdu, China)
between March and September in 2016, where 5 metastatic OSCC
specimens, 5 non-metastatic OSCC specimens and 1 non-neoplastic
oral tissue specimen were included. The clinicopathological
information of patients providing theses specimens are provided in
Table I. Tissues were fixed with 4%
paraformaldehyde for 24 h at 4°C and embedded in paraffin. Tissues
sections of 4-µm thickness were subsequently deparaffinized with
xylene and rehydrated in a graded series of ethanol (95, 90, 80 and
75%). Endogenous peroxidase activity was quenched with 0.3%
H2O2 for 10 min. Heat-induced antigen
retrieval was carried out in 0.5 mol/l of
ethylenediaminetetraacetic acid (pH 7.0) in a pressure cooker for 5
min. Nonspecific binding was blocked using 10% normal goat serum
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) at 37°C for 30
min prior to incubation with the primary antibody (dilution, 1:50;
catalog no. ab199679; Abcam) in PBS overnight. According to the
manufacture's protocol, slides were rewarmed at 37°C for 1 h and
then stained with anti-rabbit biotinylated second antibody at 37°C
for another 1 h, followed by an incubation with a
streptavidin-horseradish peroxidase system for 30 min at 37°C
(catalog no. KGSP03-KGSP04; Nanjing KeyGen Biotech Co., Ltd.). The
antigen-antibody reaction was visualized using
3,3′-diaminobenzidine, with positive cells staining brown. The
sections were subsequently counterstained with 0.2% hematoxylin for
30 sec at room temperature, then dehydrated in a series of ethanol
washes (80, 90, 95 and 100% respectively), dried, and mounted onto
coverslips. Images of representative areas of each sample were
captured under light microscopy at ×40 and ×400 magnification
(Nikon Corporation, Tokyo, Japan).
 | Table I.Clinicopathological information of
patients who provided tissue for immunohistochemistry. |
Table I.
Clinicopathological information of
patients who provided tissue for immunohistochemistry.
| Tissue type | Sex | Age, years | Pathological
diagnosis |
|---|
| Non-neoplastic | Male | 25 | Tongue tissue
(normal) |
|
| Male | 48 | SCC (Phase I–II) |
|
| Female | 44 | SCC (Phase II) |
| Non-metastatic
OSCC | Male | 69 | SCC (Phase II), with
eosinophilic infiltration |
|
| Male | 50 | SCC (Phase I) |
|
| Male | 62 | SCC (Phase I–II),
with salivary gland infiltration |
|
| Male | 61 | SCC (Phase II), with
metastasis to superior deep cervical lymphatic nodes |
|
| Male | 50 | SCC (Phase II–III),
with metastasis to superior deep cervical lymphatic nodes |
| Metastatic OSCC | Male | 63 | SCC (Phase I), with
metastasis to submental submandibular lymphatic nodes and superior,
middle and inferior deep cervical lymphatic nodes |
|
| Male | 69 | SCC (Phase I), with
metastasis to superior deep cervical lymphatic nodes |
|
| Male | 57 | SCC (Phase I and II),
with muscle layer and salivary gland infiltration; with metastasis
to superior deep cervical lymphatic nodes |
Total staining score for human OSCC and normal oral
tissues was calculated as the product of the intensity score of
HMGN2 and the percentage of HMGN2-positive cells. As a result, the
higher the total staining score, the stronger the expression of
HMGN2. Specific scoring was applied according to the following
criteria: i) The intensity scores of HMGN2 were as follows: Strong
(4); moderate (3); mild (2);
and negative (1); ii) A section was
considered positive based on brown staining in the cell nucleus,
and the mean percentage of positive cells in 5 fields of vision at
×400 magnification was scored as follows: 0 point, <5%; 1 point,
5–25%; 2 points, 26–50%; 3 points, 51–75%; and 4 points, >75%;
iii) HMGN2 expression was acquired by multiplying the intensity
score by the percentage of positive cells (points) to produce a
total score: 0–4, negative; 4–8, mild; 8–12, moderate; 12–16,
marked. D) Experiments were performed in triplicate and two
pathologists evaluated total score independently in each
experiment.
Statistical analysis
SPSS software v20.0 (IBM Corp., Armonk, NY, USA) was
adopted for data analysis. According to data type, a one-way
analysis of variance and then Dunnett-t test were used to identify
differences in HMGN2 expression among various cell lines. In
addition, the Kruskal-Wallis test followed by Student-Newman-Keuls
post hoc test were applied to evaluate significance among three
groups of tissues. P<0.05 was considered to indicate a
statistically significant difference.
Results
HMGN2 expression is increased in tumor
cell lines
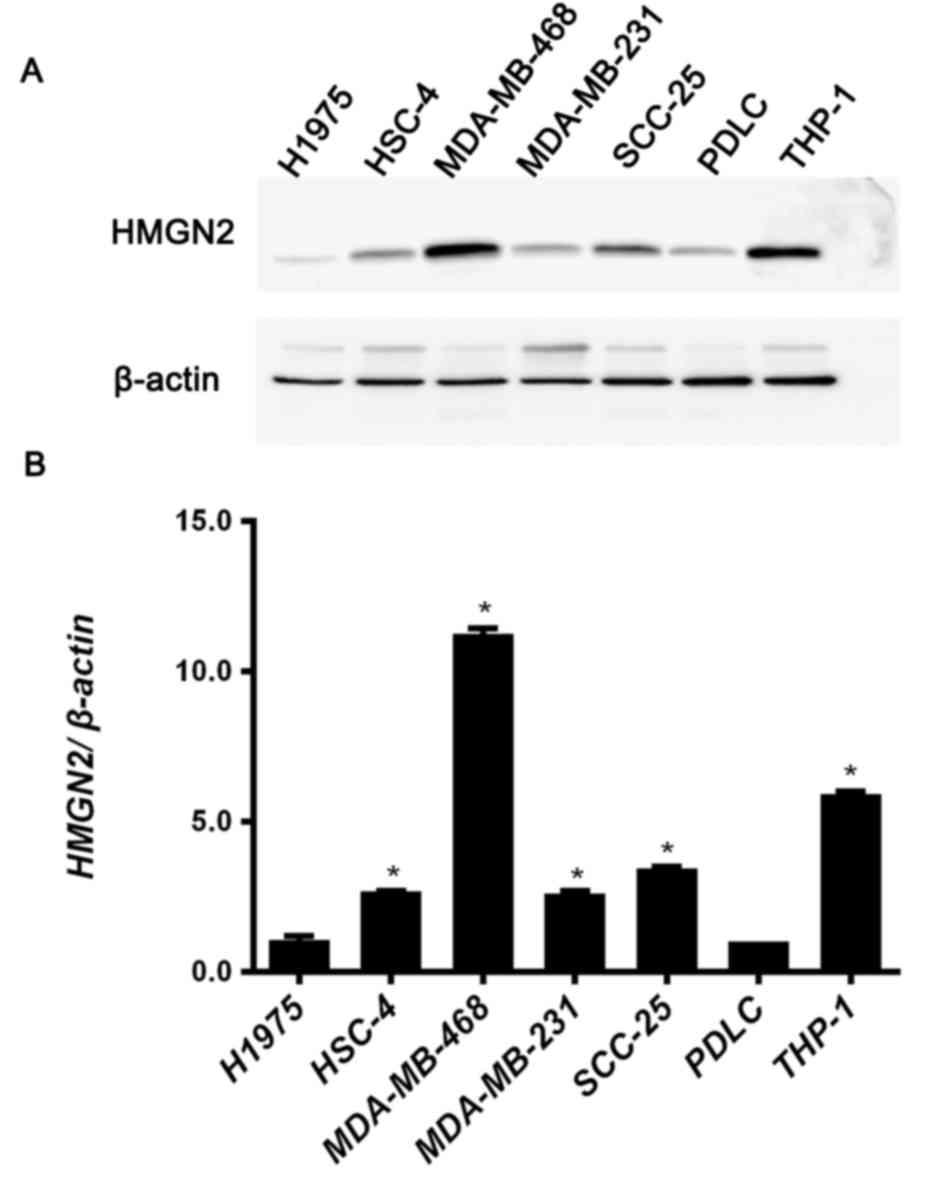
The protein expression level of HMGN2 in tumor cell
lines was analyzed using western blotting. H1975, HSC-4,
MDA-MB-468, MDA-MB-231, SCC-25 and THP-1 cells were selected for
analysis of HMGN2 expression. PDLCs were used as noncancerous
control cells. The HMGN2 expression levels in these cell lines are
presented in Fig. 1.
All tumor cells with the exception of the H1975 cell
line demonstrated increased expression of HMGN2 compared with
PDLCs. In particular, MDA-MB-468 cells and THP-1 cells exhibited
marked HMGN2 expression levels (Fig. 1A
and B). Compared with PDLCs, the expression of HMGN2 was
>10-fold greater in MDA-MB-468 cells and >5-fold greater in
THP-1 cells (Fig. 1B). The expression
level of HMGN2 in HSC-4, MDA-MB-231 and SCC-25 cells were also
increased compared with PDLCs (Fig.
1B).
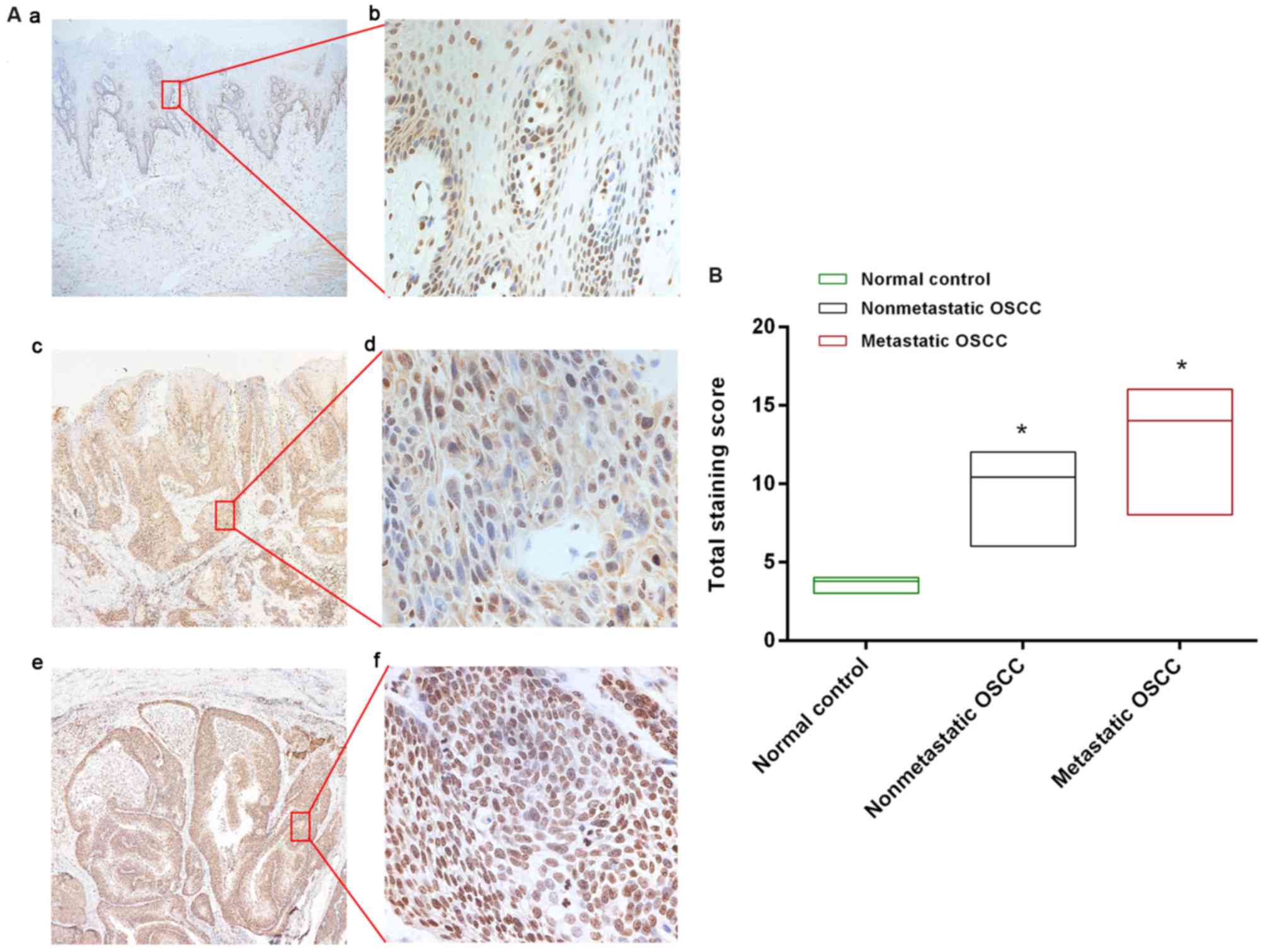
HMGN2 is overexpressed in human OSCC tissues. HMGN2
expression in human OSCC and control tissues was evaluated using
immunohistochemistry. A total of 5 metastatic and 5 non-metastatic
OSCC tissues, and 1 non-neoplastic oral tissue were included in
this analysis. Representative areas of each sample were captured,
and the number of positive (i.e., stained) cells, as well as the
staining intensity, were determined. As presented in Fig. 2, positive staining for HMGN2 was
primarily nuclear-localized and populated basal, tumor and
inflammatory cells. These results supported the hypothesis that
HMGN2 is involved in cell proliferation, inflammation and
tumorigenesis. In noncancerous control specimens, low or absent
anti-HMGN2 immunoreactivity was observed in basal cells.
Nevertheless, the overwhelming majority of squamous and
inflammatory cells exhibited markedly positive staining for
anti-HMGN2, particularly in metastatic OSCC tissues. In 5
non-metastatic OSCC tissues, 4 cases (80%) exhibited moderate
nuclear staining, and 4 (80%) metastatic OSCC tissues exhibited
strong nuclear staining.
Discussion
HMG proteins are classified into super families on
the basis of their physical and chemical properties. Each of these
proteins contains a unique structural motif that induces specific
changes in its binding sites and participates in distinct cellular
functions (10). A total of 3 HMG
protein families are defined in terms of the structure of their DNA
binding domains as well as their substrate binding specificity. The
three families include the HMGA, HMGB and HMGN family (11,12). These
HMG proteins contribute to chromatin dynamics, the transcriptional
activities of various genes and other cellular processes by
distorting, bending or otherwise modifying the structure of DNA
(13,14).
The HMGN family comprises 5 chromatin architectural
proteins that are present in higher vertebrates (13). Of these, HMGN1, 2 and 4 are expressed
ubiquitously, whereas HMGN 3 and 5 are expressed in specific
tissues (4,15,16). The
function of these proteins in transcription, DNA repair and cancer
progression have been partly established (3,4,17,18). The
results of recent studies have demonstrated that HMGN1 may be a
promising clinical biomarker for several types of cancer (19,20).
Similarly, the expression of HMGN5 (previously known as NSBP1) is
highly regulated in various types of human cancer, including
prostate, bladder, breast, lung and clear-cell renal-cell carcinoma
(21). Knockdown of HMGN5 was
demonstrated to suppress the viability and invasive ability of
human urothelial bladder cancer cells (22), which further supports the involvement
of HMGN5 in cancer progression.
HMGN2 is expressed ubiquitously in adult tissues and
is markedly expressed during embryogenesis (16,23). The
HMGN2 gene is located on chromosome 1p36.1 and contains 6 exons
(24). HMGN2 expression is
downregulated during the physiological processes of myogenesis,
erythrogenesis and chondrogenesis, as well as during embryonic
organ formation, such as kidney development (10). Abnormal expression of the HMGN2 gene
or protein has been associated with the development of neoplasms
and autoimmune diseases (25–27). However, due to the poorly defined
biological functions of this protein, further research is
required.
In the present study, HMGN2 expression was examined
in 7 tumor cell lines. The results indicated that the expression
levels of HMGN2 in the majority of tumor cell lines, particularly
MDA-MB-468 and THP-1 cells, were increased compared with PDLCs
(Fig. 1A and B). This result is in
accordance with previous results that HMGN2 was increased in
leukemia, breast cancer and small-intestine cancer cells, which
suggested an effect of HMGN2 on transcription and cell
differentiation in cancer progression (28). Based on the western blotting results
in the present study, it is speculated that HMGN2 serves an
important function in tumorigenesis (29). Oral cancer, comprising >95%
squamous cell carcinoma, is the sixth greatest public health
concern globally in terms of increasing incidence and mortality
rates (30,31). For this reason, HMGN2 expression was
examined inhuman OSCC tissues by immunohistochemistry. The
expression of HMGN2 in OSCC tissues was increased compared with
normal oral tissue. In addition, HMGN2 expression was increased in
metastatic OSCC tissues compared with that in non-metastatic
tissue. These results indicate that HMGN2 protein may be associated
with tumor metastasis.
Oncogene and tumor-associated protein overexpression
serves a key function in the progression of various forms of human
cancer (32–34). The aim of the present study was to
characterize the endogenous expression of HMGN2 in tumor cells. The
results demonstrate that the expression of HMGN2 was significantly
increased in the majority of tumor cell lines tested compared with
control PDLCs. We hypothesized that HMGN2 may serve a vital
function in the development and invasion of certain types of
cancer, having previously examined the anti-tumor mechanism of
HMGN2 protein on Tca8113 cells, an oral squamous cell carcinoma
line in which the results demonstrated that HMGN2 could induce
apoptosis in Tca8113 cells and S-phase cell cycle arrest in Tca8113
cells (35). Although additional
in vitro and in vivo studies addressing HMGN2 during
invasion and metastasis are required, the present study indicates
that HMGN2 regulates the proliferation and metastasis of various
types of human tumor cells in vitro. The main result of the
present study, that HMGN2 suppression controlled the proliferation
and metastasis of tumor cells, may have broad implications in tumor
diagnosis and therapy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81372892, 81621062
and 81520108009), 111 Project of the Ministry of Education China
(grant no. B14038), and the Research Foundation from the State Key
Laboratory of Oral Disease Sichuan University (grant no.
SKLOD201601).
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HMGN2
|
high mobility group
nucleosomal-binding domain 2
|
|
OSCC
|
oral squamous carcinoma
|
|
PDLCs
|
periodontal ligament cells
|
|
shRNAs
|
small hairpin RNAs
|
References
|
1
|
Grasser KD, Wurz A and Feix G: Isolation
and characterization of high-mobility-group proteins from maize.
Planta. 185:350–355. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bianchi ME and Agresti A: HMG proteins:
Dynamic players in gene regulation and differentiation. Curr Opin
Genet Dev. 15:496–506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerlitz G: HMGNs, DNA repair and cancer.
Biochim Biophys Acta. 1799:80–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rochman M, Taher L, Kurahashi T, Cherukuri
S, Uversky VN, Landsman D, Ovcharenko I and Bustin M: Effects of
HMGN variants on the cellular transcription profile. Nucleic Acids
Res. 39:4076–4087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimahara H, Hirano T, Ohya K, Matsuta S,
Seeram SS and Tate S: Nucleosome structural changes induced by
binding of non-histone chromosomal proteins HMGN1 and HMGN2. FEBS
Open Bio. 3:184–119, 12013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Subramanian M, Gonzalez RW, Patil H, Ueda
T, Lim JH, Kraemer KH, Bustin M and Bergel M: The
nucleosome-binding protein HMGN2 modulates global genome repair.
FEBS J. 276:6646–6657. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vestner B, Bustin M and Gruss C:
Stimulation of replication efficiency of a chromatin template by
chromosomal protein HMG-17. J Biol Chem. 273:9409–9414. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fiorillo AA, Medler TR, Feeney YB, Liu Y,
Tommerdahl KL and Clevenger CV: HMGN2 inducibly binds a novel
transactivation domain in nuclear PRLr to coordinate
Stat5a-mediated transcription. Mol Endocrinol. 25:1550–1564. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arnold LF and Baram P: In vitro culture of
periodontal ligament cells. J Dent Res. 51:953–959. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hock R, Furusawa T, Ueda T and Bustin M:
HMG chromosomal proteins in development and disease. Trends Cell
Biol. 17:72–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bustin M: Revised nomenclature for high
mobility group (HMG) chromosomal proteins. Trends Biochem Sci.
26:152–153. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Reeves R: Nuclear functions of the HMG
proteins. Biochim Biophys Acta. 1799:3–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gonzálezromero R, Eirínlópez JM and Ausió
J: Evolution of high mobility group nucleosome-binding proteins and
its implications for vertebrate chromatin specialization. Mol Biol
Evol. 32:121–131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Q and Wang Y: HMG modifications and
nuclear function. Biochim Biophys Acta. 28:1799: 28–36. 2010.
|
|
15
|
Kugler JE, Deng T and Bustin M: The HMGN
family of chromatin-binding proteins: Dynamic modulators of
epigenetic processes. Biochim Biophys Acta. 1819:652–656. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Furusawa T and Cherukuri S: Developmental
function of HMGN proteins. Biochim Biophys Acta. 1799:692010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raymond R: High mobility group (HMG)
proteins: Modulators of chromatin structure and DNA repair in
mammalian cells. DNA Repair (Amst). 36:122–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu BL and Wan Y: Research progression
about the HMGN protein in tumor. J Int Oncol. 1–281. 2016.
|
|
19
|
Wei F, Yang F, Jiang X, Yu W and Ren X:
High-mobility group nucleosome-binding protein 1 is a novel
clinical biomarker in non-small cell lung cancer. Tumor Biol.
36:9405–9410. 2015. View Article : Google Scholar
|
|
20
|
Nie Y, Yang D and Oppenheim JJ: Alarmins
and antitumor immunity. Clin Ther. 38:1042–1053. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li DQ, Hou YF, Wu J, Chen Y, Lu JS, Di GH,
Ou ZL, Shen ZZ, Ding J and Shao ZM: Gene expression profile
analysis of an isogenic tumour metastasis model reveals a
functional role for oncogene AF1Q in breast cancer metastasis. Eur
J Cancer. 42:3274–3286. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gan Y, Tan J, Yang J, Zhou Y, Dai Y, He L,
Yao K and Tang Y: Erratum to: Knockdown of HMGN5 suppresses the
viability and invasion of human urothelial bladder cancer 5,637
cells in vitro and in vivo. Med Oncol. 32:1612015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mohan G: Chromatin-binding HMGN proteins
and the neuronal differentiation of enbryonal carcinoma cells in
vitro. University of Glasgow. 2012.
|
|
24
|
Popescu N, Landsman D and Bustin M:
Mapping the human gene coding for chromosomal protein HMG-17. Human
Genet. 85:376–378. 1990. View Article : Google Scholar
|
|
25
|
Xie P, Deng LX, Gong P, Ding Y and Tang
XH: Expression of HMGB1 and HMGN2 in gingival tissues, GCF and PICF
of periodontitis patients and peri-implantitis. Braz J Microbiol.
42:1213–1219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deng LX, Wu GX, Cao Y, Fan B, Gao X, Tang
XH and Huang N: The chromosomal protein HMGN2 mediates the
LPS-induced expression of β-defensins in mice. Inflammation.
35:456–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Porkka K, Laakkonen P, Hoffman JA,
Bernasconi M and Ruoslahti E: A Fragment of the HMGN2 protein homes
to the nuclei of tumor cells and tumor endothelial cells in vivo.
Proc Natl Acad Sci USA. 99:pp. 7444–7449. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chengqian Y, Shuai S, Xue W, Yuting Z and
Jianfa Y: Pathology Do: Research progress of high mobility group N2
in tumor. J Modern Oncol. 2016.
|
|
29
|
Porkka K, Laakkonen P, Hoffman JA,
Bernasconi M and Ruoslahti E: A fragment of the HMGN2 protein homes
to the nuclei of tumor cells and tumor endothelial cells in vivo.
Proc Natl Acad Sci USA. 99:pp. 7444–7449. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neville BW and Day TA: Oral cancer and
precancerous lesions. CA Cancer J Clin. 52:195–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Choi S and Myers JN: Molecular
pathogenesis of oral squamous cell carcinoma: Implications for
therapy. J Dent Res. 87:14–32. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Andishehtadbir A, Najvani AD, Pardis S,
Ashkavandi ZJ, Ashraf MJ, Khademi B and Kamali F:
Metastasis-associated protein 1 expression in oral squamous cell
carcinomas: Correlation with metastasis and Angiogenesis/Metastaz
İlişkili protein 1 ekspresyonunun oral Skuamöz Hücreli
karsinomlarda metastaz ve anjiyogenez ile İlişkisi. Turkish J
Pathol. 31:9–15. 2015.
|
|
33
|
Li SH, Tian H, Yue WM, Li L, Li WJ, Chen
ZT, Hu WS, Zhu YC and Qi L: Overexpression of metastasis-associated
protein 1 is significantly correlated with tumor angiogenesis and
poor survival in patients with early-stage non-small cell lung
cancer. Ann Surg Oncol. 18:2048–2056. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu CC, Huang YS, Lee LY, Liang Y, Tang RP,
Chang YS, Hsieh LL and Yu JS: Overexpression and elevated plasma
level of tumor-associated antigen 90K/Mac-2 binding protein in
colorectal carcinoma. Proteomics Clin Appl. 2:1586–1595. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu A, Dong X, Liu X, Zhang P, Zhang Y, Su
N, Chen Q and Feng Y: Nucleosome-binding protein HMGN2 exhibits
antitumor activity in oral squamous cell carcinoma. Oncol Lett.
7:115–120. 2014. View Article : Google Scholar : PubMed/NCBI
|