Introduction
In previous years, lung cancer has been a leading
cause of mortality globally (1).
According to reports from the International Agency for Research on
Cancer, the morbidity and mortality rates of lung cancer have been
increasing markedly, and lung cancer has become a significant
threat to human health (2). Non-small
cell lung cancer (NSCLC) accounts for ~85% of all lung cancer cases
globally (3), with the majority
diagnosed at intermediate or advanced stages (3). Morbidity is higher among men than women
(4). Despite improved laboratory
diagnosis, surgery, chemotherapy and radiotherapy techniques, the
5-year survival rate and prognosis of patients with NSCLC remain
unfavorable (5). The main cause of
treatment failure is local recurrence (4).
Radiotherapy is an important local and regional
therapeutic technique. The role of ionizing radiation in
radiotherapy is the induction of DNA double-strand breaks (DSBs)
and the inhibition of DNA repair (6).
Thorough investigation of molecular variations that affect
responses to radiation is required to improve the understanding of
the differences in radiosensitivity between individuals. Therefore,
understanding NSCLC at the molecular level has attracted extensive
research efforts. Furthermore, identifying a specific target to
enhance radiotherapeutic efficacy and reduce its adverse effects in
NSCLC is of notable interest.
The uncontrolled proliferation of a tumor results in
the consumption of large amounts of nutritional substances
(7). Under pathological conditions,
aerobic oxidation and anaerobic metabolism cannot meet the
unlimited energetic requirements of tumor cells (8). Therefore, increasing the function of fat
metabolism is an important mechanism by which to maintain the
energy supply of tumor cells, in addition to increasing glucose
availability (9). Dysregulated lipid
metabolism is strongly associated with the development, maintenance
and metastatic progression of tumors (10,11). Based
on this, it is hypothesized that the reduction of fatty acid
synthesis may inhibit tumor cell growth and therefore that fatty
acid synthase (FASN) may be a potential target in the development
of anticancer treatments.
FASN is the pivotal enzyme required for the de
novo synthesis of fatty acids and represents one of the most
commonly overexpressed lipogenic enzymes (12), which are critical to fatty acid
synthesis and metabolism. This is thought to be essential for the
progression, metastasis, and drug and radiation resistance of
tumors (12). In recent years,
accumulating evidence has demonstrated that the overexpression FASN
is strongly associated with an unfavorable prognosis and treatment
resistance in a variety of human neoplasms, including breast
(13), bladder (14), nasopharyngeal carcinoma (15), esophageal (16) and pancreatic cancer (17). Although the roles of FASN in tumor
progression and treatment resistance have been investigated, there
have been no studies of FASN expression in NSCLC and its
association with sensitivity to ionizing irradiation.
Although the expression of FASN is upregulated in
various types of cancer, its association with radiosensitivity in
NSCLC is not well understood. Therefore, the aim of the present
study was to construct an effective short hairpin RNA (shRNA) to
knockdown FASN in an NSCLC cell line (A549), and to measure the
effect of this on cell proliferation, the cell cycle and apoptosis,
in addition to assessing radiosensitivity by the analysis of clone
formation and DNA repair proteins.
Materials and methods
Cell culture
The human NSCLC cell line A549 was obtained from the
Institute of Cancer Prevention and Treatment, Harbin Medical
University. The cells were routinely cultured in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences, Shanghai, China) at 37°C in 5% CO2
incubator.
Lentiviral vectors construction for
FASN small hairpin RNA and transfection
To construct a lentiviral vector containing small
hairpin RNA targeted to FASN, the FASN fragment was amplified from
human genomic DNA (A549 cell line) using PCR (SanTaq Plus PCR kit;
cat. no. SK2491; Sangon Biotech Co., Ltd., Shanghai, China) (30
cycles of denaturing at 94°C for 30 sec, annealing at 60°C for 30
sec, and elongation at 72°C for 30 sec). Briefly, 1 µg of each
plasmid DNA and 20 µl of Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) were mixed separately
with Optim-MEM medium (Invitrogen; Thermo Fisher Scientific, Inc.)
and incubated for 5 min at room temperature. The FASN fragment was
then inserted into AgeI and Ecor I enzyme sites of pSIH-H1-Puro
vector and sequenced. Two pairs of effective RNA interference
sequences, shRNA#3072 and shRNA#7377 to target the FASN gene
(Thermo Fisher Scientific, Inc.) were designed (DNAMAN, version
6.0) and synthesized (version A30142; Thermo Fisher Scientific,
Inc.). The sequence of shRNA#3072 was 5′-CCCAGGCTGAAGTTTACAA-3′ and
the sequence of shRNA#7377 was 5′-GGTCCTTCTACTACAAGCT-3′. At the
same time, a corresponding vector pSIH-H1-Puro-shGFP was
constructed as the negative control. Then, lentiviral vector DNAs
and packaging vectors were transfected into A549 cells.
Supernatants containing lentiviruses were harvested 72 h after
transfection, following puromycin (Invitrogen; Thermo Fisher
Scientific, Inc.) selection to obtain positive clones. Infection
efficiency was determined using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay and western blot
analysis.
RT-qPCR
Total RNA was extracted from the A549 cells using
the E.Z.N.A DNA/RNA isolation kit (Omega Bio-tek Inc., Norcross,
GA, USA). Total RNA kit I (Omega Bio-Tek, Inc., Norcross, GA, USA)
and cDNA synthesis was performed using the Transcriptor First
Strand cDNA Synthesis kit (Roche Diagnostics, Basel, Switzerland),
according to the manufacturer's protocols. Amplification conditions
consisted of pre-denaturation at 95°C for 10 min followed by 40
cycles of denaturation at 95°C for 15 sec, annealing at 55°C for 60
sec and elongation at 72°C for 45 sec. qPCR was conducted using the
FastStart Universal SYBR-Green Master (Roche Diagnostics) on a
thermocycler (Applied Biosystems 7500 Fast Real-Time PCR System;
Thermo Fisher Scientific, Inc.). DNA primers were synthesized by
Invitrogen (Thermo Fisher Scientific, Inc.) and the sequences were
as follows: FASN forward, 5′-GGACCTGACCTGCCGTCTAG-3′ and reverse,
5′-GAGGAGTGGGTGTCGCTGTT-3′; GAPDH forward,
5′-TGGTCTCCTCTGACTTCAAC-3′ and reverse, 5′-GTGAGGGTCTCTCTCTTCCT-3′.
Human GAPDH was used as an internal control. The 2−ΔΔCq
method was used to calculate the relative mRNA expression of FASN
(18).
Irradiation
Stably transfected cells and a negative control
group were irradiated at room temperature at a dose rate of 2.0
Gy/min (2 Gy for 1 min, then 4 Gy for 2 min) of 6-MV X-ray using an
ELEKTA Synergy accelerators (Uppsala, Sweden).
Cell cycle analysis
Cell cycle arrest in the presence or absence of
irradiation was measured. 1–3×106 cells were seeded onto
6-well culture plates overnight and were attached to the surface of
the plate. Following exposure to 6 Gy X-ray radiation, the cells
were harvested by trypsinization, washed twice with
phosphate-buffered saline (PBS) and fixed in 70% cold ethanol
overnight at −20°C. The cells were then washed twice with PBS.
Subsequently, the cells were resuspended in 0.5 ml propidium iodide
(PI; cat. no. 340242; BD Biosciences, San Jose, CA, USA) for
staining and incubated in the dark for 30 min at 37°C. The cell
cycle distribution was analyzed using a Coulter EPICS XL flow
cytometer (BD FACS Canto II; Beckman Coulter, Inc., CA, USA) and
MODFIT software (version: 3.2; BD Biosciences).
Apoptosis assay
To quantify the population of apoptotic cells, an
Apoptosis Detection Annexin kit (Vazyme, Nanjing, China) was used.
The cells (1–5×105) were seeded onto 6-well plates, with
or without subsequent treatment with 6 Gy X-ray. The culture medium
was collected, and the cells were harvested with trypsin and
centrifuged for 10 min at 1,200 × g at room temperature. The cells
were then resuspended in 100 µl binding buffer and incubated with 5
µl Annexin V and 5 µl PI for 10 min at room temperature, followed
by the addition of 400 µl binding buffer. Finally, the cell sample
was analyzed using a Coulter EPICS XL flow cytometer (BD FACS Canto
II; Beckman Coulter, Inc., CA, USA) with Diva software (BD
Biosciences; version 1.1.3) according to the manufacturer's
protocols.
Colony formation assay
Stable transfected cells were planted on a 6-well
culture plates at different cell densities (100, 200, 400, 1,000
and 5,000 cells per well). After 24 h, following exposure to a
single dose of 0, 2, 4 and 6 Gy X-ray radiation with a 100 cm
focus-surface distance at a dose rate of 2.0 Gy/min (2 Gy for 1
min, then 4 Gy for 2 min) at room temperature. After treatment, the
cells were allowed to grow for 10–14 days to form colonies in an
incubator at 37°C and 5% CO2, and then washed with PBS
and fixed using methanol for 15 min, and then stained using crystal
violet for 10–30 min at room temperature. Subsequently, colonies
were counted (≥50 cells were scored as clonogenic survivors) under
a light microscope (magnification, ×40). In each irradiation dose
group, surviving fraction of cells was calculated as the plating
efficiency of the irradiation cells divided by that of the
non-irradiated control. All experiments were performed
independently at least three times.
Cell proliferation analysis using cell
counting kit-8 (CCK-8)
Stably transfected A549 cells were plated onto
96-well plates at a density of 2×103 cells/100 µl/well.
At 1, 2 and 3 days after incubation, 10 µl CCK-8 solution (Beijing
Zoman Biotechnology Co., Ltd, Beijing, China) was added into each
well, and the plate was incubated for an additional 2 h in the dark
at 37°C in a humidified incubator. The absorbance values for all
wells were measured at 480 nm with a microplate reader (ELx800
Absorbance Reader, BioTek Instruments, Inc., Winooski, VT,
USA).
Western blot analysis
The cells were washed twice with PBS and lysed in
ice-cold radioimmunoprecipitation assay buffer containing
phenylmethanesulfonyl fluoride (Cell Signaling Technology, Inc.,
Danvers, MA, USA) for 30 min and centrifuged at 12,000 × g for 15
min at 4°C for isolation of total protein. The protein
concentration was determined using a bicinchoninic acid assay
(Thermo Fisher Scientific, Inc.). The proteins were then denatured
by heating at 99°C for 5 min. A total of 20–80 µg amounts of
protein were loaded onto a 6% SDS-PAGE and separated by
electrophoresis, followed by transfer onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). Following
blocking with 5% skimmed milk in Tris-buffered saline containing
0.1% Tween-20 at room temperature for 1 h, the membranes were
probed at 4°C overnight with the following primary antibodies:
Rabbit anti-FASN (cat. no. A6273; dilution, 1:500; ABclonal Biotech
Co., Ltd., Woburn, MA, USA), anti-DNA-dependent protein kinase
catalytic subunit (DNA-PKcs; cat. no. 4602; dilution, 1:500; Cell
Signaling Technology, Inc.) and mouse anti-β-actin (cat. no.
TA811000; dilution, 1:10,000; OriGene Technologies, Inc., Beijing,
China). The membranes were washed using tris buffered saline with
0.1% Tween 20 three times prior to incubation with horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat. no. A0208;
Beyotime Institute of Biotechnology, Haimen, China; 1:2,000
dilution) for 1 h at room temperature. The proteins were visualized
using a SuperSignal enhanced chemiluminescence kit (Thermo Fisher
Scientific, Inc.).
Statistical analysis
Each experiment was repeated at least three times,
and statistical analyses were performed using SPSS (version 22.0;
IBM Corp., Armonk, NY, USA). The results are expressed as the mean
± standard deviation, and statistically significant differences
were analyzed using one-way analysis of variance, followed by the
Tukey's post hoc tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Lentivirus-mediated shRNA interference
inhibits FASN expression in A549 cells
To assess the role of FASN in A549 cells, FASN
expression was knocked down using a lentivirus-mediated shRNA
approach, and the FASN mRNA and protein expression levels in
transfected cells were evaluated using RT-qPCR and western blot
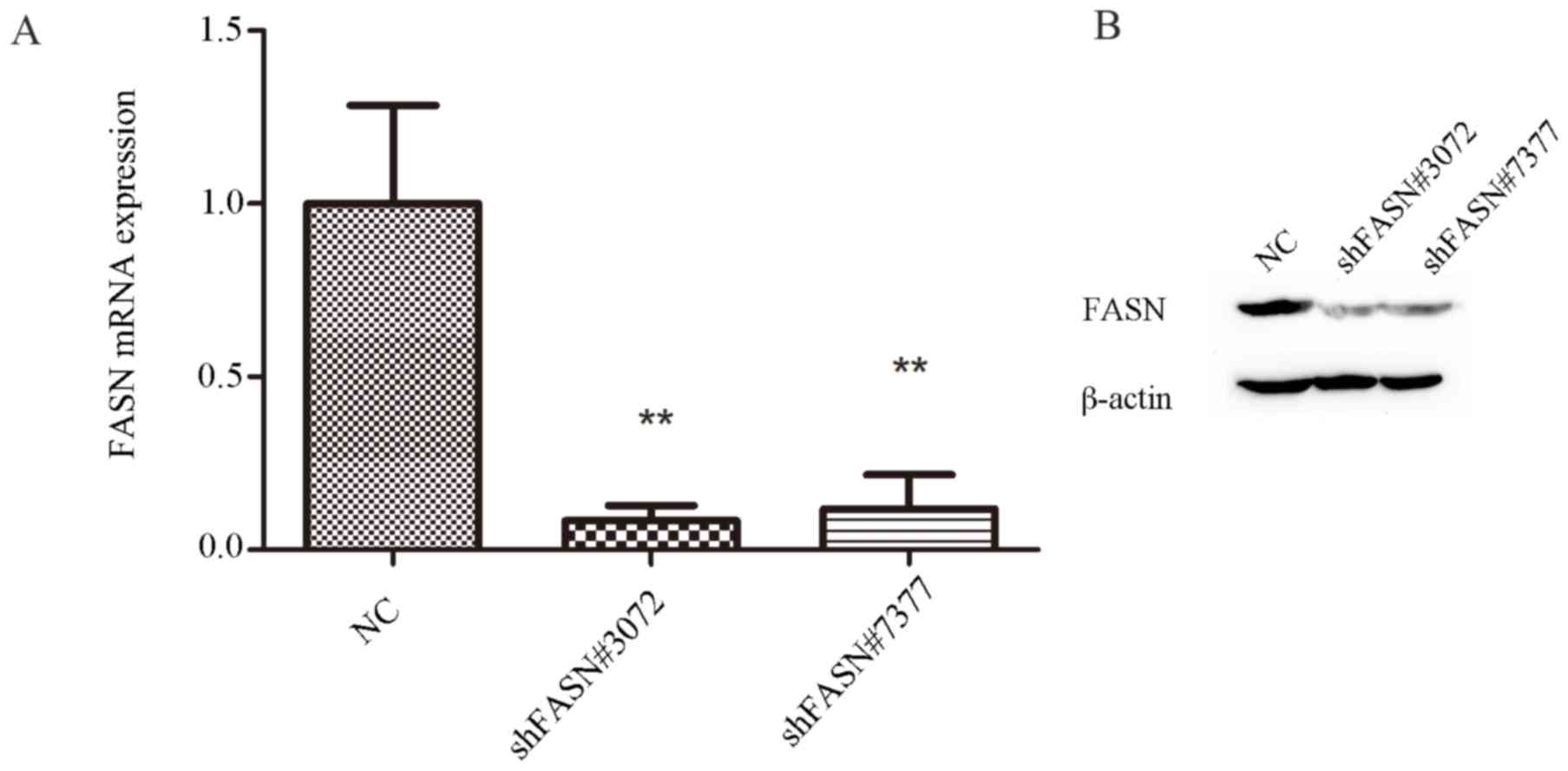
analysis. The results of RT-qPCR analysis revealed that FASN mRNA
levels were suppressed by ~92 and 88% in the cells that were
transfected with shFASN#3072 and shFASN#7377, respectively. FASN
mRNA was significantly decreased in shFASN#3072 and
shFASN#7377-transfected cells compared with the negative control
(NC) group (P<0.01; Fig. 1A). The
RT-qPCR results were consistent with the results of the western
blot analysis (Fig. 1B), which
revealed that the expression of FASN protein in cells transfected
with shFASN#3072 or shFASN#7377 was markedly diminished compared
with the NC group. These data suggested that the
lentivirus-mediated shRNA system was able to successfully inhibit
the expression of FASN in A549 cells.
Knockdown of FASN inhibits the
proliferation of A549 cells
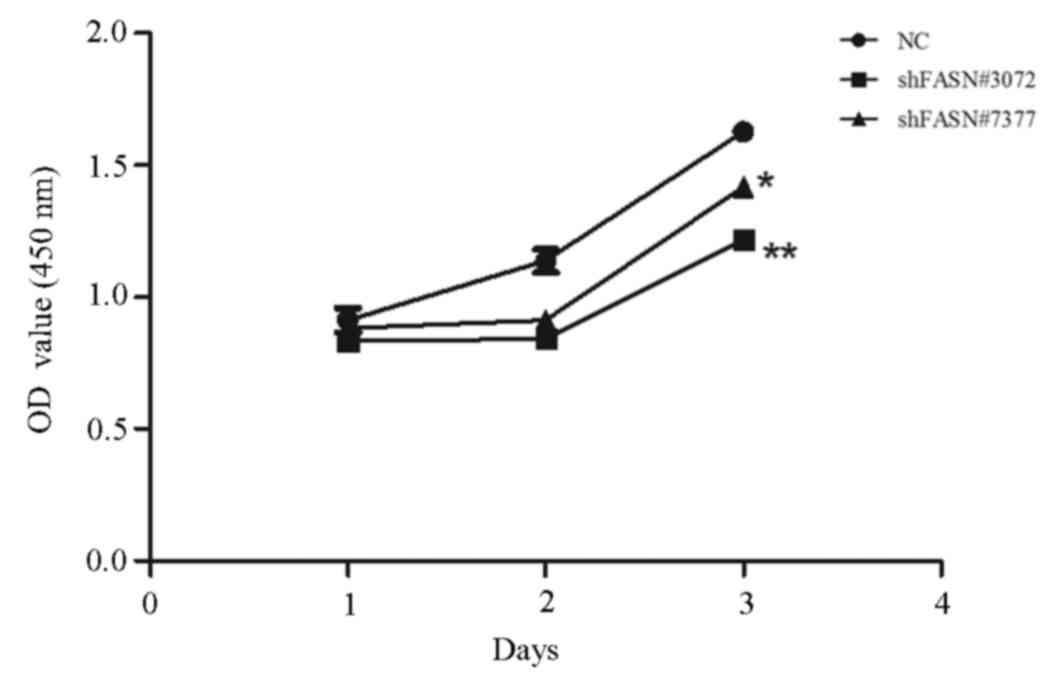
The effect of FASN knockdown on cell proliferation
was investigated using a CCK-8 assay. After 1, 2 and 3 days, the
absorbance values of the cells that were transfected with
FASN-shRNA#3072 and FASN-shRNA#7377 revealed that proliferation was
significantly inhibited in these groups compared with the NC group.
The proliferation rates of shFASN#3072- and shFASN#7377-transfected
cells at 24, 48 and 72 h were decreased by 16, 29 and 27%
(shFASN#3072; P<0.01), and 18, 17 and 17% (shFASN#7377;
P<0.05), respectively (Fig.
2).
Radiosensitivity is increased in A549
cells transfected with FASN shRNA
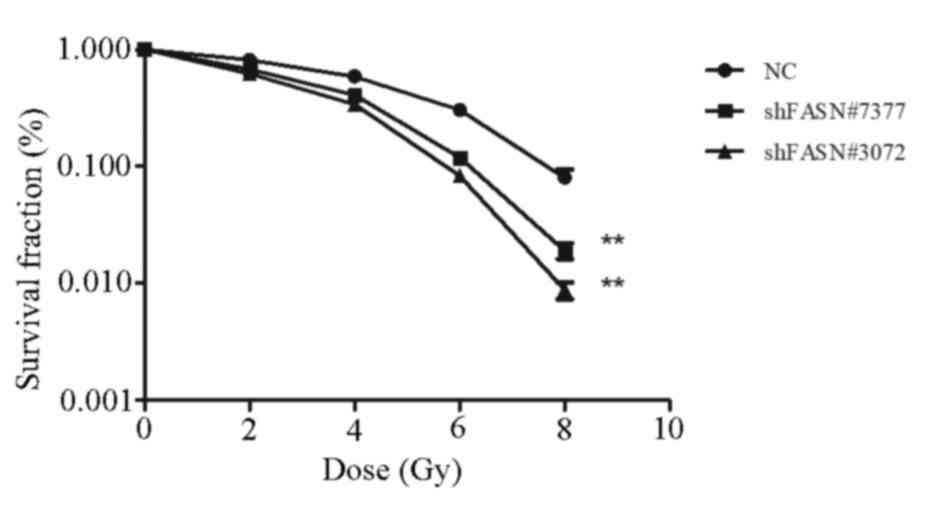
To determine the effect of FASN expression on the
radiosensitivity of A549 cells, colony formation assays were
performed following exposure of the cells to varying doses of
irradiation. As demonstrated in Fig.
3, the number of colonies formed by cells transfected with
shFASN#3072 and shFASN#7377 was significantly reduced compared with
the NC group. In addition, radiation led to a dose-dependent
decrease in the survival of A549 cells (P<0.05 for all radiation
doses). The results indicated that shRNA-FASN cells exhibited
reduced radioresistance compared with the cells in the NC
group.
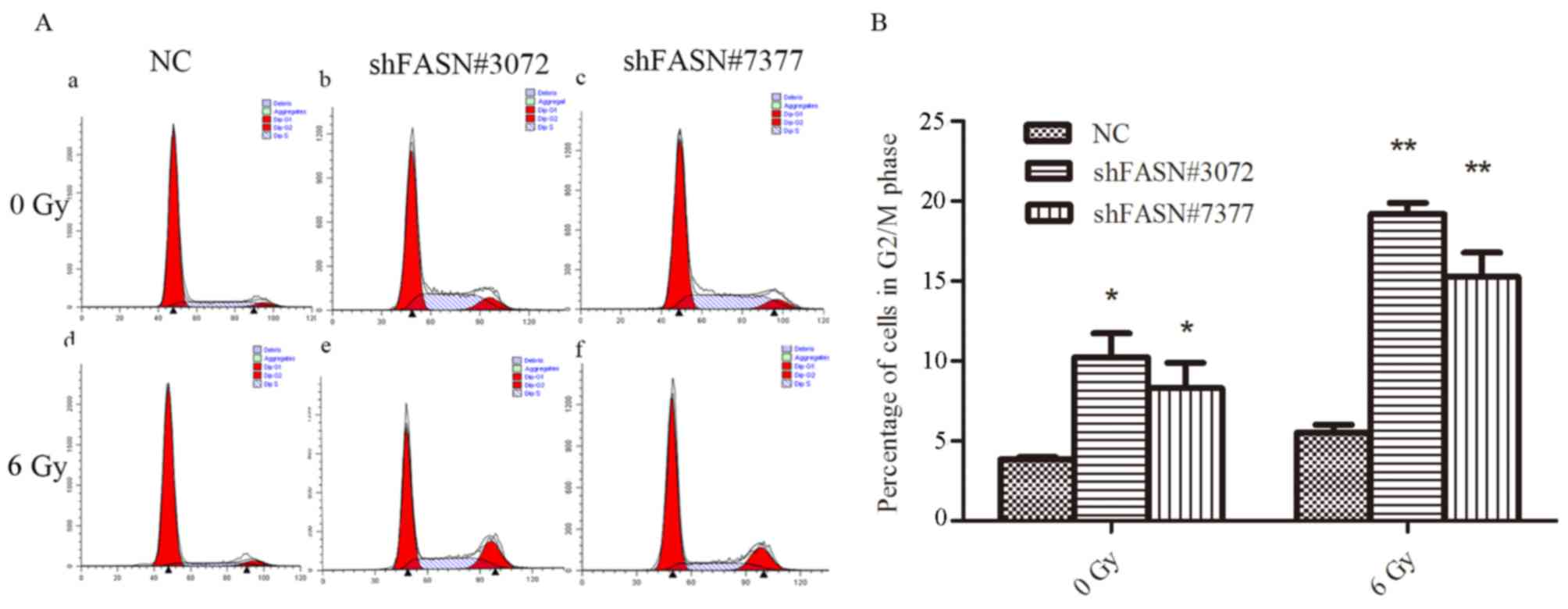
Silencing FASN increases the
percentage of cells in the G2/M phase following irradiation
Flow cytometric analysis was conducted to evaluate
changes in cell cycle progression following irradiation. As
indicated in Fig. 4, the groups
transfected with shFASN#3072 and shFASN#7377 exhibited a higher
proportion of cells in the G2/M phase compared with the NC group
(shFASN#3072 and shFASN#7377 vs. NC group, 9.93±1.56% and
8.13±1.62% vs. 3.82±0.17%; P<0.05; Fig. 4). Following exposure to 6 Gy
irradiation, the proportion of cells in the G2/M phase was
significantly increased in shFASN#3072 and shFASN#7377-transfceted
cells compared with negative control (shFASN#3072 and shFASN#7377
vs. NC group, 18.79±0.97% and 15.16±1.53% vs. 5.50±0.49%;
P<0.01; Fig. 4). These data
suggested that transfection of FASN-shRNA combined with ionizing
radiation increased cell cycle arrest at the G2/M phase compared
with the NC group.
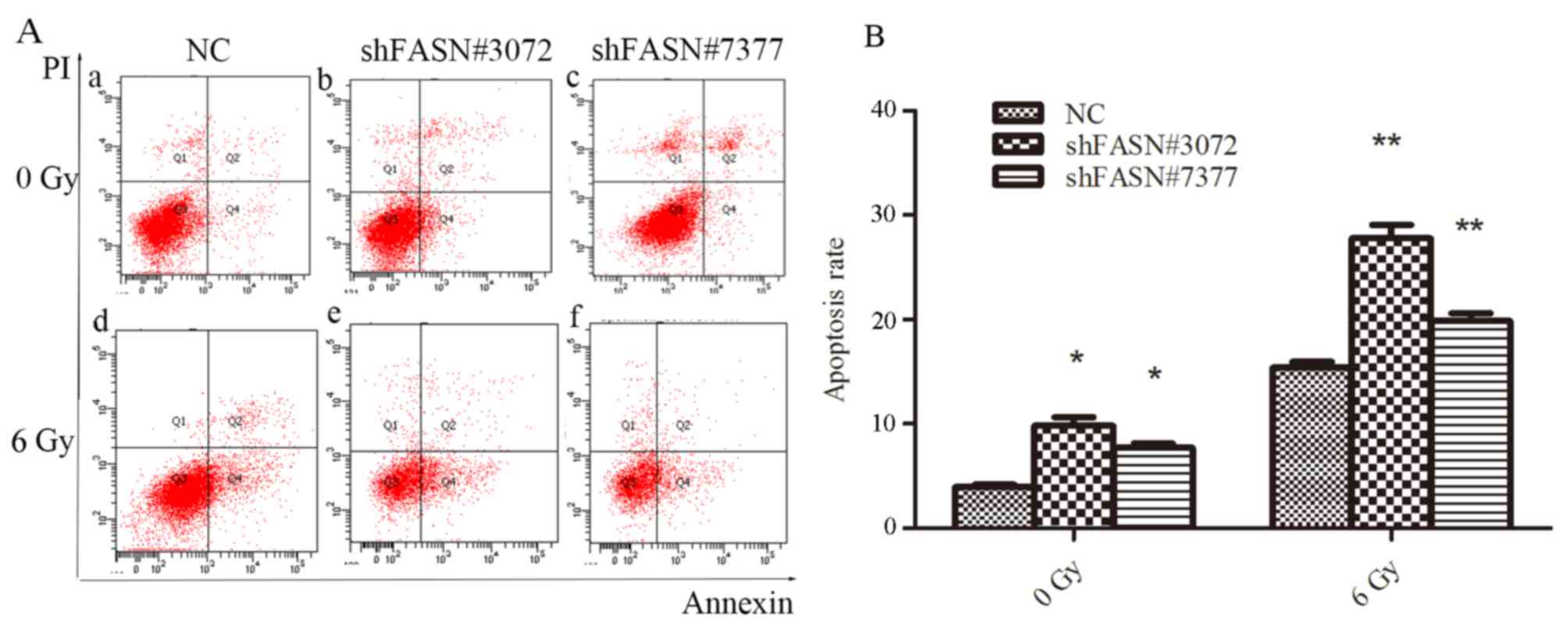
Downregulation of FASN increases
radiation-induced apoptosis of A549 cells
The rate of apoptosis following ionizing radiation
treatment was evaluated using Annexin V/PI staining. As indicated
in Fig. 5, flow cytometry revealed
that the proportion of apoptotic cells in the cells transfected
with shFASN#3072 or shFASN#7377 was higher compared with the NC
group. Following treatment with 6 Gy radiation, the rate of
apoptosis in cells transfected with shFASN#3072 or shFASN#7377 was
significantly increased compared with the NC group (P<0.01). The
percentages of apoptotic cells (upper and lower right quadrants)
for shFASN#3072- and shFASN#7377-transfected and NC cells without
radiation treatment were 9.8±1.47, 8.4±1.21 and 3.93±0.47%,
respectively and the values following treatment with 6 Gy radiation
for shFASN#3072- and shFASN#7377-transfected and NC cells were
15.38±1.00, 27.79±2.25 and 19.85±1.34%, respectively. These data
indicated that NSCLC cells transfected with FASN-shRNA that were
subjected to ionizing radiation had an increased rate of apoptosis
compared with the NC group.
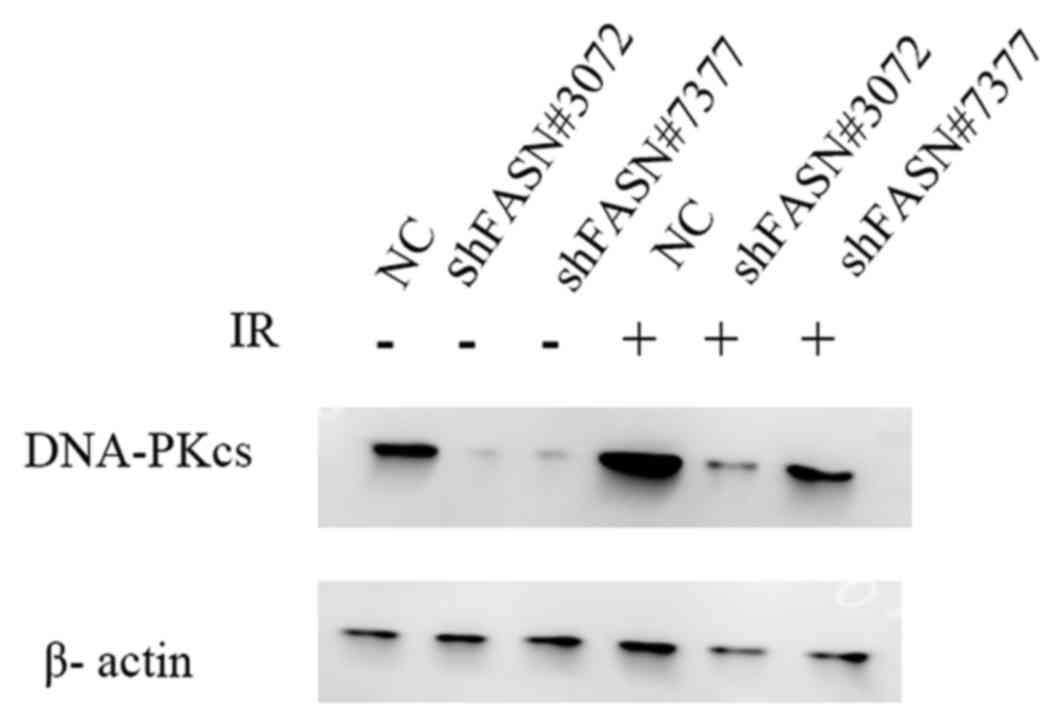
Downregulation of FASN inhibits DNA
damage repair following irradiation
To analyze whether the inhibition of FASN may affect
radiosensitivity, the expression levels of DNA-PKcs protein, a key
protein associated with the repair of DNA DSBs, following exposure
to 6 Gy irradiation were analyzed. As indicated in Fig. 6, the downregulation of FASN expression
suppressed the levels of DNA-PKcs and inhibited the increase in the
levels of these proteins following irradiation. These results
indicated that FASN-mediated radioresistance may be associated with
the expression of the DNA DSB repair protein DNA-PKcs.
Discussion
Numerous previous studies have demonstrated that
FASN, a multifunctional enzyme, has important effects on the
occurrence, progression, invasion and metastasis of tumors
(15). In mammalian metabolism, fatty
acids are either exogenously derived from the diet or endogenously
derived via de novo lipogenesis (19). Under physiological conditions, due to
a balanced diet, FASN is thought to provide the fatty acids for
growth, development and survival (20). Therefore, the latter is the primary
source of fatty acids (21). FASN is
a 250–270-kDa cytosolic protein, and its main physiological
function is to catalyze the production of palmitate, a 16-carbon
saturated fatty acid, from acetyl CoA and malonyl CoA (22). However, little is known about its
function in radiosensitivity of NSCLC. The aim of the present study
was to investigate the effect of FASN knockdown by constructing a
lentiviral vector containing shRNA that targets FASN
(pSIH-H1-Puro-shRNA-FASN). The results demonstrated that FASN is
associated with the regulation of cell proliferation, cell cycle
and apoptosis. In addition, FASN expression was revealed to have a
significant effect on radiosensitivity, where the downregulation of
FASN increased the sensitivity of A549 cells to radiation. These
results are in agreement with those of Yang et al (17), who recently reported that FASN
expression was correlated with radiation resistance and poor
clinical outcome in patients with pancreatic cancer.
FASN may be an optimal diagnostic marker and a
potential therapeutic target due to its distinctive tissue
distribution and special enzymatic activity (23,24). FASN
is often highly expressed in human cancer, whereas it is usually
undetectable or exhibits low expression in the majority of normal
human tissues (13–17). This differential tissue distribution
makes FASN an attractive target for the development of novel
anticancer techniques (13–16). Zhou et al evaluated 80 samples
of esophageal cancer, revealing that FASN is primarily localized in
the cytoplasm of esophageal cancer, but exhibited little or no
expression in normal esophageal tissues (16). Similarly, Kao et al (15) investigated FASN expression in
nasopharyngeal carcinoma using immunohistochemistry and revealed
that FASN was positively expressed in the tumoral cytoplasm, but
exhibited low or no expression in non-tumor epithelium.
Previous studies have demonstrated that the
upregulation of FASN increases tumor cell proliferation (25), decreases the rate of apoptosis
(17), and increases the resistance
to chemotherapy and radiation of tumor cells in various human
neoplasms (26). In line with this,
the present study revealed that downregulation of FASN caused a
significant reduction in the proliferation of A549 cells,
suggesting that FASN may be involved in NSCLC oncogenesis.
Furthermore, silencing of FASN led to cell cycle arrest in the G2/M
phase, suggesting that FASN promotes proliferation through
modulation of cell cycle. Furthermore, silencing of FASN was
accompanied by an increase in apoptosis. Similarly, a previous
study by Jiang et al (14)
demonstrated that downregulation of FASN led to decreased
proliferation and increased apoptosis in bladder cancer cells.
Notably, the combination of shFASN and ionizing radiation
significantly inhibited proliferation, promoted apoptosis and
increased cell cycle arrest in the G2/M phase compared with shFASN
or ionizing radiation alone.
To address the fact that a decrease in apoptosis may
lead to long-term resistance to radiotherapy, a colony formation
assay was conducted in the present study, revealing that
transfection with shFASN led to the formation of considerably fewer
colonies compared with the control group. This indicates that the
knockdown of FASN is able to markedly enhance cell susceptibility
to irradiation. At the same time, this demonstrated that a
combination of shFASN and ionizing radiation reduced clonogenic
survival compared with each treatment alone.
The present study demonstrated that the knockdown of
FASN expression increased the radiosensitivity of A549 cells.
Additionally, previous studies have indicated that FASN
overexpression contributes to the resistance to several
chemotherapeutics (17,26). This finding prompted the investigation
into whether or not FASN regulates DNA damage responses.
DNA is a major target that is damaged by ionizing
irradiation, generating a series of genomic DNA lesions, of which
DSBs are the most important (27).
There are two major mechanisms for the repair of DSBs induced by
ionizing radiation: Homologous recombination and non-homologous end
joining (NHEJ) (28). NHEJ is
regarded as the primary mechanism for the repair of
radiation-induced DSBs throughout the cell cycle (29). In healthy cells, this repair function
is crucial for cell survival, while in cancer cells this may lead
to resistance to radiation (30). The
DNA-PK proteins, which are involved in NHEJ, contain DNA-PKcs and
ku70/ku80 (31). DNA-PKcs has been
verified to initiate DNA repair directly by combining with DNA ends
in the absence of ku70/ku80 (32). As
discussed earlier, the present study assessed the association
between FASN and DNA-PKcs in order to investigate the mechanism by
which FASN affects the radiosensitivity of NSCLC cells. The results
from western blotting revealed that the inhibition of FASN
downregulated the protein levels of DNA-PKcs and markedly inhibited
the increase of the expression of this protein following
irradiation. This finding indicates that the suppression of FASN
expression may decrease the ability of NSCLC cells to repair DSBs
induced by radiation and that this effect is partly mediated by the
DNA-PKcs pathway. Further experiments to analyze the possible
molecular mechanisms of DNA DSB repair proteins in the regulation
of FASN-mediated radiosensitivity are currently being conducted in
the laboratory of the present authors.
In summary, the present study demonstrated that the
suppression of FASN combined with ionizing radiation enhanced the
radiosensitivity of NSCLC cells by inhibiting proliferation,
promoting cell cycle arrest, triggering apoptosis and decreasing
the DNA damage repair capability of A549 cells. Therefore, combined
with the observations reported in previous studies, this suggests
that FASN may be a potential target for therapeutic interventions
designed to increase the radiosensitivity of NSCLC.
References
|
1
|
Takanen S, Bangrazi C, Graziano V, Parisi
A, Resuli B, Simione L, Caiazzo R, Raffetto N and Tombolini V:
Number of mediastinal lymph nodes as a prognostic factor in PN2 non
small cell lung cancer: A single centre experience and review of
the literature. Asian Pac J Cancer Prev. 15:7559–7562. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tarone RE: On the International Agency for
Research on Cancer classification of glyphosate as a probable human
carcinogen. Eur J Cancer Prev. Nov 8–2017.(Epub ahead of
print).
|
|
3
|
Parsons A, Daley A, Begh R and Aveyard P:
Influence of smoking cessation after diagnosis of early stage lung
cancer on prognosis: Systematic review of observational studies
with meta-analysis. BMJ. 340:b55692010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer Clin. 61:69–90.
2011. View Article : Google Scholar
|
|
5
|
Fernandes AT, Mitra N, Xanthopoulos E,
Evans T, Stevenson J, Langer C, Kucharczuk JC, Lin L and Rengan R:
The impact of extent and location of mediastinal lymph node
involvement on survival in Stage III non-small cell lung cancer
patients treated with definitive radiotherapy. Int J Radiat Oncol
Biol Phys. 83:340–347. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shikazono N, Noguchi M, Fujii K,
Urushibara A and Yokoya A: The yield, processing, and biological
consequences of clustered DNA damage induced by ionizing radiation.
J Radiat Res. 50:27–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Greenstein JP: Biochemistry of cancer. New
York: Academic Press; 1954
|
|
8
|
Nakashima RA, Paggi MG and Pedersen PL:
Contributions of glycolysis and oxidative phosphorylation to
adenosine 5′-triphosphate production in AS-30D hepatoma cells.
Cancer Res. 44:5702–5706. 1984.PubMed/NCBI
|
|
9
|
Medes G, Thomas A and Weinhouse S:
Metabolism of neoplastic tissue. IV. A study of lipid synthesis in
neoplastic tissue slices in vitro. Cancer Res. 13:27–29.
1953.PubMed/NCBI
|
|
10
|
Chung YW, Han DS, Park YK, Son BK, Paik
CH, Lee HL, Jeon YC and Sohn JH: Association of obesity, serum
glucose and lipids with the risk of advanced colorectal adenoma and
cancer: a case-control study in Korea. Dig Liver Dis. 38:668–672.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Williams RR, Shorlie PD and Feinleib M:
Cancer incidence by levels of cholesterol. JAMA. 245:247–25234.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Liu JY, Wu X and Zhang JT:
Biochemistry, molecular biology, and pharmacology of fatty acid
synthase, an emerging therapeutic target and diagnosis/prognosis
marker. Int J Biochem Mol Biol. 1:69–89. 2010.PubMed/NCBI
|
|
13
|
Khan A, Aljarbou AN, Aldebasi YH, Faisal
SM and Khan MA: Resveratro suppresses the proliferation of breast
cancer cells by inhibiting fatty acid synthase signaling pathway.
Cancer Epidemiol. 38:765–772. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang B, Li EH, Lu YY, Jiang Q, Cui D,
Jing YF and Xia SJ: Inhibition of fatty acid synthase supresses
P-akt and induces apoptosis in bladder cancer. Urology.
80:484.e9–e15
|
|
15
|
Kao YC, Lee SW, Lin LC, Chen LT, Hsing CH,
Hsu HP, Huang HY, Shiue YL, Chen TJ and Li CF: Fatty acid synthase
overexpression confers an independent prognosticator and associates
with radiation resistance in nasopharyngeal carcinoma. Tumor Biol.
34:759–768. 2013. View Article : Google Scholar
|
|
16
|
Zhou Y, Niu C, Li Y, Gao B, Zheng J, Guo X
and Ma W: Fatty acid synthase expression and esophageal cancer. Mol
Biol Rep. 39:9733–9739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Liu H, Li Z, Zhao Z, Yip-Schneider
M, Fan Q, Schmidt CM, Chiorean EG, Xie J, Cheng L, et al: Role of
fatty acid synthase in gemcitabin and radiation resistance of
pancreatic cancer. Int J Biochem Mol Biol. 2:89–98. 2011.PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer. Nat Rev Cancer.
7:763–77. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shaw RJ: Glucose metabolism and cancer.
Curr Opin Cell Biol. 18:598–608. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kuhajda FP: Fatty-acid Synthase and human
cancer: New perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith S, Witkowski A and Joshi AK:
Structural and functional organization of the animal fatty acid
synthase. Prog Lipid Res. 42:289–317. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pizer ES, Thupari J, Han WF, Pinn ML,
Chrest FJ, Frehywot GL, Townsend CA and Kuhajda FP:
Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced
by fatty-acid synthase inhibition in human breast cancer cells and
xenografts. Cancer Res. 60:213–218. 2000.PubMed/NCBI
|
|
24
|
De Schrijver E, Brusselmans K, Heyns W,
Verhoeven G and Swinnen JV: RNA interference-mediated silencing of
the fatty acid synthase gene attenuates growth and induces
morphological changes and apoptosis of LNCaP prostate cancer cells.
Cancer Res. 63:3799–3804. 2003.PubMed/NCBI
|
|
25
|
Yoshii Y, Furukawa T, Oyama N, Hasegawa Y,
Kiyono Y, Nishii R, Waki A, Tsuji AB, Sogawa C, Wakizaka H, et al:
Fatty acid synthase is a key target in multiple essential tumor
functions of prostate cancer: Uptake of radiolabeled acetate as a
predictor of the targeted therapy outcome. PLoS One. 8:e645702013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Wu X, Dong Z, Luo Z, Zhao Z, Xu Y
and Zhang JT: Fatty acid synthase causes drug resistance by
inhibiting TNF-α and ceramide production. J Lipid Res. 54:776–785.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yanold J: Molecular aspects of cellular
responses to radiotherapy. Radiothery Oncol. 44:1–7. 1997.
View Article : Google Scholar
|
|
28
|
Thompson LH: Evidence that mammalian cells
possess homologous recombinational repair pathways. Mutat Res.
363:77–88. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bertolini LR, Bertolini M, Anderson GB,
Maga EA, Madden KR and Murray JD: Transient depletion of Ku70 and
Xrcc4 by RNAi as a means to manipulate the non-homologous
end-joining pathway. J Biotechnol. 128:246–257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hamer G, Roepers Gajadien HL, Van
Duyn-Goedhart A, Gademan IS, Kal HB, van Buul PP, Ashley T and de
Rooij DG: Function of DNA-protein kinase catalytic subunit during
the early meiotic prophase without Ku70 and Ku86. Biol Reprod.
68:717–721. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jeggo PA: Identification of genes involved
in repair of DNA doublestrand breaks in mammary cells. Radiat Res.
150:580–591. 1998. View
Article : Google Scholar
|
|
32
|
Yaneva M, Kowalewski T and Lieber MR:
Interaction of DNA-dependent protein kinase with DNA and with Ku:
Biochemical and atomic force microscopy. EMBO J. 16:5098–5112.
1997. View Article : Google Scholar : PubMed/NCBI
|