Introduction
Hepatoblastoma is the most common primary malignant
hepatic tumor experienced in infancy and childhood globally
(1). Chemotherapy drugs serve a vital
function in treating hepatoblastoma (2). Long-term use of chemotherapy drugs is
necessary to effectively control the condition of patients with
hepatoblastoma; however, the long-term use of chemotherapy drugs
may induce drug resistance in hepatoblastoma cells, resulting in
the decreased efficacy of chemotherapy drugs (3). Therefore, the identification of safe and
effective chemotherapy drugs for treating hepatoblastoma is
required. Recently, the use of plant-derived chemical constituents
with anti-tumor activity has been increasingly studied for their
potential use as chemotherapy drugs (4).
Chrysotoxene is a phenanthrene derivative that was
first isolated from Dendrobium (D.) chrysotoxum Lindl
(Orchidaceae) and has previously demonstrated inhibitory effects on
the growth of hepatoma and ehrlich ascites carcinoma in mice
(5). Chrysotoxene exhibited cytotoxic
activity against cultured chronic myelogenous leukemia K562 cells
using microscopic cell counting and morphological observation
(6). These two previous studies
revealed that chrysotoxene may be a potential drug used to treat
tumors, however the anti-tumor mechanism of chrysotoxene remains
unknown. The aim of the present study was to investigate the
cytotoxic effect and possible mechanism of chrysotoxene on human
hepatoblastoma HepG2 cells in vitro and in vivo.
First, the cytotoxic effect of chrysotoxene on HepG2 cells was
assessed using a Cell Counting Kit (CCK)-8 assay. Second, flow
cytometry analysis was used to investigate whether the cytotoxic
effect of chrysotoxene on HepG2 cells was associated with
apoptosis. Subsequently, the effect of chrysotoxene on apoptotic
proteins in the mitochondria-mediated apoptotic signaling pathway
were investigated using western blot analysis. Finally, the effect
and mechanism of chrysotoxene on HepG2 cell-induced tumors in nude
mice were investigated. The results of these assays demonstrated
that chrysotoxene induced the apoptosis of HepG2 cells in
vitro and in vivo via activation of the
mitochondria-mediated apoptotic signaling pathway.
Materials and methods
Chemicals and reagents
Analytical grade solvent (ethanol, petroleum ether
and chloroform) and silica-gel (200–300 mesh) were purchased from
Qingdao Haiyang Chemical Co., Ltd. (Qingdao, China). RPMI 1640
media and fetal bovine serum (FBS) were purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). Total Protein
Extraction kit (cat no. SD-001), Mitochondria Protein Extraction
kit (cat no. MP-007), and Cytoplasm Protein Extraction kit (cat no.
SM-005) were provided by Beijing BioDee Diagnostics Co., Ltd.
(Beijing, China). Dimethyl sulfoxide (DMSO) was purchased from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). CCK-8 and enhanced
Bicinchoninic Acid (BCA) protein assay kits were purchased from
Beyotime Institute of Biotechnology (Shanghai, China). The cell
staining buffer was purchased from BioPike, LLC. (Shanghai, China).
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
apoptosis assay kit was purchased from Shanghai Standard Biotech
Co., Ltd. (Shanghai, China). Primary antibodies for second
mitochondria-derived activator of caspase (Smac; Cat no. 15108;
rabbit anti-human; 1:1,000), apoptotic protease activating factor-1
(Apaf-1; Cat no. 8969; rabbit anti-human; 1:1,000), cleaved
(c)-caspase-9 (Cat no. 9501; rabbit anti-human; 1:1,000) and
β-actin (Cat no. 3700; mouse anti-human; 1:1,000) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA), whereas
antibodies against Cytochrome c (Cat no. AC909; mouse anti-human;
1:200), B cell lymphoma-2 (Bcl-2; Cat no. AB112; rabbit anti-human;
1:1,000), Bcl-2-associated X factor (Bax; Cat no. AB026; mouse
anti-human; 1:500), Survivin (Cat no. AS792; rabbit anti-human;
1:1,000), c-caspase-3 (Cat no. AC033; rabbit anti-human; 1:1,000),
Cytochrome c oxidase IV (COX IV; Cat no. AC610; rabbit anti-human;
1:1,000) and horseradish peroxidase (HRP)-conjugated secondary
antibody (goat anti-mouse: Cat no. A0216; 1:1,000. Goat
anti-rabbit: Cat no. A0208; 1:1,000) were purchased from Beyotime
Institute of Biotechnology (Shanghai, China). Enhanced
chemiluminescence detection kit for HRP were provided by Biological
Industries (Kibbutz Beit-Haemek, Israel).
Animals
A total of 20 male nude mice (five-six weeks old,
20±2 g) were obtained from the Animal Laboratory of The First
Affiliated Hospital of Chinese People's Liberation Army General
Hospital (Beijing, China) and housed in a temperature-controlled
vivarium (25°C) with a relative humidity of 65% and a 12/12-h
light-dark cycle. All animals had free access to water and food.
All assays involving animals were strictly conducted in accordance
with the National Institute of Health, Guide for the Care and Use
of Laboratory Animals (7). All assays
involving animals were performed with the approval of the Animal
Experimentation Ethics Committee of the First Affiliated Hospital
of Chinese People's Liberation Army General Hospital (protocol no.
ECFAHCPLAGH 2014251). The humane endpoint was when the tumor size
in the control group was 3 times greater compared with the
treatment group, or the humane endpoint was when the tumor size was
<2,000 mm3.
Extraction, isolation and
identification of chrysotoxene
D. chrysotoxum was obtained from www.zyctd.com in 2014, and its authenticity was
verified by Dr Ming-Ming Han based on corresponding morphological
and microscopic identification characteristics. A voucher specimen
(voucher no. GCSH201401) was stored in the Department of
Pharmaceutical Medicine Science, The First Affiliated Hospital of
Chinese PLA General Hospital for future reference at room
temperature.
D. chrysotoxum (15 kg) was extracted thrice
with refluxing 95% ethanol and the extracting solvent was combined
and evaporated under decreased pressure (−0.1 MPa) to afford a
crude extract (400 g). Subsequently, the crude extract was
subjected to column chromatography over silica-gel column (200–300
mesh), eluted with systemic gradient (10% increase) of petroleum
ether-chloroform to obtain different fractions (500 ml/fraction).
Chrysotoxene (162 mg) was obtained from fractions 149–156, and the
corresponding elution solvent was petroleum ether-chloroform (7:3).
The purity, molecular formula and chemical structure of
chrysotoxene were analyzed by high performance liquid
chromatography (HPLC), mass spectrometry and nuclear magnetic
resonance (NMR), respectively. HPLC analysis was performed on the
LC-20AT system (Shimadzu Corporation, Japan), and the
chromatographic separation was performed in an Inertsil ODS-SP
C18 column (4.6×250 mm, 5 µm) (Shimadzu Corporation),
operated at 30°C. The mobile phase, injection volume, flow rate and
detection wavelength were methanol-acetonitrile-water (60:60:165),
10 µl, 1 ml/min and 237 nm, respectively. The purity of
chrysotoxene was defined as the rate of chrysotoxene peak area to
total peak area in HPLC chromatogram. Mass spectrometry analysis
was performed on the 1290 HPLC-6500 Q-TOF (Agilent Technologies,
Inc., Santa Clara, CA, USA) with an electronic spray ion (ESI)
source under TOF mode (negative mode) to analyze the molecular
formula of chrysotoxene based on its deprotonated molecule
([M-H]−). The ESI source parameters nitrogen gas
temperature, nebulizer pressure and drying gas flow rate were
325°C, 40 psi and 10 l/min, respectively. NMR analysis was
performed on the DPX-400 (Bruker Corporation, Germany), according
to the manufacturer's protocol. Chrysotoxene was dissolved in 0.5%
DMSO to obtain required concentrations for assays.
Cell culture
HepG2 cells were obtained from the American Type
Culture Collection (Manassas, VA, USA) and cultured in RPMI-1640
medium supplemented with 10% FBS and antibiotics (100 U/ml
penicillin and 100 U/ml streptomycin). HepG2 cells were maintained
in a humidified incubator at 37°C and 5% CO2 and
sub-cultured until reaching logarithmic growth phase.
CCK-8 assay
HepG2 cells were seeded on 96-well plates at a
density of 2×104 cells/well. Following incubation for 4
h, HepG2 cells were treated with chrysotoxene (5, 10, 15, 20, 25,
30, 35 or 40 µg/ml) or 0.5% DMSO (control group). Subsequent to
incubation for 48 h at 37°C, 10 µl CCK-8 was added into each well
and cells were cultured for another 3 h prior to the optical
density (OD) of each sample being measured at 450 nm using a
Bio-Rad Laboratories, Inc. Model 680 microplate reader (Hercules,
CA, USA). The inhibitory rate of chrysotoxene against viability of
HepG2 cells was calculated using the following equation (8): Inhibitory
rate(%)=(ODcontrol-ODchrysotoxene)/ODcontrol
×100.
Flow cytometry assay
Following treatment with chrysotoxene (5, 10 or 20
µg/ml) or 0.5% DMSO for 48 h at 37°C, HepG2 cells were harvested
and washed with PBS solution. The washed HepG2 cells were
re-suspended in cell staining buffer and stained with 5 µl Annexin
V-FITC/10 µl PI for 15 min at room temperature. Subsequently, the
stained HepG2 cells were analyzed using a flow cytometer and Cell
Quest Acquisition Software v. 5.1 (BD Biosciences, Franklin Lakes,
NJ, USA).
Western blot analysis
Following treatment with chrysotoxene (5, 10 or 20
µg/ml) or 0.5% DMSO for 48 h at 37°C, HepG2 cells were harvested
and washed with PBS solution. Subsequently, the mitochondrial
protein, cytoplasmic protein and total protein of HepG2 cells were
extracted using corresponding kits, respectively, and their
concentrations were determined by enhanced BCA protein assay kit.
The mitochondrial protein and cytoplasmic protein were used to
investigate the effect of chrysotoxene on the release of Smac and
Cytochrome c from mitochondria to the cytoplasm in HepG2 cells. The
total protein was used to investigate the effect of chrysotoxene on
Bcl-2, Bax, Survivin, Apaf-1, c-caspase-9 and c-caspase-3 protein
levels in HepG2 cells. Equal amount of proteins (~40 µg) were
separated by SDS-PAGE (12% gel) and then transferred onto
polyvinylidene difluoride (PVFD) membrane. Following blocking with
5% no-fat milk, the PVDF membrane was incubated with corresponding
primary antibodies overnight at 4°C, and subsequently with
HRP-conjugated secondary antibodies for 2 h at room temperature.
Finally, the proteins were detected by chemiluminescence with the
aid of enhanced chemiluminescence detection kit for HRP. The
mitochondrial protein level was represented as protein level/COX IV
level, and the total or cytoplasmic protein level was represented
as protein level/β-actin level; COX IV and β-actin were used as
reference proteins.
Xenograft assay
Nude mice were randomly divided into 2 groups (n=10
in each group): Control and chrysotoxene groups. Nude mice were
subcutaneously injected in the right flank with HepG2 cells at a
density of 2×106 cells/mouse. When the HepG2
cell-induced tumors achieved a diameter of ~3 mm, the nude mice in
the control or chrysotoxene group were administrated orally with
0.5% DMSO or 20 mg/kg chrysotoxene once a day for 20 days,
respectively. The width and length of tumors in each mouse and the
body weight of each mouse were measured on day 5, 10, 15 and 20
using Vernier calipers and an electronic balance. The maximum
weight mice achieved prior to decapitation was <35 g.
Subsequently, nude mice were directly sacrificed by decapitation in
order to obtain the tumor tissues used for western blot assay,
which was performed as aforementioned. Based on width and length of
tumor, tumor volume was calculated using the following equation
(8): Tumor volume
(mm3)=(width2 × length)/2.
Statistical analysis
All experiments were performed in triplicate and
data are represented as mean ± standard deviation. Differences
between groups were analyzed using one-way analysis of variance
followed by Least Significance Difference multiple comparisons test
on SPSS 21.0 (IBM Corp., Armonk, NY, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Purity and identification of
chrysotoxene
The purity of chrysotoxene was >99.5% based on
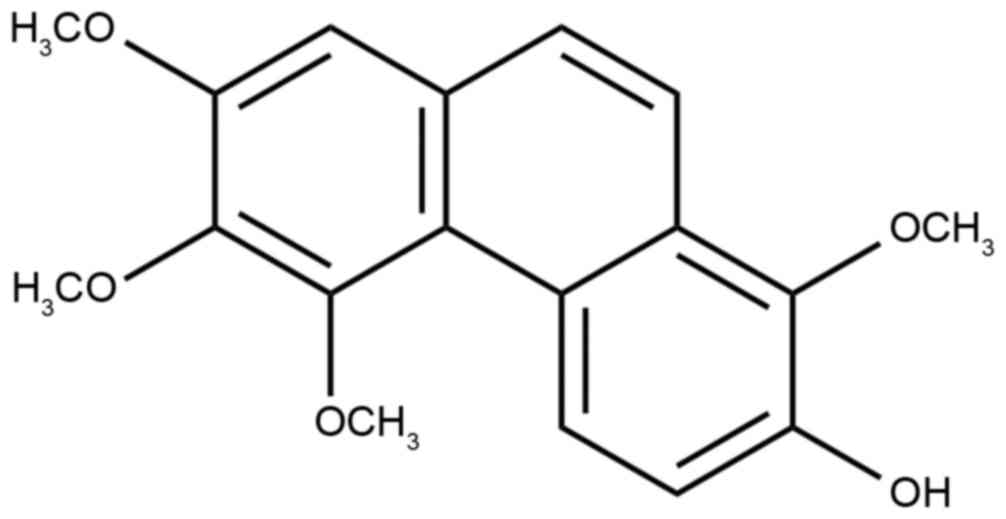
the result of area normalization method of HPLC. The chemical
structure of chrysotoxene (Fig. 1)
was identified by comparing its mass spectrometry and NMR data with
the reported literature (9,10).
Chrysotoxene exhibited cytotoxic
activity against, and induced apoptosis of, HepG2 cells
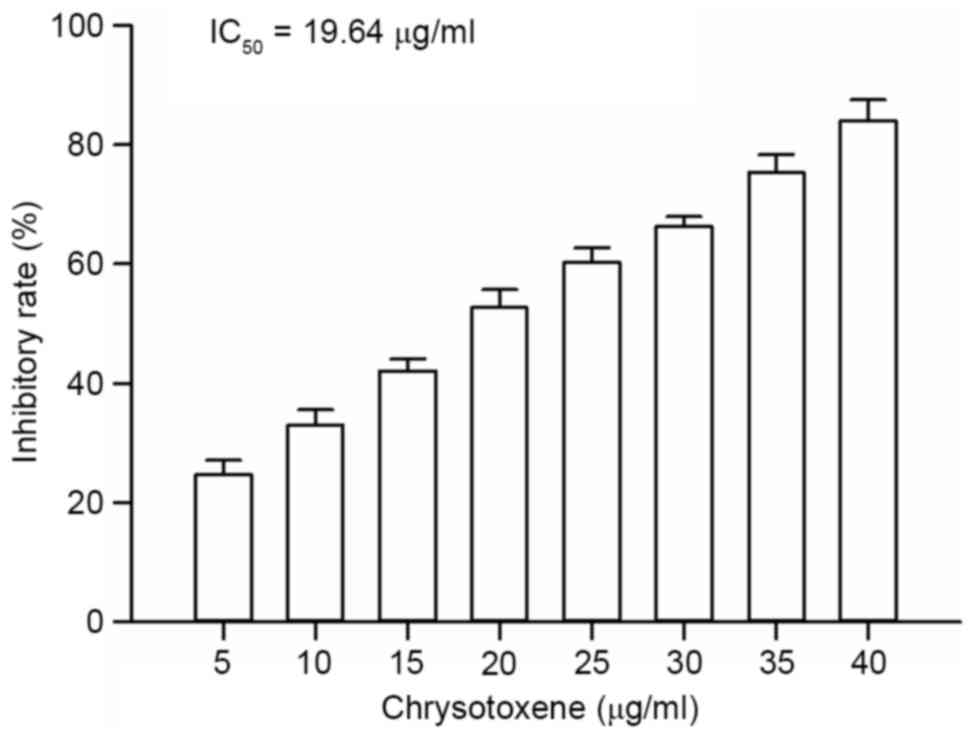
As presented in Fig.
2, chrysotoxene (5, 10, 15, 20, 25, 30, 35 or 40 µg/ml)
exhibited cytotoxic activity against HepG2 cells in a
dose-dependent manner, with inhibitory rates of between 24.67 and
84.06%; the half maximal inhibitory concentration value was 19.64
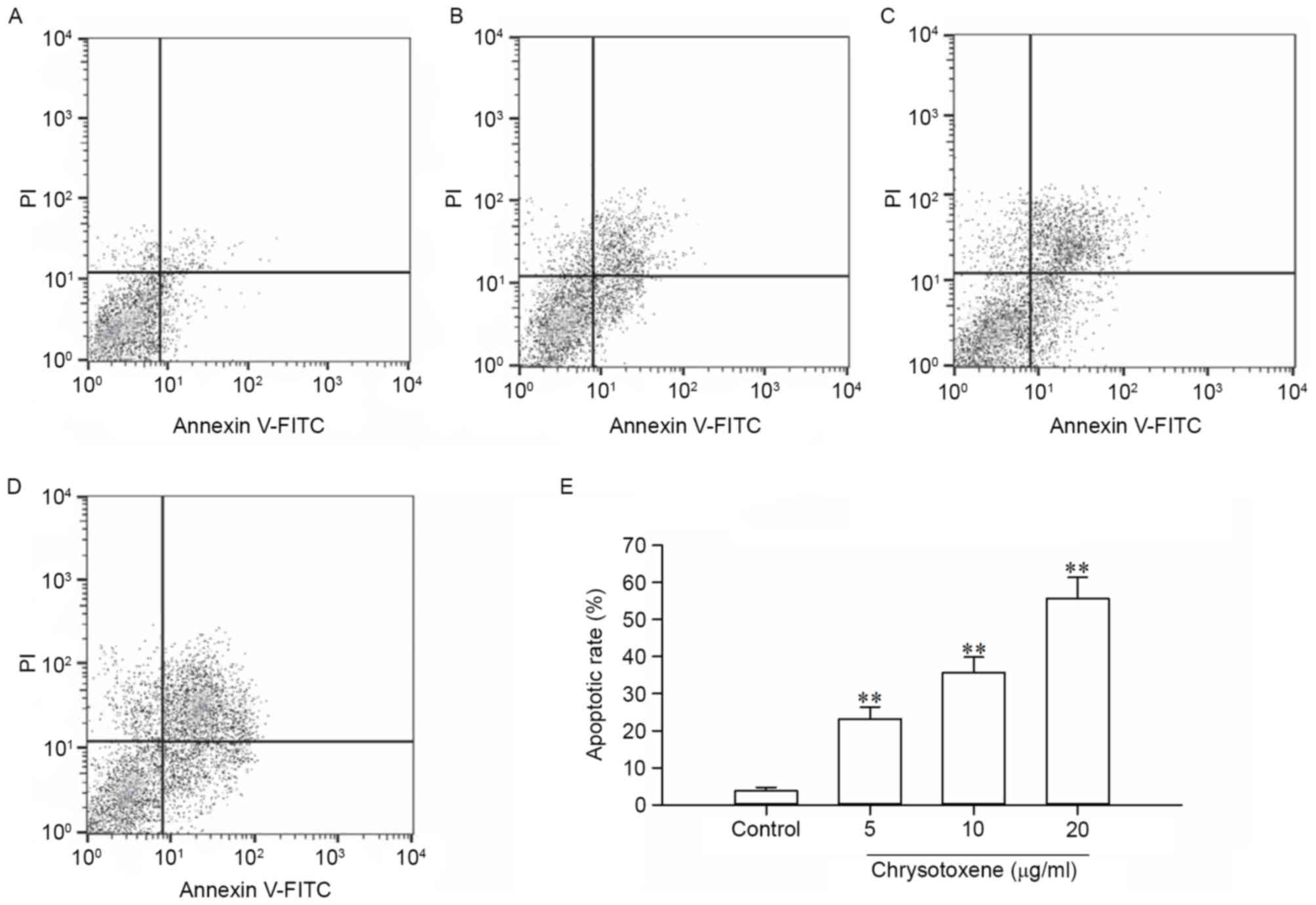
µg/ml. As presented in Fig. 3,
compared with that in the control group, chrysotoxene (5, 10 or 20
µg/ml) significantly (P<0.01) induced apoptosis of HepG2 cells
with apoptotic rates of 23.14, 35.68 and 55.61%, respectively.
These results indicated that the cytotoxic activity of chrysotoxene
against HepG2 cells was associated with apoptosis. Therefore, the
effect of chrysotoxene on apoptotic proteins in the
mitochondria-mediated apoptotic signaling pathway was further
investigated to explore the possible mechanism of chrysotoxene on
apoptosis of HepG2 cells.
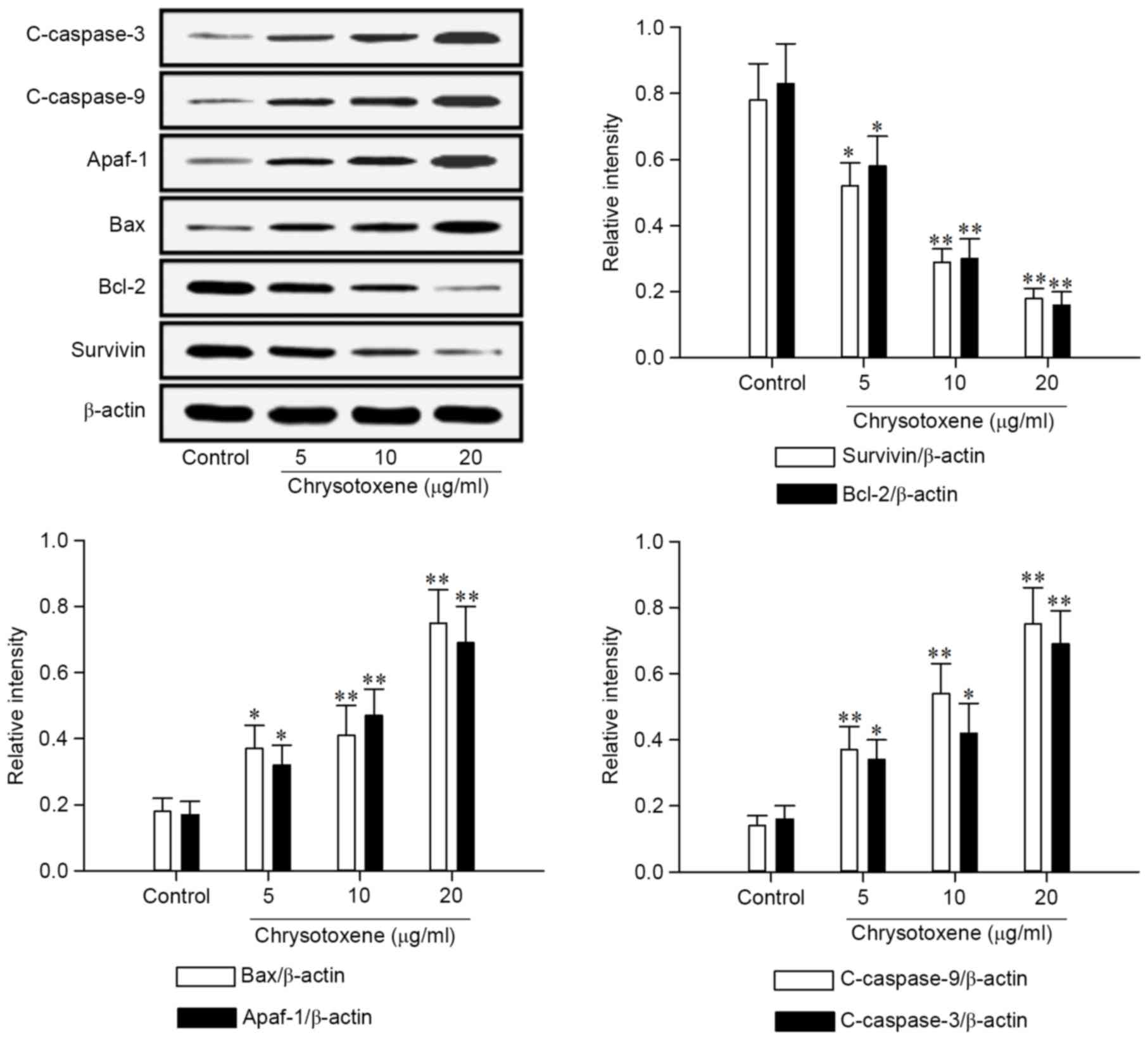
Chrysotoxene regulated apoptotic
proteins in the mitochondria-mediated apoptotic signaling
pathway
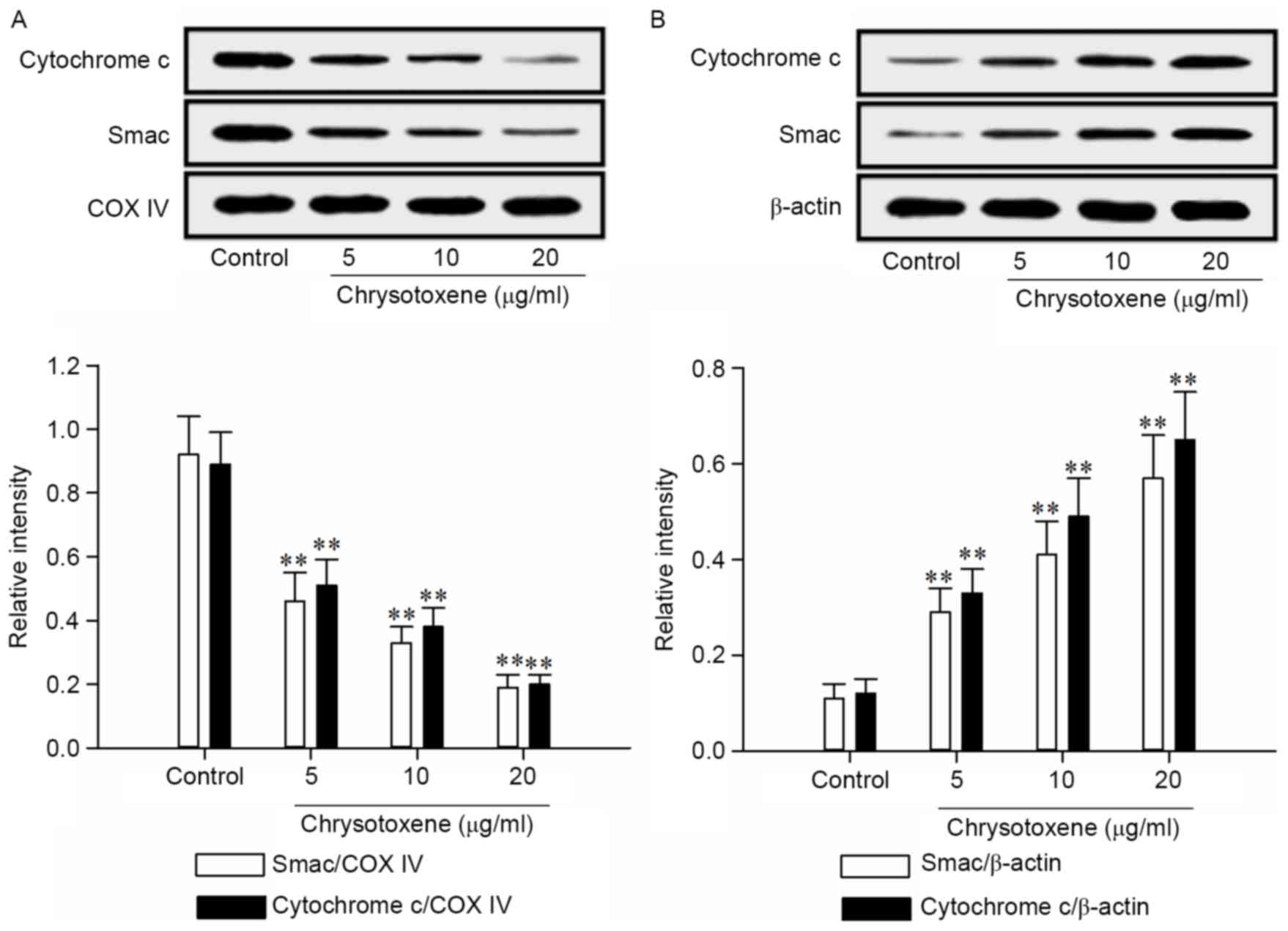
As presented in Fig.
4, chrysotoxene (5, 10 or 20 µg/ml) significantly (P<0.01)
promoted the release of Smac and Cytochrome c from the mitochondria
to the cytoplasm in HepG2 cells, compared with the control group.
Following treatment with chrysotoxene (5, 10 or 20 µg/ml), Survivin
and Bcl-2 proteins levels in HepG2 cells were significantly
(P<0.05) downregulated, and Bax, Apaf-1, c-caspase-9 and
c-caspase-3 proteins levels in HepG2 cells were significantly
(P<0.05) upregulated, compared with the control group (Fig. 5).
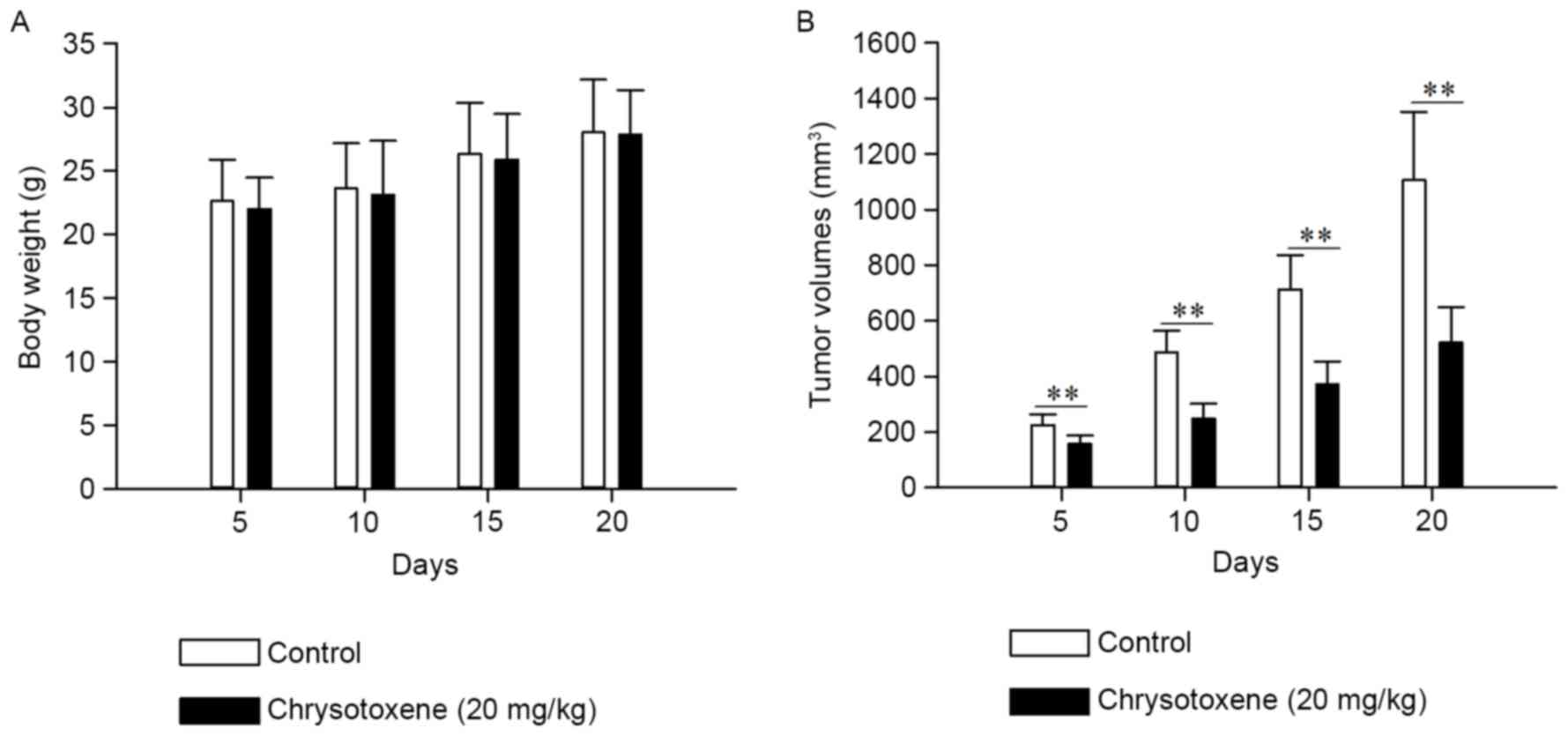
Chrysotoxene inhibited growth of HepG2
cell-induced tumors in nude mice
As presented in Fig.
6, chrysotoxene (20 mg/kg) significantly (P<0.01) inhibited
the growth of HepG2 cell-induced tumors in nude mice without
demonstrating a significant effect on the body weight, compared
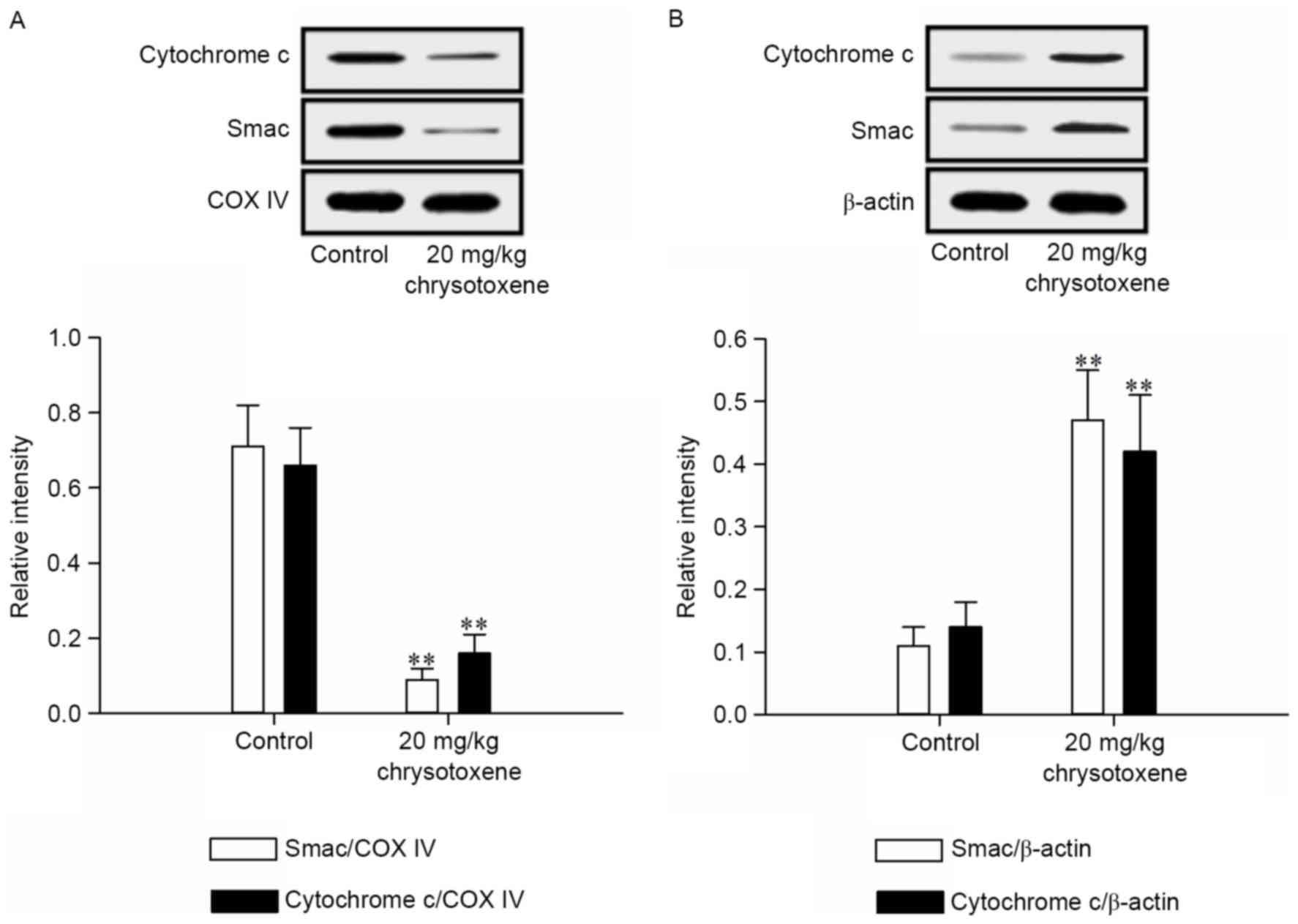
with the control group. As presented in Fig. 7, chrysotoxene (20 mg/kg) significantly
(P<0.01) promoted the release of Smac and Cytochrome c from the
mitochondria to the cytoplasm in HepG2 cell-induced tumor tissue
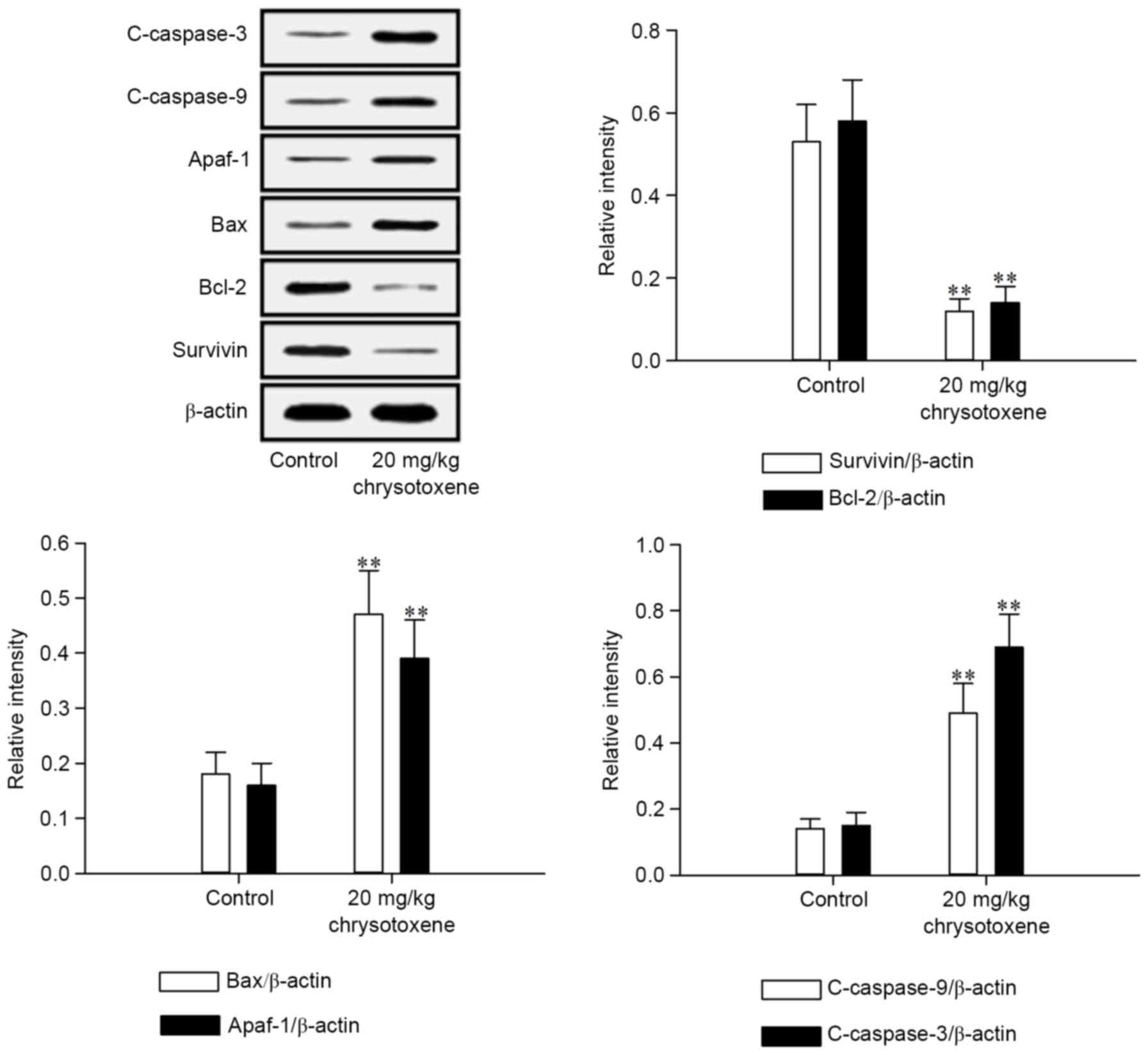
compared with those in the control group. Following treatment with
chrysotoxene (20 mg/kg), Survivin and Bcl-2 proteins levels in
HepG2 cell-induced tumor tissue were significantly (P<0.01)
downregulated, and Bax, Apaf-1, c-caspase-9 and c-caspase-3
proteins levels in HepG2 cell-induced tumor tissue were
significantly (P<0.01) upregulated, compared with those in the
control group (Fig. 8).
Discussion
In the present study, the cytotoxic activity and
possible molecular mechanism of chrysotoxene against HepG2 cells
were investigated by CCK-8, flow cytometry, western blot and
xenograft assays. CCK-8 assay is a commonly used assay to evaluate
the cytotoxic activity of chemical constituents against cancer
cells (11). Flow cytometry analysis
is a commonly used assay to explore whether the cytotoxic activity
of chemical constituents against cancer cells is associated with
apoptosis (12). The results of CCK-8
and flow cytometry assays in the present study suggested that
chrysotoxene demonstrated cytotoxic activity against HepG2 cells
and the cytotoxic activity may be induced by apoptosis (Figs. 2 and 3).
Induction of apoptosis of cancer cells is an
important pathway utilized by chemotherapy drugs to treat cancer
(13). The mitochondria-mediated
apoptotic signaling pathway serves a critical function in inducing
apoptosis of cancer cells (14).
Smac, Cytochrome c, Bcl-2, Bax, Survivin, Apaf-1, c-caspase-9 and
c-caspase-3 are primarily regulative proteins in the
mitochondria-mediated apoptotic signaling pathway (15). Apoptotic stimuli may promote the
release of Smac and Cytochrome c from the mitochondria to the
cytoplasm, which may be inhibited by Bcl-2 (16–18). Bax
is able to decrease the inhibitory effect of Bcl-2 on the release
of Smac and Cytochrome c (19).
Cytochrome c in the cytoplasm, Apaf-1 and procaspase-9, along with
deoxyadenosine triphosphate form apoptosomes, which result in the
cleavage of procaspase-9 (c-caspase-9) (20). C-caspase-9 can induce the cleavage of
procaspase-3 (c-caspase-3), which induces apoptosis of cells
(21). The cleavage of procaspase-3
caused by c-caspase-9 can be inhibited by Survivin, whose function
can be attenuated by Smac in the cytoplasm (22,23). The
results of western blot analysis in the present study indicated
that chrysotoxene induced the apoptosis of HepG2 cells by promoting
the release of Smac and Cytochrome c from the mitochondria to the
cytoplasm, downregulating Survivin and Bcl-2 protein levels, and
upregulating Bax, Apaf-1, c-caspase-9 and c-caspase-3 protein
levels (Figs. 4 and 5).
Xenograft analysis on nude mice is a commonly
applied method to assess the in vivo anti-tumor activity of
chemical constituents (24). The
results of the xenograft assay in the present study demonstrated
that chrysotoxene inhibited the growth of HepG2 cell-induced tumors
without affecting the body weight of nude mice (Fig. 6). The results of western blot analysis
of HepG2 cell-induced tumor tissues suggested that the mechanism of
action of chrysotoxene was associated with the activation of the
mitochondria-mediated apoptotic signaling pathway by regulating the
aforementioned apoptotic proteins (Figs.
7 and 8).
In conclusion, the results of the present study
demonstrated that chrysotoxene induced apoptosis of HepG2 cells
in vitro and in vivo via activation of the
mitochondria-mediated apoptotic signaling pathway. Therefore,
chrysotoxene may possess potential to be developed into an
anti-hepatoblastoma therapeutic agent. However, investigations into
the mechanism of action of chrysotoxene require further
investigation.
References
|
1
|
Sharma D, Subbarao G and Saxena R:
Hepatoblastoma. Semin Diagn Pathol. 34:192–200. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reyes JD, Carr B, Dvorchik I, Kocoshis S,
Jaffe R, Gerber D, Mazariegos GV, Bueno J and Selby R: Liver
transplantation and chemotherapy for hepatoblastoma and
hepatocellular cancer in childhood and adolescence. J Pediatr.
136:795–804. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu L, Hu Y, Duan JH and Yang XD: A novel
approach of targeted immunotherapy against adenocarcinoma cells
with nanoparticles modified by CD16 and MUC1 aptamers. J Nanomater.
2015:3169682015. View Article : Google Scholar
|
|
4
|
McAllister SD, Soroceanu L and Desprez PY:
The antitumor activity of plant-derived non-psychoactive
cannabinoids. J Neuroimmune Pharmacol. 10:255–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma GX, Xu GJ and Xu LS: Inhibitory effects
of Dendrobium chrysotoxum and its constituents on the mouse HePA
and ESC. J Chin Pharmaceut Univ. 25:188–189. 1994.
|
|
6
|
Wang TS, Lu YM, Ma GX, Pan Y, Xu GJ, Xu LS
and Wang ZT: In vitro inhibition activities of leukemia K562 cells
growth by constituents from D. chrysotoxum. Nat Prod Res Dev.
9:1–3. 1997.
|
|
7
|
The National Research Council of The
National Academy of Sciences: Guide for the care and use of
aboratory animals. Eight. Washington, DC: The National Academies
Press; 2010
|
|
8
|
Zheng Z, Qiao Z, Gong R, Wang Y, Zhang Y,
Ma Y, Zhang L, Lu Y, Jiang B, Li G, et al: Uvangoletin induces
mitochondria-mediated apoptosis in HL-60 cells in vitro and in vivo
without adverse reactions of myelosuppression, leucopenia and
gastrointestinal tract disturbances. Oncol Rep. 35:1213–1221. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma GX, Xu GJ, Xu LS, Wang ZT and Kichuchi
T: Studies on chemical constituents of Dendrobium chrysotoxum
Lindl. Acta Pharm Sin. 29:763–766. 1994.
|
|
10
|
Jiang Y, Zhong M and Peng W: Qualitative
analysis of plant-derived samples by liquid
chromatography-electrospray ionization-quadrupole-time of
flight-mass spectrometry. Trop J Pharm Res. 14:925–930. 2015.
View Article : Google Scholar
|
|
11
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Upreti D, Pathak A and Kung SK:
Development of a standardized flow cytometric method to conduct
longitudinal analyses of intracellular CD3ζ expression in patients
with head and neck cancer. Oncol Lett. 11:2199–2206. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Safarzadeh E, Sandoghchian Shotorbani S
and Baradaran B: Herbal medicine as inducers of apoptosis in cancer
treatment. Adv Pharm Bull. 4 Suppl 1:S421–S427. 2014.
|
|
14
|
Li-Weber M: Targeting apoptosis pathways
in cancer by Chinese medicine. Cancer Lett. 332:304–312. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi YG: A structure view of
mitochondria-mediated apoptosis. Nat Struct Biol. 8:394–401. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qin S, Yang C, Wang X, Xu C, Li S, Zhang B
and Ren H: Overexpression of Smac promotes cisplatin-induced
apoptosis by activating caspase-3 and caspase-9 in lung cancer A549
cells. Cancer Biother Radiopharm. 28:177–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Guo XL, Liang B, Wang XW, Fan FG, Jin J,
Lan R, Yang JH, Wang XC, Jin L and Cao Q: Glycyrrhizic acid
attenuates CCl4-induced hepatocyte apoptosis in rats via a
p53-mediated pathway. World J Gastroenterol. 19:3781–3791. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Renault TT, Teijido O, Antonsson B, Dejean
LM and Manon S: Regulation of Bax mitochondrial localization by
Bcl-2 and Bcl-x L: Keep your friends close but your enemies closer.
Int J Biochem Cell Biol. 45:64–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan S and Akey CW: Apoptosome structure,
assembly and procaspase activation. Structure. 21:501–515. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Würstle ML, Laussmann MA and Rehm M: The
central role of initiator caspase-9 in apoptosis signal
transduction and the regulation of its activation and activity on
the apoptosome. Exp Cell Res. 318:1213–1220. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang LH, Li HH, Li M, Wang S, Jiang XR, Li
Y, Ping GF, Cao Q, Liu X, Fang WH, et al: SL4, a chalcone-based
compound, induces apoptosis in human cancer cells by activation of
the ROS/MAPK signalling pathway. Cell Prolif. 48:718–728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alrieri DC: Targeting survivin in cancer.
Cancer Lett. 332:225–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khan N, Bharali DJ, Adhami VM, Siddiqui
IA, Cui H, Shabana SM, Mousa SA and Mukhtar H: Oral administration
of naturally occurring chitosan-based nanoformulated green tea
polyphenol EGCG effectively inhibits prostate cancer cell growth in
a xenograft model. Carcinogenesis. 35:415–423. 2014. View Article : Google Scholar : PubMed/NCBI
|