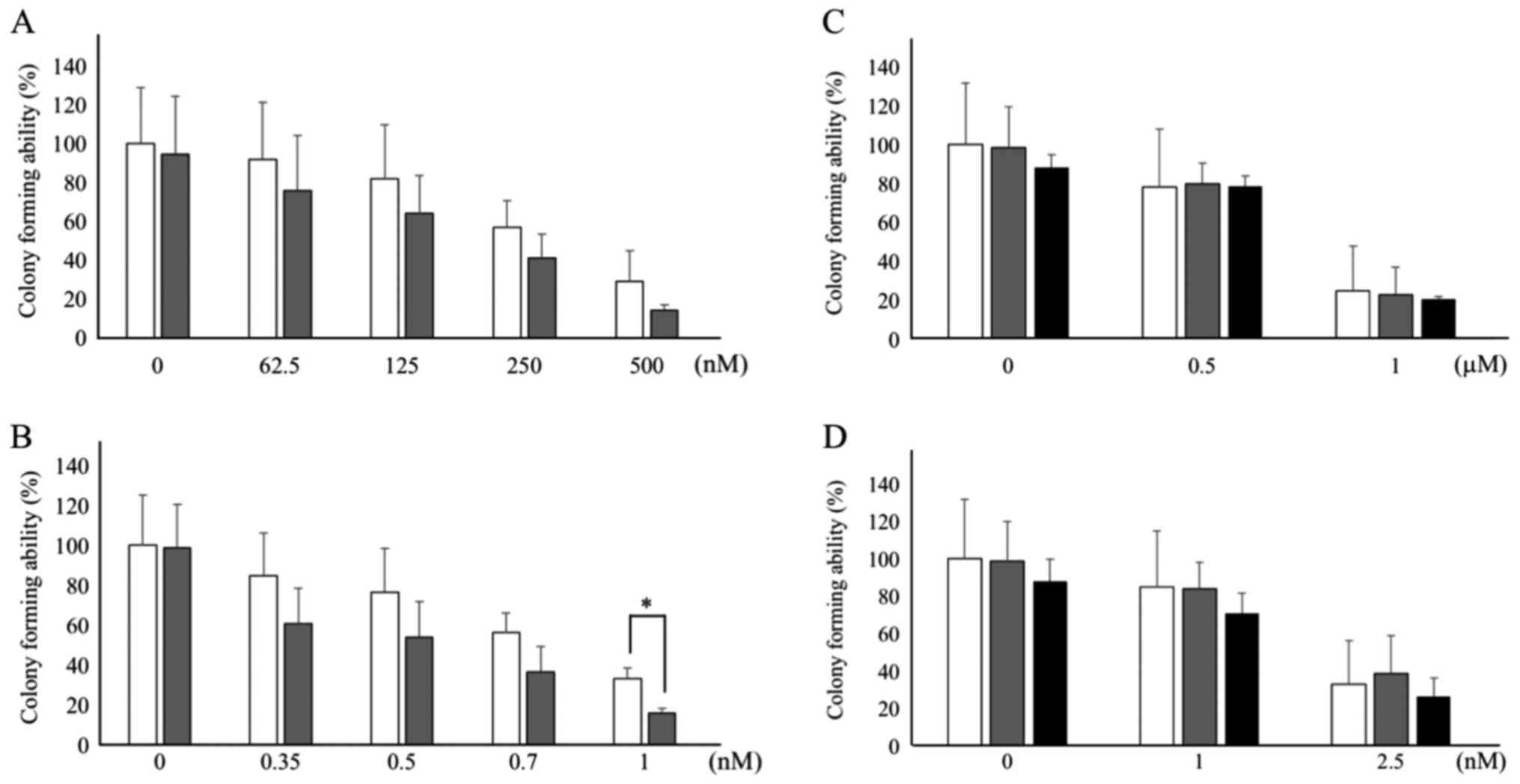
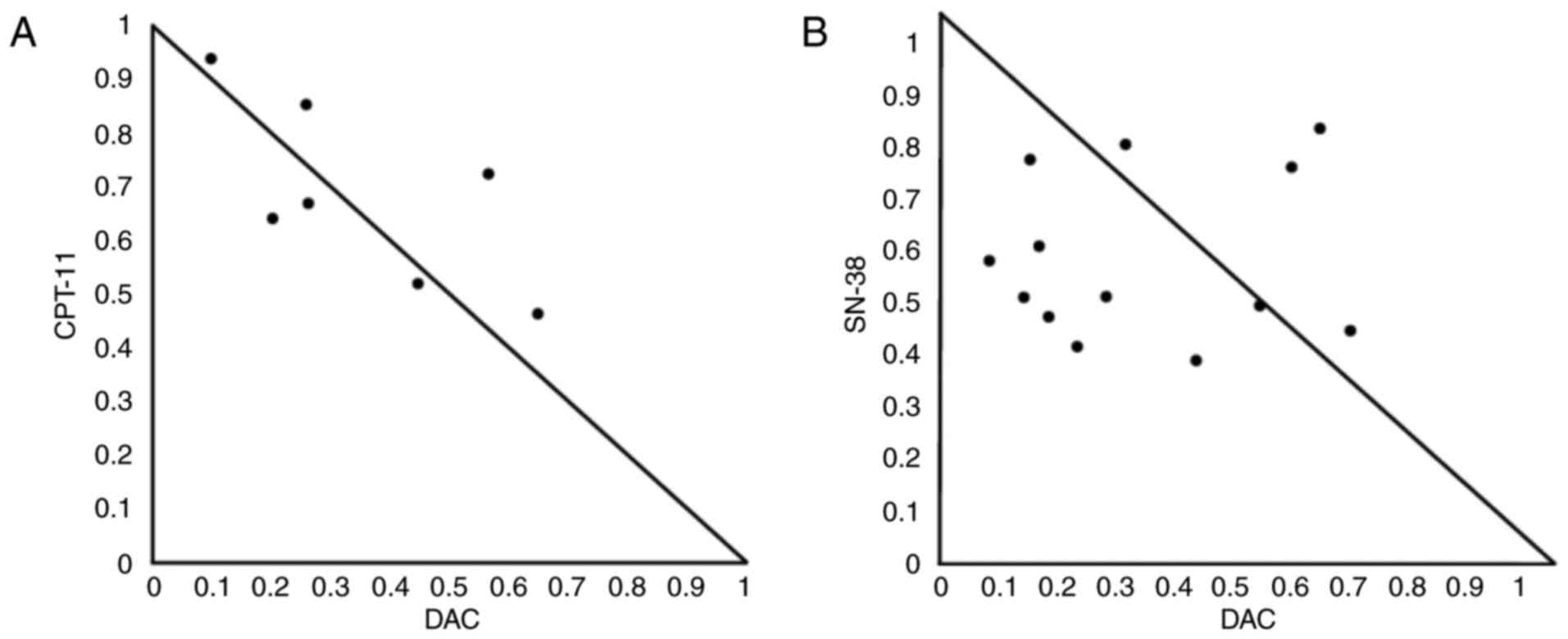
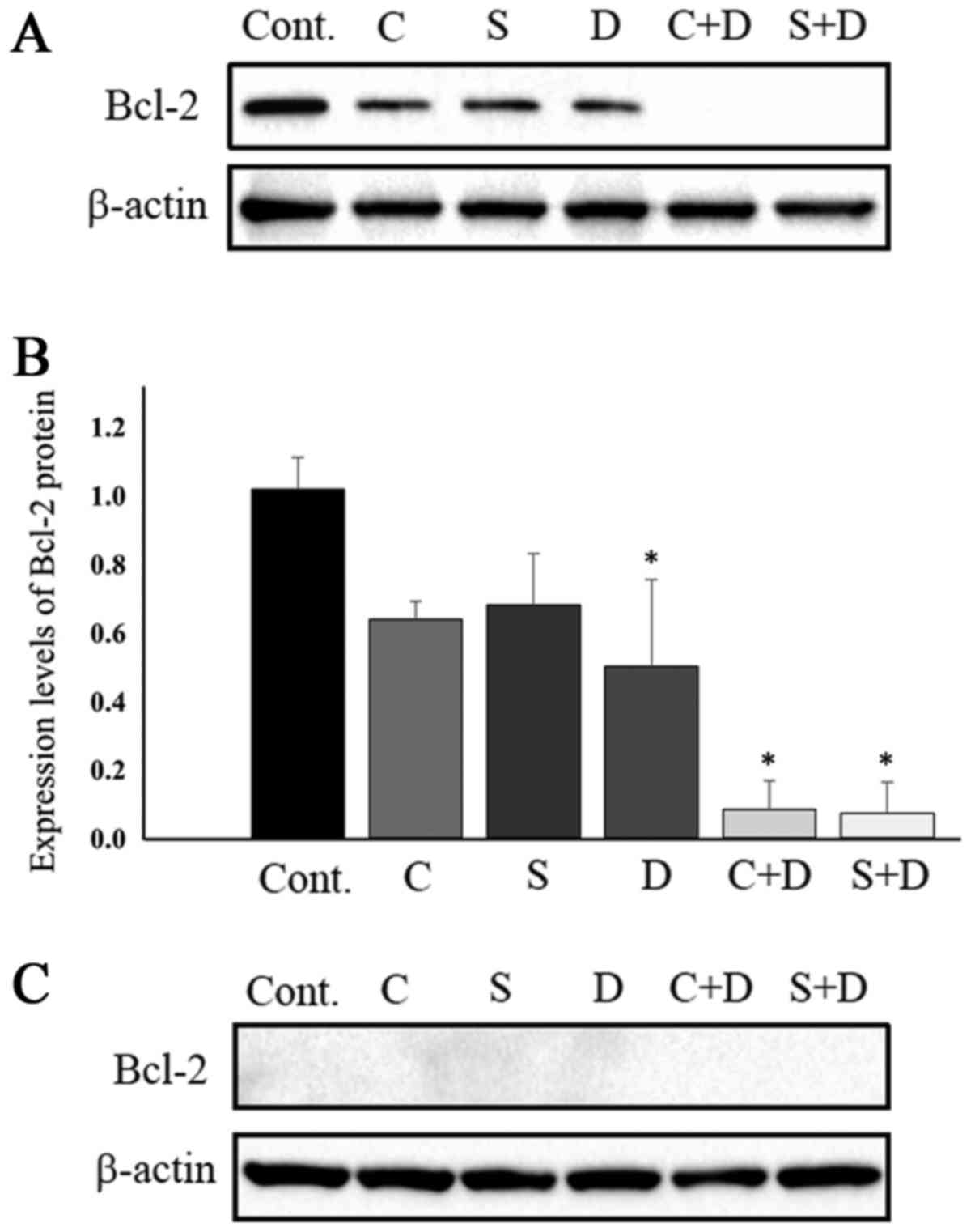
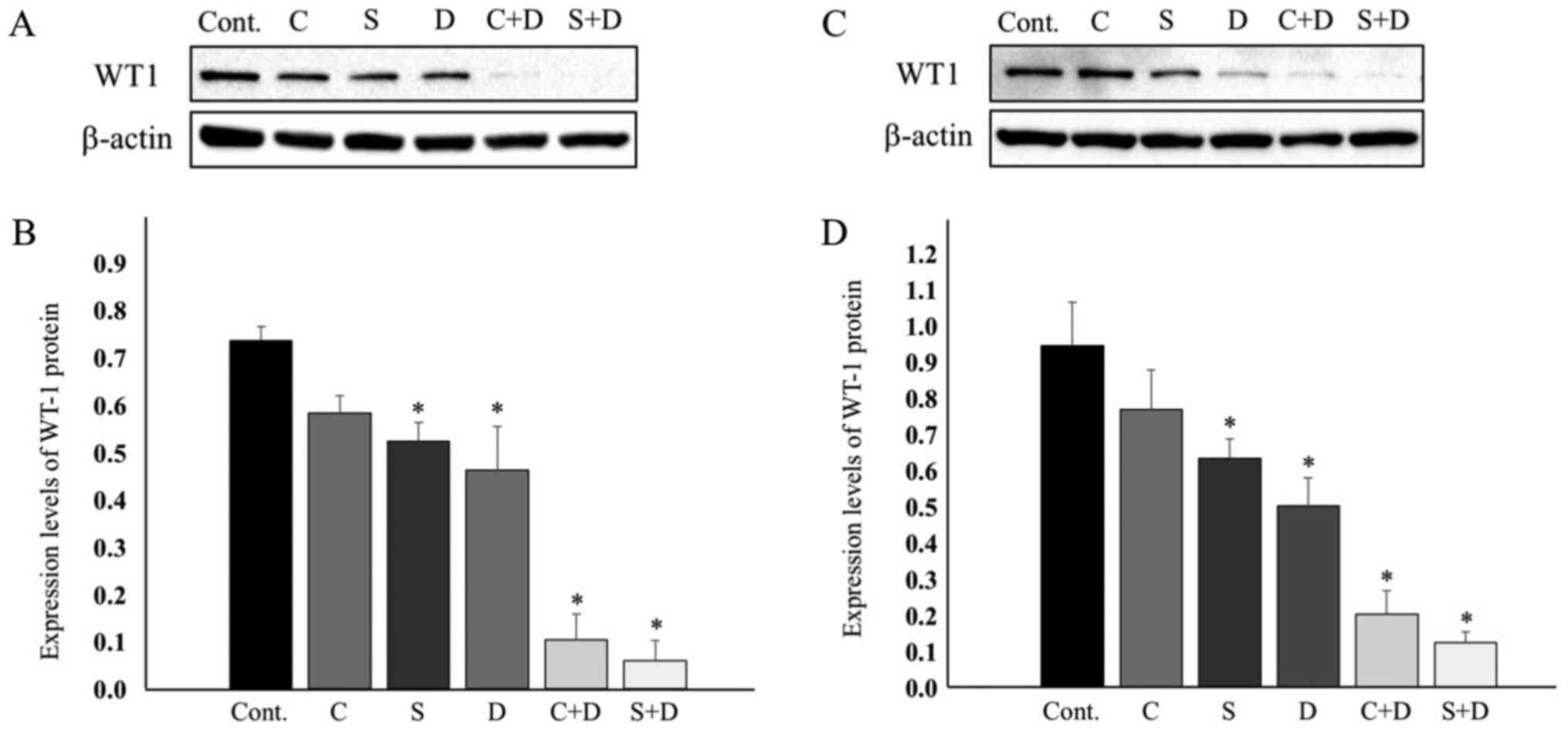
|
1
|
Stewart BW and Wild CP: International
Agency for Research on Cancer. WHO: World Cancer Report 2014.
http://www.thehealthwell.info/node/725845January
18–2018
|
|
2
|
Kawato Y, Aonuma M, Hirota Y, Kuga H and
Sato K: Intracellular roles of SN-38, a metabolite of the
camptothecin derivative CPT-11, in the antitumor effect of CPT-11.
Cancer Res. 51:4187–4191. 1991.PubMed/NCBI
|
|
3
|
Tanizawa A, Fujimori A, Fujimori Y and
Pommier Y: Comparison of topoisomerase I inhibition, DNA damage,
and cytotoxicity of camptothecin derivatives presently in clinical
trials. J Natl Cancer Inst. 11:836–842. 1994. View Article : Google Scholar
|
|
4
|
Hsiang YH, Lihou MG and Liu LF: Arrest of
replication forks by drug-stabilized topoisomerase I -DNA cleavable
complexes as a mechanism of cell killing by camptotecin. Cancer
Res. 49:5077–5082. 1989.PubMed/NCBI
|
|
5
|
Te Poele RH and Joel SP:
Schedule-dependent cytotoxicity of SN-38 in p53 wild-type and
mutant colon adenocarcinoma cell lines. Br J Cancer. 81:1285–1293.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Allen WL and Johnston PG: Role of genomic
markers in colorectal cancer treatment. J Int Oncol. 20:4545–4552.
2005.
|
|
7
|
Paz MF, Fraga MF, Avila S, Guo M, Pollan
M, Herman JG and Esteller M: A systematic profile of DNA
methylation in human cancer cell lines. Cancer Res. 63:1114–1121.
2003.PubMed/NCBI
|
|
8
|
Gnyszka A, Jastrzebski Z and Flis S: DNA
methyltransferase inhibitors and their emerging role in epigenetic
therapy of cancer. Anticancer Res. 33:2989–2996. 2013.PubMed/NCBI
|
|
9
|
Tsai HC, Li H, Van Neste L, Cai Y, Robert
C, Rassool FV, Shin JJ, Harbom KM, Beaty R, Pappou E, et al:
Transient low doses of DNA-demethylating agents exert durable
antitumor effects on hematological and epithelial tumor cells.
Cancer Cell. 21:430–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishiguro M, Iida S, Uetake H, Morita S,
Makino H, Kato K, Takagi Y, Enomoto M and Sugihara K: Effect of
combined therapy with low-dose 5-aza-2′-deoxycytidine and
irinotecan on colon cancer cell line HCT-15. Ann Surg Oncol.
14:1752–1762. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikehata M, Ogawa M, Yamada Y, Tanaka S,
Ueda K and Iwakawa S: Different effects of epigenetic modifiers on
the cytotoxicity induced by 5-fluorouracil, irinotecan or
oxaliplatin in colon cancer cells. Biol Pharm Bull. 37:67–73. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oji Y, Inohara H, Nakazawa M, Nakano Y,
Akahani S, Nakatsuka S, Koga S, Ikeba A, Abeno S, Honjo Y, et al:
Overexpression of the Wilms' tumor gene WT1 in head and neck
squamous cell carcinoma. Cancer Sci. 94:523–529. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okada K, Hakata S, Terashima J, Gamou T,
Habano W and Ozawa S: Combination of the histone deacetylase
inhibitor depsipeptide and 5-fluorouracil upregulates major
histocompatibility complex class II and p21 genes and activates
caspase-3/7 in human colon cancer HCT-116 cells. Oncol Rep.
36:1875–1885. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kamesaki S, Kamesaki H, Jorgensen TJ,
Tanizawa A, Pommier Y and Cossman J: Bcl-2 protein inhibits
etoposide-induced apoptosis through its effects on events
subsequent to topoisomerase II-induced DNA strand breaks and their
repair. Cancer Res. 18:4251–4256. 1993.
|
|
16
|
Dole M, Nuñez G, Merchant AK, Maybaum J,
Rode CK, Bloch CA and Castle VP: Bcl-2 inhibits
chemotherapy-induced apoptosis in neuroblastoma. Cancer Res.
12:3253–3259. 1994.
|
|
17
|
Lou YF, Zou ZZ, Chen PJ, Huang GB, Li B,
Zheng DQ, Yu XR and Luo XY: Combination of Gefitinib and DNA
methylation inhibitor decitabine exerts synergistic anti-cancer
activity in colon cancer cells. PLoS One. 9:e977192014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Call KM, Glaser T, Ito CY, Buckler AJ,
Pelletier J, Haber DA, Rose EA, Kral A, Yeger H, Lewis WH, et al:
Isolation and characterization of a zinc finger polypeptide gene at
the human chromosome 11 Wilms' tumor locus. Cell. 60:509–520. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haber DA, Sohn RL, Buckler AJ, Pelletier
J, Call KM and Housman DE: Alternative splicing and genomic
structure of the Wilms tumor gene WT1. Proc Natl Acad Sci USA.
88:pp. 9618–9622. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hewitt SM, Hamada S, McDonnell TJ,
Rauscher FJ III and Saunders GF: Regulation of the proto-oncogenes
bcl-2 and c-myc by the Wilms' tumor suppressor gene WT1. Cancer
Res. 55:5386–5389. 1995.PubMed/NCBI
|
|
21
|
Mayo MW, Wang CY, Drouin SS, Madrid LV,
Marshall AF, Reed JC, Weissman BE and Baldwin AS: WT1 modulates
apoptosis by transcriptionally upregulating the bcl-2
proto-oncogene. EMBO J. 18:3990–4003. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Inoue K, Ogawa H, Sonoda Y, Kimura T,
Sakabe H, Oka Y, Miyake S, Tamaki H, Oji Y, Yamagami T, et al:
Aberrant overexpression of the Wilms' tumor gene (WT1) in human
leukemia. Blood. 89:1405–1412. 1997.PubMed/NCBI
|
|
23
|
Oji Y, Miyoshi S, Maeda H, Hayashi S,
Tamaki H, Nakatsuka S, Yao M, Takahashi E, Nakano Y, Hirabayashi H,
et al: Overexpression of the Wilms' tumor gene WT1 in de novo lung
cancers. Int J Cancer. 100:297–303. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Oji Y, Yamamoto H, Nomura M, Nakano Y,
Ikeba A, Nakatsuka S, Abeno S, Kiyotoh E, Jomgeow T, Sekimoto M, et
al: Overexpression of the Wilms' tumor gene WT1 in colorectal
adenocarcinoma. Cancer Sci. 94:712–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Loeb DM, Evron E, Patel CB, Sharma PM,
Niranjan B, Buluwela L, Weitzman SA, Korz D and Sukumar S: Wilms'
tumor suppressor gene (WT1) is expressed in primary breast tumors
despite tumor-specific promoter methylation. Cancer Res.
61:921–925. 2001.PubMed/NCBI
|
|
26
|
Oji Y, Nakamori S, Fujikawa M, Nakatsuka
S, Yokota A, Tatsumi N, Abeno S, Ikeba A, Takashima S, Tsujie M, et
al: Overexpression of the Wilms' tumor gene WT1 in pancreatic
ductal adenocarcinoma. Cancer Sci. 95:583–587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miyoshi Y, Ando A, Egawa C, Taguchi T,
Tamaki Y, Tamaki H, Sugiyama H and Noguchi S: High expression of
Wilms' tumor suppressor gene predicts poor prognosis in breast
cancer patients. Clin Cancer Res. 8:1167–1171. 2002.PubMed/NCBI
|
|
28
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chou TC: Drug combination studies and
their synergy quantification using Chou-Talalay method. Cancer Res.
70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohmori T, Podack ER, Nishio K, Takahashi
M, Miyahara Y, Takeda Y, Kubota N, Funayama Y, Ogasawara H, Ohira
T, et al: Apoptosis of lung cancer cells caused by some anti-cancer
agents (MMC, CPT-11, ADM) is inhibited by BCL-2. Biochem Biophys
Res Commun. 192:30–36. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Palissot V, Belhoussine R, Carpentier Y,
Sebille S, Morjani H, Manfait M and Dufer J: Resistance to
apoptosis induced by topoisomerase I inhibitors in
multidrug-resistant HL60 leukemic cells. Biochem Biophys Res
Commun. 245:918–922. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Appleton K, Mackay HJ, Judson I, Plumb JA,
McCormick C, Strathdee G, Lee C, Barrett S, Reade S, Jadayel D, et
al: Phase I and pharmacodynamic trial of the DNA methyltransferase
inhibitor decitabine and carboplatin in solid tumors. J Clin Oncol.
25:4603–4609. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oki Y, Kondo Y, Yamamoto K, Ogura M, Kasai
M, Kobayashi Y, Watanabe T, Uike N, Ohyashiki K, Okamoto S, et al:
Phase I/II study of decitabine in patients with myelodysplastic
syndrome: A multi-center study in Japan. Cancer Sci. 103:1839–1847.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tatsumi N, Oji Y, Tsuji N, Tsuda A,
Higashio M, Aoyagi S, Fukuda I, Ito K, Nakamura J, Kitamura Y, et
al: Wilms' tumor gene WT1-shRNA as a potent apoptosis-inducing
agent for solid tumors. Int J Oncol. 32:701–711. 2008.PubMed/NCBI
|