Introduction
Hepatocellular carcinoma (HCC) is the most common
primary liver cancer and has been the third most common cause of
death from cancer globally in recent years (1,2). Several
clinical protocols, including conventional chemotherapy, liver
transplantation, surgical resection, radio frequency ablation, and
transcatheter arterial chemoembolization, have been used to treat
HCC (2,3). However, these treatments are often not
effective, as indicated by frequent recurrence and low objective
response rates (4). Therefore, it is
necessary to develop novel effective and safe therapeutic agents in
order to improve the efficacy of HCC treatment.
It has been demonstrated that angiogenesis is
involved in the progression of human HCC and that angiogenesis is a
process that serves a significant role in the aggressiveness of HCC
(5). Angiogenesis is induced when
hepatic stellate cells and tumor inflammatory cells start to
release factors such as vascular endothelial growth factor (VEGF)
(6). VEGF is the strongest vasoactive
factor and induces the formation of new capillaries from nearby
normal existing ones, thereby promoting tumor growth and tumor
stroma formation (7). Therefore, VEGF
has an important role in the occurrence and development of HCC and
treatment strategies for liver cancer by targeting VEGF have become
a hot spot.
Curcumin is a polyphenol compound extracted from the
rhizomes of Curcuma longa that has been demonstrated to
exert effective antiangiogenic, anti-inflammatory and antioxidant
effects (8–10). Curcumin exerts antitumor effects in
several types of cancer, including hepatic carcinoma, by modulating
tumor growth factor-β, protein kinase B, and caspase-3 expression
(11–14). However, the antiangiogenic effect of
curcumin by VEGF in HCC remains obscure. To explore this, the
present study examined the antitumor effect of curcumin in HCC. The
results demonstrated that curcumin inhibited HCC proliferation
in vitro and in vivo by inhibiting VEGF expression
and the phosphoinositide 3-kinase (PI3K)/AKT serine/threonine
kinase 1 (AKT) signaling pathway.
Materials and methods
Reagents
Curcumin was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). A Cell Counting Kit-8 (CCK-8) was
purchased from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). An Annexin V/fluorescein isothiocyanate (FITC) kit was
purchased from Vazyme Biotech Co., Ltd. (Nanjing, China). VEGF
antibodies (cat no. sc-7269), used for western blotting and
immunohistochemistry, were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). PI3K (cat no. ab151549) and AKT (cat no.
ab38449) antibodies were purchased from Abcam (Cambridge, USA). The
polymer horseradish peroxidase (HRP) detection systems, used for
immunohistochemistry and western blotting, were purchased from
Zhongshan Golden Bridge Biotechnology Co., Ltd (Beijing, China) and
Cellular Signaling Technology (Danvers, MA). Recombinant human VEGF
(rhVEGF) was from R&D Systems, Inc. (Minneapolis, MN, USA).
Cell culture
The H22 HCC cell line was purchased from the Cell
Bank of Type Culture Collection of the Chinese Academy of Sciences,
Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco's modified Eagle's
medium supplemented with 10% fetal calf serum (Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin, and
100 µg/ml streptomycin in a humidified incubator at 37°C with 5%
CO2.
Animals
Nude male mice (4–5 weeks old) were purchased from
the Model Animal Research Center of Nanjing University (Nanjing,
China) and kept under controlled conditions with a 12-h light/dark
cycle with access to food and water ad libitum. All animal work was
conducted in compliance with guidelines for ethical animal research
and approved by the Animal Ethics Committee of Xiamen University
(Xiamen, China).
Cell viability assay
H22 cells were treated with different concentrations
of curcumin (0, 5, 10, 20, 40 and 80 µm) for 12, 24, or 48 h. In
other experiments, H22 cells were stimulated by VEGF (20 ng/ml) for
24 h in serum-free medium, and curcumin (20, 40, and 80 µm) was
added at the same time. Thereafter, a CCK-8 assay was used to
determine cell viability, as per the manufacturer's protocol.
Apoptosis assay
H22 cells were treated with different concentrations
of curcumin (0, 20, 40, and 80 µm) for 24 h. Apoptosis was
quantitatively measured by Annexin V/propidium iodide (PI) double
staining which was carried out at room temperature for 2 h. Cell
apoptosis was evaluated using FACSVerse™ (Beckman Coulter, Inc.,
Brea, CA, USA), and data were analyzed using FlowJo V10 (Flowjo
LLC, Ashland, OR, USA).
Tumor xenografts and in vivo curcumin
treatment
H22 cells (2×105) were subcutaneously
injected into nude mice (4–5 weeks old, n=15). Seven days after
tumor cell inoculation, the mice were randomly divided into three
groups of five and treated with vehicle (0.1% DMSO) or curcumin at
a dose of 50, 100 mg/kg daily for two weeks. At day 24, the tumors
were removed and weighed.
Histopathology
Tumor tissues from the xenograft model mice were
collected and fixed in formalin at 4°C for 48 h, decalcified in 10%
ethylenediamine tetraacetic acid, and embedded in paraffin for
histopathological analysis. Serial paraffin sections (5 µm) were
stained with hematoxylin and eosin at room temperature for 10
min.
Immunohistochemistry
Tumor samples were fixed with formalin at 4°C for 48
h, embedded in paraffin, and sectioned into 5 µm sections. Sections
were dewaxed and dehydrated in a descending series of ethanol
dilutions: 100% ethanol (2 times, 2 min each), 95% ethanol (2
times, 2 min each) and 70% ethanol (1 time, 2 min). Once
rehydrated, antigen retrieval was performed using a citrate buffer,
and endogenous peroxidase activity was blocked at room temperature
for 10 min using 3.0% hydrogen peroxide, and the sections were
blocked at room temperature for 30 min using 10% goat serum, then
incubated with the primary antibodies directed against VEGF (1:200)
at 4°C overnight. Primary antibodies were detected using a
horseradish peroxidase-conjugated anti-mouse secondary antibody
(1:1,000; cat no. SP-9002; Zhongshan Golden Bridge Biotechnology
Co., Ltd) for 30 min at 37°C. Following rinsing with PBS three
times, the sections were exposed for 7 min to diaminobenzidine
(DAB) + chromogen, then dehydrated in ethanol and xylene and
mounted onto glass slides. The staining intensity and average
percentage of positive cells were assayed for 10 independent fields
using a light microscope at a magnification of ×400.
Western blot analysis
Tumor tissues were washed with PBS three times, then
ground and lysed in RIPA cell lysis buffer (1% NP-40, 0.5% sodium
deoxycholate, 0.1% SDS in PBS). Following centrifugation at a speed
of 14,000 × g for 5 min at 4°C, the supernatants were kept frozen
at −80°C until use. Protein concentrations were measured using the
BCA protein quantification Kit (Beyotime, Haimen, China) according
to the manufacturer's protocol. A total of 50 µg of denatured
protein was separated using 10% SDS-PAGE and transferred onto
polyvinylidene fluoride membranes. The membranes were blocked with
10% non-fat milk at room temperature for 1 h and then incubated
with primary antibodies for VEGF, PI3K, AKT (1:1,000) and mouse
monoclonal anti-β-actin (1:5,000) at 4°C overnight, followed by
incubation with appropriate HRP-conjugated anti-mouse secondary
antibodies (1:1,000; cat nos. 7074 and 7076; Cell Signaling
Technology, Inc., Danvers, MA, USA) for 1 h at room temperature.
Protein signals were detected using the BeyoECL plus kit (Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's protocol. Finally, the densities of the bands were
quantified using Image J software (version 1.38; National
Institutes of Health, Bethesda, MD, USA) (15). Equivalent protein loading and transfer
efficiency were verified by staining for β-actin.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the tumor tissues was isolated using
TRIzol (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's protocol. RETROscript™ Reverse Transcription kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1 µg RNA
were used for cDNA synthesis. SYBR Green One-Step qRT-PCR kit
(Thermo Fisher Scientific, Inc.) and a 7900 HT Fast Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.) were
used for qPCR. Data were normalized to the internal control GAPDH
and presented as an expression fold change using the
2−ΔΔCq method (16).
Thermocycling conditions were as follows: 95°C for 3 min, followed
by 40 cycles at 94°C for 30 sec, 60°C for 30 sec and 72°C for 60
sec. The gene-specific primers were selected according to the
literature. The primers were synthesized by Sangon Biological
Engineering Technology Services Co., Ltd. (Shanghai, China). The
primer sequences were as follows: PI3K, forward
5′-CGTTTCTGCTTTGGGACAAC-3′ and reverse 5′-CCTGATGATGGTGGAG-3′; AKT,
forward 5′-TGAGAGAAGCCACGCTGTC-3′ and reverse
5′-CGGAGAACAAACTGGATGAA-3′; VEGF, forward
5′-TAGACGTTCCCTGCCAGCAA-3′ and reverse
5′-AGCATCCGAGGAAAACATAAAATCTT-3′; and GAPDH, forward
5′-AACGGATTTGGTCGTATTGGG-3′ and reverse
5′-TCGCTCCTGGAAGATGGTGAT-3′.
Statistical analysis
Data were expressed as mean ± standard deviation.
Statistics were presented in Prisms 6.1 software (GraphPad
Software, Inc., La Jolla, CA, USA). All data were analyzed using
one-way analysis of variance followed by the Tukey test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Curcumin inhibits proliferation and
induces apoptosis in HCC cells
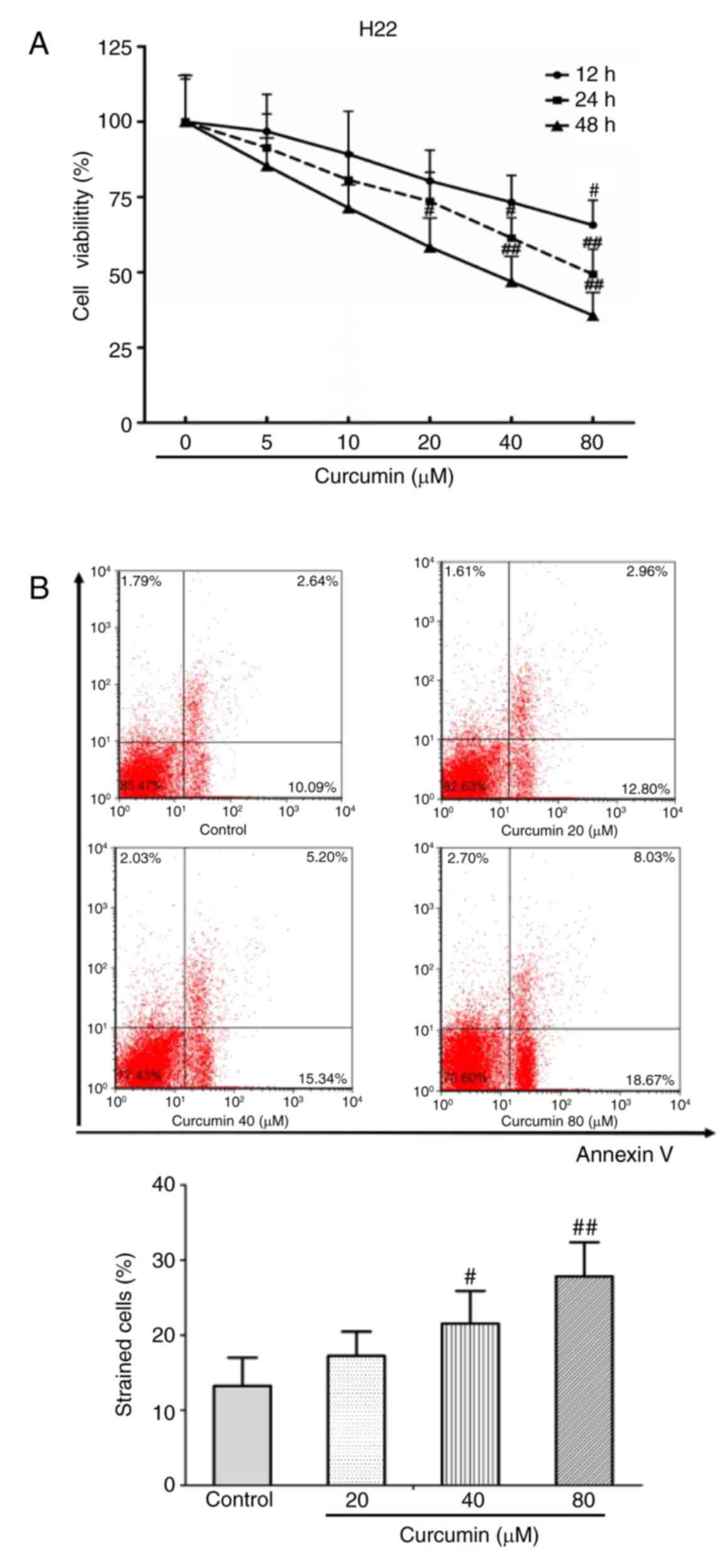
To investigate the inhibitory effect of curcumin on
HCC cells, a CCK-8 assay was performed to detect cell viability
in vitro. The results demonstrated that curcumin inhibited
H22 cell viability in a dose-dependent manner, and particularly at
a very high dose of 80 µM (P<0.01; Fig. 1A). In addition, Annexin V/PI staining
was performed to determine the apoptosis rate of H22 cells.
Curcumin treatment significantly induced H22 cell apoptosis,
especially at concentrations of 40 and 80 µm (P<0.05 and
P<0.01 compared with untreated control respectively; Fig. 1B).
Curcumin inhibits tumor growth in a
xenograft model in vivo
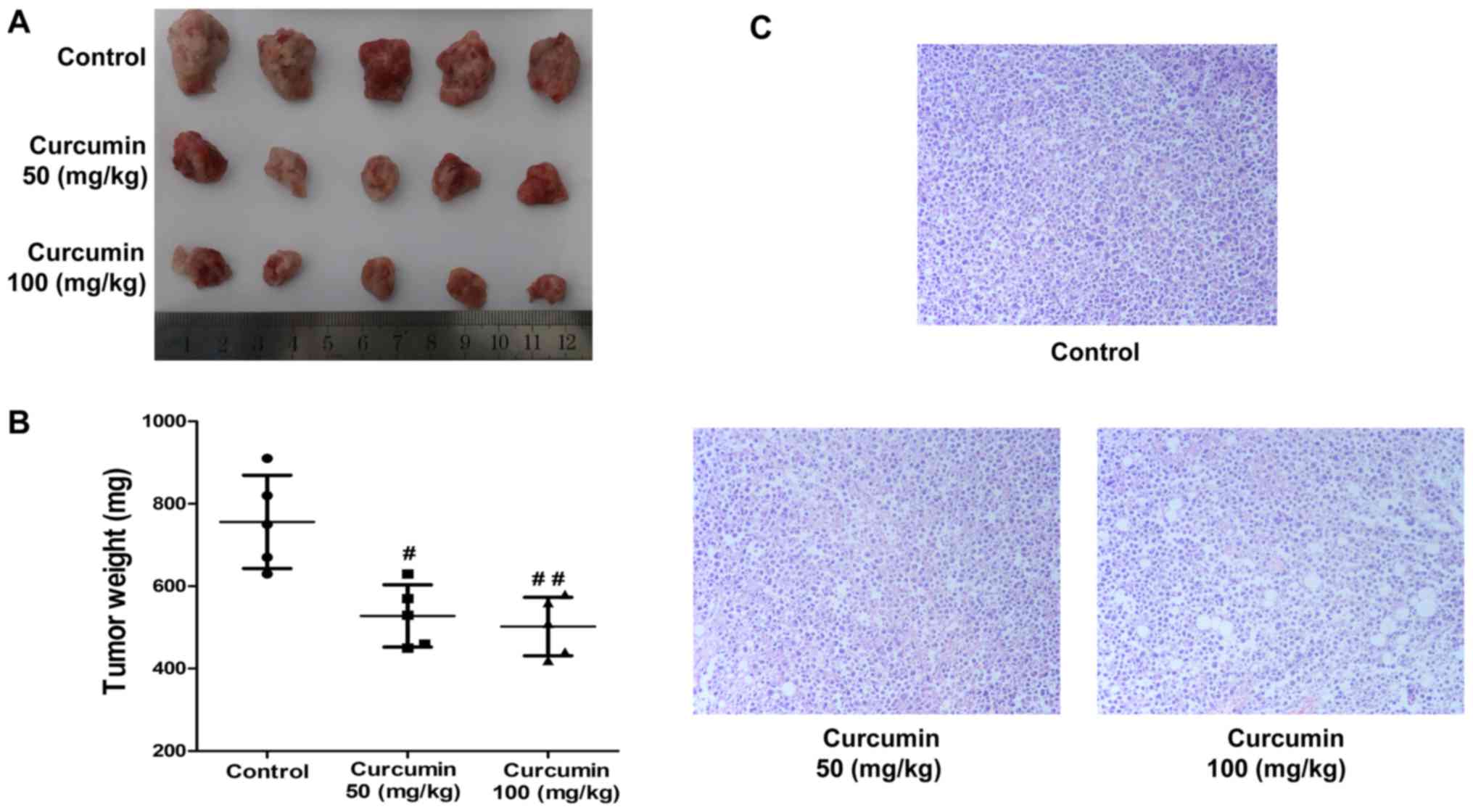
To further explore the anticancer activities of
curcumin in vivo, a xenograft model was established in mice
using H22 cells and treated with 50 or 100 mg/kg of curcumin by
intragastric administration for two weeks. The results demonstrated
that curcumin treatment resulted in a dramatic inhibition of H22
xenograft tumor growth compared with the vehicle-treated control
group, particularly at a very high dose (50 mg/kg, P<0.05; 100
mg/kg, P<0.01; n=5; Fig. 2A). The
tumor weight in the curcumin-treated groups were significantly
decreased compared with the vehicle-treated control group (50
mg/kg, P<0.05; 100 mg/kg, P<0.01; n=5; Fig. 2B). Furthermore, histopathological
analysis indicated that tumor cells in the curcumin-treated groups
were visibly sparser compared with those in the vehicle-treated
control group (Fig. 2C).
Curcumin inhibits VEGF protein
expression and PI3K/AKT signaling in vivo
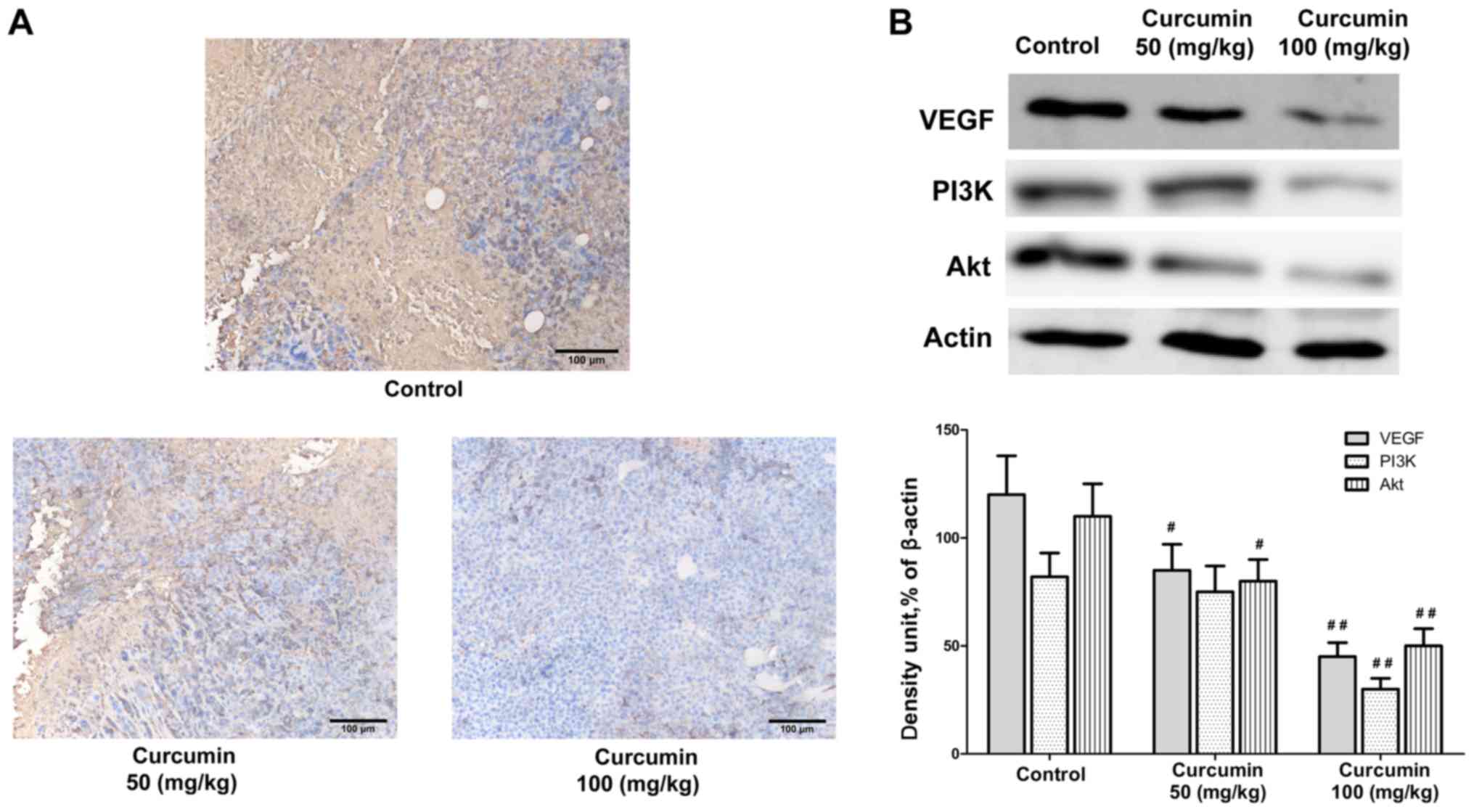
Next, the mechanisms by which curcumin exerts its
antitumor effect in vivo were explored. Immunohistochemistry
analysis indicated that curcumin treatment impaired the expression
of VEGF in the tumor tissues obtained from the tumor-bearing mice
in a dose dependent manner (50 mg/kg, P<0.05; 100 mg/kg,
P<0.01; Fig. 3). Previous studies
have implicated that VEGF expression is mediated via PI3K/AKT
signaling pathway. Results from the western blot analysis of the
tumor tissues indicated that protein expression levels of PI3K and
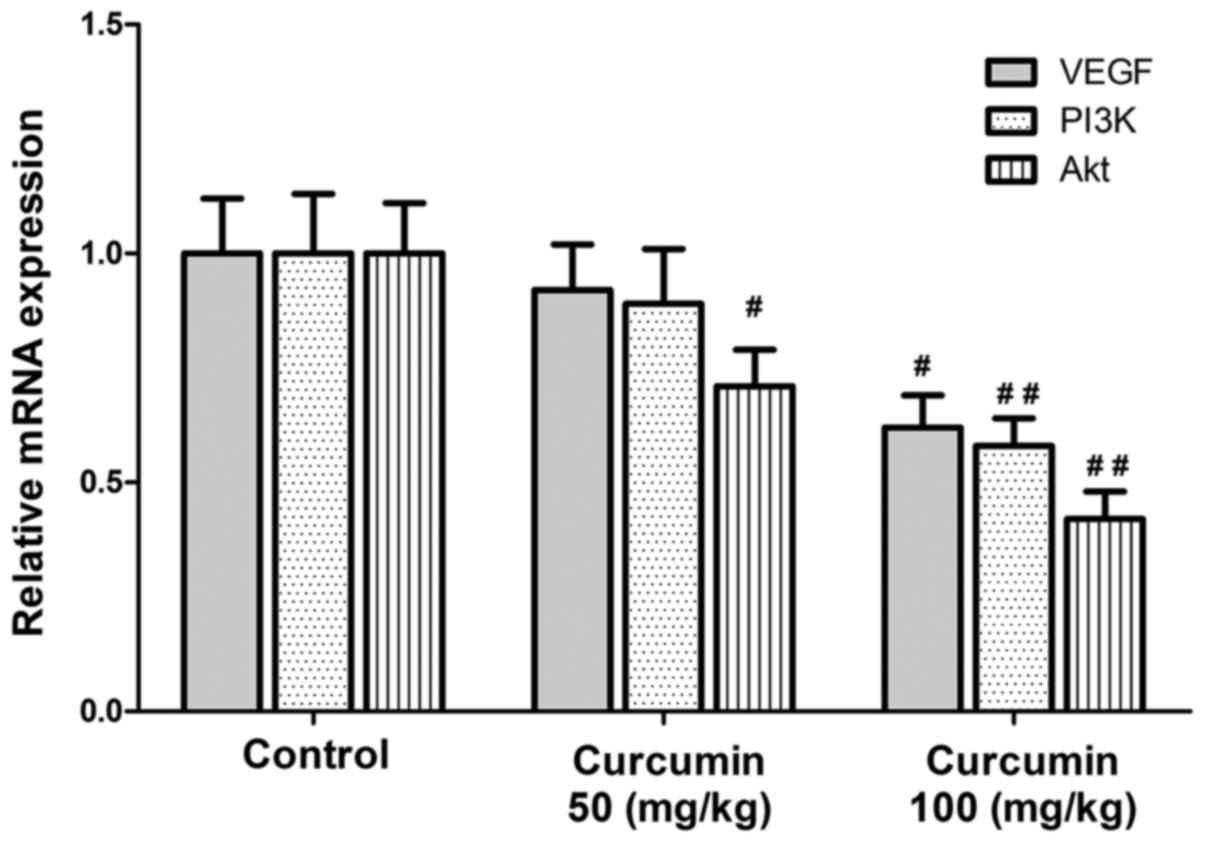
AKT were inhibited following curcumin treatment (Fig. 3). In addition, the mRNA expression
levels of VEGF, PI3K and AKT were also significantly downregulated
by curcumin treatment (Fig. 4). These
findings suggested that the anticancer activities of curcumin in
vivo were associated with the downregulation of VEGF expression
in human HCC cells, and most likely mediated through the PI3K/AKT
signaling pathway.
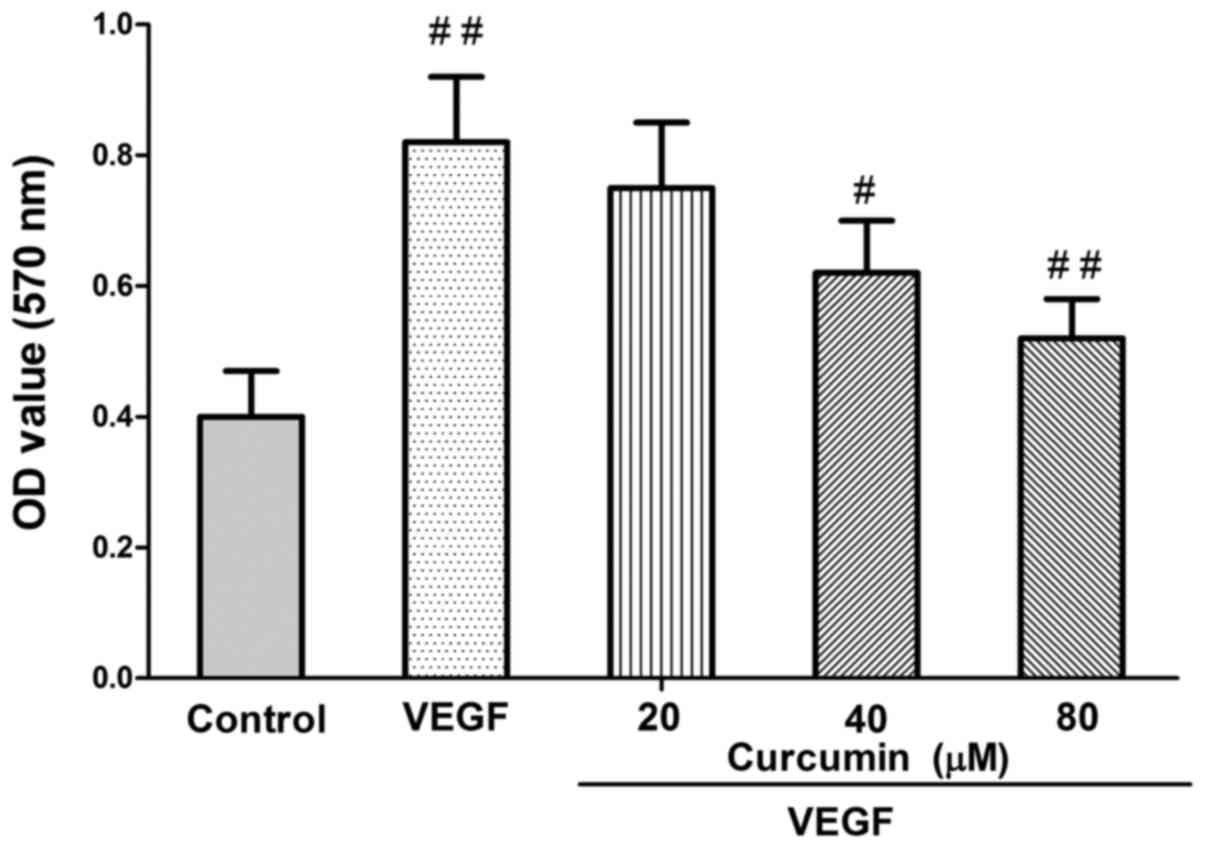
VEGF mediates the curcumin effect on
HCC cell viability
Finally, in order to examine whether VEGF mediates
the curcumin effect in inhibiting HCC cell viability, recombinant
human VEGF (rhVEGF) was used. Compared with different
concentrations of curcumin-treated cells, rhVEGF-treated group
caused an increase in HCC cell growth (Fig. 5). Curcumin treatment resulted in a
significant reduction of VEGF-induced proliferation (P<0.01;
Fig. 5).
Discussion
In the present study, proliferation and apoptosis of
H22 cells were detected with CCK-8 assay and Annexin V/PI double
staining, respectively, following treatment with different
concentrations of curcumin. The results indicated that curcumin
significantly inhibited cell growth and induced apoptosis of H22
cells in a dose-dependent manner in vitro. In addition,
curcumin treatment significantly inhibited tumor growth, and
significantly reduced VEGF protein expression and PI3K/AKT
signaling, in vivo in a xenograft mouse model.
HCC is the primary form of liver cancer and
represents the sixth most common cancer worldwide. As part of its
multifaceted molecular pathogenesis, angiogenesis has a significant
role in the aggressiveness of HCC (17,18).
Therefore, improved knowledge of oncogenic processes and the
signaling pathways that regulate angiogenesis progression are
important and drive the development of molecularly targeted
therapies (6).
Curcumin, a major constituent of C. longa,
has been demonstrated to act as a potential therapeutic agent in
diseases like cancer involving a variety of biological mechanisms,
including cell cycle regulation, induction of apoptosis, inhibition
of metastasis, oncogene expression, and tumorigenesis (8,19–21). A potent inhibition by curcumin of the
transcription factor nuclear factor (NF)-κB, and the resultant
downstream gene products, including MYC proto-oncogene (c-myc),
BCL2 apoptosis regulator (Bcl-2), nitric oxide synthase,
cyclooxygenase-2, cyclin D1, matrix metallopeptidase9, tumor
necrosis factor-α, and interleukins, was reported to be effective
against most types of cancer (22).
In addition, curcumin has been reported to exert its tumor
suppressing effect via an antiangiogenic effect (23). However, it remains unknown whether
curcumin could be a new therapeutic agent targeting HCC due to its
antiangiogenic mechanism. In the present study, curcumin
significantly inhibited HCC growth in vitro and in
vivo by targeting VEGF expression and the PI3K/AKT signaling
pathway.
One of the major limitations regarding the present
work is that the exact mechanism by which curcumin targets VEGF in
HCC cells remains unclear. Therefore, it will be very important to
perform additional studies in the future to explore this specific
mechanism.
The present results are the first to demonstrate
that curcumin inhibited HCC growth in vitro and in
vivo through targeting VEGF expression, and may provide
important information about curcumin as a potential therapeutic
agent for the treatment of HCC. However, further in vitro
and in vivo studies will be needed to understand the exact
mechanism of curcumin targeting HCC.
Acknowledgements
The present study was supported by the Project of
Xiamen Haicang District Scientific and Technological Plan (grant
no. 350205Z54011), Medical Innovations Topic in Fujian Province
(grant no. 2012-CXB-29), the Project of Xiamen Scientific and
Technological Plan (grant no. 3502Z20174023) and the Science and
Technology Project of Natural Science Foundation of Fujian Province
(grant no. 2016J01639).
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
VEGF
|
vascular endothelial growth factor
|
|
CCK-8
|
Cell Counting Kit-8
|
|
FITC
|
fluorescein isothiocyanate
|
|
HRP
|
horseradish peroxidase
|
|
PI
|
propidium iodide
|
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li M, Zhang W, Wang B, Gao Y, Song Z and
Zheng QC: Ligand-based targeted therapy: A novel strategy for
hepatocellular carcinoma. Int J Nanomedicine. 11:5645–5669. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Forner A, Reig ME, de Lope CR and Bruix J:
Current strategy for staging and treatment: The BCLC update and
future prospects. Semin Liver Dis. 30:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peters GJ and Honeywell RJ: Drug transport
and metabolism of novel anticancer drugs. Expert Opin Drug Metab
Toxicol. 11:661–663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berretta M, Rinaldi L, Di Benedetto F,
Lleshi A, De Re V, Facchini G, De Paoli P and Di Francia R:
Angiogenesis inhibitors for the treatment of hepatocellular
carcinoma. Front Pharmacol. 7:4282016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di J, Gao K, Qu D, Yang J and Zheng J:
Rap2B promotes angiogenesis via PI3K/AKT/VEGF signaling pathway in
human renal cell carcinoma. Tumour Biol. 39:10104283177016532017.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jurenka JS: Anti-inflammatory properties
of curcumin, a major constituent of Curcuma longa: A review of
preclinical and clinical research. Altern Med Rev. 14:141–153.
2009.PubMed/NCBI
|
|
9
|
Jiao D, Wang J, Lu W, Tang X, Chen J, Mou
H and Chen QY: Curcumin inhibited HGF-induced EMT and angiogenesis
through regulating c-Met dependent PI3K/Akt/mTOR signaling pathways
in lung cancer. Mol Ther Oncolytics. 3:160182016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Haryuna TS, Munir D, Maria A and
Bashiruddin J: The antioxidant effect of curcumin on cochlear
fibroblasts in rat models of diabetes mellitus. Iran J
Otorhinolaryngol. 29:197–202. 2017.PubMed/NCBI
|
|
11
|
Zhang CY, Zhang L, Yu HX, Bao JD and Lu
RR: Curcumin inhibits the metastasis of K1 papillary thyroid cancer
cells via modulating E-cadherin and matrix metalloproteinase-9
expression. Biotechnol Lett. 35:995–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin J, Deng H, Jin L, Pandey P, Quinn J,
Cantin S, Rynkiewicz MJ, Gorga JC, Bibbins F, Celatka CA, et al:
Design, synthesis, and biological evaluation of peptidomimetic
inhibitors of factor XIa as novel anticoagulants. J Med Chem.
49:7781–7791. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen WC, Lai YA, Lin YC, Ma JW, Huang LF,
Yang NS, Ho CT, Kuo SC and Way TD: Curcumin suppresses
doxorubicin-induced epithelial-mesenchymal transition via the
inhibition of TGF-β and PI3K/AKT signaling pathways in
triple-negative breast cancer cells. J Agric Food Chem.
61:11817–11824. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abouzied MM, Eltahir HM, Abdel Aziz MA,
Ahmed NS, Abd El-Ghany AA, Abd El-Aziz EA and Abd El-Aziz HO:
Curcumin ameliorate DENA-induced HCC via modulating TGF-β, AKT, and
caspase-3 expression in experimental rat model. Tumour Biol.
36:1763–1771. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Whittaker S, Marais R and Zhu AX: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mogler C, König C, Wieland M, Runge A,
Besemfelder E, Komljenovic D, Longerich T, Schirmacher P and
Augustin HG: Hepatic stellate cells limit hepatocellular carcinoma
progression through the orphan receptor endosialin. EMBO Mol Med.
9:741–749. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu A, Huang JJ, Jin XJ, Li JP, Tang YJ,
Huang XF, Cui HJ, Xu WH and Sun GB: Curcumin suppresses
invasiveness and vasculogenic mimicry of squamous cell carcinoma of
the larynx through the inhibition of JAK-2/STAT-3 signaling
pathway. Am J Cancer Res. 5:278–288. 2014.PubMed/NCBI
|
|
20
|
Zhu AX: Systemic therapy of advanced
hepatocellular carcinoma: How hopeful should we be? Oncologist.
11:790–800. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Farhangi B, Alizadeh AM, Khodayari H,
Khodayari S, Dehghan MJ, Khori V, Heidarzadeh A, Khaniki M,
Sadeghiezadeh M and Najafi F: Protective effects of dendrosomal
curcumin on an animal metastatic breast tumor. Eur J Pharmacol.
758:188–196. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar P, Kadakol A, Shasthrula PK, Mundhe
NA, Jamdade VS, Barua CC and Gaikwad AB: Curcumin as an adjuvant to
breast cancer treatment. Anticancer Agents Med Chem. 15:647–656.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semin Cancer Biol. 35 Suppl:S224–S243.
2015. View Article : Google Scholar : PubMed/NCBI
|