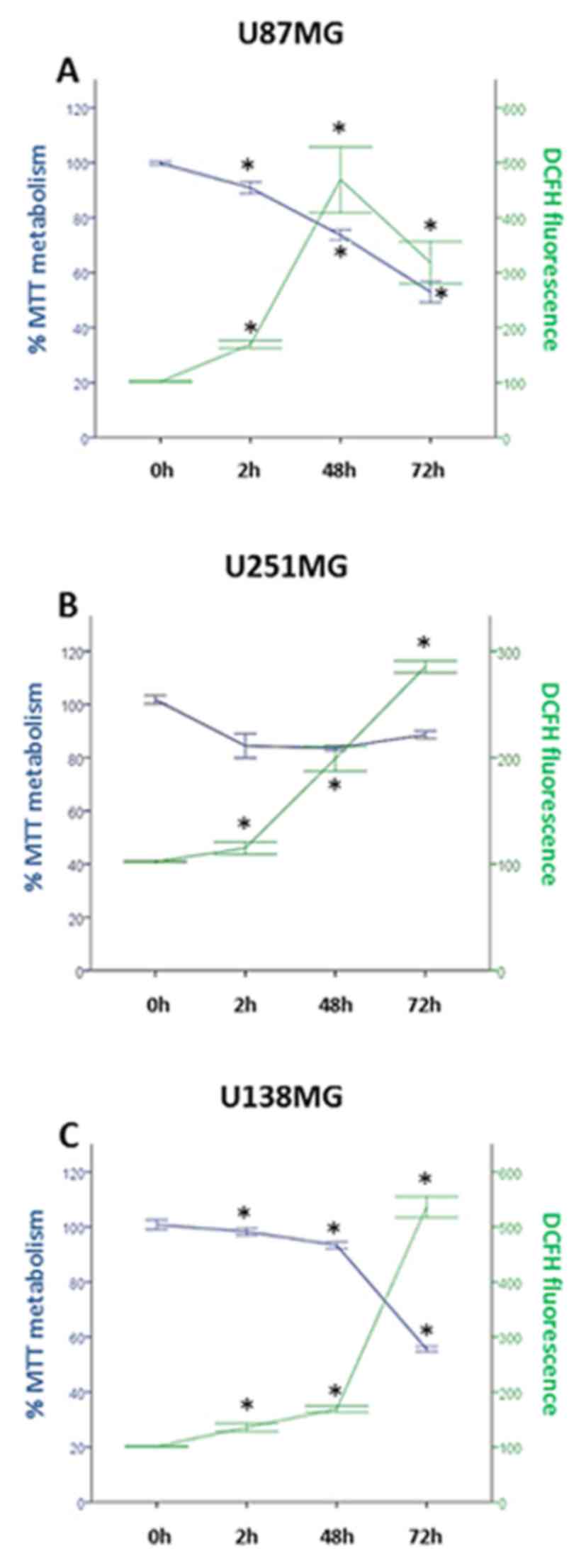
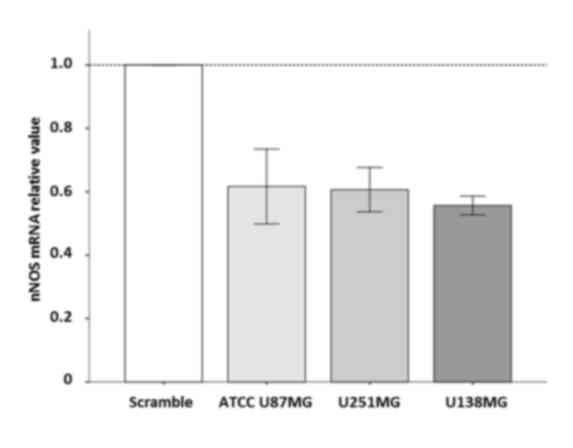
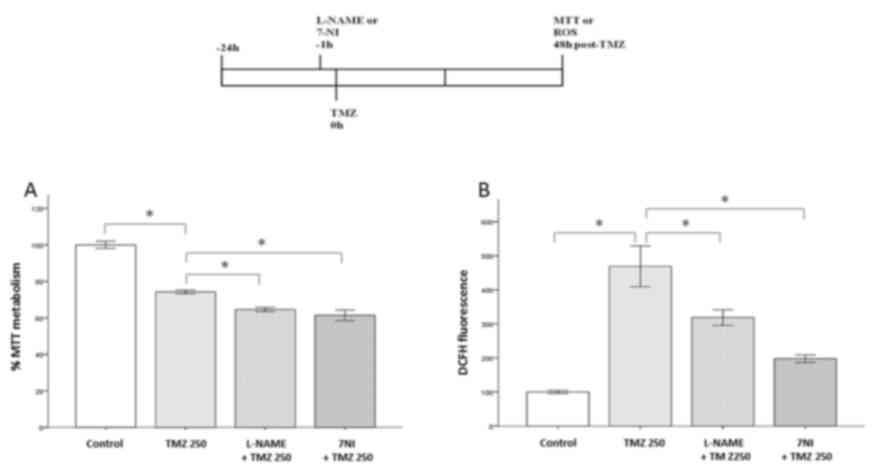
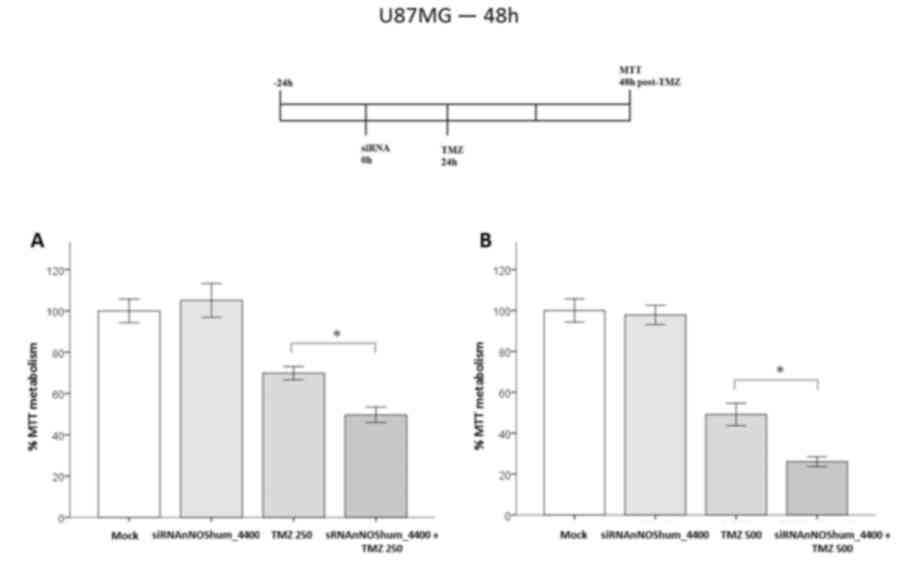
|
1
|
Lassman AB: Molecular biology of gliomas.
Curr Neurol Neurosci Rep. 4:228–233. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maher EA, Furnari FB, Bachoo RM, Rowitch
DH, Louis DN, Cavenee WK and DePinho RA: Malignant glioma: Genetics
and biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Henson JW: Treatment of glioblastoma
multiforme: A new standard. Arch Neurol. 63:337–341. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ostrom QT, Gittleman H, Farah P, Ondracek
A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 15
Suppl 2:ii1–ii56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stupp R, Hegi ME, Gilbert MR and
Chakravarti A: Chemoradiotherapy in malignant glioma: Standard of
care and future directions. J Clin Oncol. 25:4127–4136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pedretti M, Verpelli C, Mårlind J, Bertani
G, Sala C, Neri D and Bello L: Combination of temozolomide with
immunocytokine F16-IL2 for the treatment of glioblastoma. Br J
Cancer. 103:827–836. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sathornsumetee S and Rich JN: New
treatment strategies for malignant gliomas. Expert Rev Anticancer
Ther. 6:1087–1104. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kardeh S, Ashkani-Esfahani S and Alizadeh
AM: Paradoxical action of reactive oxygen species in creation and
therapy of cancer. Eur J Pharmacol. 735:150–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Conti A, Guli C, La Torre D, Tomasello C,
Angileri FF and Aguennouz M: Role of inflammation and oxidative
stress mediators in gliomas. Cancers (Basel). 2:693–712. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim SH, Kwon CH and Nakano I:
Detoxification of oxidative stress in glioma stem cells: Mechanism,
clinical relevance, and therapeutic development. J Neurosci Res.
92:1419–1424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moncada S and Bolaños JP: Nitric oxide,
cell bioenergetics and neurodegeneration. J Neurochem.
97:1676–1689. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo CX and Zhu DY: Research progress on
neurobiology of neuronal nitric oxide synthase. Neurosci Bull.
27:23–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomsen LL and Miles DW: Role of nitric
oxide in tumour progression: Lessons from human tumours. Cancer
Metastasis Rev. 17:107–118. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bakshi A, Nag TC, Wadhwa S, Mahapatra AK
and Sarkar C: The expression of nitric oxide synthases in human
brain tumours and peritumoral areas. J Neurol Sci. 155:196–203.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukumura D and Jain RK: Role of nitric
oxide in angiogenesis and microcirculation in tumors. Cancer
Metastasis Rev. 17:77–89. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanriover N, Ulu MO, Isler C, Durak H, Oz
B, Uzan M and Akar Z: Neuronal nitric oxide synthase expression in
glial tumors: Correlation with malignancy and tumor proliferation.
Neurol Res. 30:940–944. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Swaroop GR, Kelly PA, Bell HS, Shinoda J,
Yamaguchi S and Whittle IR: The effects of chronic nitric oxide
synthase suppression on glioma pathophysiology. Br J Neurosurg.
14:543–548. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roche AK, Cook M, Wilcox GL and Kajander
KC: A nitric oxide synthesis inhibitor (L-NAME) reduces licking
behavior and Fos-labeling in the spinal cord of rats during
formalin-induced inflammation. Pain. 66:331–341. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Southan GJ and Szabó C: Selective
pharmacological inhibition of distinct nitric oxide synthase
isoforms. Biochem Pharmacol. 51:383–394. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huesken D, Lange J, Mickanin C, Weiler J,
Asselbergs F, Warner J, Meloon B, Engel S, Rosenberg A, Cohen D, et
al: Design of a genome-wide siRNA library using an artificial
neural network. Nat Biotechnol. 23:995–1001. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Titze-de-Almeida SS, Lustosa CF, Horst CH,
Bel ED and Titze-de-Almeida R: Interferon Gamma potentiates the
injury caused by MPP(+) on SH-SY5Y cells, which is attenuated by
the nitric oxide synthases inhibition. Neurochem Res. 39:2452–2464.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dotsch J, Harmjanz A, Christiansen H,
Hänze J, Lampert F and Rascher W: Gene expression of neuronal
nitric oxide synthase and adrenomedullin in human neuroblastoma
using real-time PCR. Int J Cancer. 88:172–175. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kwon MJ, Oh E, Lee S, Roh MR, Kim SE, Lee
Y, Choi YL, In YH, Park T, Koh SS and Shin YK: Identification of
novel reference genes using multiplatform expression data and their
validation for quantitative gene expression analysis. PLoS One.
4:e61622009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sandhu LC, Warters RL and Dethlefsen LA:
Fluorescence studies of Hoechst 33342 with supercoiled and relaxed
plasmid pBR322 DNA. Cytometry. 6:191–194. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jia W, Jackson-Cook C and Graf MR:
Tumor-infiltrating, myeloid-derived suppressor cells inhibit T cell
activity by nitric oxide production in an intracranial rat glioma +
vaccination model. J Neuroimmunol. 223:20–30. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muntané J and La Mata MD: Nitric oxide and
cancer. World J Hepatol. 2:337–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sikora AG, Gelbard A, Davies MA, Sano D,
Ekmekcioglu S, Kwon J, Hailemichael Y, Jayaraman P, Myers JN, Grimm
EA and Overwijk WW: Targeted inhibition of inducible nitric oxide
synthase inhibits growth of human melanoma in vivo and synergizes
with chemotherapy. Clin Cancer Res. 16:1834–1844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sang DP, Li RJ and Lan Q: Quercetin
sensitizes human glioblastoma cells to temozolomide in vitro via
inhibition of Hsp27. Acta Pharmacol Sin. 35:832–838. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jakubowicz-Gil J, Langner E, Badziul D,
Wertel I and Rzeski W: Apoptosis induction in human glioblastoma
multiforme T98G cells upon temozolomide and quercetin treatment.
Tumour Biol. 34:2367–2378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oliva CR, Moellering DR, Gillespie GY and
Griguer CE: Acquisition of chemoresistance in gliomas is associated
with increased mitochondrial coupling and decreased ROS production.
PLoS One. 6:e246652011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang WB, Wang Z, Shu F, Jin YH, Liu HY,
Wang QJ and Yang Y: Activation of AMP-activated protein kinase by
temozolomide contributes to apoptosis in glioblastoma cells via p53
activation and mTORC1 inhibition. J Biol Chem. 285:40461–40471.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bady P, Diserens AC, Castella V, Kalt S,
Heinimann K, Hamou MF, Delorenzi M and Hegi ME: DNA fingerprinting
of glioma cell lines and considerations on similarity measurements.
Neuro Oncol. 14:701–711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barretina J, Caponigro G, Stransky N,
Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV,
Sonkin D, et al: The cancer cell line encyclopedia enables
predictive modelling of anticancer drug sensitivity. Nature.
483:603–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 world health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reni M, Mazza E, Zanon S, Gatta G and
Vecht CJ: Central nervous system gliomas. Crit Rev Oncol Hematol.
113:213–234. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goldbrunner RH, Wagner S, Roosen K and
Tonn JC: Models for assessment of angiogenesis in gliomas. J
Neurooncol. 50:53–62. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stylli SS, Luwor RB, Ware TM, Tan F and
Kaye AH: Mouse models of glioma. J Clin Neurosci. 22:619–626. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng SY, Huang HJ, Nagane M, Ji XD, Wang
D, Shih CC, Arap W, Huang CM and Cavenee WK: Suppression of
glioblastoma angiogenicity and tumorigenicity by inhibition of
endogenous expression of vascular endothelial growth factor. Proc
Natl Acad Sci USA. 93:pp. 8502–8507. 1996; View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Doblas S, He T, Saunders D, Pearson J,
Hoyle J, Smith N, Lerner M and Towner RA: Glioma morphology and
tumor-induced vascular alterations revealed in seven rodent glioma
models by in vivo magnetic resonance imaging and angiography. J
Magn Reson Imaging. 32:267–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kirschner S, Murle B, Felix M, Arns A,
Groden C, Wenz F, Hug A, Glatting G, Kramer M, Giordano FA and
Brockmann MA: Imaging of orthotopic glioblastoma xenografts in mice
using a clinical CT scanner: Comparison with Micro-CT and
histology. PLoS One. 11:e01659942016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu X, Dong C, Shi J, Ma T, Jin Z, Jia B,
Liu Z, Shen L and Wang F: Radiolabeled novel mAb 4G1 for
immunoSPECT imaging of EGFRvIII expression in preclinical
glioblastoma xenografts. Oncotarget. 8:6364–6375. 2017.PubMed/NCBI
|
|
46
|
Rogers S, Hii H, Huang J, Ancliffe M,
Gottardo NG, Dallas P, Lee S and Endersby R: A novel technique of
serial biopsy in mouse brain tumour models. PLoS One.
12:e01751692017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Arcella A, Oliva MA, Staffieri S, Aalberti
S, Grillea G, Madonna M, Bartolo M, Pavone L, Giangaspero F,
Cantore G and Frati A: In vitro and in vivo effect of human
lactoferrin on glioblastoma growth. J Neurosurg. 123:1026–1035.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nitta Y, Shimizu S, Shishido-Hara Y,
Suzuki K, Shiokawa Y and Nagane M: Nimotuzumab enhances
temozolomide-induced growth suppression of glioma cells expressing
mutant EGFR in vivo. Cancer Med. 5:486–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gromeier M, Lachmann S, Rosenfeld MR,
Gutin PH and Wimmer E: Intergeneric poliovirus recombinants for the
treatment of malignant glioma. Proc Natl Acad Sci USA. 97:pp.
6803–6808. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kang KB, Wang TT, Woon CT, Cheah ES, Moore
XL, Zhu C and Wong MC: Enhancement of glioblastoma radioresponse by
a selective COX-2 inhibitor celecoxib: Inhibition of tumor
angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol
Phys. 67:888–896. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jin J, Choi SH, Lee JE, Joo JD, Han JH,
Park SY and Kim CY: Antitumor activity of 7-O-succinyl macrolactin
A tromethamine salt in the mouse glioma model. Oncol Lett.
13:3767–3773. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gravina GL, Mancini A, Marampon F,
Colapietro A, Delle Monache S, Sferra R, Vitale F, Richardson PJ,
Patient L, Burbidge S and Festuccia C: The brain-penetrating CXCR4
antagonist, PRX177561, increases the antitumor effects of
bevacizumab and sunitinib in preclinical models of human
glioblastoma. J Hematol Oncol. 10:52017. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhong X, Zhao H, Liang S, Zhou D, Zhang W
and Yuan L: Gene delivery of apoptin-derived peptide using an
adeno-associated virus vector inhibits glioma and prolongs animal
survival. Biochem Biophys Res Commun. 482:506–513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Blaise GA, Gauvin D, Gangal M and Authier
S: Nitric oxide, cell signaling and cell death. Toxicology.
208:177–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Brunelli L, Yermilov V and Beckman JS:
Modulation of catalase peroxidatic and catalatic activity by nitric
oxide. Free Radic Biol Med. 30:709–714. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Cobbs CS, Whisenhunt TR, Wesemann DR,
Harkins LE, Van Meir EG and Samanta M: Inactivation of wild-type
p53 protein function by reactive oxygen and nitrogen species in
malignant glioma cells. Cancer Res. 63:8670–8673. 2003.PubMed/NCBI
|
|
57
|
Xu W, Liu LZ, Loizidou M, Ahmed M and
Charles IG: The role of nitric oxide in cancer. Cell Res.
12:311–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang P, Wang YZ, Kagan E and Bonner JC:
Peroxynitrite targets the epidermal growth factor receptor, Raf-1,
and MEK independently to activate MAPK. J Biol Chem.
275:22479–22486. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Oyoshi T, Nomoto M, Hirano H and Kuratsu
J: Pathodynamics of nitric oxide production within implanted glioma
studied with an in vivo microdialysis technique and
immunohistochemistry. J Pharmacol Sci. 91:15–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Broholm H, Rubin I, Kruse A, Braendstrup
O, Schmidt K, Skriver EB and Lauritzen M: Nitric oxide synthase
expression and enzymatic activity in human brain tumors. Clin
Neuropathol. 22:273–281. 2003.PubMed/NCBI
|
|
61
|
Agnihotri S, Burrell KE, Wolf A, Jalali S,
Hawkins C, Rutka JT and Zadeh G: Glioblastoma, a brief review of
history, molecular genetics, animal models and novel therapeutic
strategies. Arch Immunol Ther Exp (Warsz). 61:25–41. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Lenting K, Verhaak R, Ter Laan M,
Wesseling P and Leenders W: Glioma: Experimental models and
reality. Acta Neuropathol. 133:263–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Resende FF, Bai X, Del Bel EA, Kirchhoff
F, Scheller A and Titze-de-Almeida R: Evaluation of
TgH(CX3CR1-EGFP) mice implanted with mCherry-GL261 cells as an in
vivo model for morphometrical analysis of glioma-microglia
interaction. BMC Cancer. 16:722016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen J, McKay RM and Parada LF: Malignant
glioma: Lessons from genomics, mouse models, and stem cells. Cell.
149:36–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cloughesy TF, Cavenee WK and Mischel PS:
Glioblastoma: From molecular pathology to targeted treatment. Annu
Rev Pathol. 9:1–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kegelman TP, Hu B, Emdad L, Das SK, Sarkar
D and Fisher PB: In vivo modeling of malignant glioma: The road to
effective therapy. Adv Cancer Res. 121:261–330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y and Jiang T: Understanding high
grade glioma: Molecular mechanism, therapy and comprehensive
management. Cancer Lett. 331:139–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Kato T, Natsume A, Toda H, Iwamizu H,
Sugita T, Hachisu R, Watanabe R, Yuki K, Motomura K, Bankiewicz K
and Wakabayashi T: Efficient delivery of liposome-mediated
MGMT-siRNA reinforces the cytotoxity of temozolomide in
GBM-initiating cells. Gene Ther. 17:1363–1371. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shervington A and Patel R: Silencing DNA
methyltransferase (DNMT) enhances glioma chemosensitivity.
Oligonucleotides. 18:365–374. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wen X, Huang A, Liu Z, Liu Y, Hu J, Liu J
and Shuai X: Downregulation of ROCK2 through nanocomplex sensitizes
the cytotoxic effect of temozolomide in U251 glioma cells. PLoS
One. 9:e920502014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sales TT, Resende FF, Chaves NL,
Titze-De-Almeida SS, Báo SN, Brettas ML and Titze-De-Almeida R:
Suppression of the Eag1 potassium channel sensitizes glioblastoma
cells to injury caused by temozolomide. Oncol Lett. 12:2581–2589.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cruickshanks N, Shervington L, Patel R,
Munje C, Thakkar D and Shervington A: Can hsp90alpha-targeted siRNA
combined with TMZ be a future therapy for glioma? Cancer Invest.
28:608–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jakubowicz-Gil J, Langner E, Badziul D,
Wertel I and Rzeski W: Silencing of Hsp27 and Hsp72 in glioma cells
as a tool for programmed cell death induction upon temozolomide and
quercetin treatment. Toxicol Appl Pharmacol. 273:580–589. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Paul-Samojedny M, Pudelko A, Kowalczyk M,
Fila-Daniłow A, Suchanek-Raif R, Borkowska P and Kowalski J:
Combination therapy with AKT3 and PI3KCA siRNA enhances the
antitumor effect of temozolomide and carmustine in T98G
glioblastoma multiforme cells. BioDrugs. 30:129–144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Qian C, Li P, Yan W, Shi L, Zhang J, Wang
Y, Liu H and You Y: Downregulation of osteopontin enhances the
sensitivity of glioma U251 cells to temozolomide and cisplatin by
targeting the NF-κB/Bcl-2 pathway. Mol Med Rep. 11:1951–1955. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Tivnan A, Zakaria Z, O'Leary C, Kögel D,
Pokorny JL, Sarkaria JN and Prehn JH: Inhibition of multidrug
resistance protein 1 (MRP1) improves chemotherapy drug response in
primary and recurrent glioblastoma multiforme. Front Neurosci.
9:2182015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Wang Q, Du J, Xu B, Xu L, Wang X, Liu J
and Wang J: Silence of bFGF enhances chemosensitivity of glioma
cells to temozolomide through the MAPK signal pathway. Acta Biochim
Biophys Sin (Shanghai). 48:501–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Titze-de-Almeida R, David C and
Titze-de-Almeida SS: The race of 10 synthetic RNAi-based drugs to
the pharmaceutical market. Pharm Res. 34:1339–1363. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
de Boer AG and Gaillard PJ: Drug targeting
to the brain. Annu Rev Pharmacol Toxicol. 47:323–355. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Lonser RR, Sarntinoranont M, Morrison PF
and Oldfield EH: Convection-enhanced delivery to the central
nervous system. J Neurosurg. 122:697–706. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cohen ZR, Ramishetti S, Peshes-Yaloz N,
Goldsmith M, Wohl A, Zibly Z and Peer D: Localized RNAi
therapeutics of chemoresistant grade IV glioma using
hyaluronan-grafted lipid-based nanoparticles. ACS Nano.
9:1581–1591. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Danhier F, Messaoudi K, Lemaire L, Benoit
JP and Lagarce F: Combined anti-Galectin-1 and anti-EGFR
siRNA-loaded chitosan-lipid nanocapsules decrease temozolomide
resistance in glioblastoma: In vivo evaluation. Int J Pharm.
481:154–161. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Tsujiuchi T, Natsume A, Motomura K, Kondo
G, Ranjit M, Hachisu R, Sugimura I, Tomita S, Takehara I, Woolley
M, et al: Preclinical evaluation of an O(6)-methylguanine-DNA
methyltransferase-siRNA/liposome complex administered by
convection-enhanced delivery to rat and porcine brains. Am J Transl
Res. 6:169–178. 2014.PubMed/NCBI
|
|
84
|
Golan T, Khvalevsky EZ, Hubert A, Gabai
RM, Hen N, Segal A, Domb A, Harari G, David EB, Raskin S, et al:
RNAi therapy targeting KRAS in combination with chemotherapy for
locally advanced pancreatic cancer patients. Oncotarget.
6:24560–24570. 2015. View Article : Google Scholar : PubMed/NCBI
|