Introduction
Yes-associated protein 1 (YAP1) and its
transcriptional co-activator with PDZ-binding domain taffazin (TAZ)
form the backbone of the Hippo pathway kinase cascade, which is
involved in regulation of tissue homeostasis, organ size,
regeneration and tumorigenesis (1).
In mammals, the Hippo pathway is comprised of the core kinase
complexes mammalian Ste2-like kinase 1 and 2 (MST1 and MST2) and
large tumor suppressor kinase 1 and 2 (LATS1 and LATS2) (2). Upon activation of the Hippo pathway, the
inhibitory MST/LSTS kinases phosphorylate YAP1 and TAZ. This
phosphorylation leads to the nuclear exclusion of YAP1 and TAZ,
which are then sequestered and undergo ubiquitin-mediated
proteasomal degradation in the cytoplasm to suppress the expression
of YAP1- and TAZ-regulated genes (3,4). If
molecular events, including crosstalk with oncogenic signaling
pathways, trigger the dysregulation of the Hippo pathway, YAP1/TAZ
are translocated into the nucleus (5). There, they interact with four
transcriptional enhancer associated domain (TEAD) transcription
factors, TEAD1-4, and promote cell proliferation and inhibit
apoptosis (6).
The Hippo pathway was first hypothesized to be
important in human cancer when tissue overgrowth was observed in
Drosophila melanogaster flies with mutations in the Hippo
pathway (7–9). A number of studies have indicated that
human tumors use these biological properties of YAP1 to foster
their own proliferation, progression and metastasis (4,10,11). Increased activation of YAP1 has been
identified in a broad range of carcinomas including lung cancer,
colorectal cancer, ovarian cancer, prostate cancer, melanoma and
glioblastoma, and has often been associated with poor prognosis
(12–18). However, the exact function of YAP1 in
the tumorigenesis of certain types of cancer remains obscure,
despite its oncogenic behavior in several types of cancer. In
breast cancer, nuclear YAP1 expression does not differ
significantly between normal breast and ductal carcinoma breast
tissues (14). In hematological
malignancies, including lymphoma and multiple myeloma, the
expression of YAP1 is markedly downregulated (19).
In colon cancer, several studies reported that YAP1
is overexpressed, contributes to tumorigenesis and is associated
with poor prognosis (20–22). However, Yuen et al (23) demonstrated that TAZ was the only
prognostic marker in colorectal cancer. The functions of YAP1 and
Hippo pathway genes have not yet been fully investigated in a large
cohort of patients with colon cancer. Therefore, in the present
study, the expression levels of Hippo pathway-associated genes
including YAP1, TAZ, TEAD1, TEAD2, TEAD3, TEAD4, MST1, MST2,
LATS1 and LATS2 were investigated and their clinical
significance evaluated in a population of 458 patients with colon
adenocarcinoma (COAD) using data obtained from The Cancer Genome
Atlas (TCGA) research network (https://cancergenome.nih.gov/).
Materials and methods
Gene expression profiling
Level 3 mRNA expression data from 41 normal samples
and 458 COAD samples were obtained from TCGA database (https://portal.gdc.cancer.gov/). Raw data were
initially analyzed using R software (v.3.2.5) (24). Chip data were normalized using the
RankNormalize module in GenePattern (https://genepattern.broadinstitute.org). GeneNeighbors
and ClassNeighbors, modules programmed in GenePattern, were used to
select genes closely associated with YAP1 (25). cBioportal (http://www.cbioportal.org/) and Firebrowse (http://firebrowse.org) were used to analyze mRNA
expression and alterations in Hippo pathway genes.
Functional enrichment analysis
Differentially expressed genes were imported into
the Database for Annotation, Visualization and Integrated Discovery
(DAVID; http://david.abcc.ncifcrf.gov/) (26) for Gene Ontology (GO)-based functional
enrichment analysis. Gene Set Enrichment Analysis (GSEA) was
utilized to identify mRNAs predicted to associate with pathways in
C2 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway gene sets
(27,28). GO categories encompass three domains:
Biological processes, cellular components and molecular
functions.
Survival analysis
Cutoff Finder (http://molpath.charite.de/cutoff) was used to
determine threshold values in mRNA and protein expression of COAD
using log-rank tests (29).
Cumulative event (mortality) rate was calculated using the
Kaplan-Meier method, with the time to the first event as the
outcome variable. The probability of recurrence and calculated risk
for recurrence were determined by actuarial analysis. The criteria
for statistical analysis were the date of surgery and the date of
mortality.
Statistical analysis
The distributions of characteristics between the two
groups were compared using unpaired Student's t-test for continuous
variables (or the Kolmogorov-Smirnov test when the expected
frequency within any cell was <5) and the χ2 test (or
Fisher's exact test when the expected frequency within any cell was
<5) for categorical variables. The distributions of
characteristics between >3 groups were compared using analysis
of variance and Newman-Keuls post-hoc test. Survival curves were
compared by the log-rank test for various recurrence factors and
Cox's model for multivariate analysis. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using the GraphPad Prism software (version
5.0; GraphPad Prism Software, La Jolla, CA, USA) and the
Statistical Package for Social Sciences v.13.0 for Windows (SPSS,
Inc., Chicago, IL, USA).
Results
Cross-cancer mRNA expression of
YAP1
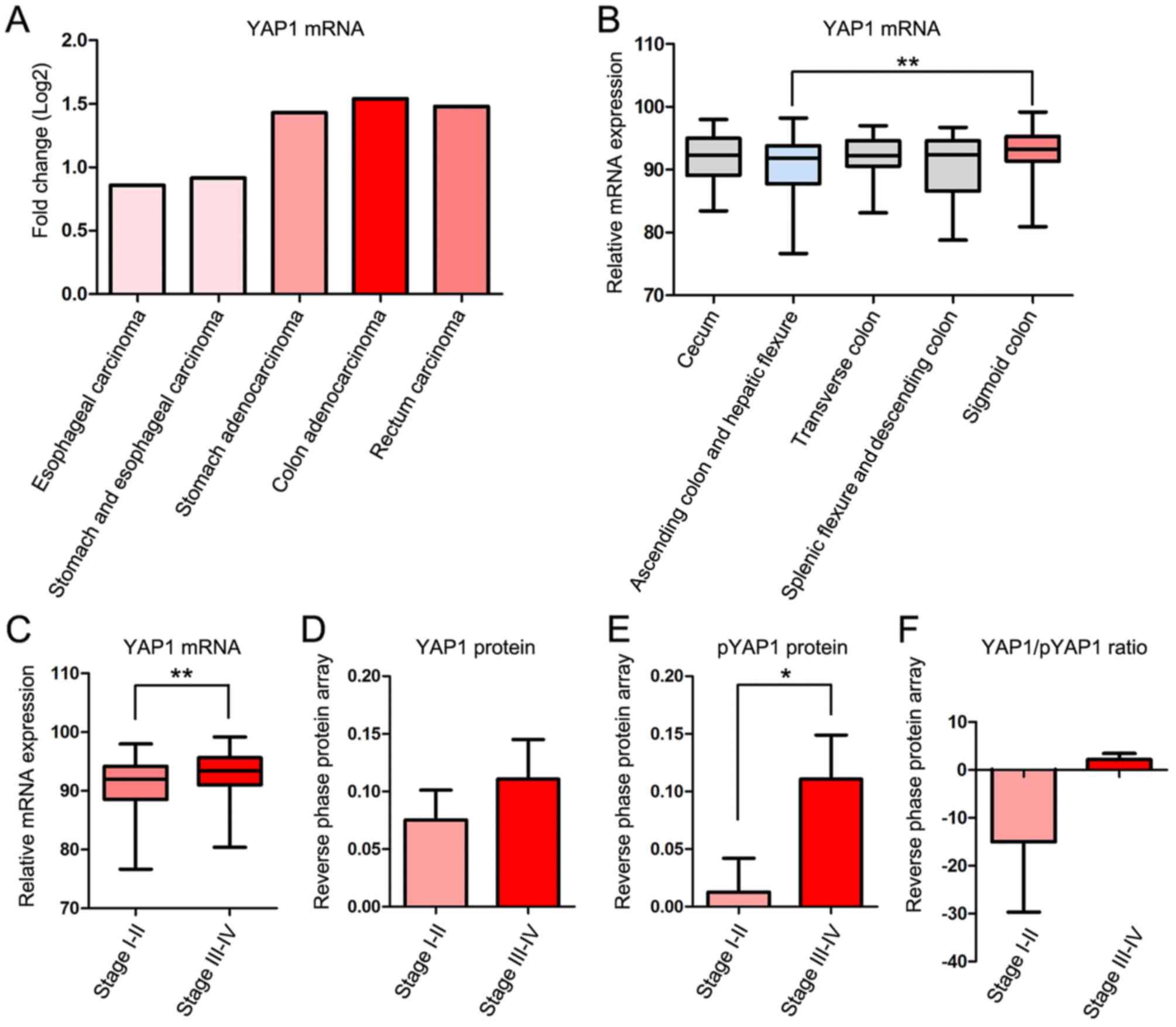
The fold change of YAP1 mRNA expression compared
with 41 normal control samples in cases of COAD was higher than
that in four other gastrointestinal cancer types; Esophageal
carcinoma, stomach and esophageal carcinoma, stomach adenocarcinoma
and rectal carcinoma in TCGA database. Clinicopathological
information for the patients is presented in Table I. YAP1 mRNA expression was increased
in COAD, stomach adenocarcinoma and rectal carcinoma, but decreased
in esophageal carcinoma and ‘stomach and esophageal carcinoma’
combined compared with normal samples (Fig. 1A).
 | Table I.Clinicopathological information of
the patients with colon adenocarcinoma. |
Table I.
Clinicopathological information of
the patients with colon adenocarcinoma.
| Feature | Patients, n
(%) |
|---|
| Number | 458 (100.0) |
| Sex | 458 (100.0) |
|
Female | 216 (47.2) |
|
Male | 242 (52.8) |
| Age, years | 458 (100.0) |
|
≤65 | 189 (41.3) |
|
>65 | 269 (58.7) |
| Anatomic
subdivision | 442 (96.5) |
|
Ascending colon | 87 (19.0) |
|
Cecum | 108 (24.0) |
|
Descending colon | 20 (4.4) |
| Hepatic
flexure | 27 (5.9) |
|
Rectosigmoid junction | 1 (0.2) |
| Sigmoid
colon | 152 (33.2) |
| Splenic
flexure | 7 (1.5) |
|
Transverse colon | 40 (8.7) |
| Histological
type | 453 (98.9) |
| Colon
adenocarcinoma | 391 (85.4) |
| Colon
mucinous adenocarcinoma | 62 (13.5) |
| Vital status | 458 (100.0) |
|
Alive | 356 (77.7) |
|
Dead | 102 (22.3) |
| Postoperative
Treatment | 390 (85.1) |
|
Yes | 147 (32.1) |
| No | 243 (53.0) |
| Pathologic stage
(TNM) | 448 (97.8) |
| I | 76 (16.6) |
| II | 178 (38.9) |
|
III | 129 (28.2) |
| IV | 65 (14.2) |
| Lymphatic
invasion | 414 (90.4) |
|
Absent | 250 (54.6) |
|
Present | 164 (35.8) |
| Perineural
invasion | 179 (39.0) |
|
Absent | 133 (29.0) |
|
Present | 46 (10.0) |
| Venous
invasion | 397 (86.8) |
|
Absent | 301 (65.7) |
|
Present | 96 (21.1) |
mRNA expression of Hippo pathway genes
in COAD
The Hippo pathway genes included in the present
study were YAP1, TAZ, TEAD1, TEAD2, TEAD3, TEAD4, MST1, MST2, LATS1
and LATS2 (Table II). The fold
change in mRNA expression in COAD compared with normal control
samples identified that YAP1, TAZ, TEAD1, TEAD2, TEAD4, MST1 and
MST2 were highly expressed in COAD compared with normal control
samples, with TEAD4 and MST1 exhibiting the largest shifts.
However, TEAD3, LATS1 and LATS2 exhibited lower expression in COAD
than in normal control samples.
 | Table II.Hippo pathway genes in colon
adenocarcinoma. |
Table II.
Hippo pathway genes in colon
adenocarcinoma.
| Symbol | Gene name | Chromosome
location | Fold change,
Log | Alteration, % |
|---|
| YAP1 | Yes associated
protein 1 | 11q22.1 | 1.54 | 7.0 |
| TAZ | Tafazzin | Xq28 | 1.78 | 4.0 |
| TEAD1 | TEA domain
transcription factor 1 | 11p15.3 | 1.11 | 4.0 |
| TEAD2 | TEA domain
transcription factor 2 | 19q13.33 | 1.52 | 7.0 |
| TEAD3 | TEA domain
transcription factor 3 | 6p21.31 | 0.87 | 5.0 |
| TEAD4 | TEA domain
transcription factor 4 | 12p13.33 | 4.91 | 6.0 |
| MST1 | Macrophage
stimulating 1 | 3p21.31 | 4.04 | 1.6 |
| MST2 | STK3,
serine/threonine kinase 3 | 8q22.2 | 1.97 | 20.0 |
| LATS1 | Large tumor
suppressor kinase 1 | 6q25.1 | 0.89 | 7.0 |
| LATS2 | Large tumor
suppressor kinase 2 | 13q12.11 | 0.83 | 10.0 |
mRNA and protein expression of YAP1 in
various Tumor-Node-Metastasis (TNM) stages of COAD
To examine the association between YAP1 expression
and the location and progression of COAD, the expression of YAP1
was studied according to the location and TNM stage of COAD
(30). YAP1 mRNA expression was
significantly increased in the sigmoid colon compared with the
ascending colon and hepatic flexure (Fig.
1B) and in TNM stages III–IV compared with stages I–II
(Fig. 1C). Protein expression of YAP1
was markedly increased in TNM stages III–IV compared with stages
I–II (Fig. 1D). The
serine-1217-phosphorylated form of YAP1 (pYAP), which is inactive
and localized in the cytoplasm, was significantly increased in TNM
stages III–IV compared with stages I–II (Fig. 1E). However, the YAP/pYAP ratio, which
represents YAP1 activity, was increased in TNM stages III–IV
compared with stages I–II (Fig.
1F).
YAP1 mRNA and protein expression in T,
N and M stages
To investigate the association between YAP1
expression and progression of COAD in more detail, the mRNA and
protein expression of YAP1 was examined in each of the T, N and M
stages. In the T stage, YAP1/pYAP was significantly increased in
T3-4 compared with T1-2 stages (Fig.
2A-D). In the M stage, YAP1 mRNA and protein expression were
significantly increased in N1-2 compared with N0 (Fig. 2E-H). Although mRNA expression and
protein expression of YAP1 were higher in M1 than in M0, the
differences were not significant (Fig.
2I-L).
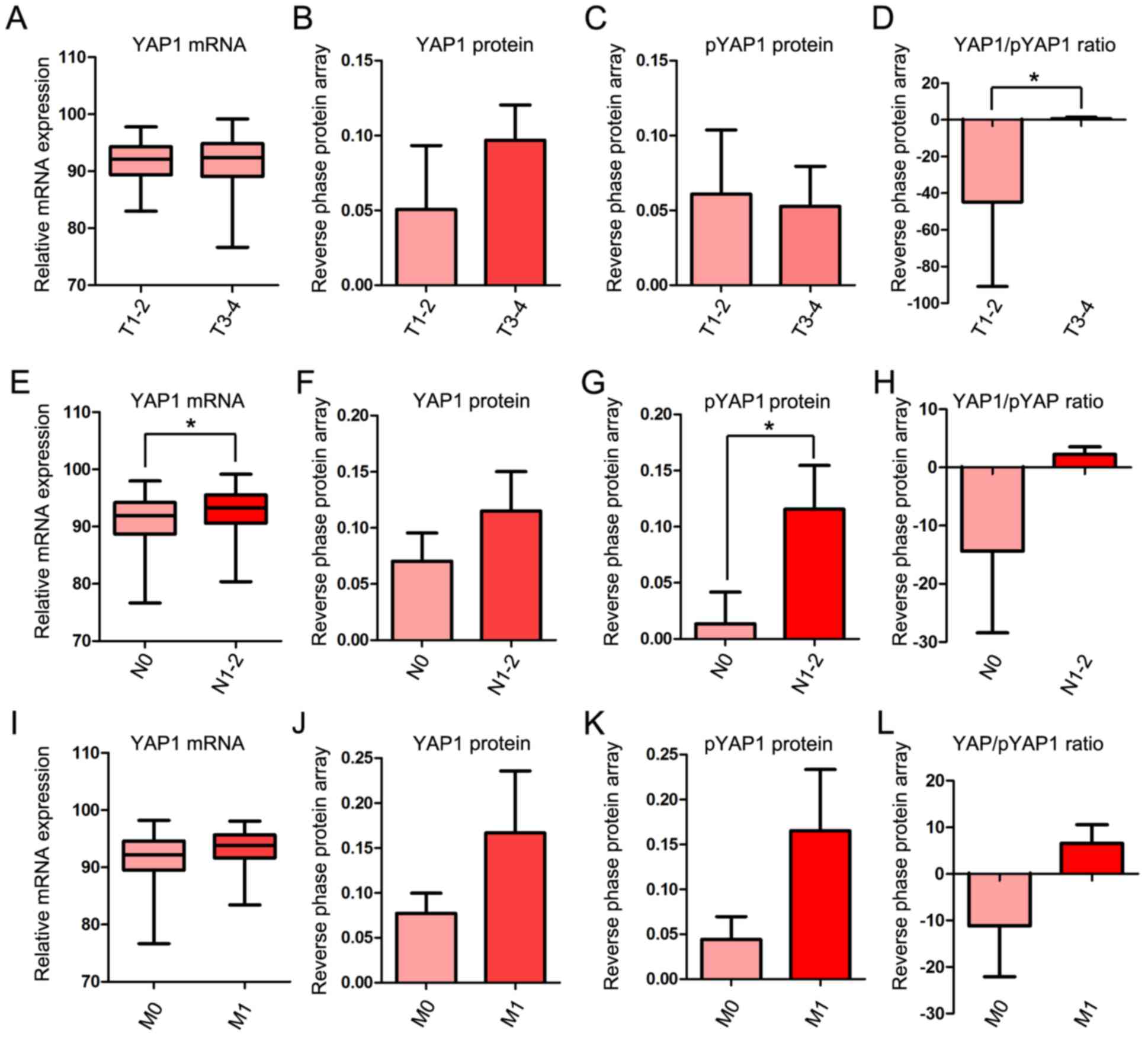 | Figure 2.YAP1 mRNA and protein expression in
TNM stages. (A) Relative mRNA expression of YAP1, (B) protein level
of YAP1, (C) protein level of pYAP and (D) YAP1/pYAP ratio between
T1-2 and T3-4. (E) Relative mRNA expression of YAP1, (F) protein
level of YAP1, (G) protein level of pYAP and (H) YAP1/pYAP ratio
between N0 and N1-2. (I) Relative mRNA expression of YAP1, (J)
protein level of YAP1, (K) protein level of pYAP and (L) YAP1/pYAP
ratio between M0 and M1. mRNA and protein expression data of YAP1
in COAD obtained from The Cancer Genome Atlas data portal.
*P<0.05. YAP1, yes-associated protein 1; pYAP, phosphorylated
form of YAP1; TNM, Tumor-Node-Metastasis; COAD, colon
adenocarcinoma. |
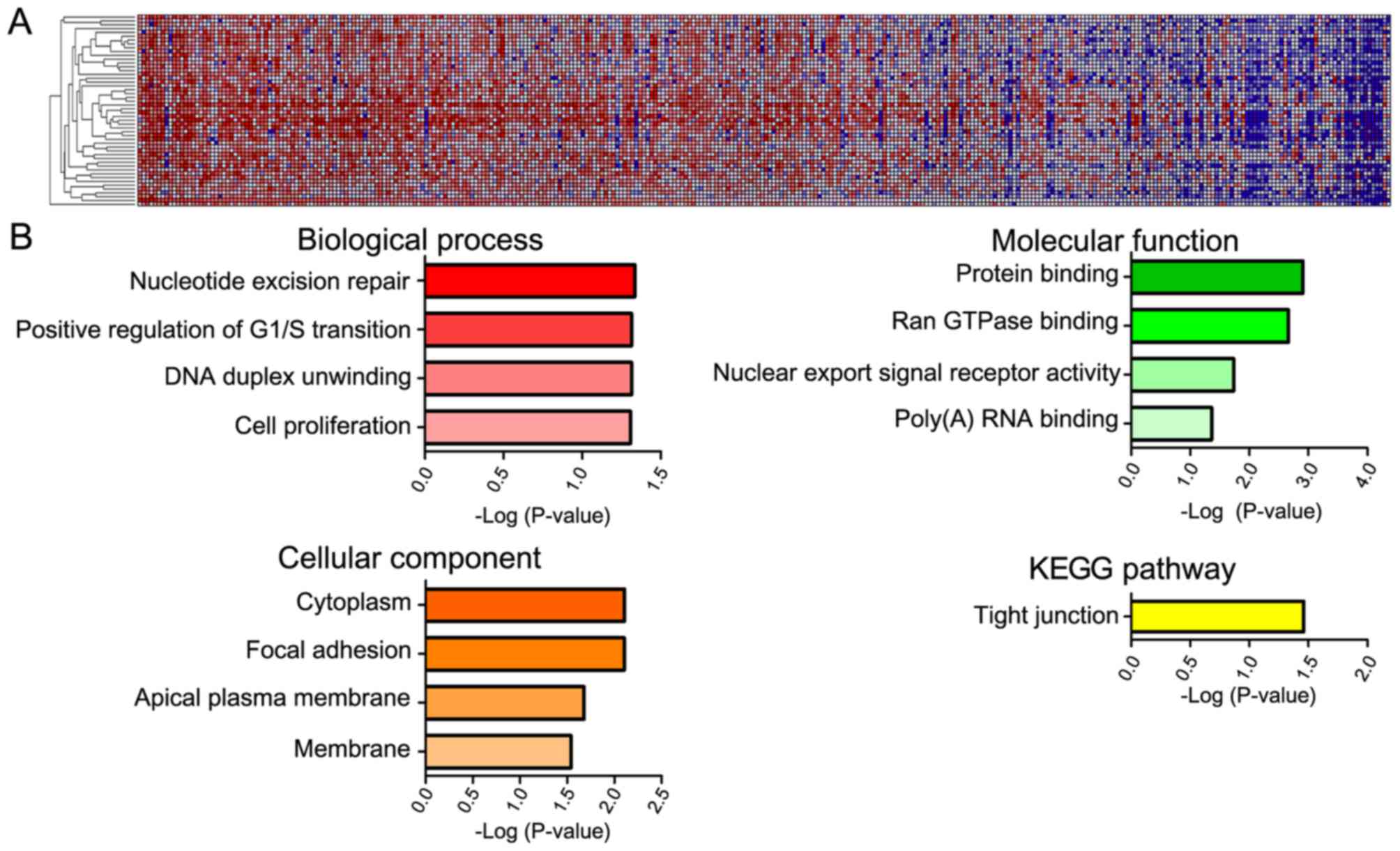
GeneNeighbors analysis of YAP1
The 100 genes most closely associated with YAP1 were
selected using the GeneNeighbors program (Fig. 3) and were classified using DAVID. The
genes were sorted into three groups: GO terms that differed
significantly served functions in: i) Biological processes, ii)
cellular components, and iii) molecular functions. Genes that were
highly expressed in COAD and associated with biological processes
were mainly associated with DNA duplication (positive regulation of
G1/S transition, nucleotide excision repair and DNA
duplex unwinding) (Fig. 3B). As for
cellular components, highly expressed genes in COAD were primarily
associated with the cytoplasm and membrane (focal adhesion, apical
plasma membrane and membrane). Regarding molecular functions, genes
that were expressed at a high level in COAD were primarily
associated with protein binding (Ran GTPase-binding and poly (A)
RNA binding) and nuclear export. In addition, when genes were
analyzed according to cell signaling pathway (KEGG), the tight
junction pathway was the most significant.
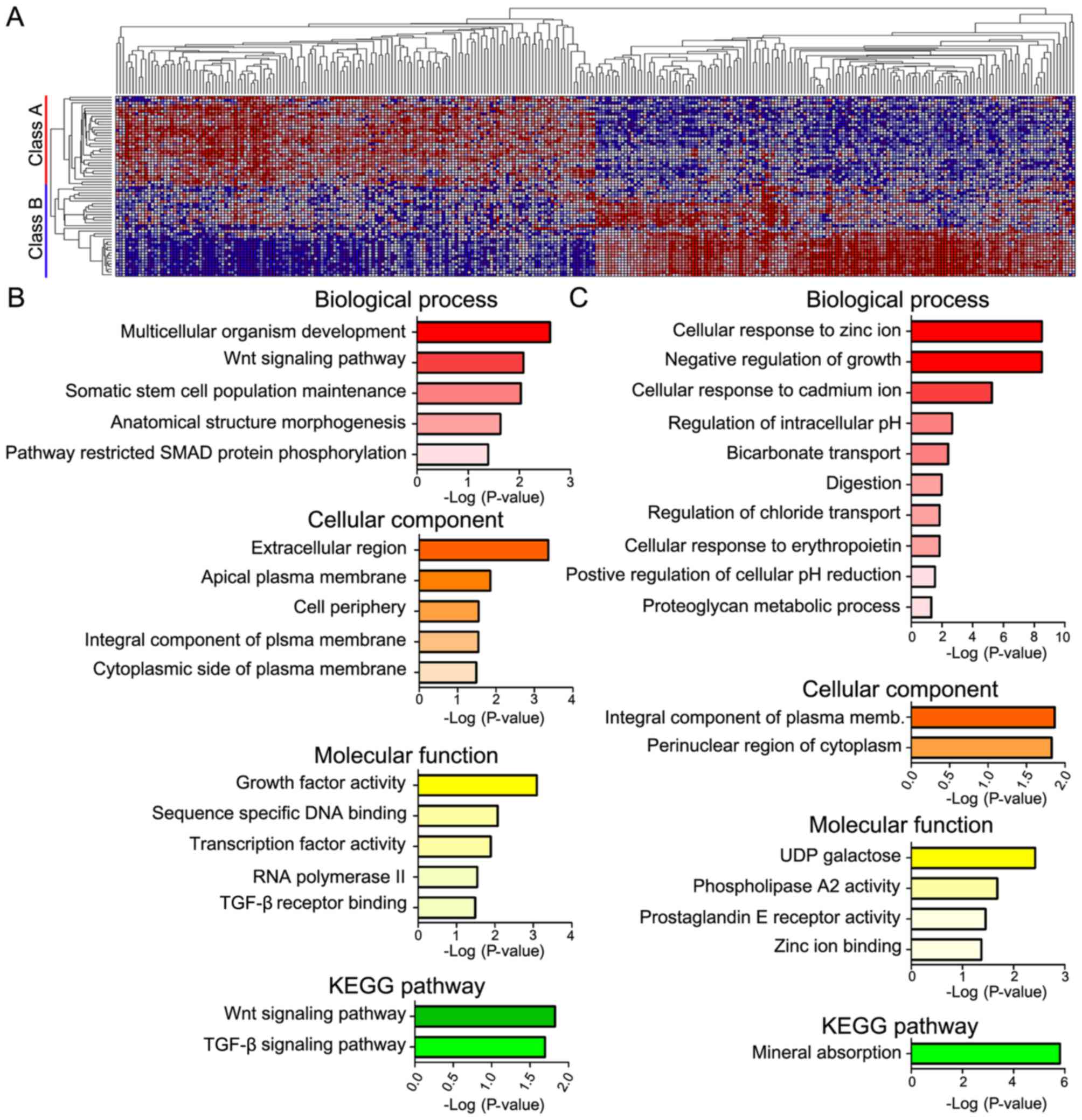
ClassNeighbors analysis of YAP1
Analysis using ClassNeighbors divided the COAD
samples in to two classes: Class A contained the most marked 10% of
YAP1-upregulated COAD samples and class B contained the most marked
10% of YAP1-downregulated COAD samples (Fig. 4A). Of the 20,502 probe sets, the 150
genes that were most significantly associated and most highly
expressed in classes A and B were selected (Table III). DAVID analysis classified these
genes into groups based on the GO terms: i) Biological processes,
ii) cellular components and iii) molecular functions, as well as
iv) KEGG pathways (Fig. 4). Genes
highly expressed in class A were mainly associated with development
(multicellular organism development, somatic stem cell population
maintenance and anatomical structure morphogenesis) and pathways
(Wnt signaling pathway, pathway restricted SMAD protein
phosphorylation) in biological processes; the plasma membrane
(apical plasma membrane, integral component of plasma membrane and
cytoplasmic side of plasma membrane) in cellular components;
activity (growth factor activity and transcriptional activity) and
binding [sequence-specific DNA binding and transforming growth
factor-β (TGF-β) receptor binding] in molecular function; and
signaling pathways (Wnt signaling, TGF-β signaling) in KEGG
pathways. Genes highly expressed in class B were mostly associated
with ions (cellular response to zinc ion and cadmium ion, and
negative regulation of growth) in biological process; integral
component of plasma membrane and pronuclear region of cytoplasm in
cellular components; activity (phospholipase A2 activity and
prostaglandin E receptor activity) in molecular function, mineral
absorption in KEGG pathways.
 | Table III.DAVID analysis of ClassNeighbors. |
Table III.
DAVID analysis of ClassNeighbors.
| A, Class A |
|---|
|
|---|
| Term | Count | % | P-value |
|---|
| Biological
process |
|
|
|
| GO:
0030178-Negative regulation of Wnt signaling pathway | 6 |
4.5 | <0.01 |
| GO:
0001942-Hair follicle development | 4 |
3.0 | <0.01 |
| GO:
0001580-Detection of chemical stimulus involved in sensory
perception of bitter taste | 4 |
3.0 | <0.01 |
| GO:
0007275-Multicellular organism development | 11 |
8.2 | <0.01 |
| GO:
0090090-Negative regulation of canonical Wnt signaling pathway | 6 |
4.5 | <0.01 |
| GO:
0016055-Wnt signaling pathway | 6 |
4.5 | 0.01 |
| GO:
0035019-Somatic stem cell population maintenance | 4 |
3.0 | 0.01 |
| GO:
0060279-Positive regulation of ovulation | 2 |
1.5 | 0.01 |
| GO:
0046882-Negative regulation of follicle-stimulating hormone
secretion | 2 |
1.5 | 0.02 |
| GO:
0007411-Axon guidance | 5 |
3.7 | 0.02 |
| GO:
0009653-Anatomical structure morphogenesis | 4 |
3.0 | 0.02 |
| GO:
0070858-Negative regulation of bile acid biosynthetic process | 2 |
1.5 | 0.03 |
| GO:
0030154-Cell differentiation | 8 |
6.0 | 0.04 |
| GO:
0001755-Neural crest cell migration | 3 |
2.2 | 0.04 |
| GO:
0042423-Catecholamine biosynthetic process | 2 |
1.5 | 0.04 |
| GO:
0046881-Positive regulation of follicle-stimulating hormone
secretion | 2 |
1.5 | 0.04 |
| GO:
0010862-Positive regulation of pathway-restricted SMAD protein
phosphorylation | 3 |
2.2 | 0.04 |
| GO:
0009072-Aromatic amino acid family metabolic process | 2 |
1.5 | 0.05 |
| GO:
0021516-Dorsal spinal cord development | 2 |
1.5 | 0.05 |
| GO:
0000122-Negative regulation of transcription from RNA polymerase II
promoter | 10 |
7.5 | 0.05 |
| GO:
0010470-Regulation of gastrulation | 2 |
1.5 | 0.05 |
| Cellular
component |
|
|
|
| GO:
0005576-Extracellular region | 24 | 17.9 | <0.01 |
| GO:
0005615-Extracellular space | 21 | 15.7 | <0.01 |
| GO:
0016324-Apical plasma membrane | 7 |
5.2 | 0.01 |
| GO:
0071944-Cell periphery | 3 |
2.2 | 0.03 |
| GO:
0005887-Integral component of plasma membrane | 17 | 12.7 | 0.03 |
| GO:
0009898-Cytoplasmic side of plasma membrane | 3 |
2.2 | 0.03 |
| Molecular
function |
|
|
|
| GO:
0008083-Growth factor activity | 7 |
5.2 | <0.01 |
| GO:
0043565-Sequence-specific DNA binding | 10 |
7.5 | 0.01 |
| GO:
0003700-Transcription factor activity, sequence-specific DNA
binding | 14 | 10.4 | 0.01 |
| GO:
0016714-Oxidoreductase activity, acting on paired donors, with
incorporation or reduction of molecular oxygen, reduced pteridine
as one donor, and incorporation of one atom of oxygen | 2 |
1.5 | 0.03 |
| GO:
0000981-RNA polymerase II transcription factor activity,
sequence-specific DNA binding | 5 |
3.7 | 0.03 |
| GO:
0005160-Transforming growth factor beta receptor binding | 3 |
2.2 | 0.03 |
| GO:
0000978-RNA polymerase II core promoter proximal region
sequence-specific DNA binding | 7 |
5.2 | 0.03 |
| KEGG |
|
|
|
|
hsa04310: Wnt signaling
pathway | 5 |
3.7 | 0.02 |
|
hsa04350: TGF-β signaling
pathway | 4 |
3.0 | 0.02 |
|
hsa04970: Salivary
secretion | 4 |
3.0 | 0.02 |
|
hsa04530: Tight junction | 4 |
3.0 | 0.07 |
|
hsa04550: Signaling pathways
regulating pluripotency of stem cells | 4 |
3.0 | 0.07 |
|
hsa04151: PI3K-Akt signaling
pathway | 6 |
4.5 | 0.09 |
|
| B, Class
B |
|
| Term | Count | % | P-value |
|
| Biological
process |
|
|
|
| GO:
0071294-Cellular response to zinc ion | 7 |
5.0 | <0.01 |
| GO:
0045926-Negative regulation of growth | 7 |
5.0 | <0.01 |
| GO:
0071276-Cellular response to cadmium ion | 5 |
3.6 | <0.01 |
| GO:
0051453-Regulation of intracellular pH | 4 |
2.9 | <0.01 |
| GO:
0015701-Bicarbonate transport | 4 |
2.9 | <0.01 |
| GO:
0007586-Digestion | 4 |
2.9 | 0.01 |
| GO:
2001225-Regulation of chloride transport | 2 |
1.4 | 0.01 |
| GO:
0036018-Cellular response to erythropoietin | 2 |
1.4 | 0.01 |
| GO:
0032849-Positive regulation of cellular pH reduction | 2 |
1.4 | 0.03 |
| GO:
1902476-Chloride transmembrane transport | 4 |
2.9 | 0.03 |
| GO:
0006029-Proteoglycan metabolic process | 2 |
1.4 | 0.05 |
| GO:
0007189-Adenylate cyclase-activating G protein-coupled receptor
signaling pathway | 3 |
2.1 | 0.05 |
| Cellular
component |
|
|
|
| GO:
0005887-Integral component of plasma membrane | 19 | 13.6 | 0.01 |
| GO:
0048471-Perinuclear region of cytoplasm | 11 |
7.9 | 0.01 |
| GO:
0042589-Zymogen granule membrane | 2 |
1.4 | 0.08 |
| GO:
0005886-Plasma membrane | 38 | 27.1 | 0.08 |
| Molecular
function |
|
|
|
| GO:
0008499-UDP-galactose:β-N-acetylglucosamine
β-1,3-galactosyltransferase activity | 3 |
2.1 | <0.01 |
| GO:
0004623-Phospholipase A2 activity | 3 |
2.1 | 0.02 |
| GO:
0004957-Prostaglandin E receptor activity | 2 |
1.4 | 0.04 |
| GO:
0008270-Zinc ion binding | 15 | 10.7 | 0.04 |
| GO:
0005254-Chloride channel activity | 3 |
2.1 | 0.06 |
| GO:
0046983-Protein dimerization activity | 4 |
2.9 | 0.09 |
| GO:
0004089-Carbonate dehydratase activity | 2 |
1.4 | 0.10 |
| KEGG |
|
|
|
|
hsa04978: Mineral
absorption | 7 |
5.0 | <0.01 |
|
hsa04972: Pancreatic
secretion | 6 |
4.3 | <0.01 |
|
hsa04975: Fat digestion and
absorption | 4 |
2.9 | <0.01 |
|
hsa04924: Renin secretion | 3 |
2.1 | 0.09 |
|
hsa00830: Retinol
metabolism | 3 |
2.1 | 0.10 |
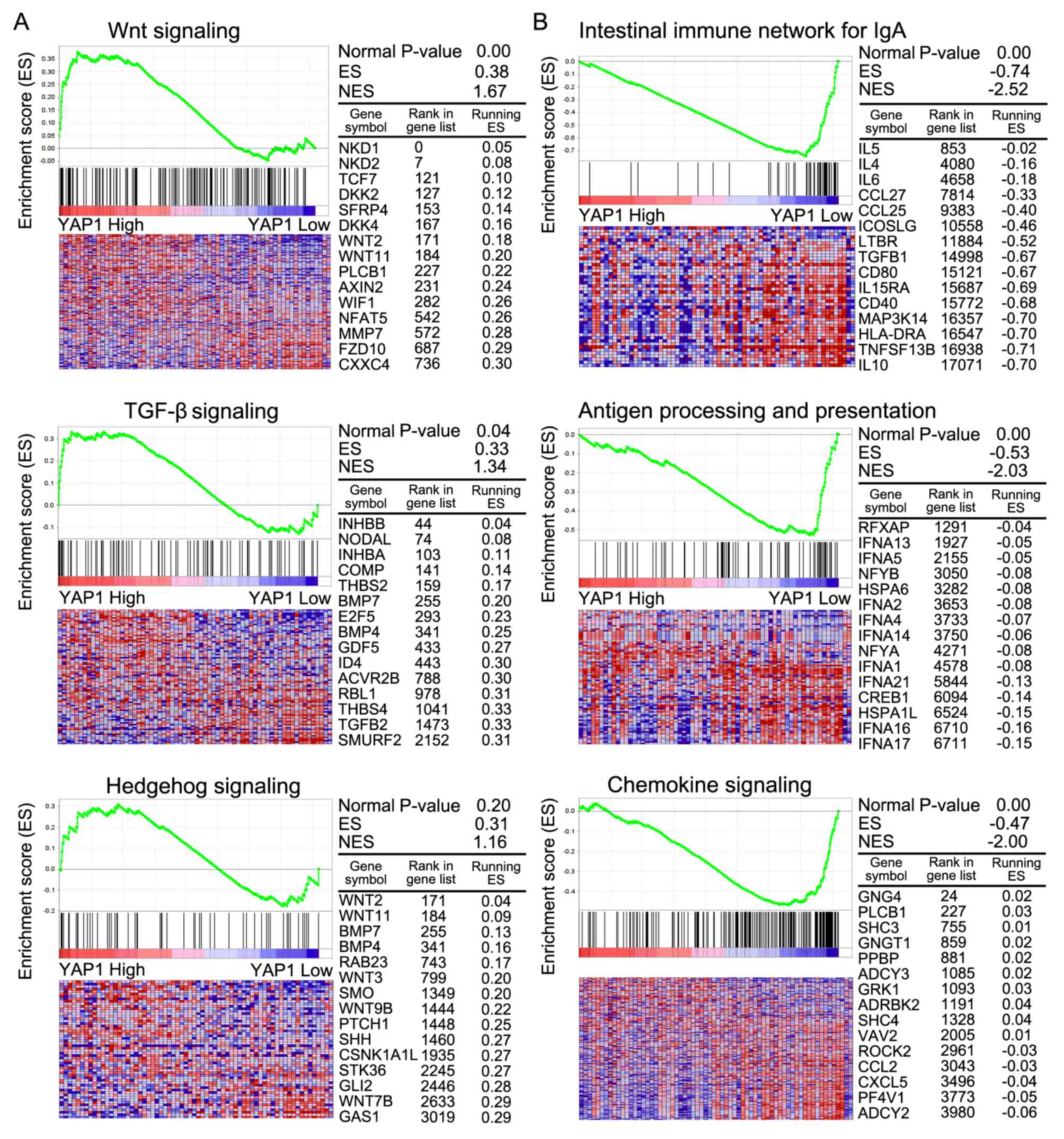
GSEA analysis
GSEA was conducted to compare more specifically the
significantly enriched pathways between classes A and B (Table IV). In class A, pathways involving
Wnt signaling, TGF-β signaling and Hedgehog signaling were
significantly enriched compared with class B. In class B, pathways
were mainly involved in the intestinal immune network for
immunoglobulin A, antigen processing and presentation and chemokine
signaling. In class A, Wnt and TGF-β signaling were associated with
cancer progression (Fig. 5A).
Immune-associated signaling pathways were associated with class B
(Fig. 5B).
 | Table IV.Gene set enrichment analysis of class
A and class B. |
Table IV.
Gene set enrichment analysis of class
A and class B.
| A, Class A |
|---|
|
|---|
| KEGG pathway | Size | ES | NES | NOM P-value |
|---|
| RNA polymerase | 29 | 0.54 | 1.76 | <0.01 |
| Melanoma | 71 | 0.42 | 1.69 | <0.01 |
| Wnt signaling
pathway | 150 | 0.38 | 1.67 | <0.01 |
| Basal cell
carcinoma | 55 | 0.41 | 1.53 | 0.01 |
| Basal transcription
factors | 35 | 0.40 | 1.37 | 0.10 |
| TGF-β signaling
pathway | 85 | 0.33 | 1.34 | 0.04 |
| Homologous
recombination | 26 | 0.40 | 1.30 | 0.11 |
| ECM receptor
interaction | 83 | 0.30 | 1.22 | 0.11 |
| Hedgehog signaling
pathway | 56 | 0.31 | 1.16 | 0.20 |
| Adherens
junction | 73 | 0.30 | 1.15 | 0.20 |
| Spliceosome | 114 | 0.26 | 1.10 | 0.23 |
|
| B, Class
B |
|
| Name | Size | ES | NES | NOM
P-value |
|
| Intestinal immune
network for IgA production | 46 | −0.74 | −2.52 | <0.01 |
| Hematopoietic cell
lineage | 84 | −0.66 | −2.50 | <0.01 |
| Allograft
rejection | 35 | −0.71 | −2.30 | <0.01 |
| Primary
immunodeficiency | 35 | −0.70 | −2.27 | <0.01 |
| Antigen processing
and presentation | 81 | −0.53 | −2.03 | <0.01 |
| Chemokine signaling
pathway | 188 | −0.47 | −2.00 | <0.01 |
Survival analysis
To determine the prognostic significance of Hippo
pathway genes in patients with COAD, the association between mRNA
expression of Hippo pathway genes and overall survival was
evaluated using Kaplan-Meier curves (Fig.
6). High mRNA expression of YAP1, TAZ, TEAD4 and LATS2 was
significantly associated with poor prognosis in COAD.
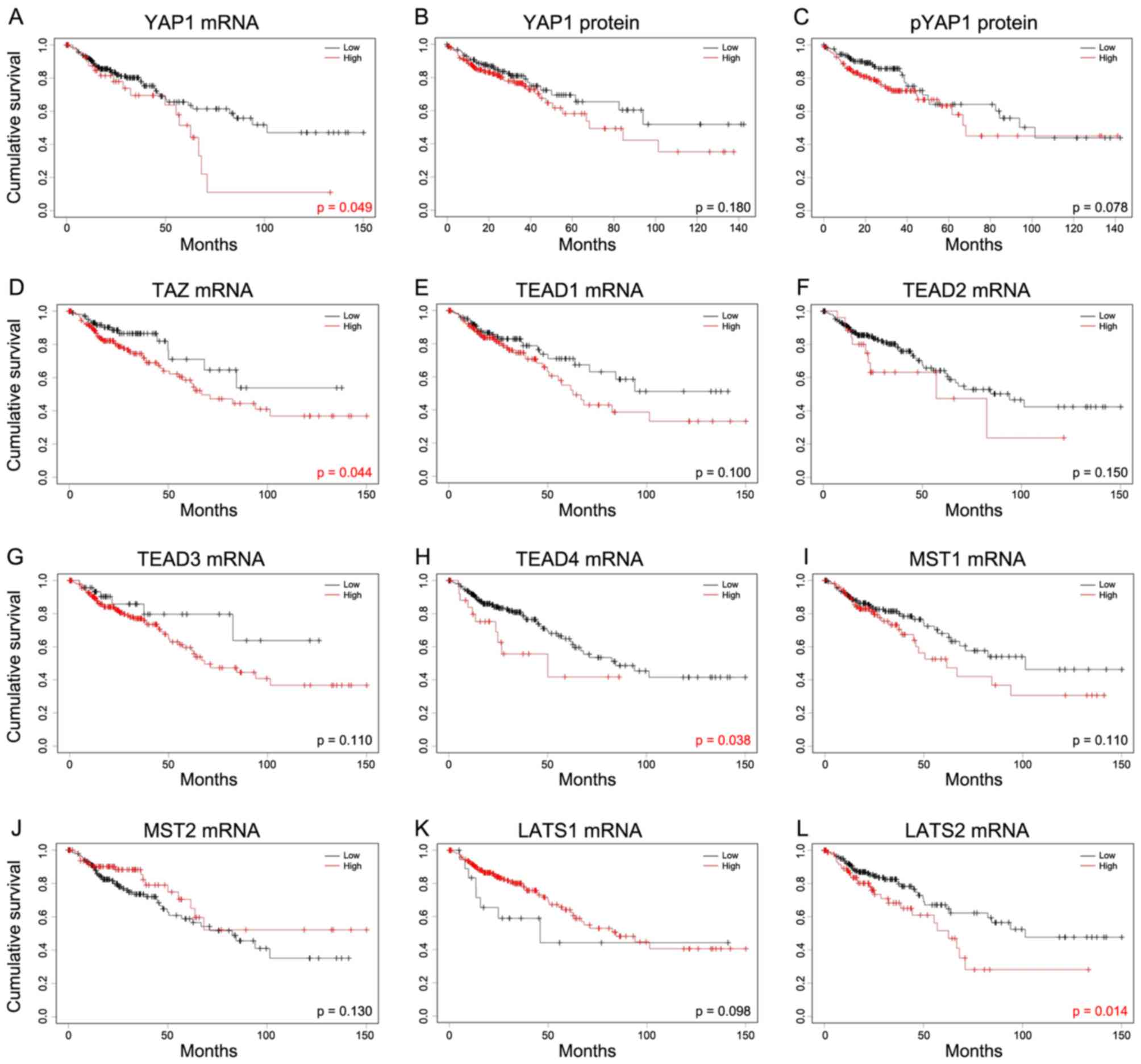 | Figure 6.Kaplan-Meier analysis of the
association between Hippo pathway genes and overall survival. In
order to investigate the Hippo pathway, survival was studied
against levels of (A) YAP1 mRNA, (B) YAP1 protein and (C) pYAP1
protein. The mRNA levels of (D) TAZ, (E) TEAD1, (F) TEAD2, (G)
TEAD3, (H) TEAD4, (I) MST1, (J) MST2, (K) LATS1 and (L) LATS2 were
also assessed. Cutoff Finder was used to determine cut-off values
in mRNA and protein expression of colon adenocarcinoma using
log-rank tests. mRNA of YAP1, TAZ, TEAD4 and LATS2 exhibited the
poor prognosis. YAP1, yes-associated protein 1; pYAP,
phosphorylated form of YAP1; TAZ, taffazin; TEAD, transcriptional
enhancer associated domain; MST, macrophage stimulating; LATS,
large tumor suppressor kinase. |
Discussion
In the present study, the expression of YAP1
mRNA in cases of COAD was identified to be higher than that in
other types of gastrointestinal tract cancer. YAP1 mRNA
expression was significantly increased in the sigmoid colon
compared with the ascending colon and hepatic flexure, and in
advanced TNM stages. YAP1 protein was highly expressed in advanced
TNM stages, and pYAP1 levels were high; however, YAP1/pYAP1 was
also higher in the advanced TNM stages. When YAP1 expression was
compared separately for each of the TNM stages, YAP1/pYAP1 was only
significantly elevated in advanced T stages, but was markedly
higher in the advanced N and M stages. YAP1 mRNA and pYAP1
levels were significantly elevated in the advanced N stage. YAP1
was mainly associated with cell proliferation and development. WNT
and TGF-β signaling were significantly enriched in the high
YAP1-expression group, as assessed by GSEA. Finally, YAP1, TAZ,
TEAD4 and LATS2 mRNA expression were associated with
poor overall survival rates in patients with COAD.
Evidence indicates that the right and left sides of
the colon exhibit significantly different histological and
molecular characters (31–34). At the molecular level, genes are
significantly differentially expressed between right- and
left-sided colon cancer (33). In the
present study, expression of YAP1 mRNA in COAD was
significantly higher in the sigmoid colon than in the ascending
colon and hepatic flexure. Although levels of pYAP1 were increased
in advanced TNM stages, the YAP1/pYAP1 ratio, which represents the
nuclear activity of YAP1, was consistently higher in advanced TNM
stages, particularly in the advanced T stage.
Increases in the expression of YAP1 and other
Hippo pathway genes have been investigated in multiple types of
cancer, including cancer of the liver, colon, lung and prostate
(12–14,35,36). In
liver cancer, YAP1 was identified as an independent
prognostic marker for overall and disease-free survival (36). In ovarian cancer, YAP1 has a
marked association with poor prognosis (16). In colon cancer, several studies have
reported that Hippo pathway genes were overexpressed and associated
with poor prognosis (20–23). Wang et al (21) reported that co-overexpression of
YAP1 and TAZ is an independent predictor of prognosis
for patients. Liang et al (22) demonstrated that YAP1 and
TEAD1 were upregulated and MST1 and LATS2 were
down regulated in colorectal cancer. Yuen et al (23) reported that TAZ exhibited
greater prognostic value than YAP1 in colorectal cancer. In
accordance with previous findings, the present study demonstrated
that YAP1 and TAZ were highly expressed and
associated with poor overall survival in COAD (Fig. 6). In addition, the present study
identified that TEAD4 was significantly associated with poor
prognosis (P=0.038; Fig. 6H). Among
upstream components, MST1/2 was highly expressed, whereas
LATS1/2 was expressed at a low level. Although LATS2
exhibited low expression in the present study, LATS2 was
associated with poor overall survival. In the hippo pathway,
YAP1, TAZ, TEAD4 and LATS2 genes may be able to serve
as molecular markers in COAD.
To verify the function and mechanism of YAP1
in COAD, bioinformatics analysis was conducted. GeneNeighbors
analysis revealed that cell proliferation and protein
binding-associated genes were associated with YAP1 in 458
samples from patients with COAD (Fig.
3). Additionally, ClassNeighbors analysis classified
YAP1-expressing COAD samples into class A, which expresses
genes associated with development, stem cell maintenance and growth
factor activity, and class B, which expresses genes associated with
the negative regulation of growth, cellular response to ions and
mineral absorption. Class A genes enhance development and cell
growth-associated functions, whereas class B genes enhance the
suppression of cell growth and mineral interaction-associated
functions. GSEA was performed to compare pathways that were
enriched between classes A and B. In class A, pathways involved in
tight junction, Wnt signaling, TGF-β signaling and adherens
junctions pathways exhibited greater activity than those in class
B. In class B, pathways involved in primary immunodeficiency,
intestinal immune network for immunoglobulin A production and
regulation of autophagy were enriched. The Hippo pathway is able to
interact with other oncogenic signaling pathways, including Wnt,
TGF-β, Sonic hedgehog, Notch and epidermal growth factor
receptor/KRAS proto-oncogene, GTPase pathways, to modify more
downstream components (37,38). In the present study, YAP1
expression was associated with Wnt signaling and TGF-β signaling,
which are associated with cancer progression (1,39).
Additionally, GSEA also identified that YAP1 was associated
with RNA polymerase, basal transcription factors, ECM receptor
interaction and adherens junction in COAD.
In conclusion, the expression and clinical
significance of Hippo pathway genes in COAD was investigated using
a cohort of 458 patients obtained from TGCA. YAP1 mRNA was
highly expressed in sigmoid colon cancer. YAP1 activity was
consistently higher in advanced TNM stages, particularly in the
advanced T stage. YAP1 was associated with proliferation and
development, and was significantly associated with Wnt and TGF-β
signaling, as indicated by bioinformatics analysis. High mRNA
expression of YAP1 and its associated genes, TAZ,
TEAD4 and LATS2, was significantly associated with poor
patient prognosis in COAD. However, further study is required to
confirm the prognostic value of TAZ, TEAD4 and LATS2,
and the underlying molecular mechanisms of their functions in
COAD.
References
|
1
|
Ikushima H and Miyazono K: TGFbeta
signalling: A complex web in cancer progression. Nat Rev Cancer.
10:415–424. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
3
|
Johnson R and Halder G: The two faces of
Hippo: Targeting the Hippo pathway for regenerative medicine and
cancer treatment. Nat Rev Drug Discov. 13:63–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piccolo S, Dupont S and Cordenonsi M: The
biology of YAP/TAZ: Hippo signaling and beyond. Physiol Rev.
94:1287–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santucci M, Vignudelli T, Ferrari S, Mor
M, Scalvini L, Bolognesi ML, Uliassi E and Costi MP: The Hippo
pathway and YAP/TAZ-TEAD protein-protein interaction as targets for
regenerative medicine and cancer treatment. J Med Chem.
58:4857–4873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Justice N, Roegiers F, Jan LY and Jan YN:
Lethal giant larvae acts together with numb in notch inhibition and
cell fate specification in the Drosophila adult sensory organ
precursor lineage. Curr Biol. 13:778–783. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu T, Wang W, Zhang S, Stewart RA and Yu
W: Identifying tumor suppressors in genetic mosaics: The Drosophila
lats gene encodes a putative protein kinase. Development.
121:1053–1063. 1995.PubMed/NCBI
|
|
9
|
Tapon N, Harvey KF, Bell DW, Wahrer DC,
Schiripo TA, Haber D and Hariharan IK: salvador Promotes both cell
cycle exit and apoptosis in Drosophila and is mutated in human
cancer cell lines. Cell. 110:467–478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui ZL, Han FF, Peng XH, Chen X, Luan CY,
Han RC, Xu WG and Guo XJ: YES-associated protein 1 promotes
adenocarcinoma growth and metastasis through activation of the
receptor tyrosine kinase Axl. Int J Immunopathol Pharmacol.
25:989–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pei T, Li Y, Wang J, Wang H, Liang Y, Shi
H, Sun B, Yin D, Sun J, Song R, et al: YAP is a critical oncogene
in human cholangiocarcinoma. Oncotarget. 6:17206–17220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim
J, Xie J, Ikenoue T, Yu J, Li L, et al: Inactivation of YAP
oncoprotein by the Hippo pathway is involved in cell contact
inhibition and tissue growth control. Genes Dev. 21:2747–2761.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in Drosophila and
mammals. Cell. 130:1120–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Steinhardt AA, Gayyed MF, Klein AP, Dong
J, Maitra A, Pan D, Montgomery EA and Anders RA: Expression of
Yes-associated protein in common solid tumors. Hum Pathol.
39:1582–1589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, George J, Deb S, Degoutin JL,
Takano EA, Fox SB; AOCS Study group, ; Bowtell DD and Harvey KF:
The Hippo pathway transcriptional co-activator, YAP, is an ovarian
cancer oncogene. Oncogene. 30:2810–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hall CA, Wang R, Miao J, Oliva E, Shen X,
Wheeler T, Hilsenbeck SG, Orsulic S and Goode S: Hippo pathway
effector Yap is an ovarian cancer oncogene. Cancer Res.
70:8517–8525. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nallet-Staub F, Marsaud V, Li L, Gilbert
C, Dodier S, Betaille V, Sudol M, Herlyn M and Mauviel A:
Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in
cutaneous melanoma. J Invest Dermatol. 134:123–132. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhat KP, Salazar KL, Balasubramaniyan V,
Wani K, Heathcock L, Hollingsworth F, James JD, Gumin J, Diefes KL,
Kim SH, et al: The transcriptional coactivator TAZ regulates
mesenchymal differentiation in malignant glioma. Genes Dev.
25:2594–2609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cottini F, Hideshima T, Xu C, Sattler M,
Dori M, Agnelli L, ten Hacken E, Bertilaccio MT, Antonini E, Neri
A, et al: Rescue of Hippo coactivator YAP1 triggers DNA
damage-induced apoptosis in hematological cancers. Nat Med.
20:599–606. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Xie C, Li Q, Xu K and Wang E:
Clinical and prognostic significance of Yes-associated protein in
colorectal cancer. Tumour Biol. 34:2169–2174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Shi S, Guo Z, Zhang X, Han S, Yang
A, Wen W and Zhu A: Overexpression of YAP and TAZ is an independent
predictor of prognosis in colorectal cancer and related to the
proliferation and metastasis of colon cancer cells. PLoS One.
8:e655392013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang K, Zhou G, Zhang Q, Li J and Zhang
C: Expression of hippo pathway in colorectal cancer. Saudi J
Gastroenterol. 20:188–194. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yuen HF, McCrudden CM, Huang YH, Tham JM,
Zhang X, Zeng Q, Zhang SD and Hong W: TAZ expression as a
prognostic indicator in colorectal cancer. PLoS One. 8:e542112013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
R Core Team, . R: A Language and
Environment for Statistical Computing. R Foundation for Statistical
Computing; Vienna, Austria: 2016
|
|
25
|
Golub TR, Slonim DK, Tamayo P, Huard C,
Baasenbeek M, Mersirov JP, Coller H, Loh ML, Downing JR, Caligiuri
MA, et al: Molecular classification of cancer: Class discovery and
class prediction by gene expression monitoring. Science.
286:531–537. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID gene functional classification tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanehisa M, Goto S, Sato Y, Furumichi M
and Tanabe M: KEGG for integration and interpretation of
large-scale molecular data sets. Nucleic Acids Res. 40:D109–D114.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kanehisa M and Goto S: KEGG: Kyoto
Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Budczies J, Klauschen F, Sinn BV, Győrffy
B, Schmitt WD, Darb-Esfahani S and Denkert C: Cutoff Finder: A
comprehensive and straightforward Web application enabling rapid
biomarker cutoff optimization. PLoS One. 7:e518622012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hari DM, Leung AM, Lee JH, Sim MS, Vuong
B, Chiu CG and Bilchik AJ: AJCC cancer staging manual 7th edition
criteria for colon cancer: Do the complex modifications improve
prognostic assessment? J Am Coll Surg. 217:181–190. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Benedix F, Kube R, Meyer F, Schmidt U,
Gastinger I and Lippert H; Colon/Rectum Carcinomas (Primary Tumor)
Study Group, : Comparison of 17,641 patients with right- and
left-sided colon cancer: Differences in epidemiology, perioperative
course, histology and survival. Dis Colon Rectum. 53:57–64. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Papagiorgis P, Oikonomakis I,
Karapanagiotou I, Wexner SD and Nikiteas N: The impact of tumor
location on the histopathologic expression of colorectal cancer. J
Buon. 11:317–321. 2006.PubMed/NCBI
|
|
33
|
Glebov OK, Rodriguez LM, Nakahara K,
Jenkins J, Cliatt J, Humbyrd CJ, DeNobile J, Soballe P, Simon R,
Wright G, et al: Distinguishing right from left colon by the
pattern of gene expression. Cancer Epidemiol Biomarkers Prev.
12:755–762. 2003.PubMed/NCBI
|
|
34
|
Azzoni C, Bottarelli L, Campanini N, Di
Cola G, Bader G, Mazzeo A, Salvemini C, Morari S, Di Mauro D,
Donadei E, et al: Distinct molecular patterns based on proximal and
distal sporadic colorectal cancer: Arguments for different
mechanisms in the tumorigenesis. Int J Colorectal Dis. 22:115–126.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT,
Znder L, Lowe SW, Poon RT and Luk JM: Yes-associated protein is an
independent prognostic marker in hepatocellular carcinoma. Cancer.
115:4576–4585. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Irvine KD: Integration of intercellular
signaling through the Hippo pathway. Semin Cell Dev Biol.
23:812–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao B, Li L and Guan KL: Hippo signaling
at a glance. J Cell Sci. 123:4001–4006. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
DiMeo TA, Anderson K, Phadke P, Fan C,
Perou CM, Naber S and Kuperwasser C: A novel lung metastasis
signature links Wnt signaling with cancer cell self-renewal and
epithelial-mesenchymal transition in basal-like breast cancer.
Cancer Res. 69:5364–5373. 2009. View Article : Google Scholar : PubMed/NCBI
|