Introduction
Glioma is the most common malignant tumor in the
central nervous system (CNS). It occurs in any part of the CNS and
exhibits highly aggressive and malignant behavior. The survival
rates of glioma remain at a low level after the traditional
treatment, including surgical resection, radiotherapy, and
chemotherapy (1,2). Tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) has become one of the hotspots in
the last several years (3). TRAIL has
shown great anticancer activity by selectively binding to death
receptor 4 (DR4) and death receptor 5 (DR5) and inducing tumor cell
apoptosis, while healthy cells remain affected (3–6). However,
blood-brain barrier (BBB), as a protective mechanism for CNS
tumors, prevents TRAIL from reaching tumor sites (7,8). TRAIL
cannot be applied in CNS cancer therapy owing to its short
half-life (9,10). TRAIL was combined with stem cells to
address these issues. Both neural stem cells (NSCs) and mesenchymal
stem cells (MSCs) were engineered to express TRAIL and restrict
glioma cells, showing bright prospects (11–15).
However, SCs had some limitations, such as uncertain
differentiation and difficulties in acquisition from adults.
Endothelial progenitor cells (EPCs) are the
precursors of endothelial cells, with CD31 and CD34 as their
specific surface markers. They were first isolated in 1997 from
human peripheral blood (16,17). EPCs are recruited in the
neovascularization of tumor and involved in tumor vascular network
(18,19). Owing to these characteristics, EPCs
have the potential to be involved in anti-tumor therapies. Most
previous studies explored anti-angiogenic treatment by suppressing
the mobilization and homing of EPCs to tumors (20). Increasing attention has been paid to
the use of EPCs as a vector for treatment (21,22).
Recent studies have demonstrated that EPCs can move across the BBB
from peripheral blood and participate in the angiogenesis of
gliomas (23,24). Compared with NSCs and MSCs, EPCs can
be obtained from peripheral blood, which means that EPCs can be
easily acquired from adults. This makes the autotransplantation of
EPCs possible and avoids immunological rejection. Therefore, EPCs
have been recognized as an ideal vector to deliver antineoplastic
molecules to CNS tumors. However, whether the TRAIL-expressing EPCs
home to glioma cells through the BBB and induce cell apoptosis
needs further investigation.
In this study, EPCs engineered with a lentivirus
encoding TRAIL were generated and used to induce apoptosis of a
glioma cell line SHG44. First, the Transwell assay was used to
verify that EPCs could home to glioma cells. Then, the co-culture
system of TRAIL-overexpressing EPCs and SHG44 cells was
established, and the apoptotic state of SHG44 cells was examined.
Interestingly, the apoptosis percentage and change in protein
levels of caspase-8 and −3, as well as poly ADP-ribose polymerase
(PARP), in SHG44 cells were found to increase remarkably. Taken
together, the study demonstrated that the TRAIL-modified EPCs could
be a feasible approach for glioma therapy.
Materials and methods
Cell cultures
Human glioma cell line, SHG44 cells, were purchased
from Cell Bank of the Chinese Academy of Sciences and cultured in
Dulbecco's modified Eagle's medium (DMEM)/high-glucose medium
(HyClone; GE Healthcare, Logan, UT, USA) with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 0.5 mm glutamine, and 100 U/ml penicillin/streptomycin at
37°C in a humidified atmosphere containing 5% CO2 and
95% air.
EPCs were extracted from the blood of neonatal
Sprague-Dawley rats by the density gradient method, as previously
described (25), and cultured in
endothelial cell growth medium-2 (EGM-2; Lonza, Group, Ltd., Basel,
Switzerland) culture medium with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.), 0.5 mm glutamine, and 100 U/ml
penicillin/streptomycin in a 5% CO2 atmosphere at
37°C.
Immunocytochemical analysis
EPCs were washed twice with phosphate-buffered
saline (PBS) and fixed with 4% paraformaldehyde at 4°C overnight.
After removing paraformaldehyde and washing the cells with PBS, 3%
H2O2 was used to inactivate endogenous
peroxidase for 10 min. The cells were incubated with 5% bovine
serum albumin (BSA) for 1 h at room temperature to block the
nonspecific binding. Then, the primary antibodies (CD31 and CD34,
from Abcam, Cambridge, MA, USA) were added onto cells and incubated
at 4°C for 12 h. Only PBS with 5% BSA was used as negative control.
The cells were washed with PBS three times to remove unbound
antibody and then incubated with secondary antibody conjugated to
horseradish peroxidase (HRP; ZSGF-BIO, China) for 30 min at room
temperature. After removing the secondary antibody by washing with
PBS, the cells were incubated with 3,3′-diaminobenzidine developer,
counterstained with hematoxylin, and observed under a
microscope.
Immunofluorescence
EPCs were washed with PBS and fixed with 4%
paraformaldehyde after 3 days of lentiviral transfection. The cells
were blocked with 5% BSA for 30 min and then incubated with primary
antibodies (TRAIL, DR4, and DR5; Abcam) at 4°C for 12 h. After the
cells were washed with PBS, secondary antibodies were used for
staining (Alexa 594 conjugated; Thermo Fisher Scientific, Inc.;
Alexa 405 conjugated; Wuhan Sanying Biotechnology, Wuhan, China).
Cells were observed using laser scanning confocal microscopy (Nikon
Corporation, Tokyo, Japan).
Angiogenesis assay
BD Matrigel basement membrane matrix (BD
Biosciences, Franklin Lakes, NJ, USA) was melted at 0°C, implanted
in a 24-pore culture plate, and then placed in a 37°C incubator for
1 h. EPCs were inoculated on the surface of solidified Matrigel
(5×104 cells per well) and cultured in a 5%
CO2 atmosphere at 37°C. The cells were observed under a
microscope after 6 h, and images were taken under magnification,
×40 and ×100.
Lentiviral infection
The lentivirus with the TRAIL sequence was delivered
to overexpress the TRAIL protein in EPCs. The negative control
virus carrying green fluorescent protein (GFP) only was used as a
control. Both viruses were constructed and packed by Shanghai
GeneChem Co., Ltd., Shanghai, China. EPCs were infected using
lentiviruses with a multiplicity of infection of 10 on the second
day after the first passage of EPCs.
RNA extraction, cDNA synthesis, and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
After a week of lentiviral infection, total RNA was
extracted from EPCs in both the groups using TRIzol reagent
(Solarbio, Beijing, China), chloroform, and isopropyl alcohol.
Then, 1 µg of extracted RNA was reverse transcribed into strand
cDNA after removing genomic DNA using a PrimeScript Reverse
Transcription Reagent kit with gDNA Eraser (TaKaRa Bio Co., Ltd.,
Otsu, Japan). Real-time quantitative polymerase chain reaction
(PCR) was performed on Roche LightCycler (Roche Applied Science,
Rotkreuz, Switzerland) using the same reagent kit with cDNA
synthesis for the desired gene. The primers were designed and
synthesized by Sangon Biotech as follows:
TRAIL sense, 5′-AACTGGGACCAGAGGAAGAAGCAA-3′ and
antisense, 5′-ATGCCCACTCCTTGATGATTCCCA-3′;
Glyceraldehyde-3-phosphate dehydrogenase sense,
5′-TCCTGCACCACCAACTGCTTAG-3′ and antisense,
5′-TCCTGCACCACCAACTGCTTAG-3′.
Co-culture
SHG44 cells were cultured with TRAIL-expressing EPCs
as the experimental group, or with EPCs expressing only GFP as the
control group. Both groups were in the same culture environment:
EGM-2 culture medium was used with 10% FBS, 0.5 mM glutamine, and
100 U/ml penicillin/streptomycin in a 5% CO2 atmosphere
at 37°C.
Transwell migration assay
In vitro cell migration assays were performed
using 24-well Transwell chambers (12-µm pores; Corning Inc.,
Corning, NY, USA). Nontransfected EPCs and TRAIL-transfected EPCs
(2×104 cells per well) were cultured in the top chamber,
whereas SHG44 cells, as a positive group, were cultured in the
lower chamber. DMEM medium with 10% FBS only in the lower chamber
was used as the control group. After 24 h of cultivation, the cells
on the upper side were removed and the migrated cells were fixed in
4% paraformaldehyde, stained with 4′,6-diamidino-2-phenylindole
(DAPI) solution, and examined using a high-content screening system
(HCS; Thermo Fisher Scientific, Inc.).
Propidium iodide and Annexin V
assays
The cells were gently washed once with PBS on the
third day of co-culture and then stained with Annexin V-kFluor594
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) for 15 min at
room temperature away from light. The cells were observed under a
fluorescence microscope after replacing the staining solution with
PBS. The flow cytometry assay was performed using Annexin V-APC
(Nanjing KeyGen Biotech Co., Ltd.) and propidium iodide (PI)
(Nanjing KeyGen Biotech Co., Ltd.), whose fluorescence signals were
excited at 633 and 488 nm and collected at 660 and 610 nm,
respectively, to determine the degree of apoptosis of SHG44 cells
further. More than 10,000 cells per sample group were collected and
divided into EPCs and SHG44 cells on the basis of GFP fluorescence
intensity. The percentage of Annexin V-positive SHG44 cells was
calculated as an indicator of apoptosis.
Co-immunoprecipitation
The cells from co-culture were homogenized in cell
lysis buffer (Solarbio), supplemented with 1 mM
phenylmethanesulfonyl fluoride (PMSF) and complete protease
inhibitor mixture (Solarbio). The homogenate was centrifuged at
11,000 rpm for 15 min at 4°C. The supernatant was incubated with
anti-TRAIL (Abcam) crosslinked beads at 4°C overnight with
rotation. The Pierce Crosslink Magnetic IP kit instruction was used
for the pretreatment of beads and immunoprecipitation. The
associated proteins were detected using western blot analysis.
Homogenates from co-culture cells were used as positive
controls.
Quantitative immunoblot analysis
The cells in the two groups were collected on third
and fifth days of co-culture, homogenized in 1X cell lysis buffer
(Solarbio) supplemented with 1 mM PMSF and complete protease
inhibitor mixture (Solarbio) for 30 min on ice, and then
centrifuged at 11,000 rpm for 10 min at 4°C. The supernatant was
measured using a Bicinchoninic Acid Protein Assay kit (Solarbio).
The measurement samples were combined with 5X SDS loading buffer
and boiled at 100°C for 10 min. For each protein, equal amounts of
samples (20–100 µg) from each group were analyzed using sodium
dodecyl sulfate-polyacrylamide gel electrophoresis as previously
described (26). After proteins were
transferred onto a polyvinylidene difluoride membrane, the membrane
was incubated with 5% BSA at room temperature for 2 h to block the
nonspecific protein site and then corresponded with primary
antibodies [TRAIL from Abcam; DR4 and DR5 from Abcam; vascular
endothelial growth factor receptor 2 (VEGF-R2), caspase-8 and −3,
and PARP from Cell Signaling Technology, Inc. (Danvers, MA, USA);
β-actin from ZSGF-BIO] at 4°C overnight. This step was followed by
incubation with HRP-conjugated secondary antibodies (ZSGF-BIO).
Visualization was achieved using a SuperSignal West Pico Trial kit
(Thermo Fisher Scientific, Inc.).
Statistical analysis
Data were expressed as mean ± standard deviation
(SD). One-way analysis of variance was used for comparisons among
multiple groups, followed by the Student post hoc two-tailed test.
The unpaired Student t-test was performed for comparisons between
the means of two groups. GraphPad 6.0 (GraphPad Software, Inc., La
Jolla, CA, USA) was used for all the statistical analyses.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Generation of TRAIL-expressing
EPCs
Blood cells were extracted from neonatal
Sprague-Dawley rats, and the specific surface markers CD31 and CD34
were detected by immunohistochemical analysis (27). Most cells revealed positive reactions
to CD31 and CD34 (Fig. 1A and B). The
angiogenic ability of EPCs was also identified. The cells
inoculated on the Matrigel surface formed tube-like structures
within 6 h, as shown in representative images (Fig. 1C). Thus, EPCs were isolated
successfully from the blood of neonatal rats.
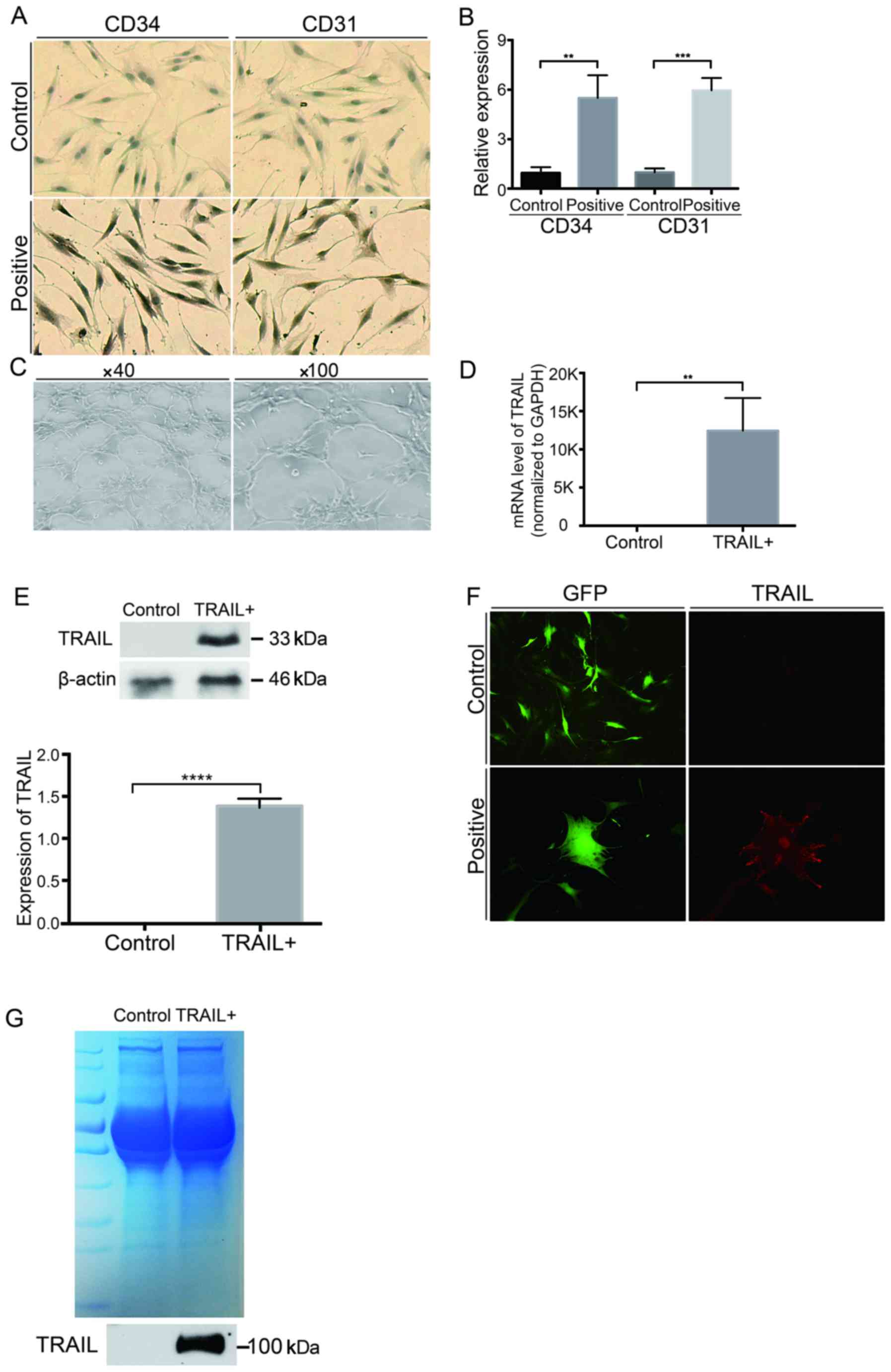 | Figure 1.Construction of TRAIL-expressing
EPCs. (A and B) Immunocytochemical staining showed that the cells
were CD31 and CD34 positive (magnification, ×100) (P<0.05,
compared with the control group). (C) EPCs formed capillary-like
networks after being seeded onto the Matrigel surface for 6 h. (D
and E) EPCs were infected with lentiviruses encoding GFP or TRAIL.
The mRNA and protein levels of TRAIL in EPCs were determined using
qPCR (D) (P<0.05, compared with the control group) and western
blot analysis. β-Actin was used as a loading control (E, P<0.05,
compared with the control group). (F) EPCs harboring GFP showed
green fluorescence, and EPCs harboring TRAIL showed red
fluorescence (magnification, ×200). (G) The sTRAIL was detected
using western blot and Coomassie blue staining. Data are presented
as means ± SD. Three individual experiments were performed for each
group. **P<0.01, ***P<0.0001 and ****P<0.001, compared
with the control group. TRAIL, tumor necrosis factor-related
apoptosis-inducing ligand; EPCs, endothelial progenitor cells; GFP,
green fluorescent protein qPCR, quantitative polymerase chain
reaction; sTRAIL, soluble extracellular TRAIL; SD, standard
deviation. |
The isolated EPCs were infected and screened using
lentivirus encoding TRAIL. Then, the expression level of TRAIL on
EPCs was detected. Total mRNA was also extracted from both
TRAIL-transfected EPCs and negative controls. Quantitative PCR was
performed to quantify the mRNA levels. The TRAIL mRNA level in
infected EPCs significantly increased compared with the control
group (P<0.05) (Fig. 1D).
Furthermore, the TRAIL protein level was also enhanced, as
determined by western blot analysis (P<0.05) (Fig. 1E). The location of TRAIL was
determined by immunofluorescence staining. TRAIL was found to be
mainly expressed on the cell surface and partly in the cytoplasm
and nucleus (Fig. 1F). The soluble
extracellular TRAIL (sTRAIL) was also detected by western blot
analysis. It was found that the sTRAIL existed in the trimeric form
in the culture medium of the TRAIL-positive group (Fig. 1G). These results showed that TRAIL was
overexpressed in the EPCs after lentiviral transfection, and the
overexpressed TRAIL was further distributed both on the cell
membrane and in the culture medium.
Overexpression of TRAIL did not affect
the migration of EPCs toward SHG44 cells
A Transwell migration assay was performed to assess
the directional migration of EPCs. The number of migrated cells
increased significantly when SHG44 cells were cultured in the lower
chamber, as evident from statistics and HCS of the images
(P<0.05) (Fig. 2A and B). However,
similar numbers of the migrated cells were found in the
TRAIL-transfected EPCs and controls, indicating that the
overexpression of TRAIL did not affect the homing of EPCs to SHG44
cells (P>0.05) (Fig. 2A and B).
Furthermore, the overexpression of TRAIL did not alter the protein
levels of VEGF-R2 in the TRAIL-transfected EPCs compared with the
controls (P>0.05) (Fig. 2C). These
findings indicated that EPCs with the overexpression of TRAIL had
the similar ability for directional migration and angiogenesis,
which could be used in the following experiments.
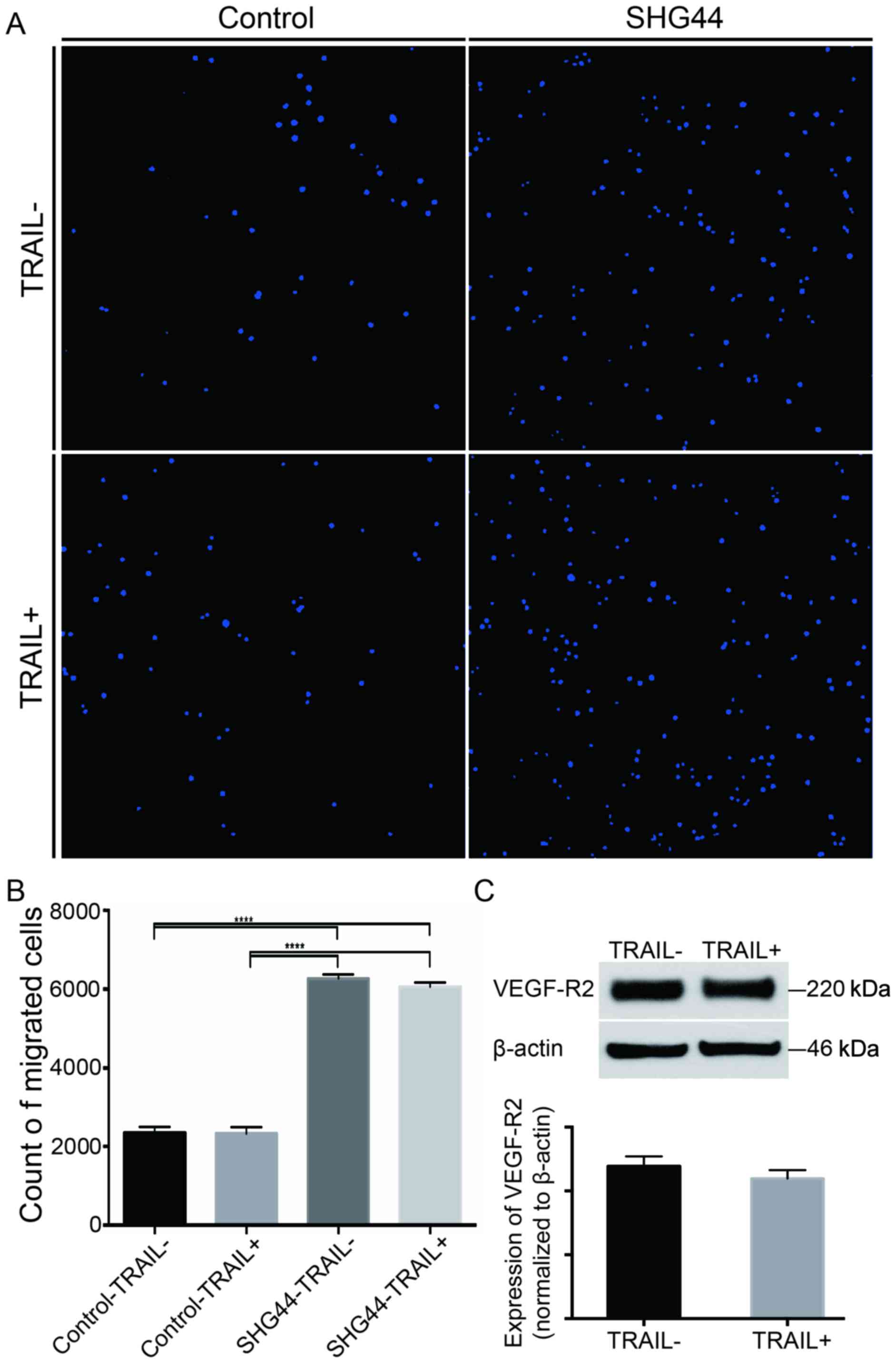 | Figure 2.Transwell migration assay of EPCs.
(A) Migrated EPCs were stained using DAPI, and images were obtained
using HCS of 25 continuous visual fields. (B) Numbers of migrated
cells were counted using HCS. The migrated EPCs increased obviously
when SHG44 cells were cultured in the lower chamber (magnification,
×100) (P<0.05, compared with the control group). No significant
difference was found between EPCs with or without the
overexpression of TRAIL (P>0.05). (C) Expression of VEGF-R2 on
control and TRAIL + EPCs. β-Actin was used as a loading control.
Data are presented as means ± SD. Three individual experiments were
performed for each group. ****P<0.0001, compared with the
control group. EPCs, endothelial progenitor cells; DAPI,
4′,6-diamidino-2-phenylindole, HCS, high-content screening; SD,
standard deviation. |
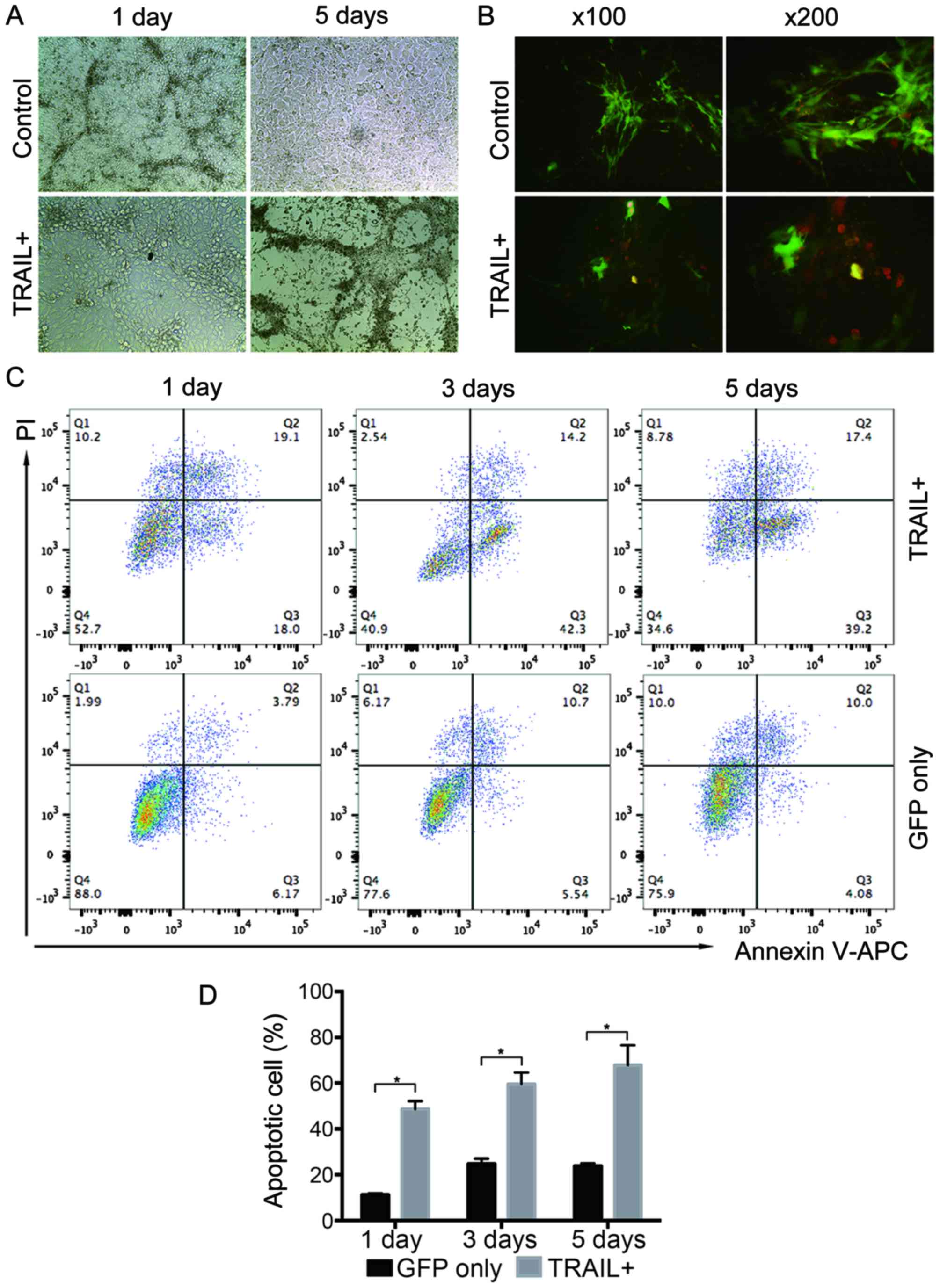
Apoptosis of SHG44 in co-culture
system with EPCs
A co-culture system in which SHG44 cells were
co-cultured with EPCs harboring TRAIL or GFP was established to
determine the effect of TRAIL-overexpressing EPCs on SHG44 cells.
After 5 days of continuous observation, it was found that the
number of SHG44 cells in the TRAIL-expressing EPC-treated group
obviously reduced compared with that in the GFP-harboring EPCs
(Fig. 3A). Annexin V-kFluor594 was
used on the fifth day of co-culture to detect cell apoptosis. More
Annexin V-kFluor594-positive cells (red fluorescence) were found
around the TRAIL-expressing EPCs (green fluorescence) (Fig. 3B) in the TRAIL-expressing EPC-treated
group compared with the GFP group. It indicated that the
TRAIL-expressing EPCs could induce SHG44 cell apoptosis. Then, the
flow cytometry assay was used to measure the apoptotic rate of
SHG44 cells. The cells were collected and stained with Annexin
V-APC/PI on the first, third, and fifth days after co-culturing.
The apoptotic rate of SHG44 cells significantly increased by
co-culturing with the TRAIL-expressing EPCs, compared with that of
the cells harboring GFP, after separating EPCs and SHG44 cells
based on GFP fluorescence intensity, (Fig. 3C and D). These findings indicated that
TRAIL-positive EPCs could increase SHG44 cell apoptosis.
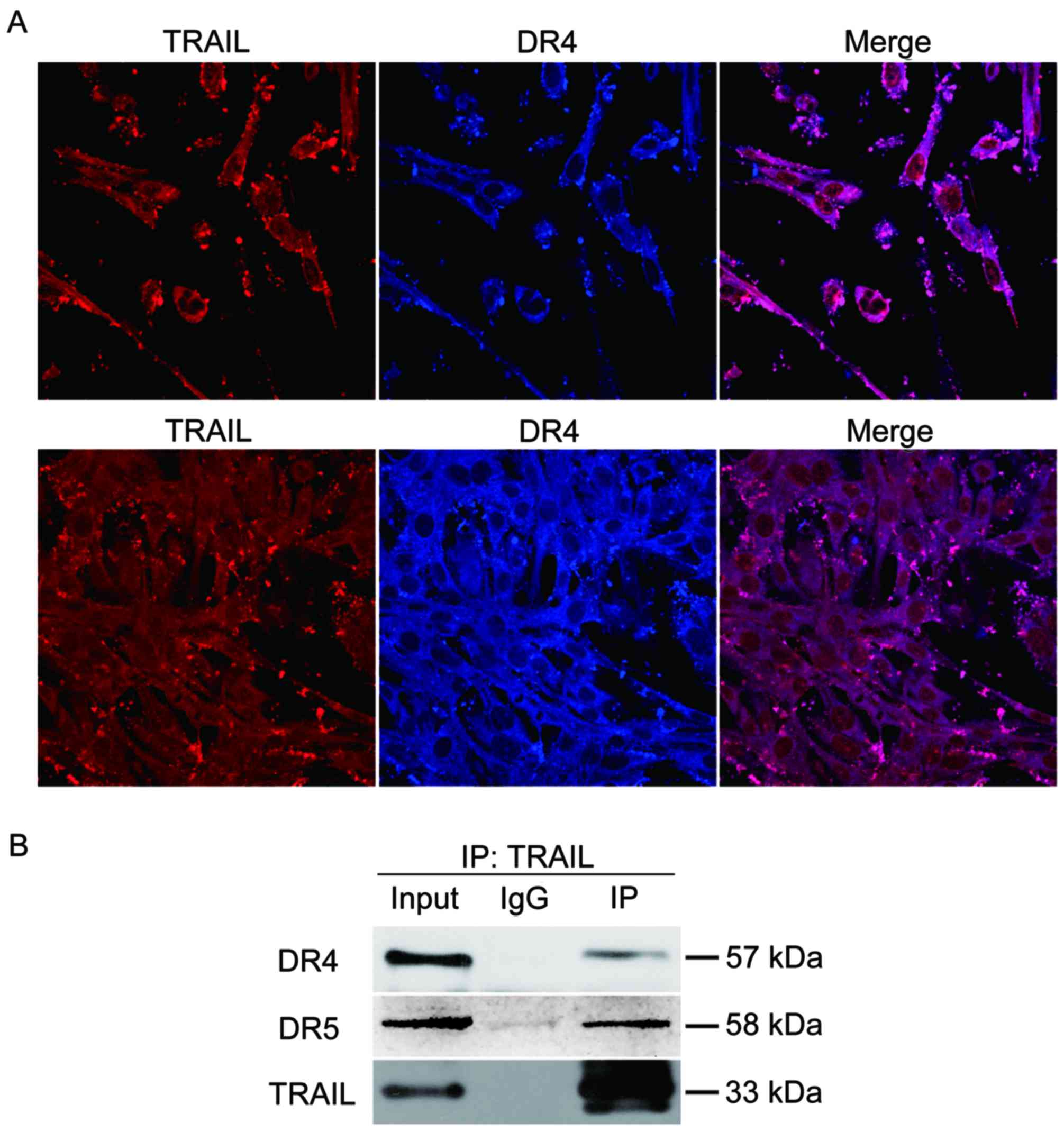
TRAIL bound with DR4/5 on glioma
cells
The study demonstrated that TRAIL-positive EPCs
could induce SHG44 cell apoptosis obviously. Next,
immunofluorescent co-localization and co-immunoprecipitation were
used to verify the interaction of TRAIL and death receptor so as to
explore the apoptotic mechanism further. Double-channel confocal
imaging revealed that TRAIL showed red fluorescence, TRAIL
co-localized with DR4 and DR5 (DR4 and DR5) showed blue
fluorescence (Fig. 4A). The total
proteins from the co-culture system were also extracted to test the
interaction between TRAIL and DR4/5 by immunoblotting. It was found
that TRAIL co-immunoprecipitated with DR4/5 in SHG44 cells
(Fig. 4B).
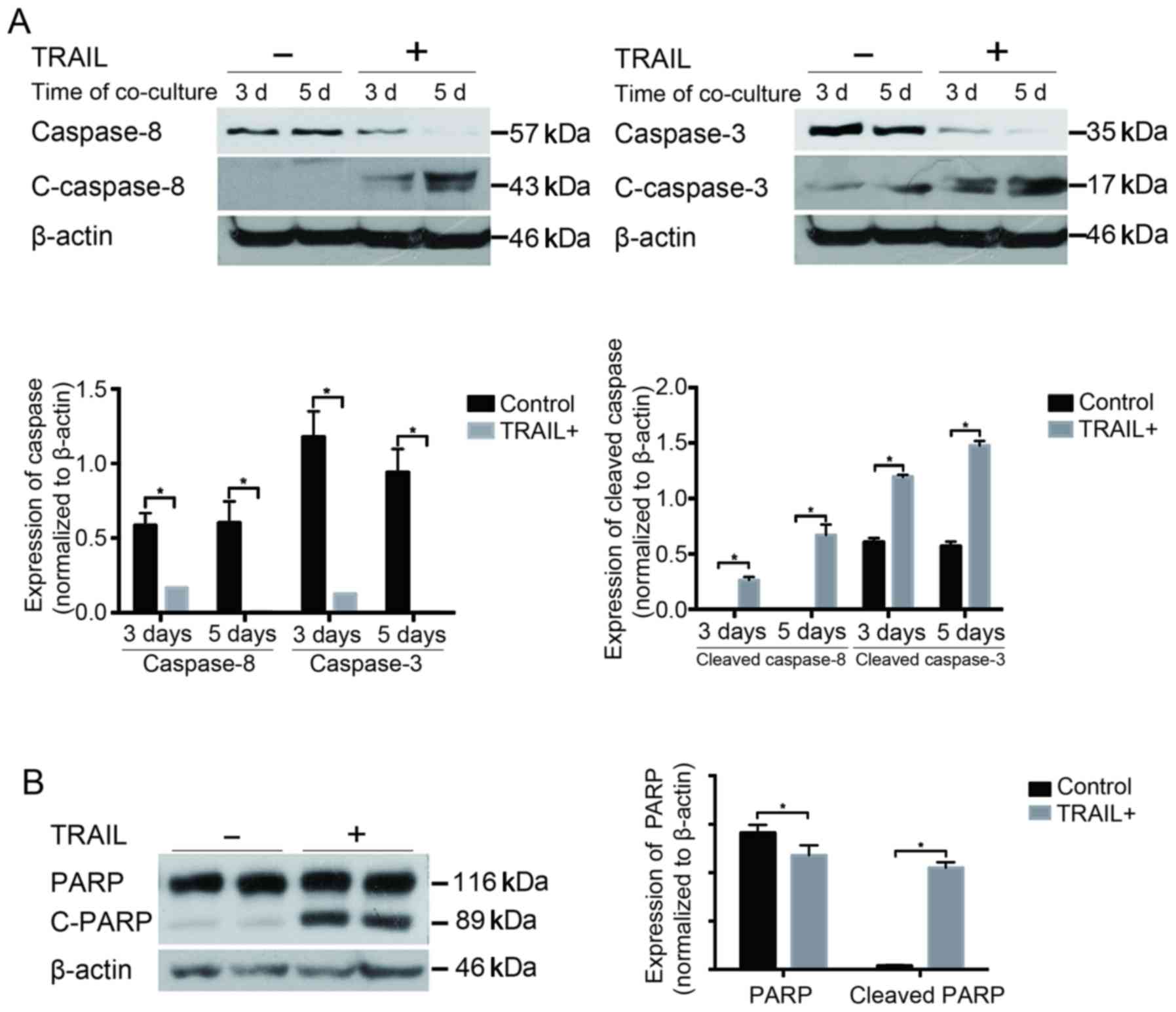
TRAIL enhanced the activation of
caspases and PARP
Next, the expression levels of pro/cleaved
caspase-8, pro/caspase-3, and PARP were examined. The expression
levels of cleaved caspase-8 and −3 significantly increased and were
further enhanced with time in the co-culture system, whereas the
expression levels of pro-caspase-8 and −3 decreased significantly,
compared with the GFP group (P<0.05) (Fig. 5A). Similar changes were observed in
the cleaved PARP. In contrast, the amount of full-length PARP was
much less in the TRAIL-positive group than in the GFP group
(P<0.05) (Fig. 5B), indicating
that PARP was largely activated under the effect of TRAIL on
EPCs.
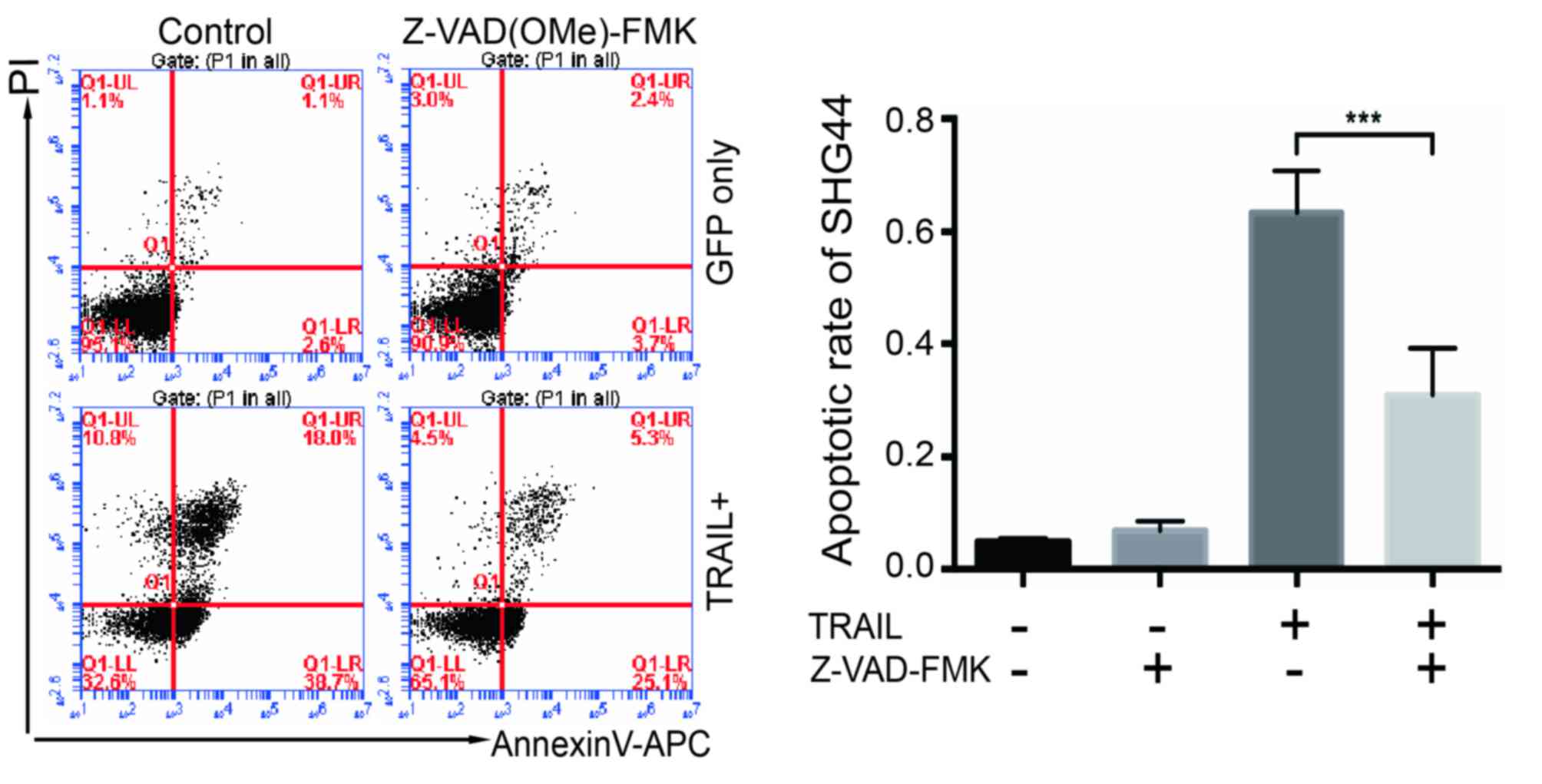
Apoptosis in the co-culture system
could be reversed by a caspase inhibitor
The caspase inhibitor Z-VAD(OMe)-FMK was used for 5
days of co-culture, and the apoptosis of SHG44 cells was detected
to verify the involvement of TRAIL-expressing EPCs further.
Fig. 6 shows that the apoptotic rate
was significantly reversed when Z-VAD(OMe)-FMK was added at the
concentration of 20 µmol/l (Fig.
6).
Discussion
Gliomas, the most common malignant tumors in CNS,
exhibit highly aggressive behavior. The therapeutic effect on
glioma is still not improved drastically despite various new
therapies reported for this tumor. In this study, the extracted
EPCs from the blood of neonatal Sprague-Dawley rats were identified
by detecting specific surface markers CD31 and CD34 as well as the
capability of vasculogenesis. TRAIL was found to increase the
apoptosis of SHG44 cells significantly. This trend could be
reversed by caspase-8 inhibitor. The mechanism underlying this
phenomenon might be related to the increased pro-apoptotic protein
levels of cleaved caspase-3, caspase-8, and PARP.
EPCs can self-proliferate and differentiate into the
endothelial lineage and express CD31, CD34, CD133, and VEGFR-2 on
their surface (28,29). They have the capability of
vasculogenesis and are involved in tumor vessel neogenesis. Many
studies investigated the relationship between EPCs and tumors
(22). Most previous studies focused
on anti-angiogenic treatment by suppressing the mobilization and
homing of EPCs to tumors (20). Only
a few studies used EPCs as a vector for treatment (21). EPCs harboring specific matrix
metallopeptidase-12 (MMP-12) could inhibit melanoma growth
(30). On the contrary, EPCs
releasing CD40 ligand could induce the increase in cleaved
caspase-3 and −7 in metastatic breast cancer (31). These findings proved that EPCs could
be used for tumor treatment. Growing evidence indicated that EPCs
could move across the BBB and home to brain tumors (23). EPCs can serve as a medium for
anti-glioma therapy. The Transwell assay indicated that EPCs had
the capability of homing to glioma cells, as previously reported.
The present study also proved that lentiviral infection had little
influence on the migration ability of EPCs. Therefore, it was
feasible to use the TRAIL-expressing EPCs as a therapeutic vector
after lentiviral infection.
TRAIL induces apoptosis through binding to DR4 or
DR5 on the surface of cells, recruiting and activating caspase-8
via the death domains of DRs and adaptor protein Fas-associated
death domain (FADD) (9,32). Activated caspase-8 cleaves
pro-caspase-3, inducing cell apoptosis (33,34). The
low bioavailability of TRAIL limits its application despite its
immense potential for anti-tumor therapy. Attempts were made to
address this limitation through enhancing the stability of TRAIL
(35–38) and concentrating TRAIL in the tumor
site (39–42). In this study, EPCs were used as a
vector for TRAIL, continuously producing TRAIL besides tumor cells.
The findings indicated that TRAIL delivered by EPCs could bind to
DR4 or DR5 on the surface of glioma cells. Correspondingly, the
activated caspase-8 and −3, and cleaved PARP increased obviously.
In other words, TRAIL-expressing EPCs could promote the apoptosis
of SHG44 cells through activating the death receptor pathway.
In conclusion, the present study demonstrated that
TRAIL-overexpressing EPCs could migrate toward SHG44 cells and
induce apoptosis by activating the death receptor pathway. The
findings suggested that TRAIL and EPCs could be combined for glioma
therapy.
Acknowledgements
The present study is supported by the National
Natural Science Foundation of China (grant no. 81100928) and the
Henan Medical Science Research Project (grant no. 2011020010). The
present study was supported by grants from the National Natural
Science Fund of China (no. 342600531547), the National Natural
Science Foundation of China (nos. 81270270 and 81470524, for WZ),
PhD Educational Award from the Ministry of Education (no.
20134101110013, for WZ), and the national Key R&D Program for
‘Key Projects’ from the Ministry of Science and Technology (no.
2016YFA0501800, for WZ).
References
|
1
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 4
17 Suppl:iv1–iv62. 2015. View Article : Google Scholar
|
|
2
|
Wang X, Chen JX, Zhou Q, Liu YH, Mao Q,
You C, Chen N, Xiong L, Duan J and Liu L: Statistical report of
central nervous system tumors histologically diagnosed in the
Sichuan province of China from 2008 to 2013: A West China Glioma
Center report. Ann Surg Oncol. 23 Suppl 5:S946–S953. 2016.
View Article : Google Scholar
|
|
3
|
Lim B, Allen JE, Prabhu VV, Talekar MK,
Finnberg NK and El-Deiry WS: Targeting TRAIL in the treatment of
cancer: New developments. Expert Opin Ther Targets. 19:1171–1185.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kichev A, Rousset CI, Baburamani AA,
Levison SW, Wood TL, Gressens P, Thornton C and Hagberg H: Tumor
necrosis factor-related apoptosis-inducing ligand (TRAIL) signaling
and cell death in the immature central nervous system after
hypoxia-ischemia and inflammation. J Biol Chem. 289:9430–9439.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bo Y, Guo G and Yao W: MiRNA-mediated
tumor specific delivery of TRAIL reduced glioma growth. J
Neurooncol. 112:27–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Förster A, Falcone FH, Gibbs BF, Preussner
LM, Fiebig BS, Altunok H, Seeger JM, Cerny-Reiterer S, Rabenhorst
A, Papenfuss K, et al: Anti-Fas/CD95 and tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) differentially
regulate apoptosis in normal and neoplastic human basophils. Leuk
Lymphoma. 54:835–842. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo L, Fan L, Pang Z, Ren J, Ren Y, Li J,
Chen J, Wen Z and Jiang X: TRAIL and doxorubicin combination
enhances anti-glioblastoma effect based on passive tumor targeting
of liposomes. J Control Release. 154:93–102. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gao JQ, Lv Q, Li LM, Tang XJ, Li FZ, Hu YL
and Han M: Glioma targeting and blood-brain barrier penetration by
dual-targeting doxorubincin liposomes. Biomaterials. 34:5628–5639.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Holoch PA and Griffith TS: TNF-related
apoptosis-inducing ligand (TRAIL): A new path to anti-cancer
therapies. Eur J Pharmacol. 625:63–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelley SK and Ashkenazi A: Targeting death
receptors in cancer with Apo2L/TRAIL. Curr Opin Pharmacol.
4:333–339. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bagci-Onder T, Agarwal A, Flusberg D,
Wanningen S, Sorger P and Shah K: Real-time imaging of the dynamics
of death receptors and therapeutics that overcome TRAIL resistance
in tumors. Oncogene. 32:2818–2827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Perlstein B, Finniss SA, Miller C,
Okhrimenko H, Kazimirsky G, Cazacu S, Lee HK, Lemke N, Brodie S,
Umansky F, et al: TRAIL conjugated to nanoparticles exhibits
increased anti-tumor activities in glioma cells and glioma stem
cells in vitro and in vivo. Neuro Oncol. 15:29–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Redjal N, Zhu Y and Shah K: Combination of
systemic chemotherapy with local stem cell delivered S-TRAIL in
resected brain tumors. Stem Cells. 33:101–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Balyasnikova IV, Prasol MS, Ferguson SD,
Han Y, Ahmed AU, Gutova M, Tobias AL, Mustafi D, Rincón E, Zhang L,
et al: Intranasal delivery of mesenchymal stem cells significantly
extends survival of irradiated mice with experimental brain tumors.
Mol Ther. 22:140–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi SA, Yun JW, Joo KM, Lee JY, Kwak PA,
Lee YE, You JR, Kwon E, Kim WH, Wang KC, et al: Preclinical
biosafety evaluation of genetically modified human adipose
tissue-derived mesenchymal stem cells for clinical applications to
brainstem glioma. Stem Cells Dev. 25:897–908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asahara T and Kawamoto A: Endothelial
progenitor cells for postnatal vasculogenesis. Am J Physiol Cell
Physiol. 287:C572–C579. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Asahara T, Murohara T, Sullivan A, Silver
M, van der Zee R, Li T, Witzenbichler B, Schatteman G and Isner JM:
Isolation of putative progenitor endothelial cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang J, Chen X, Wang S, Xie T, Du X, Liu
H, Wang S, Li X, Chen J, Zhang B, et al: The expression of
P2X7 receptors in EPCs and their potential role in the
targeting of EPCs to brain gliomas. Cancer Biol Ther. 16:498–510.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varma NR, Shankar A, Iskander A, Janic B,
Borin TF, Ali MM and Arbab AS: Differential biodistribution of
intravenously administered endothelial progenitor and cytotoxic
T-cells in rat bearing orthotopic human glioma. BMC Med Imaging.
13:172013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flamini V, Jiang WG, Lane J and Cui YX:
Significance and therapeutic implications of endothelial progenitor
cells in angiogenic-mediated tumour metastasis. Crit Rev Oncol
Hematol. 100:177–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marçola M and Rodrigues CE: Endothelial
progenitor cells in tumor angiogenesis: Another brick in the wall.
Stem Cells Int. 2015:8326492015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
de la Puente P, Muz B, Azab F and Azab AK:
Cell trafficking of endothelial progenitor cells in tumor
progression. Clin Cancer Res. 19:3360–3368. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang L, Chen L, Wang Q, Wang L, Wang H,
Shen Y, Li X, Fu Y, Shen Y and Yu Y: Circulating endothelial
progenitor cells are involved in VEGFR-2-related endothelial
differentiation in glioma. Oncol Rep. 32:2007–2014. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang SH, Xiang P, Wang HY, Lu YY, Luo YL
and Jiang H: The characteristics of bone marrow-derived endothelial
progenitor cells and their effect on glioma. Cancer Cell Int.
12:322012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kang SD, Carlon TA, Jantzen AE, Lin FH,
Ley MM, Allen JD, Stabler TV, Haley NR, Truskey GA and Achneck HE:
Isolation of functional human endothelial cells from small volumes
of umbilical cord blood. Ann Biomed Eng. 41:2181–2192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao W, Waggoner J, Zhang ZG, Lam CK, Han
P, Qian J, Schroder PM, Mitton B, Kontrogianni-Konstantopoulos A,
Robia SL and Kranias EG: The anti-apoptotic protein HAX-1 is a
regulator of cardiac function. Proc Natl Acad Sci USA. 106:pp.
20776–20781. 2009; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang G, Tao L, Shen S and Chen L: Hypoxia
induced CCL28 promotes angiogenesis in lung adenocarcinoma by
targeting CCR3 on endothelial cells. Sci Rep. 6:271522016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peichev M, Naiyer AJ, Pereira D, Zhu Z,
Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA and Rafii
S: Expression of VEGFR-2 and AC133 by circulating human CD34(+)
cells identifies a population of functional endothelial precursors.
Blood. 95:952–958. 2000.PubMed/NCBI
|
|
29
|
Fadini GP, Losordo D and Dimmeler S:
Critical reevaluation of endothelial progenitor cell phenotypes for
therapeutic and diagnostic use. Circ Res. 110:624–637. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Laurenzana A, Biagioni A, D'Alessio S,
Bianchini F, Chillà A, Margheri F, Luciani C, Mazzanti B,
Pimpinelli N, Torre E, et al: Melanoma cell therapy: Endothelial
progenitor cells as shuttle of the MMP12 uPAR-degrading enzyme.
Oncotarget. 5:3711–3727. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Purwanti YI, Chen C, Lam DH, Wu C, Zeng J,
Fan W and Wang S: Antitumor effects of CD40 ligand-expressing
endothelial progenitor cells derived from human induced pluripotent
stem cells in a metastatic breast cancer model. Stem Cells Transl
Med. 3:923–935. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Miguel D, Lemke J, Anel A, Walczak H
and Martinez-Lostao L: Onto better TRAILs for cancer treatment.
Cell Death Differ. 23:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozören N and El-Deiry WS: Defining
characteristics of Types I and II apoptotic cells in response to
TRAIL. Neoplasia. 4:551–557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rudner J, Jendrossek V, Lauber K, Daniel
PT, Wesselborg S and Belka C: Type I and type II reactions in
TRAIL-induced apoptosis-results from dose-response studies.
Oncogene. 24:130–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rozanov DV, Savinov AY, Golubkov VS,
Rozanova OL, Postnova TI, Sergienko EA, Vasile S, Aleshin AE, Rega
MF, Pellecchia M and Strongin AY: Engineering a leucine
zipper-TRAIL homotrimer with improved cytotoxicity in tumor cells.
Mol Cancer Ther. 8:1515–1525. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berg D, Lehne M, Müller N, Siegmund D,
Münkel S, Sebald W, Pfizenmaier K and Wajant H: Enforced covalent
trimerization increases the activity of the TNF ligand family
members TRAIL and CD95L. Cell Death Differ. 14:2021–2034. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schneider B, Münkel S,
Krippner-Heidenreich A, Grunwald I, Wels WS, Wajant H, Pfizenmaier
K and Gerspach J: Potent antitumoral activity of TRAIL through
generation of tumor-targeted single-chain fusion proteins. Cell
Death Dis. 1:e682010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siegemund M, Pollak N, Seifert O, Wahl K,
Hanak K, Vogel A, Nussler AK, Göttsch D, Münkel S, Bantel H, et al:
Superior antitumoral activity of dimerized targeted single-chain
TRAIL fusion proteins under retention of tumor selectivity. Cell
Death Dis. 3:e2952012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mitchell MJ, Wayne E, Rana K, Schaffer CB
and King MR: TRAIL-coated leukocytes that kill cancer cells in the
circulation. Proc Natl Acad Sci USA. 111:pp. 930–935. 2014;
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Loi M, Becherini P, Emionite L, Giacomini
A, Cossu I, Destefanis E, Brignole C, Di Paolo D, Piaggio F, Perri
P, et al: sTRAIL coupled to liposomes improves its pharmacokinetic
profile and overcomes neuroblastoma tumour resistance in
combination with Bortezomib. J Control Release. 192:157–166. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yan C, Li S, Li Z, Peng H, Yuan X, Jiang
L, Zhang Y, Fan D, Hu X, Yang M and Xiong D: Human umbilical cord
mesenchymal stem cells as vehicles of CD20-specific TRAIL fusion
protein delivery: A double-target therapy against non-Hodgkin's
lymphoma. Mol Pharm. 10:142–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Seifert O, Pollak N, Nusser A, Steiniger
F, Rüger R, Pfizenmaier K and Kontermann RE: Immuno-LipoTRAIL:
Targeted delivery of TRAIL-functionalized liposomal nanoparticles.
Bioconjug Chem. 25:879–887. 2014. View Article : Google Scholar : PubMed/NCBI
|