Introduction
Due to the high mortality and recurrence, bladder
cancer (BC) remains one of the most frequently appearing cancer of
the urinary system (1). In America,
~79,030 new patients with BC will be diagnosed in 2017, including
60,490 males and 18,540 females (2).
Although there has been huge progress in therapeutic methods, such
as cystectomy and adjuvant treatments, BC occupies one of the most
common cancers with a high mortality and recurrence rate worldwide
(1). Among the patients with
recurrent BC, more than one-tenth continue to develop into higher
phases, such as muscle invasion and metastasis (3–5). The
discovery of cancer at an advanced stage could lead to a poor
prognosis. It is a prioritized event to explore the exact mechanism
of BC to improve the sensitivity and specificity of early
diagnosis. In recent years, evidence accumulation in the knowledge
of molecular biology and application of bioinformatics has provided
good opportunities to understand BC comprehensively via
bioinformatic analysis, such as the application of microRNAs
(miRNAs) (6–8).
miRNAs, a type of short non-coding RNA ~22
nucleotides in length, facilitate mRNA degradation by
sequence-specific combination with mRNA, providing new strategies
on diagnosis and treatment of cancers (9–12). miRNAs
have been found to participate in the expression regulation of hub
genes related to the development and progression of tumors,
including BC (13–16). The abnormally expressed and
genetically altered miRNAs have been reported to be involved in the
biological process of BC, including miR-199a-5p, miR-1-3p, miR-9,
miR-182 and miR-200b (17–19).
miR-183-5p, located on chromosome 7q32.2, has been
studied in various types of cancers, such as human breast cancer
(20), esophageal squamous cell
carcinoma (21), esophageal squamous
cell carcinoma (22), gastric cancer
(23), human pancreatic
adenocarcinoma (24) and melanoma
(25). The expression of miR-183 in
BC patients was higher in tissues (26–28) and
serum (29) and showed a moderate
value of the BC diagnosis (27).
However, no functional study of miR-183-5p in BC has been reported
previously. Most of the published studies on miR-183-5p tended to
focus on the higher expression of miR-183-5p in samples from
patients with BC, including tissues, urine and serum, than in
samples from normal individuals, or the diagnostic efficiency of
miR-183-5p. However, the clinical significance, as well as the
promising target genes of miR-183-5p in BC, needs to be explored
(26–29). In addition, the studies mentioned
above were based on a small scale of BC patient cohorts, and big
data analysis was needed to uncover the real role of miR-183-5p in
BC.
Therefore, in the present study, we first attempted
to investigate the clinical significance of the expression level
and genetic alteration of miR-183-5p in BC based on the data from
The Cancer Genome Atlas (TCGA; https://cancergenome.nih.gov/), cBioPortal for Cancer
Genomics (http://www.cbioportal.org/), Gene
Expression Omnibus (GEO, https://www.ncbi.nlm.nih.gov/geo/), and YM500v3
(http://driverdb.tms.cmu.edu.tw/ym500v3/). In addition,
we identified potential target genes of miR-183-5p via differential
expressed genes calculated by RNA-seq data from TCGA, predicting
platforms and gene profiling post miR-183-5p overexpression in
vitro. Further bioinformatic analyses, including the enrichment
of functional annotation and biological pathway analyses, were
performed to explore the possible roles of miR-183-5p in the
tumorigenesis and progression of BC.
Materials and methods
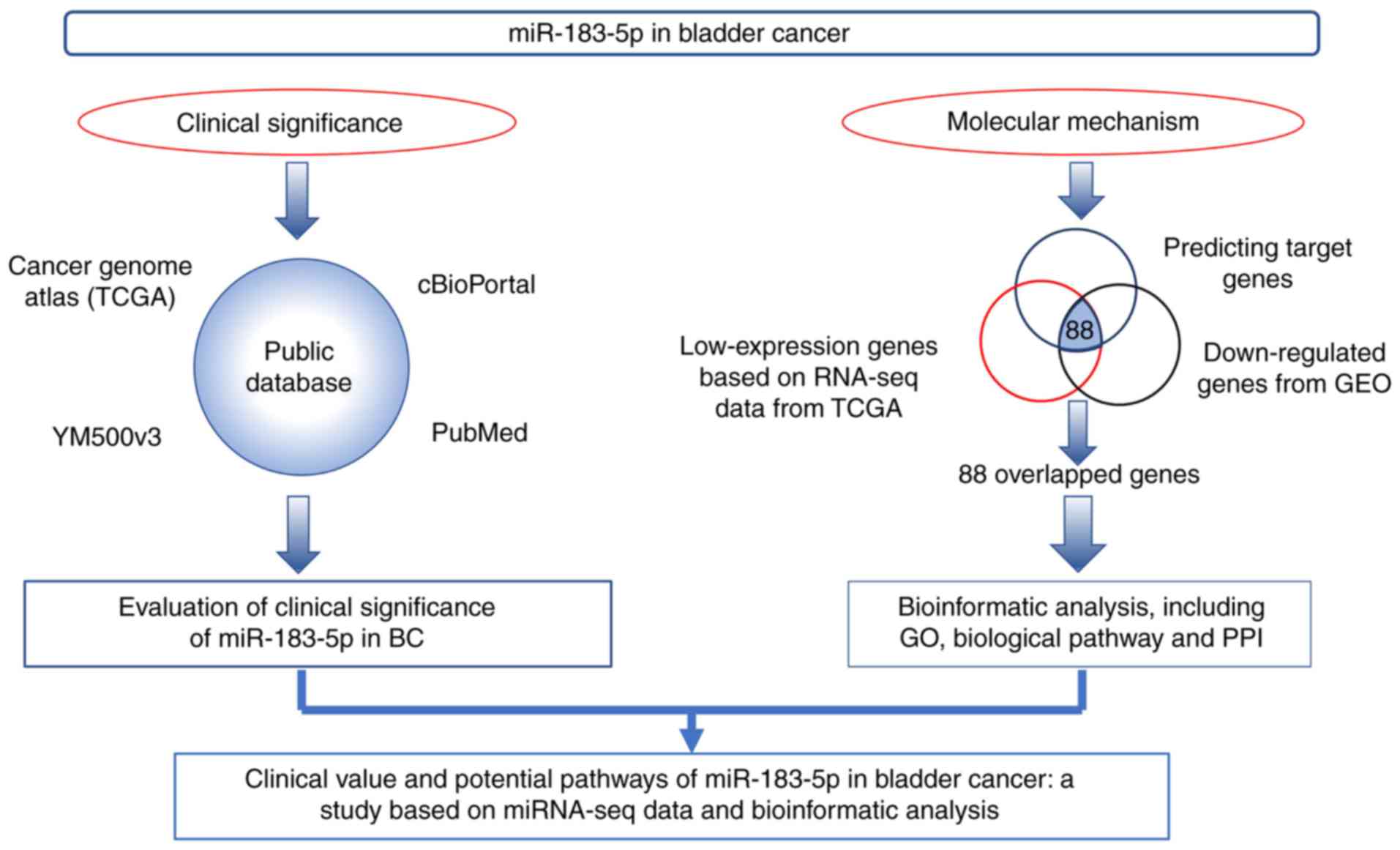
The work flow of the present study is shown in
Fig. 1. Firstly, we evaluated the
clinical significance of miR-183-5p in BC based on data from TCGA,
cBioPortal, YM500v3 and PubMed. Secondly, the potential molecular
mechanism of miR-183-5p in BC was explored via bioinformatic
analysis with potential target genes overlapped with predicting
target genes, low-expression genes based on RNA-seq data from TCGA
and downregulated genes from Gene Expression Omnibus (GEO).
Data acquisition and analysis
The miRNA sequencing data of miR-183 and clinical
information of patients with BC were obtained from TCGA and
contained 412 cases and 19 para-carcinoma tissues of counterparts
as controls (30,31). Only 409 cases possessed the miRNA
sequencing data and clinical information. The clinical information
and follow-up data of these cases, including sex, body mass index
(BMI), primary therapy outcome, pack number of cigarettes smoked
per year, primary therapy outcome, diagnosis subtype,
lymphovascular invasion, pathologic T stage, pathologic N stage,
pathologic M stage, pathologic stage and new tumor events after
initial treatment were also downloaded to analyze the correlation
between miR-183 expression and clinical parameters (Table I). Receiver operator characteristic
curve (ROC) and Kaplan-Meier (K-M) analyses were used to assess the
diagnostic and prognostic roles of miR-183.
 | Table I.Correlation between miR-183
expression and clinical parameters. |
Table I.
Correlation between miR-183
expression and clinical parameters.
|
| miR-183
expression |
|---|
|
|
|
|---|
| Clinical
parameters | n | Mean ± SD | t | P-value |
|---|
| Tissue |
|
| −7.489 | <0.001 |
|
Adjacent | 19 |
8.975±2.409 |
|
|
|
Tumor | 403 | 13.155±1.562 |
|
|
| Sex |
|
| −0.151 | 0.880 |
|
Male | 298 | 13.148±1.561 |
|
|
|
Female | 105 | 13.175±1.572 |
|
|
| BMI |
|
| 0.591 | 0.555 |
|
≤25 | 146 | 13.188±1.381 |
|
|
|
>25 | 209 | 13.088±1.683 |
|
|
| Primary therapy
outcome |
|
| 0.899 | 0.370 |
|
CR+PR+SD | 187 | 13.175±1.533 |
|
|
| PD | 42 | 12.938±1.613 |
|
|
| Pack number of
cigarettes smoked per year |
|
| 1.513 | 0.132 |
|
<39.003 | 132 | 13.225±1.486 |
|
|
|
≥39.003 | 88 | 12.886±1.824 |
|
|
| Primary therapy
outcome |
|
| 0.899 | 0.370 |
|
CRR+PRR+SD | 187 | 13.175±1.533 |
|
|
| PD | 42 | 12.938±1.613 |
|
|
| Diagnosis
subtype |
|
| −3.353 | 0.001 |
|
Non-papillary | 270 | 12.993±1.700 |
|
|
|
Papillary | 129 | 13.486±1.186 |
|
|
| Lymphovascular
invasion |
|
| −1.051 | 0.294 |
| No | 130 | 13.054±1.668 |
|
|
|
Yes | 147 | 13.253±1.488 |
|
|
| Pathologic T
stage |
|
| 2.106 | 0.036 |
|
T0-T2 | 123 | 13.333±1.502 |
|
|
|
T3-T4 | 247 | 12.966±1.614 |
|
|
| Pathologic N
stage |
|
| 1.369 | 0.172 |
|
N0-N1 | 278 | 13.208±1.553 |
|
|
|
N2-N3 | 84 | 12.948±1.430 |
|
|
| Pathologic M
stage |
|
| −0.925 | 0.356 |
| M0 | 193 | 13.515±1.317 |
|
|
| M1 | 10 | 13.921±1.975 |
|
|
| Pathologic
stage |
|
| 2.533 | 0.012 |
| Stage
I–II | 132 | 13.420±1.445 |
|
|
| Stage
III–IV | 269 | 13.017±1.604 |
|
|
| New tumor event
after initial treatment |
|
| 0.864 | 0.388 |
| No | 224 | 13.168±1.488 |
|
|
|
Yes | 69 | 12.987±1.619 |
|
|
Furthermore, the expression data and prognostic
analysis of miR-183-5p were provided from database of YM500v3
(http://driverdb.tms.cmu.edu.tw/ym500v3/). The genetic
alteration of miR-183 was validated from the cBioPortal database
(http://www.cbioportal.org/).
Meta-analysis of miR-183 expression
based on the data from TCGA, GEO and the literature
A resourceful GEO database could provide strong
support in mining the expression data of miRNAs in human cancers.
Therefore, we also searched the GEO database to mine the miR-183-5p
expression level in BC. The following terms were used for
searching: (bladder OR urothelial OR urinary OR urogenital) AND
(cancer OR carcinoma OR tumor OR neoplasm* OR malignant*). The
miR-183-5p expression data were extracted from BC and relevant
controls.
A comprehensive search in several main literary
databases worldwide, including PubMed, Chinese VIP, CNKI, WanFang
database, SinoMed, Embase, Web of science, Science Direct and Wiley
Online Library, was performed for the validation of the miR-183
expression level, up to July 1, 2017. The following terms were used
for searching: (bladder OR urothelial OR urinary OR urogenital) AND
(cancer OR carcinoma OR tumor OR neoplasm* OR malignant*) AND
(MicroRNA183 OR miRNA183 OR miR183 OR miR-183 OR miRNA-183 OR
microRNA-183 OR ‘microRNA183’ OR ‘miRNA1’ OR ‘miR183’ OR miR-183-5p
OR miRNA-183-5p OR microRNA-183-5p).
Prediction of the prospective target
genes of miR-183-5p
The prediction of miR-183-5p target genes was
conducted with different bioinformatics tools, including miRWalk,
MicroT4, miRanda, miRBridge, miRDB, miRMap, miRNAMap, PICTAR2,
PITA, RNA22, RNAhybrid, TargetScan. Only 7,421 genes appearing for
over 4 times among 12 platforms were regarded as potential target
genes of miR-183-5p.
Potentially related genes of
miR-183-5p in BC cells as detected by microarray
According to the information provided by GSE24782
microarray, a few human cancer cell lines, including BOY, T24,
A498, PC3, DU145, FaDu, SAS, HSC3 and IMC3, were transfected with
different miRNAs (miR-183-5p, miR-218, miR-145, miR-1 and miR-874).
Based on the functional mechanism of miRNA, low-expression genes
with a fold change (FC) <0.85 after transfected with miR-183-5p
in BOY and T24 cells were regarded as potential target genes of
miR-183-5p. A total of 3,163 genes were selected for further
analysis.
Selection of low-expression genes
based on RNA-seq dataset from TCGA
The RNA-seq dataset from TCGA contained 60,483
mRNAs, which were then used for the analysis of the differential
expression with the R package of edgeR (32,33).
Differentially expressed genes with the expression data missing in
>10% samples were excluded. Due to the high expression of
miR-183 in tumor tissues, low-expression genes were selected as the
candidates of target genes of miR-183-5p. Therefore, 2,918
low-expression genes with log2 FC<-1 were included.
Protein-protein interaction (PPI)
analysis, Gene ontology (GO) and biological pathway
PPI was conducted to identify the hub genes by
STRING: Functional protein association networks (https://string-db.org/). Furthermore, to study the
prospective biological effects of miR-183-5p in BC, the potential
target genes of miR-183-5p were sent for GO and biological pathway
analyses, which was performed via FunRich: Functional Enrichment
analysis tool (34,35) (http://www.funrich.org/).
Moreover, to validate the reliability of the
potential target genes of miR-183-5p, correlation analysis between
the mRNA expression data of the prospective target genes of
miR-183-5p enriched in the first pathway of the biological pathway
and miR-183-5p expression data were performed. The comparisons of
potential target genes between BC and non-cancerous tissues were
also carried out.
Statistical analysis
The miR-183-5p expression data are displayed as the
means ± standard deviation (SD). Student's t-test for independent
samples was performed to evaluate the relationship between miR-183
and clinical parameters. Pearson correlation analysis and Student's
t-test were performed for the verification of genes significantly
enriched in biological pathways. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical significance of miR-183
Due to the lack of expression data of mature
miR-183-5p in the dataset of TCGA, the comparison of the stem-loop
mir-183 expression level between BC and adjacent non-cancerous
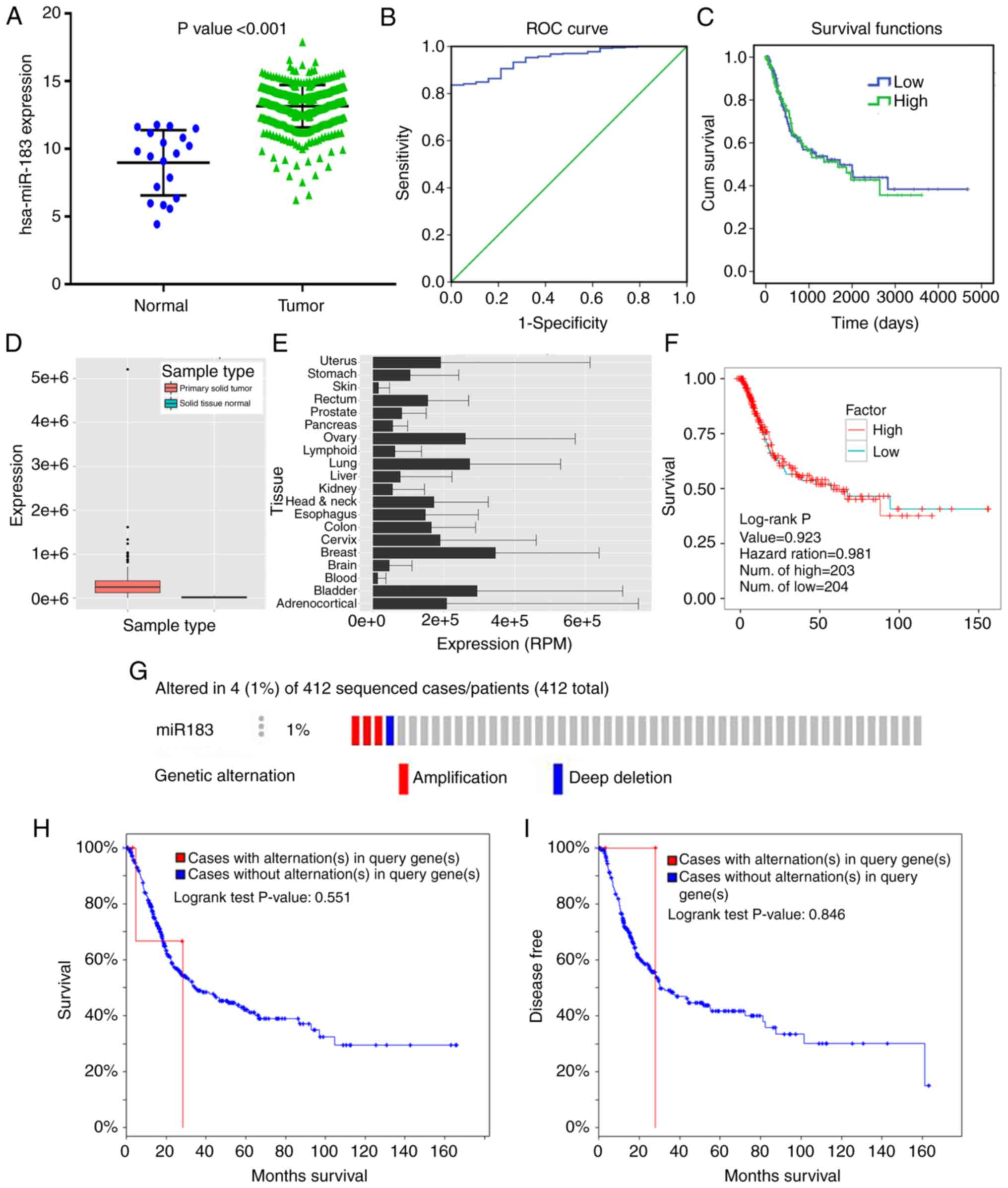
tissues was provided. The miR-183 expression level in BC tissues
was notably higher than that in adjacent tissues (Fig. 2A; Table
I). High expression of miR-183 was notably associated with the
papillary subtype (t=−3.353, P=0.001), low pathologic T stage
(t=2.106, P=0.036), and early pathologic stage (t=2.533, P=0.012).
However, no remarkable differences were found between miR-183
expression and sex, BMI, primary therapy outcome, pack number of
cigarettes smoked per year (mean), primary therapy outcome,
lymphovascular invasion present, pathologic N stage, pathologic M
stage or new tumor event after initial treatment (Table I).
ROC curve analysis showed that the area under the
curve (AUC) was 0.948 (95% CI: 0.919–0.977) with 83.6% sensitivity
and 100% specificity (Fig. 2B),
indicating that a high expression level of miR-183 may be an ideal
marker for BC diagnosis. Furthermore, the K-M curve uncovered that
no significant difference in the survival time was noted between
patients in the low and high miR-183 expression groups (P=0.861)
(Fig. 2C).
The expression of miR-183-5p in primary solid tumors
were much higher than that in solid normal tissues (Fig. 2D), which was consistent with the
result from TCGA (Fig. 2A). The
miR-183-5p expression data in various cancers were provided in
YM500v3 (Fig. 2E). Compared with
other cancers, BC exhibited higher expression levels of miR-183-5p.
This finding again confirmed that there was no significant
prognostic value of miR-183-5p in patients with BC (Fig. 2F).
The data from cBioPortal revealed that miR-183
contained two kinds of genetic alterations, including amplification
and deep deletion, accounting for 1% (4/412) of patients with BC
(Fig. 2G). In addition, no prominent
correlation was found between miR-183 alteration and the outcome of
BC patients, including overall survival or disease-free survival
(Fig. 2H and I).
miR-183-5p expression level from GEO
and literatures
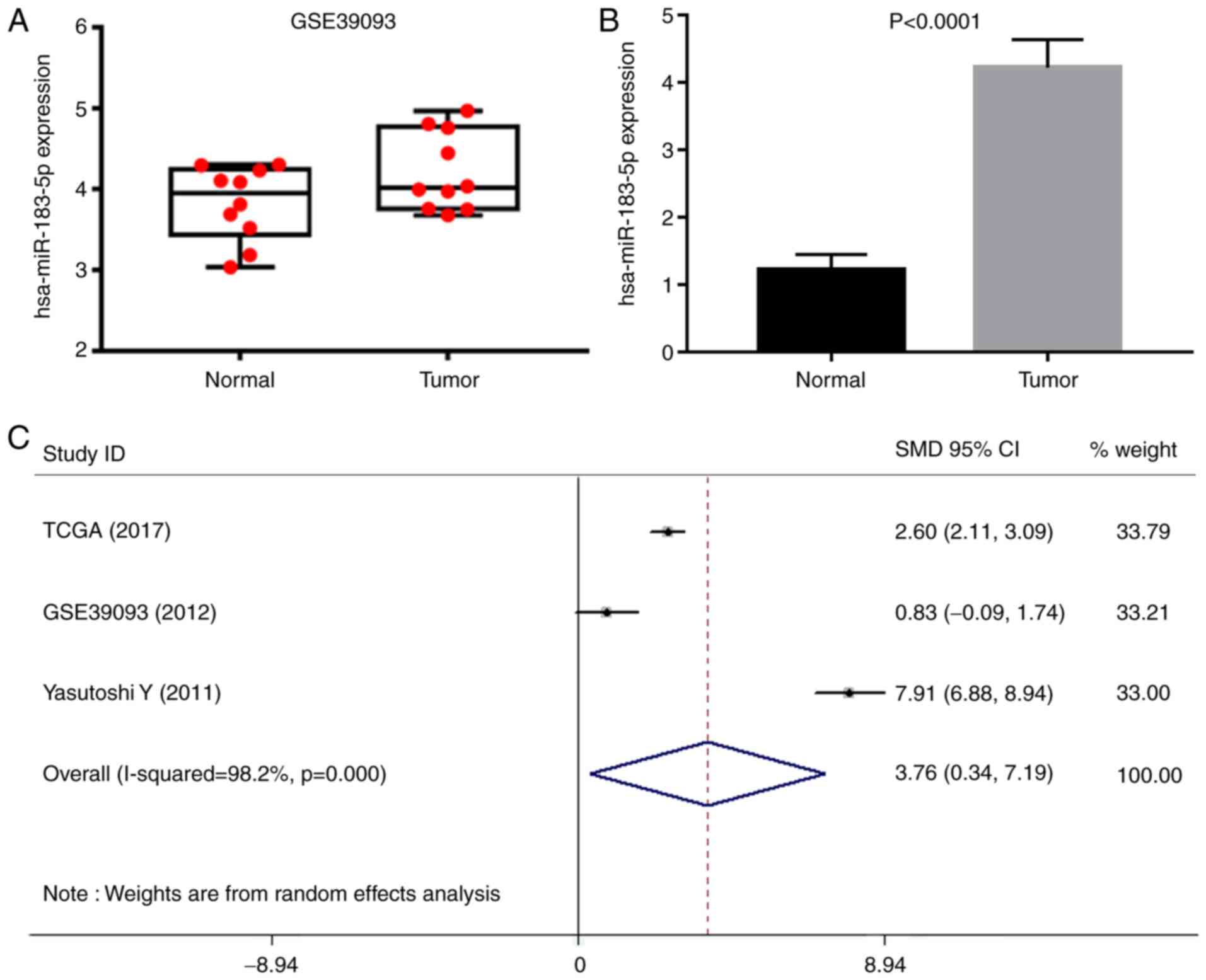
A total of 16 microarrays were obtained from GEO,
including GSE20414, GSE20418, GSE2564, GSE31616, GSE31617,
GSE36121, GSE39067, GSE39093, GSE40355, GSE48008, GSE50894,
GSE59483, GSE81201, GSE83586, GSE84525 and GSE86411. Eventually,
only GSE39093 was eligible, and the data were used for
recalculation. The expression level of miR-183-5p (4.216±0.485) in
10 BC tissue samples was moderately higher than that in the 10
normal counterparts (3.825±0.460, P>0.05) (Fig. 3A). A comprehensive search was
performed in several literature databases; however, only one study
provided normalized expression data. In detail, the expression of
miR-183-5p in 104 urothelial carcinomas (4.225±0.414) was
significantly higher than that in 31 normal bladder epitheliums
(1.224±0.224, P<0.0001) (Fig. 3B)
(27). All of the eligible expression
data of miR-183-5p extracted from TCGA, one GEO microarray and one
literature research provided an overall SMD of 3.76 (0.34–7.19) as
analyzed by STATA software (Fig. 3C),
suggesting that miR-183-5p was more highly expressed in samples
from BC than in those from normal counterparts. The source of
patients with BC and different detection methods may lead to strong
heterogeneity.
Identification of the potential target
genes of miR-183-5p
In the present study, the overlapped genes from 3
subsets of genes, including predicted target genes, related
potential target genes of miR-183-5p from GEO and low-expression
genes from TCGA, were regarded as the potential target genes of
miR-183-5p. Finally, 88 overlapped genes were obtained (Table II).
 | Table II.A total of 88 potential target genes
of miR-183-5p. |
Table II.
A total of 88 potential target genes
of miR-183-5p.
| Ensemble ID | Gene name | Ensemble ID | Gene name | Ensemble ID | Gene name |
|---|
|
ENSG00000163431 | LMOD1 |
ENSG00000138685 | FGF2 |
ENSG00000140450 | ARRDC4 |
|
ENSG00000118496 | FBXO30 |
ENSG00000136842 | TMOD1 |
ENSG00000135269 | TES |
|
ENSG00000102271 | KLHL4 |
ENSG00000184985 | SORCS2 |
ENSG00000163661 | PTX3 |
|
ENSG00000113196 | HAND1 |
ENSG00000169554 | ZEB2 |
ENSG00000108797 | CNTNAP1 |
|
ENSG00000100784 | RPS6KA5 |
ENSG00000144655 | CSRNP1 |
ENSG00000163083 | INHBB |
|
ENSG00000104447 | TRPS1 |
ENSG00000167483 | FAM129C |
ENSG00000105835 | NAMPT |
|
ENSG00000140090 | SLC24A4 |
ENSG00000064309 | CDON |
ENSG00000083067 | TRPM3 |
|
ENSG00000017427 | IGF1 |
ENSG00000159167 | STC1 |
ENSG00000152217 | SETBP1 |
|
ENSG00000183454 | GRIN2A |
ENSG00000152102 | FAM168B |
ENSG00000104313 | EYA1 |
|
ENSG00000143878 | RHOB |
ENSG00000134531 | EMP1 |
ENSG00000151929 | BAG3 |
|
ENSG00000105974 | CAV1 |
ENSG00000166974 | MAPRE2 |
ENSG00000007312 | CD79B |
|
ENSG00000173334 | TRIB1 |
ENSG00000105784 | RUNDC3B |
ENSG00000138944 | KIAA1644 |
|
ENSG00000188385 | JAKMIP3 |
ENSG00000173068 | BNC2 |
ENSG00000182168 | UNC5C |
|
ENSG00000095794 | CREM |
ENSG00000188803 | SHISA6 |
ENSG00000169083 | AR |
|
ENSG00000106829 | TLE4 |
ENSG00000169504 | CLIC4 |
ENSG00000163788 | SNRK |
|
ENSG00000187098 | MITF |
ENSG00000058272 | PPP1R12A |
ENSG00000004799 | PDK4 |
|
ENSG00000113448 | PDE4D |
ENSG00000172399 | MYOZ2 |
ENSG00000145861 | C1QTNF2 |
|
ENSG00000126351 | THRA |
ENSG00000115252 | PDE1A |
ENSG00000151892 | GFRA1 |
|
ENSG00000163328 | GPR155 |
ENSG00000164741 | DLC1 |
ENSG00000169946 | ZFPM2 |
|
ENSG00000178662 | CSRNP3 |
ENSG00000066382 | MPPED2 |
ENSG00000101333 | PLCB4 |
|
ENSG00000198961 | PJA2 |
ENSG00000181773 | GPR3 |
ENSG00000163171 | CDC42EP3 |
|
ENSG00000018408 | WWTR1 |
ENSG00000140416 | TPM1 |
ENSG00000107968 | MAP3K8 |
|
ENSG00000058668 | ATP2B4 |
ENSG00000073910 | FRY |
ENSG00000136267 | DGKB |
|
ENSG00000018625 | ATP1A2 |
ENSG00000107562 | CXCL12 |
ENSG00000126524 | SBDS |
|
ENSG00000136158 | SPRY2 |
ENSG00000112320 | SOBP |
ENSG00000091831 | ESR1 |
|
ENSG00000134201 | GSTM5 |
ENSG00000142627 | EPHA2 |
ENSG00000079308 | TNS1 |
|
ENSG00000118922 | KLF12 |
ENSG00000058866 | DGKG |
ENSG00000131016 | AKAP12 |
|
ENSG00000186354 | C9orf47 |
ENSG00000162616 | DNAJB4 |
ENSG00000131018 | SYNE1 |
|
ENSG00000078687 | TNRC6C |
ENSG00000157368 | IL34 |
ENSG00000146151 | HMGCLL1 |
|
ENSG00000067900 | ROCK1 |
|
|
|
|
Bioinformatic analyses of the target
genes of miR-183-5p
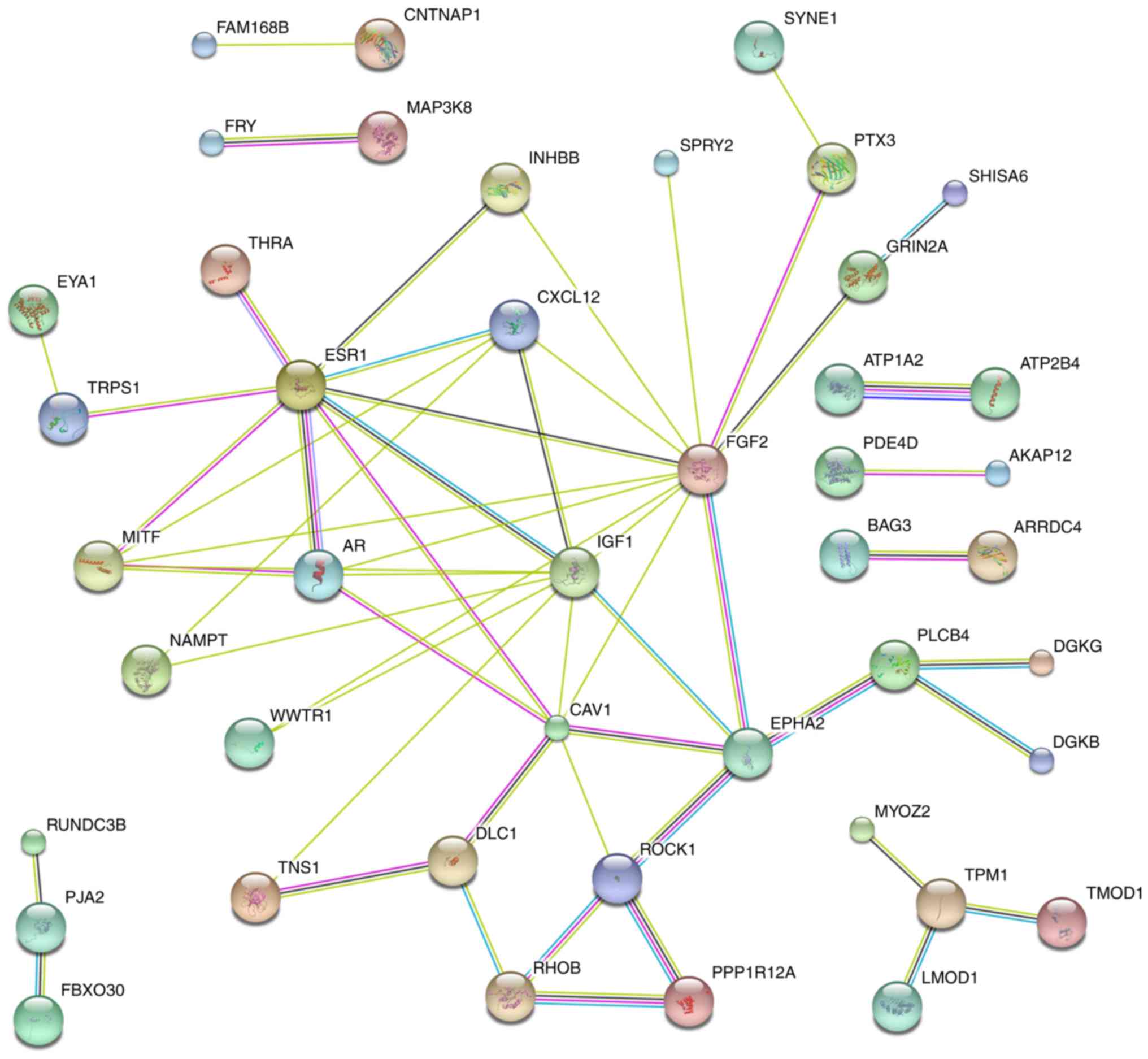
As shown in Fig. 4,
hub genes, which contained >3 connected lines, were identified,
including estrogen receptor 1 (ESR1), melanogenesis-associated
transcription factor (MITF), androgen receptor (AR), C-X-C motif
chemokine ligand 12 (CXCL12), insulin-like growth factor 1 (IGF1),
caveolin 1 (CAV1), DLC1 Rho GTPase activating protein (DLC1),
fibroblast growth factor 2 (FGF2), EPH receptor A2 (EPHA2), Rho
associated coiled-coil containing protein kinase 1 (ROCK1), Ras
homolog family member B (RHOB), phospholipase C beta 4 (PLCB4) and
tropomyosin 1 (TPM1) (single genes without connections are not
shown) (Fig. 4).
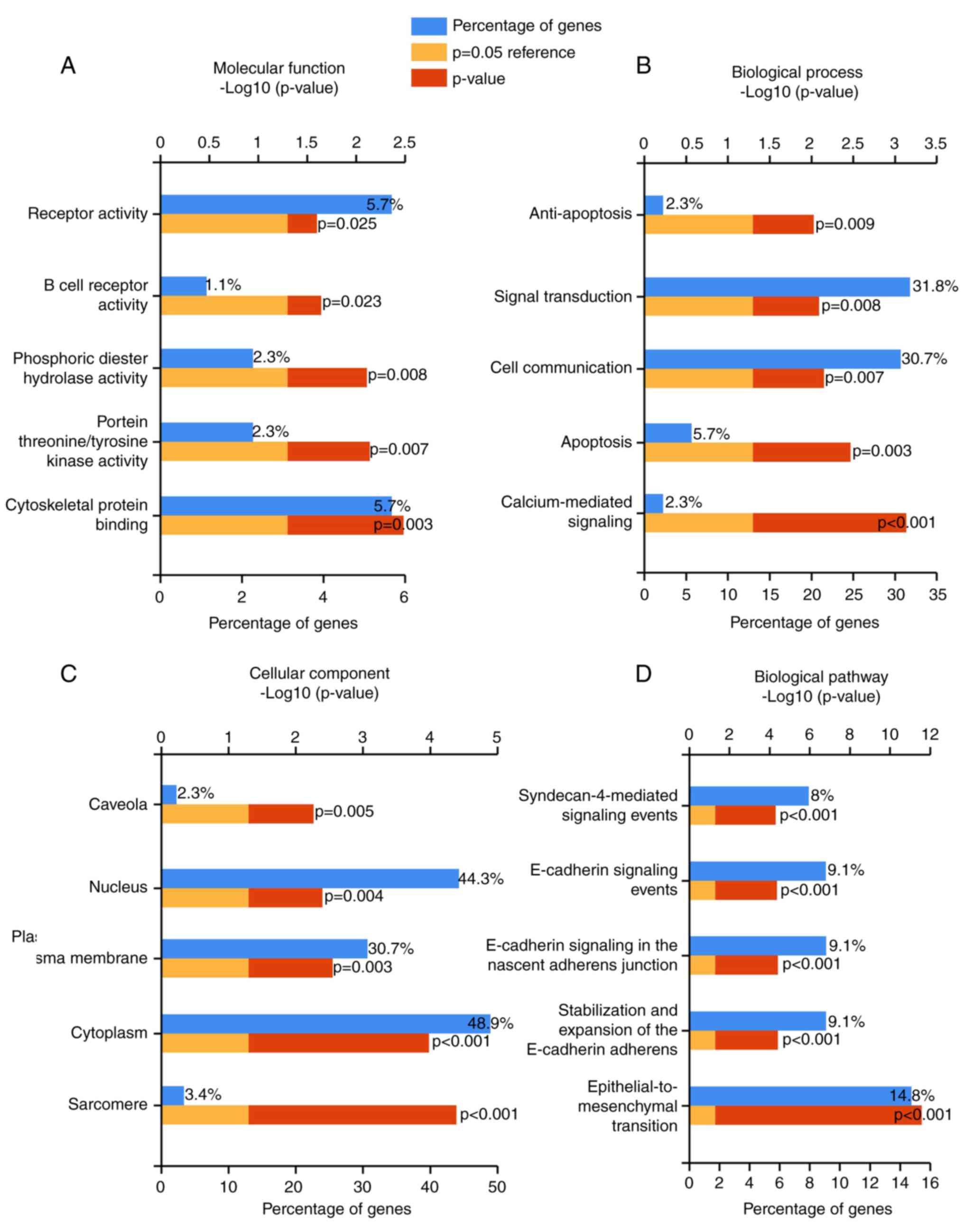
The overlapped genes were also categorized in GO and
biological pathway analyses using the software of FunRich:
Functional Enrichment analysis tool. The top 5 pathways in
molecular functions (MFs), such as cytoskeletal protein binding,
protein threonine/tyrosine kinase activity, were displayed in
Fig. 5A and Table III. In addition, the top 5 pathways
of biological processes (BPs) (Fig.
5B) and cellular component (CC) were also noted (P-value
<0.05) (Fig. 5C; Table III). Via the biological pathway
analysis, we discovered that the epithelial-to-mesenchymal
transition pathway was the most significantly enriched (P-value
<0.00001) (Fig. 5D; Table III).
 | Table III.Pathways of potential target genes of
miR-183-5p in bladder cancer. |
Table III.
Pathways of potential target genes of
miR-183-5p in bladder cancer.
| Pathway | No. of genes in the
dataset | Percentage of
genes | Fold
enrichment | P-value
(Hypergeometric test) | Gene |
|---|
| MF: Cytoskeletal
protein binding | 5 | 5.682 | 5.021 | 0.003 | TPM1; KLHL4; LMOD1;
TMOD1; MAPRE2 |
| MF: Protein
threonine/tyrosine kinase activity | 2 | 2.273 | 15.679 | 0.007 | MAP3K8; TRIB1 |
| MF: Phosphoric
diester hydrolase activity | 2 | 2.273 | 15.139 | 0.008 | PDE1A; PDE4D |
| MF: B cell receptor
activity | 1 | 1.136 | 44.049 | 0.023 | CD79B |
| MF: Receptor
activity | 5 | 5.682 | 3.032 | 0.025 | CDON; GFRA1; UNC5C;
CNTNAP1; SORCS2 |
| BP:
Calcium-mediated signaling | 2 | 2.273 | 48.744 | 0.001 | PLCB4; TRPM3 |
| BP: Apoptosis | 5 | 5.682 | 4.976 | 0.003 | CSRNP1; CSRNP3;
BAG3; SNRK; DLC1 |
| BP: Cell
communication | 27 | 30.682 | 1.589 | 0.007 | STC1; CDC42EP3;
GPR3; PLCB4; SPRY2; EPHA2; MAP3K8; RHOB; AR; CDON; CXCL12; DGKB;
GRIN2A; PDE1A; PDE4D; TNS1; UNC5C; CNTNAP1; GPR155; IGF1; ROCK1;
SORCS2; FGF2; INHBB; TRIB1; MYOZ2; RUNDC3B |
| BP: Signal
transduction | 28 | 31.818 | 1.556 | 0.008 | STC1; CDC42EP3;
GPR3; PLCB4; SPRY2; EPHA2; MAP3K8; RHOB; AR; CDON; CXCL12; DGKB;
GFRA1; GRIN2A; PDE1A; PDE4D; TNS1; UNC5C; CNTNAP1; GPR155; IGF1;
ROCK1; SORCS2; FGF2; INHBB; TRIB1; MYOZ2; RUNDC3B |
| BP:
Anti-apoptosis | 2 | 2.273 | 13.720 | 0.009 | NAMPT; IGF1 |
| CC: Sarcomere | 3 | 3.409 | 43.816 | <0.001 | TPM1; SYNE1;
MYOZ2 |
| CC: Cytoplasm | 43 | 48.864 | 1.653 | <0.001 | STC1; ATP2B4;
CDC42EP3; SPRY2; THRA; AKAP12; ATP1A2; CLIC4; FBXO30; MAP3K8; TLE4;
TPM1; AR; DGKB; DGKG; GFRA1; GRIN2A; KLHL4; LMOD1; MITF; NAMPT;
PDE1A; PDE4D; PPP1R12A; SBDS; SETBP1; TMOD1; BAG3; HAND1; IGF1;
MAPRE2; ROCK1; SYNE1; TRPM3; ESR1; EYA1; TRIB1; WWTR1; MYOZ2;
CD79B; GSTM5; RPS6KA5; DLC1 |
| CC: Plasma
membrane | 27 | 30.682 | 1.696 | 0.003 | ATP2B4; GPR3;
SPRY2; AKAP12; ATP1A2; CLIC4; EPHA2; RHOB; AR; CAV1; CDON; DGKB;
DGKG; GFRA1; GRIN2A; PPP1R12A; TNS1; UNC5C; CNTNAP1; GPR155;
SORCS2; TRPM3; ESR1; FGF2; TES; SLC24A4; CD79B |
| CC: Nucleus | 39 | 44.318 | 1.458 | 0.004 | STC1; ZFPM2;
ATP2B4; PLCB4; THRA; TRPS1; AKAP12; ATP1A2; CLIC4; CSRNP1; CSRNP3;
TLE4; TPM1; ZEB2; AR; BNC2; CREM; GFRA1; MITF; NAMPT; PDE4D;
PPP1R12A; SBDS; SETBP1; TMOD1; CNTNAP1; HAND1; MAPRE2; SYNE1;
TRPM3; ESR1; EYA1; FGF2; TRIB1; KLF12; WWTR1; SNRK; RPS6KA5;
DLC1 |
| CC: Caveola | 2 | 2.273 | 18.292 | 0.005 | CAV1; DLC1 |
| Biological pathway:
Epithelial-to-mesenchymal transition | 13 | 14.773 | 15.365 | <0.001 | ZFPM2; AKAP12;
CLIC4; ZEB2; BNC2; CAV1; CXCL12; SOBP; TNS1; IGF1; SYNE1; PTX3;
WWTR1 |
| Biological pathway:
Stabilization and expansion of the E-cadherin adherens
junction | 8 | 9.091 | 6.364 | <0.001 | SPRY2; EPHA2; TLE4;
AR; GFRA1; MITF; IGF1; ROCK1 |
| Biological pathway:
E-cadherin signaling in the nascent adherens junction | 8 | 9.091 | 6.364 | <0.001 | SPRY2; EPHA2; TLE4;
AR; GFRA1; MITF; IGF1; ROCK1 |
| Biological pathway:
E-cadherin signaling events | 8 | 9.091 | 6.250 | <0.001 | SPRY2; EPHA2; TLE4;
AR; GFRA1; MITF; IGF1; ROCK1 |
| Biological pathway:
Syndecan-4-mediated signaling events | 7 | 7.955 | 7.328 | <0.001 | TLE4; AR; CAV1;
CXCL12; MITF; ROCK1; FGF2 |
Validation of potential target genes enriched in the
epithelial-to-mesenchymal transition pathway by mRNA expression
data from TCGA. The mRNA expression data of potential target genes
enriched in epithelial-to-mesenchymal transition pathway in BC and
non-carcinomatous tissues, including zinc finger protein, FOG
family member 2 (ZFPM2), A-kinase anchoring protein 12 (AKAP12),
chloride intracellular channel 4 (CLIC4), zinc finger E-box binding
homeobox 2 (ZEB2), basonuclin 2 (BNC2), caveolin 1 (CAV1), C-X-C
motif chemokine ligand 12 (CXCL12), sine oculis binding protein
homolog (SOBP), tensin 1 (TNS1), insulin-like growth factor 1
(IGF1), spectrin repeat containing nuclear envelope protein 1
(SYNE1), pentraxin 3 (PTX3), WW domain containing transcription
regulator 1 (WWTR1), were extracted from TCGA. The correlation of
the expression level between miR-183 and potential target genes in
BC tissues was evaluated by Pearson correlation analysis. The
results showed that miR-183 expression in BC tissues were all
notably correlated with the expression levels of prospective target
genes, respectively (P-value <0.001) (Fig. 6). Furthermore, Student' t test was
executed to compare the expression levels of candidate genes
between the BC and non-carcinomatous tissues. Consequently,
candidate gene expression data were all remarkably higher in BC
than in non-carcinomatous tissues (Fig.
6; Table IV).
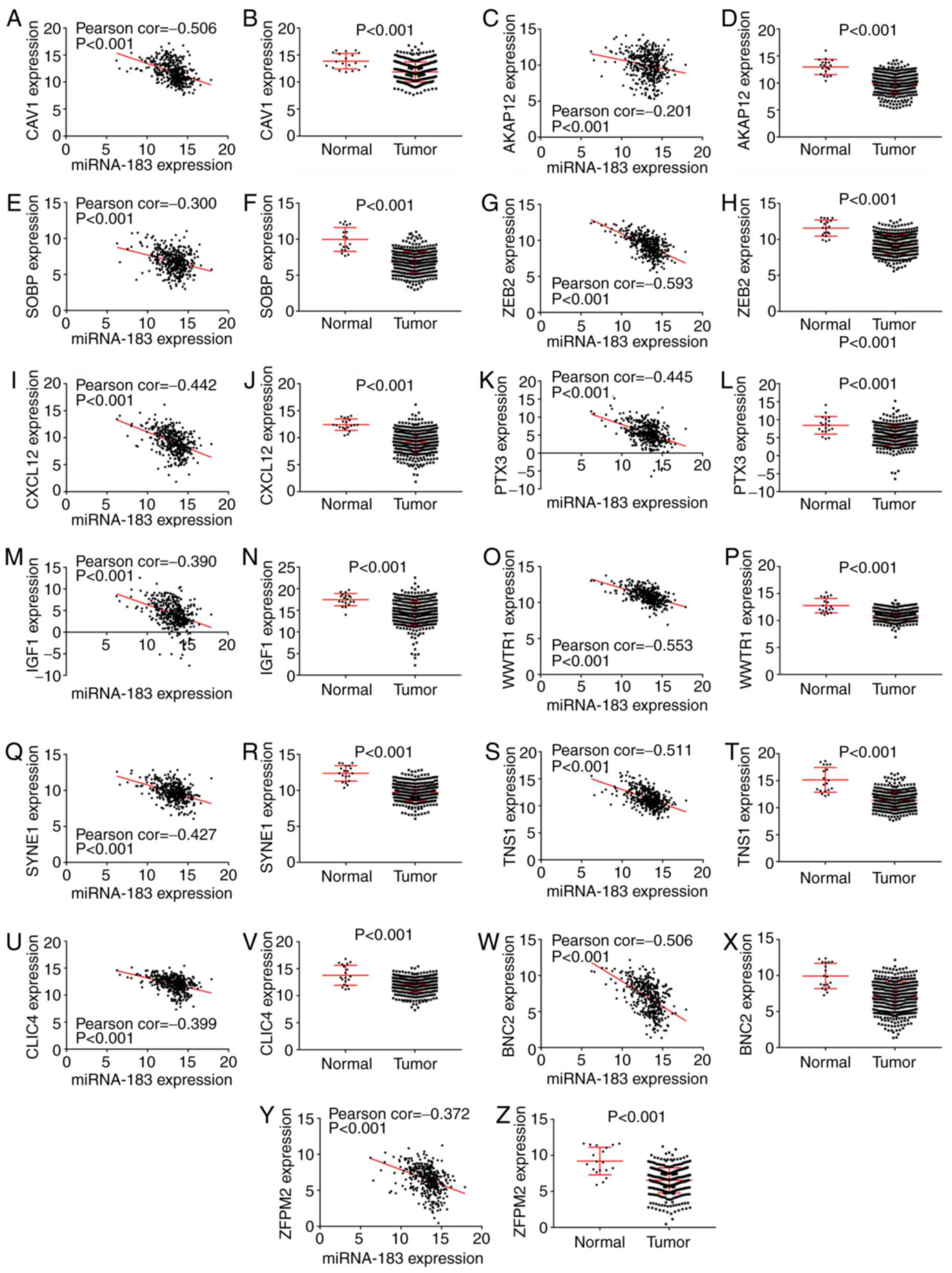 | Figure 6.Validation of the potential target
genes enriched in the epithelial-to-mesenchymal transition pathway
by mRNA expression data from The Cancer Genome Atlas (TCGA),
including (A and B) caveolin 1 (CAV1), (C and D) A-kinase anchoring
protein 12 (AKAP12), (E and F) sine oculis binding protein homolog
(SOBP), (G and H) zinc finger E-box binding homeobox 2 (ZEB2), (I
and J) C-X-C motif chemokine ligand 12 (CXCL12), (K and L)
pentraxin 3 (PTX3), (M and N) insulin-like growth factor 1 (IGF1),
(O and P) WW domain containing transcription regulator 1 (WWTR1),
(Q and R) spectrin repeat containing nuclear envelope protein 1
(SYNE1), (S and T) tensin 1 (TNS1), (U and V) chloride
intracellular channel 4 (CLIC4), (W and X) basonuclin 2 (BNC2) and
(Y and Z) zinc finger protein, FOG family member 2 (ZFPM2). |
 | Table IV.Comparison of the expression levels
of potential target genes between normal bladder tissues and
bladder cancer (BC) tissues. |
Table IV.
Comparison of the expression levels
of potential target genes between normal bladder tissues and
bladder cancer (BC) tissues.
| Gene | Group | N | Mean | Std. Deviation | P-value | Featurea |
|---|
| CAV1 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 13.87475 | 1.427754 |
|
|
|
| Tumor | 399 | 11.86889 | 1.857218 |
|
|
| AKAP12 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 12.96746 | 1.407823 |
|
|
|
| Tumor | 399 | 9.992088 | 1.793414 |
|
|
| SOBP |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 9.964385 | 1.65852 |
|
|
|
| Tumor | 399 | 6.790578 | 1.474642 |
|
|
| ZEB2 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 11.57411 | 1.129419 |
|
|
|
| Tumor | 399 | 9.299417 | 1.35383 |
|
|
| CXCL12 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 12.41901 | 1.059525 |
|
|
|
| Tumor | 399 | 9.179279 | 2.088323 |
|
|
| PTX3 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 8.439694 | 2.440688 |
|
|
|
| Tumor | 399 | 5.515195 | 2.690033 |
|
|
| IGF1 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 7.488964 | 1.422601 |
|
|
|
| Tumor | 399 | 4.200803 | 2.727906 |
|
|
| WWTR1 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 12.77304 | 1.334257 |
|
|
|
| Tumor | 399 | 10.95718 | 0.966398 |
|
|
| SYNE1 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 12.37864 | 1.079004 |
|
|
|
| Tumor | 399 | 9.730599 | 1.186041 |
|
|
| TNS1 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 15.15798 | 2.307397 |
|
|
|
| Tumor | 399 | 11.36795 | 1.597026 |
|
|
| CLIC4 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 13.79678 | 1.851609 |
|
|
|
| Tumor | 399 | 12.06632 | 1.372628 |
|
|
| BNC2 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 9.935324 | 1.7555 |
|
|
|
| Tumor | 399 | 6.986046 | 2.130077 |
|
|
| ZFPM2 |
|
|
|
| <0.001 | Down |
|
| Normal | 19 | 9.218381 | 1.91825 |
|
|
|
| Tumor | 399 | 6.539535 | 1.752474 |
|
|
Discussion
In the present study, the clinical role of
miR-183-5p in BC was first evaluated according to the data from
miRNA sequencing (TCGA, cBioPortal and YM5003v). miR-183-5p in BC
contained two types of genetic alteration, gene amplification and
deep deletion. Furthermore, pathway analyses uncovered that
miR-183-5p could deeply affect multiple pathways and
‘epithelial-to-mesenchymal transition’ was the most remarkable one
as defined by the FunRich: Functional Enrichment analysis tool,
containing several genes ZFPM2, AKAP12, CLIC4, ZEB2, BNC2, CAV1,
CXCL12, SOBP, TNS1, IGF1, SYNE1, PTX3 and WWTR1; among these genes,
CAV1, CXCL12 and IGF1 were also identified as hub genes of
miR-183-5p in BC by STRING: Functional protein association
networks.
It has been fully demonstrated that miRNA expression
levels could become reliable markers for the diagnosis and
prognosis of cancers (36).
miR-183-5p is one of the attractive cancer-relative miRNAs that has
been reported to play important roles in various cancers with
remarkably high or low expression levels compared with normal
tissues. A remarkably high expression of miR-183-5p that could
promote the proliferation and restrain apoptosis in cells through
targeting PDCD4 was reported in human breast cancer tissues
compared with that in the adjacent non-cancerous tissues (20). A similar phenomenon and function of
miR-183-5p was also reported in esophageal squamous cell carcinoma
and glioblastoma multiforme (21–23).
Upregulation of oncogenic miR-183-5p was reported to enhance the
ability of proliferation and metastasis in human pancreatic
adenocarcinoma cells probably via targeting the SOCS-6 gene
(24). A notable lower expression of
miR-183-5p was found in nasopharyngeal carcinoma and cervical
cancer tissues, where miR-183-5p could act as the tumor suppressor
by targeting MTA1 and MMP-9, respectively (37,38).
Reduced expression of miR-183-5p was discovered in pancreatic
ductal adenocarcinoma tissues; and significantly associated with
tumor grade, metastasis and TNM stage. Patients with low expression
of miR-183-5p tended to have notably worse overall survival than
those with high expression of miR-183-5p expression (38). Patients with low-expression of
miR-183-5p tended to have a poor overall survival. miR-183-5p was
involved in cell proliferation by regulating the expression of
Bmi-1 (39). Low-expression
miR-183-5p was found in melanoma tissues and cells and was
correlated with poor overall survival, while high-expression of
miR-183-5p caused remarkable inhibition of cell growth in
vitro and in vivo (25).
Thus, miR-183-5p could play different roles in various cancers.
To date, only several studies were reported
concerning the research of miR-183-5p in BC. It was validated that
miR-183-5p expression in samples from patients with BC, including
tissues (26,40–42), urine
(27,43) and serum (29), were all prominently higher than that
those in normal counterparts. In the present study, we revealed the
consistent results in tissue samples.
Eissa et al performed ROC analysis of
miR-183-5p expression in urine samples. However, only the
sensitivity (71.3%) and specificity (88.9%) were provided (43). Yamada et al showed the result
of ROC analysis (AUC=0.817; 95% CI: 0.752–0.872) with 74.0%
sensitivity and 77.3% specificity, indicating a moderate diagnostic
efficiency of miR-183-5p expression in urine, and they also found a
significant correlation between miR-183-5p expression and clinical
parameters, including tumor grade and pathological stage (P-value
<0.05) (27). In our study, ROC
curve analysis showed a robust diagnostic efficiency of miR-183-5p
in BC tissues (AUC=0.948; 95% CI: 0.919–0.977). The expression of
miR-183-5p was markedly related to the diagnosis subtype,
pathologic T stage and pathologic stage (P-value <0.05).
However, K-M curve analysis showed no prognostic efficiency of
miR-183-5p, based on the data from TCGA, YM500v3 and
cBioPortal.
miR-183-5p was conformed to affect the biological
behavior of cells by targeting different kind of proteins in
various types of cancers, such as regulating cell proliferation by
targeting PDCD4 in breast (44) and
esophageal cancer (21), and
esophageal squamous cell carcinoma (22), inhibiting tumorigenesis by targeting
MTA1 in nasopharyngeal carcinoma (37), promoting cell proliferation by
targeting NEFL in glioblastoma multiforme (45), and suppressing retinoblastoma cell
growth by targeting LRP6 (46).
However, no study has reported the possible molecular mechanisms of
miR-183-5p in BC. In the present, the potential target genes of
miR-183-5p were obtained by the overlap of genes from 3 datasets,
including the available predicting online tools, low-expression
genes from TCGA and low-expression genes from microarray data after
miR-183-5p mimic transfection.
The potential molecular mechanism of miR-183-5p in
BC was explored. In total, 7,421 genes were predicted using the
online predicting tool of miRwalk2.0. The low-expression genes from
the RNA-seq data of TCGA with log2 FC <-1 were regarded as
potential target genes of miR-183-5p, and 2,918 genes were
involved. The GSE24782 microarray, which contained the data of
differential-expressed genes after the overexpression of miR-183-5p
in BOY and T24 cells, was reassessed to identify the downregulated
genes with a fold change <0.85; 3,163 eligible genes were
included. Consequently, 88 overlapped genes were identified and
were more prone to be the target genes of miR-183-5p in BC. Next,
bioinformatics analyses discovered that these potential target
genes were enriched in several pathways involved in the
tumorigenesis and development of BC. Forty-three genes were
enriched in the pathway of cytoplasm, which ranked the 2nd most
significant pathways in the cellular component (CC). The most
significant pathway indicated in molecular function (MF) was the
pathway of cytoskeletal protein binding. Several significant
pathways relative to signal transduction or cell communication were
involved in biological process (BP), such as calcium-mediated
signaling, cell communication and signal transduction. The pathway
of epithelial-to-mesenchymal transition was the most enriched
pathway in the biological pathway, playing a crucial role in tumor
progression with genes of ZFPM2, AKAP12, CLIC4, ZEB2, BNC2, CAV1,
CXCL12, SOBP, TNS1, IGF1, SYNE1, PTX3 and WWTR1. The expression of
these 13 target genes was negatively correlated with the expression
of miR-183-5p in BC tissues. miR-183-5p was conformed to target the
tumor suppressor AKAP12 and plays an important role in human
hepatocarcinogenesis (47). CAV1,
CXCL12 and IGF1 were also identified as hub genes of miR-183-5p in
BC which may deeply affect the generation and development of BC.
However, rigorous experiments in vitro and in vivo
are needed to confirm the potential molecular mechanism.
In summary, miR-183-5p may play critical roles in
the tumorigenesis and development of BC; however, the clinical
function of miR-183-5p in BC urgently requires exploration.
Furthermore, several crucial pathways for miR-183-5p in BC were
predicted by bioinformatics analysis. However, validation of the
real molecular mechanisms of miR-183-5p in BC by well-designed and
rigorous functional experiments is still needed.
Acknowledgements
The present study was supported by the Guangxi
Natural Science Fund for Innovation Research Team
(2013GXNSFFA019002, 2016GXNSFGA38006), the Guangxi Collaborative
Innovation Center for genomic and personalized medicine (201319),
the Promoting Project of Basic Capacity for Young and Middle-aged
University Teachers in Guangxi (KY2016YB090), and the Innovation
Project of Guangxi Graduate Education (YCBZ2017044).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ouyang H, Zhou Y, Zhang L and Shen G:
Diagnostic value of microRNAs for urologic cancers: A systematic
review and meta-analysis. Medicine. 94:e12722015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bao Z, Zhang W and Dong D: A potential
prognostic lncRNA signature for predicting survival in patients
with bladder urothelial carcinoma. Oncotarget. 8:10485–10497. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berrondo C, Flax J, Kucherov V, Siebert A,
Osinski T, Rosenberg A, Fucile C, Richheimer S and Beckham CJ:
Expression of the long non-coding RNA HOTAIR correlates with
disease progression in bladder cancer and is contained in bladder
cancer patient urinary exosomes. PLoS One. 11:e01472362016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kang M, Jeong CW, Kwak C, Kim HH and Ku
JH: Preoperative neutrophil-lymphocyte ratio can significantly
predict mortality outcomes in patients with non-muscle invasive
bladder cancer undergoing transurethral resection of bladder tumor.
Oncotarget. 8:12891–12901. 2017.PubMed/NCBI
|
|
6
|
Urquidi V, Netherton M, Gomes-Giacoia E,
Serie DJ, Eckel-Passow J, Rosser CJ and Goodison S: A microRNA
biomarker panel for the non-invasive detection of bladder cancer.
Oncotarget. 7:86290–86299. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu X, Wang X, Fu B, Meng L and Lang B:
Differentially expressed genes and microRNAs in bladder carcinoma
cell line 5637 and T24 detected by RNA sequencing. Int J Clin Exp
Pathol. 8:12678–12687. 2015.PubMed/NCBI
|
|
8
|
Falzone L, Candido S, Salemi R, Basile MS,
Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M and
Libra M: Computational identification of microRNAs associated to
both epithelial to mesenchymal transition and NGAL/MMP-9 pathways
in bladder cancer. Oncotarget. 7:72758–72766. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng Y, Zhang X and Li P, Yang C, Tang J,
Deng X, Yang X, Tao J, Lu Q and Li P: MiR-200c promotes bladder
cancer cell migration and invasion by directly targeting RECK. Onco
Targets Ther. 9:5091–5099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Egawa H, Jingushi K, Hirono T, Ueda Y,
Kitae K, Nakata W, Fujita K, Uemura M, Nonomura N and Tsujikawa K:
The miR-130 family promotes cell migration and invasion in bladder
cancer through FAK and Akt phosphorylation by regulating PTEN. Sci
Rep. 6:205742016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ding M, Li Y, Wang H, Lv Y, Liang J, Wang
J and Li C: Diagnostic value of urinary microRNAs as non-invasive
biomarkers for bladder cancer: A meta-analysis. Int J Clin Exp Med.
8:15432–15440. 2015.PubMed/NCBI
|
|
12
|
Zhang X, Zhang Y, Liu X, Fang A, Li P, Li
Z, Liu T, Yang Y, Du L and Wang C: MicroRNA-203 is a prognostic
indicator in bladder cancer and enhances chemosensitivity to
cisplatin via apoptosis by targeting Bcl-w and Survivin. PLoS One.
10:e01434412015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Morais DR, Reis ST, Viana N, Piantino CB,
Massoco C, Moura C, Dip N, Silva IA, Srougi M and Leite KR: The
involvement of miR-100 in bladder urothelial carcinogenesis
changing the expression levels of mRNA and proteins of genes
related to cell proliferation, survival, apoptosis and chromosomal
stability. Cancer Cell Int. 14:1192014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu WB, Wang W, Du YH, Li H, Xia SJ and Liu
HT: MicroRNA-3713 regulates bladder cell invasion via MMP9. Sci
Rep. 6:323742016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng S, Liu J, Zhang Y, Lin Y, Liu Q, Li
H, Huang J and Zhang P: Association detection between genetic
variants in the microRNA binding sites of toll-like receptors
signaling pathway genes and bladder cancer susceptibility. Int J
Clin Exp Pathol. 7:8118–8126. 2014.PubMed/NCBI
|
|
16
|
Wang H, Zhang W, Zuo Y, Ding M, Ke C, Yan
R, Zhan H, Liu J and Wang J: miR-9 promotes cell proliferation and
inhibits apoptosis by targeting LASS2 in bladder cancer. Tumour
Biol. 36:9631–9640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou M, Wang S, Hu L, Liu F, Zhang Q and
Zhang D: miR-199a-5p suppresses human bladder cancer cell
metastasis by targeting CCR7. BMC Urol. 16:642016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shang A, Yang M, Shen F, Wang J, Wei J,
Wang W, Lu W and Wang C and Wang C: MiR-1-3p suppresses the
proliferation, invasion and migration of bladder cancer cells by
up-regulating SFRP1 expression. Cell Physiol Biochem. 41:1179–1188.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pignot G, Cizeron-Clairac G, Vacher S,
Susini A, Tozlu S, Vieillefond A, Zerbib M, Lidereau R, Debre B,
Amsellem-Ouazana D and Bieche I: microRNA expression profile in a
large series of bladder tumors: Identification of a 3-miRNA
signature associated with aggressiveness of muscle-invasive bladder
cancer. Int J Cancer. 132:2479–2491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu W, Gao T, Shen J, Sun Y, Zheng X, Wang
J, Ma J, Hu XY, Li J and Hu MJ: MicroRNA-183 inhibits apoptosis and
promotes proliferation and invasion of gastric cancer cells by
targeting PDCD4. Int J Clin Exp Med. 7:2519–2529. 2014.PubMed/NCBI
|
|
21
|
Yang M, Liu R, Li X, Liao J, Pu Y, Pan E,
Yin L and Wang Y: miRNA-183 suppresses apoptosis and promotes
proliferation in esophageal cancer by targeting PDCD4. Mol Cells.
37:873–880. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren LH, Chen WX, Li S, He XY, Zhang ZM, Li
M, Cao RS, Hao B, Zhang HJ, Qiu HQ and Shi RH: MicroRNA-183
promotes proliferation and invasion in oesophageal squamous cell
carcinoma by targeting programmed cell death 4. Br J Cancer.
111:2003–2013. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li C, Deng L, Zhi Q, Meng Q, Qian A, Sang
H, Li X and Xia J: MicroRNA-183 functions as an oncogene by
regulating PDCD4 in gastric cancer. Anticancer Agents Med Chem.
16:447–455. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miao F, Zhu J, Chen Y, Tang N, Wang X and
Li X: MicroRNA-183-5p promotes the proliferation, invasion and
metastasis of human pancreatic adenocarcinoma cells. Oncol Lett.
11:134–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun Y, Cheng H, Wang G, Yu G, Zhang D,
Wang Y, Fan W and Yang W: Deregulation of miR-183 promotes melanoma
development via lncRNA MALAT1 regulation and ITGB1 signal
activation. Oncotarget. 8:3509–3518. 2017.PubMed/NCBI
|
|
26
|
Han Y, Chen J, Zhao X, Liang C, Wang Y,
Sun L, Jiang Z, Zhang Z, Yang R, Chen J, et al: MicroRNA expression
signatures of bladder cancer revealed by deep sequencing. PLoS One.
6:e182862011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamada Y, Enokida H, Kojima S, Kawakami K,
Chiyomaru T, Tatarano S, Yoshino H, Kawahara K, Nishiyama K, Seki N
and Nakagawa M: MiR-96 and miR-183 detection in urine serve as
potential tumor markers of urothelial carcinoma: Correlation with
stage and grade, and comparison with urinary cytology. Cancer Sci.
102:522–529. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei S, Bing Z, Yao Y, Master SR and Gupta
P: Higher expression of miR-182 in cytology specimens of high-grade
urothelial cell carcinoma: A potential diagnostic marker. Acta
Cytol. 59:109–112. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scheffer AR, Holdenrieder S, Kristiansen
G, von Ruecker A, Müller SC and Ellinger J: Circulating microRNAs
in serum: Novel biomarkers for patients with bladder cancer? World
J Urol. 32:353–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robertson AG, Kim J, Al-Ahmadie H,
Bellmunt J, Guo G, Cherniack AD, Hinoue T, Laird PW, Hoadley KA,
Akbani R, et al: Comprehensive molecular characterization of
muscle-invasive bladder cancer. Cell. 171:540–556.e25. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li QQ, Hsu I, Sanford T, Railkar R, Balaji
N, Sourbier C, Vocke C, Balaji KC and Agarwal PK: Protein kinase D
inhibitor CRT0066101 suppresses bladder cancer growth in vitro and
xenografts via blockade of the cell cycle at G2/M. Cell Mol Life
Sci. Oct;25;2017.(Epub ahead of print). doi:
10.1007/s00018-017-2681-z.
|
|
32
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeng JH, Liang L, He RQ, Tang RX, Cai XY,
Chen JQ, Luo DZ and Chen G: Comprehensive investigation of a novel
differentially expressed lncRNA expression profile signature to
assess the survival of patients with colorectal adenocarcinoma.
Oncotarget. 8:16811–16828. 2017.PubMed/NCBI
|
|
34
|
Benito-Martin A and Peinado H: FunRich
proteomics software analysis, let the fun begin. Proteomics.
15:2555–2556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pathan M, Keerthikumar S, Ang CS, Gangoda
L, Quek CY, Williamson NA, Mouradov D, Sieber OM, Simpson RJ, Salim
A, et al: FunRich: An open access standalone functional enrichment
and interaction network analysis tool. Proteomics. 15:2597–2601.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu F, Zhang H, Su Y, Kong J, Yu H and Qian
B: Up-regulation of microRNA-183-3p is a potent prognostic marker
for lung adenocarcinoma of female non-smokers. Clin Transl Oncol.
16:980–985. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang G, Wang S and Li C: MiR-183
overexpression inhibits tumorigenesis and enhances DDP-induced
cytotoxicity by targeting MTA1 in nasopharyngeal carcinoma. Tumour
Biol. 39:10104283177038252017.PubMed/NCBI
|
|
38
|
Fan D, Wang Y, Qi P, Chen Y, Xu P, Yang X,
Jin X and Tian X: MicroRNA-183 functions as the tumor suppressor
via inhibiting cellular invasion and metastasis by targeting MMP-9
in cervical cancer. Gynecol Oncol. 141:166–174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou L, Zhang WG, Wang DS, Tao KS, Song WJ
and Dou KF: MicroRNA-183 is involved in cell proliferation,
survival and poor prognosis in pancreatic ductal adenocarcinoma by
regulating Bmi-1. Oncol Rep. 32:1734–1740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen YH, Wang SQ, Wu XL, Shen M, Chen ZG,
Chen XG, Liu YX, Zhu XL, Guo F, Duan XZ, et al: Characterization of
microRNAs expression profiling in one group of Chinese urothelial
cell carcinoma identified by Solexa sequencing. Urol Oncol.
31:219–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ichimi T, Enokida H, Okuno Y, Kunimoto R,
Chiyomaru T, Kawamoto K, Kawahara K, Toki K, Kawakami K, Nishiyama
K, et al: Identification of novel microRNA targets based on
microRNA signatures in bladder cancer. Int J Cancer. 125:345–352.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Friedman JM, Liang G, Liu CC, Wolff EM,
Tsai YC, Ye W, Zhou X and Jones PA: The putative tumor suppressor
microRNA-101 modulates the cancer epigenome by repressing the
polycomb group protein EZH2. Cancer Res. 69:2623–2629. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eissa S, Matboli M, Hegazy MG, Kotb YM and
Essawy NO: Evaluation of urinary microRNA panel in bladder cancer
diagnosis: Relation to bilharziasis. Transl Res. 165:731–739. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng Y, Xiang G, Meng Y and Dong R:
MiRNA-183-5p promotes cell proliferation and inhibits apoptosis in
human breast cancer by targeting the PDCD4. Reprod Biol.
16:225–233. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang ZY, Xiong J, Zhang SS, Wang JJ, Gong
ZJ and Dai MH: Up-regulation of microRNA-183 promotes cell
proliferation and invasion in glioma by directly targeting NEFL.
Cell Mol Neurobiol. 36:1303–1310. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang J, Wang X, Li Z, Liu H and Teng Y:
MicroRNA-183 suppresses retinoblastoma cell growth, invasion and
migration by targeting LRP6. FEBS J. 281:1355–1365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goeppert B, Schmezer P, Dutruel C, Oakes
C, Renner M, Breinig M, Warth A, Vogel MN, Mittelbronn M, Mehrabi
A, et al: Down-regulation of tumor suppressor A kinase anchor
protein 12 in human hepatocarcinogenesis by epigenetic mechanisms.
Hepatology. 52:2023–2033. 2010. View Article : Google Scholar : PubMed/NCBI
|