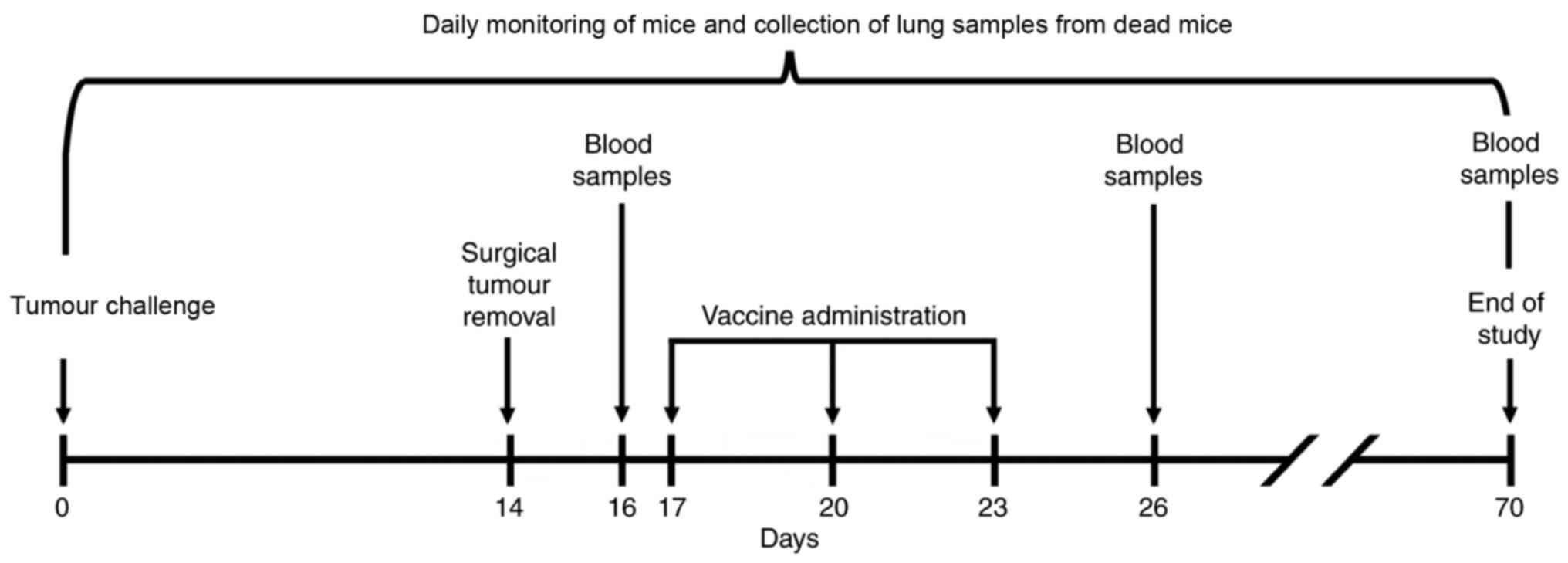
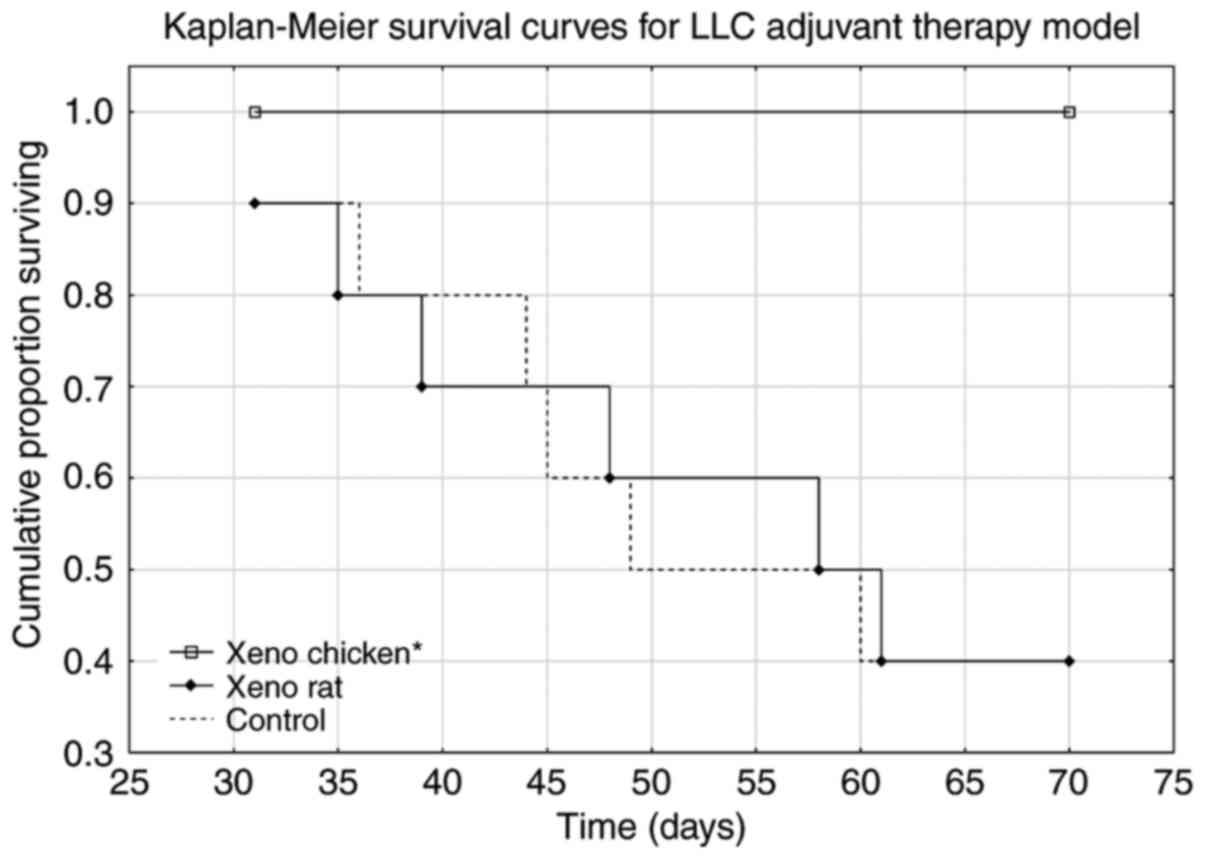
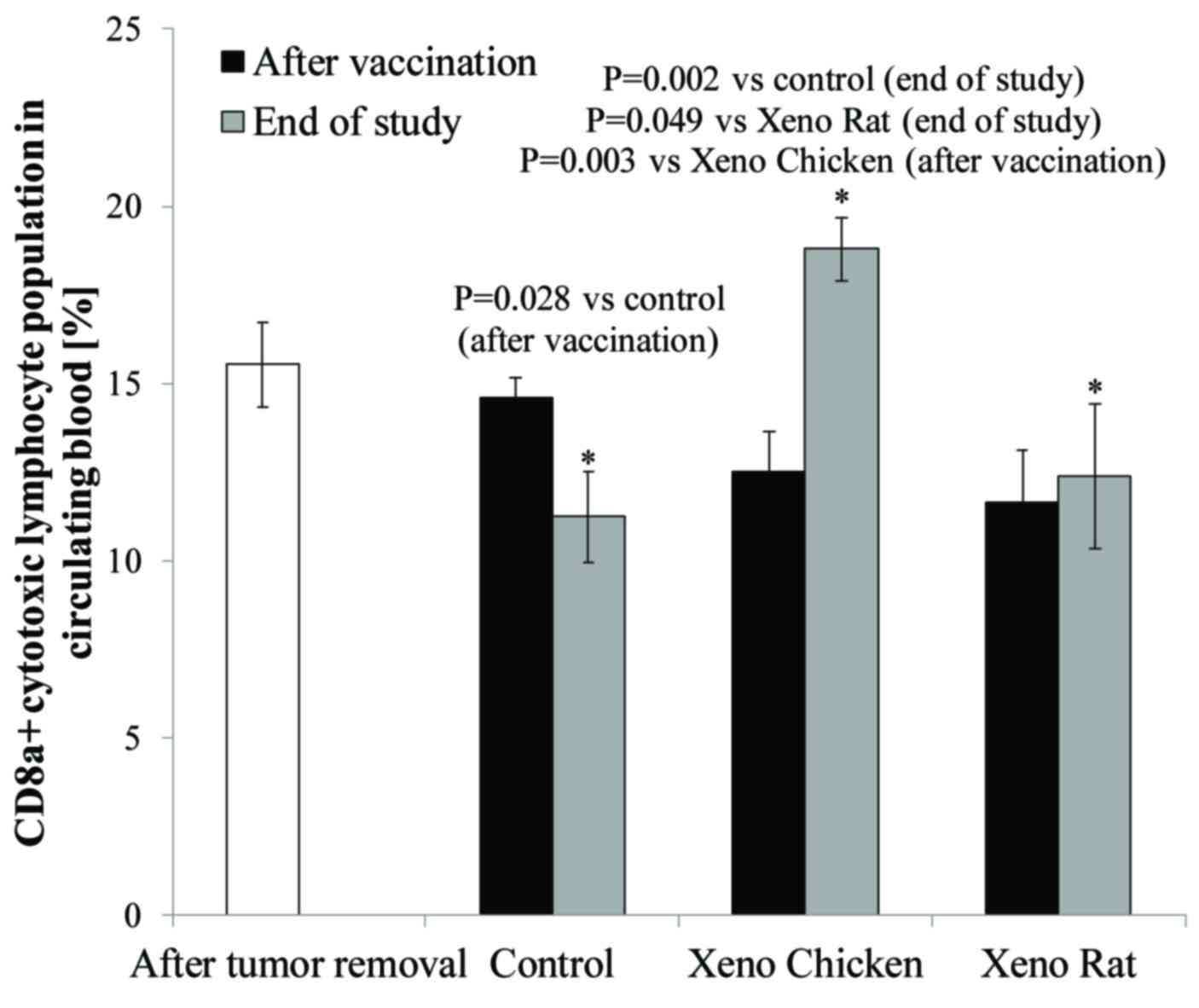
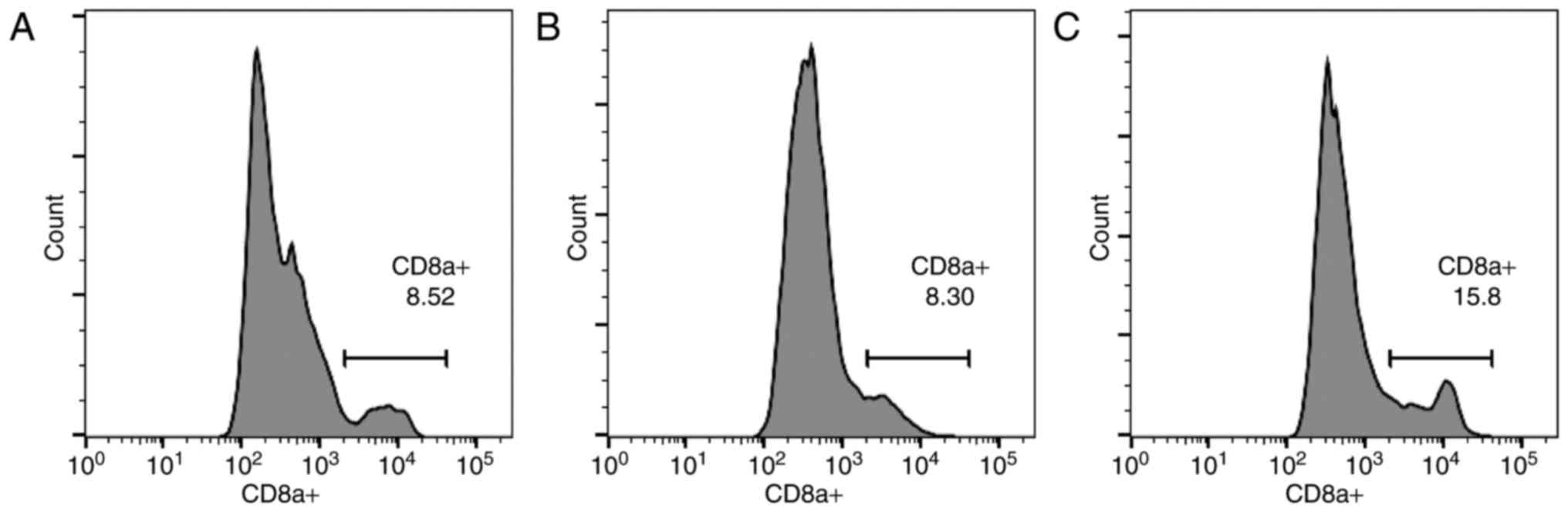
|
1
|
Mittal D, Gubin MM, Schreiber RD and Smyth
MJ: New insights into cancer immunoediting and its three component
phases-elimination, equilibrium and escape. Curr Opin Immunol.
27:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France:
2013
|
|
3
|
Zitvogel L, Tesniere A and Kroemer G:
Cancer despite immunosurveillance: Immunoselection and
immunosubversion. Nat Rev Immunol. 6:715–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galluzzi L, Vacchelli E, Bravo-San Pedro
JM, et al: Classification of current anticancer immunotherapies.
Oncotarget. 5:12472–12508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strioga M, Schijns V, Powell DJ Jr,
Pasukoniene V, Dobrovolskiene N and Michalek J: Dendritic cells and
their role in tumor immunosurveillance. Innate Immun. 19:98–111.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strioga MM, Felzmann T, Powell DJ Jr,
Ostapenko V, Dobrovolskiene NT, Matuskova M, Michalek J and Schijns
VE: Therapeutic dendritic cell-based cancer vaccines: The state of
the art. Crit Rev Immunol. 33:489–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strioga MM, Darinskas A, Pasukoniene V,
Mlynska A, Ostapenko V and Schijns V: Xenogeneic therapeutic cancer
vaccines as breakers of immune tolerance for clinical application:
To use or not to use? Vaccine. 32:4015–4024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weber LW, Bowne WB, Wolchok JD, Srinivasan
R, Qin J, Moroi Y, Clynes R, Song P, Lewis JJ and Houghton AN:
Tumor immunity and autoimmunity induced by immunization with
homologous DNA. J Clin Invest. 102:1258–1264. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Overwijk WW, Lee DS, Surman DR, Irvine KR,
Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA and
Restifo NP: Vaccination with a recombinant vaccinia virus encoding
a ‘self’ antigen induces autoimmune vitiligo and tumor cell
destruction in mice: requirement for CD4(+) T
lymphocytes. Proc Natl Acad Sci USA. 96:pp. 2982–2987. 1999;
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei YQ, Wang QR, Zhao X, Yang L, Tian L,
Lu Y, Kang B, Lu CJ, Huang MJ, Lou YY, et al: Immunotherapy of
tumors with xenogeneic endothelial cells as a vaccine. Nat Med.
6:1160–1166. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steitz J, Brück J, Steinbrink K, Enk A,
Knop J and Tüting T: Genetic immunization of mice with human
tyrosinase-related protein 2: Implications for the immunotherapy of
melanoma. Int J Cancer. 86:89–94. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kornberg TB and Krasnow MA: The Drosophila
genome sequence: Implications for biology and medicine. Science.
287:2218–2220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nilsson S, Helou K, Walentinsson A,
Szpirer C, Nerman O and Ståhl F: Rat-mouse and rat-human
comparative maps based on gene homology and high-resolution
zoo-FISH. Genomics. 74:287–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overwijk WW, Tsung A, Irvine KR, Parkhurst
MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA and
Restifo NP: gp100/pmel 17 is a murine tumor rejection antigen:
Induction of ‘self’-reactive, tumoricidal T cells using
high-affinity, altered peptide ligand. J Exp Med. 188:277–286.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soong RS, Trieu J, Lee SY, He L, Tsai YC,
Wu TC and Hung CF: Xenogeneic human p53 DNA vaccination by
electroporation breaks immune tolerance to control murine tumors
expressing mouse p53. PLoS One. 8:e569122013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fong L, Brockstedt D, Benike C, Breen JK,
Strang G, Ruegg CL and Engleman EG: Dendritic cell-based
xenoantigen vaccination for prostate cancer immunotherapy. J
Immunol. 167:7150–7156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wepsic HT: Overview of oncofetal antigens
in cancer. Ann Clin Lab Sci. 13:261–266. 1983.PubMed/NCBI
|
|
19
|
Lim SH, Zhang Y and Zhang J: Cancer-testis
antigens: The current status on antigen regulation and potential
clinical use. Am J Blood Res. 2:29–35. 2012.PubMed/NCBI
|
|
20
|
Malati T: Tumour markers: An overview.
Indian J Clin Biochem. 22:17–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohue Y, Wada H, Oka M and Nakayama E:
Antibody response to cancer/testis (CT) antigens: A prognostic
marker in cancer patients. Oncoimmunology. 3:e9700322014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Symchych TV, Fedosova NI, Karaman OM,
Yevstratieva LM, Lisovenko HS, Voyejkova IM and Potebnia HP: The
anticancer efficiency of the xenogeneic vaccine and the indication
for its use. Exp Oncol. 36:79–84. 2014.PubMed/NCBI
|
|
23
|
Seledtsova GV, Shishkov AA, Kaschenko EA,
Goncharov AG, Gazatova ND and Seledtsov VI: Xenogeneic cell-based
vaccine therapy for stage III melanoma: Safety, immune-mediated
responses and survival benefits. Eur J Dermatol. 26:138–143.
2016.PubMed/NCBI
|
|
24
|
Seledtsova GV, Shishkov AA, Kaschenko EA
and Seledtsov VI: Xenogeneic cell-based vaccine therapy for
colorectal cancer: Safety, association of clinical effects with
vaccine-induced immune responses. Biomed Pharmacother.
83:1247–1252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Voeykova IM, Fedosova NI, Karaman OM,
Yudina OY, Didenko GV, Lisovenko GS, Evstratieva LM and Potebnya
GP: Use of xenogeneic vaccine modified with embryonal nervous
tissue antigens in the treatment of B16-melanoma-bearing mice. Exp
Oncol. 36:24–28. 2014.PubMed/NCBI
|
|
26
|
Directive 2010/63/EU of the European
Parliament and of the Council of 22 September 2010 on the
protection of animals used for scientific purposes. 33–79.
2010.
|
|
27
|
Potebnya GP VI, Yudina OYu, Fedosova NI,
Karaman OM, Didenko GV, Yevstratyeva LM, Lisovenko GS and Chekhun
VF: The way to generate cancer vaccine. UKRPATENT: Ukraine:
2013
|
|
28
|
Isokawa K, Rezaee M, Wunsch A, Markwald RR
and Krug EL: Identification of transferrin as one of multiple
EDTA-extractable extracellular proteins involved in early chick
heart morphogenesis. J Cell Biochem. 54:207–218. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Symchych TV, Fedosova NI, Karaman ОМ,
Yevstratieva LM, Lisovenko HS, Voyeykova IM and Potebnia HP:
Anticancer effectiveness of vaccination based on xenogeneic embryo
proteins applied in different schedules. Exp Oncol. 37:197–202.
2015.PubMed/NCBI
|
|
30
|
Niu PG, Zhang YX, Shi DH, Liu Y, Chen YY
and Deng J: Cardamonin inhibits metastasis of lewis lung carcinoma
cells by decreasing mTOR activity. PLoS One. 10:e01277782015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bland JM and Altman DG: The logrank test.
BMJ. 328:10732004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Judge GD, William EG, Hill RC and Lee TC:
The theory and practise of econometrics. John Wiley & Sons; New
York: pp. 7391980
|
|
34
|
Box GEP and Cox DR: An analysis of
transformations. J R Stat Soc. 26:211–252. 1964.
|
|
35
|
Gosset WS: The probable error of a mean.
Biometrica. 6:1–25. 1908. View Article : Google Scholar
|
|
36
|
Mann HB and Whitney DR: On a test of
whether one of two random variables is stochastically larger than
the other. Ann Math Stat. 18:50–60. 1947. View Article : Google Scholar
|
|
37
|
Schirrmacher V, Fournier P and Schlag P:
Autologous tumor cell vaccines for post-operative active-specific
immunotherapy of colorectal carcinoma: Long-term patient survival
and mechanism of function. Expert Rev Vaccines. 13:117–130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Laufer I, Iorgulescu JB, Chapman T, Lis E,
Shi W, Zhang Z, Cox BW, Yamada Y and Bilsky MH: Local disease
control for spinal metastases following ‘separation surgery’ and
adjuvant hypofractionated or high-dose single-fraction stereotactic
radiosurgery: Outcome analysis in 186 patients. J Neurosurg Spine.
18:207–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kraśko JA, Žilionytė K, Darinskas A,
Strioga M, Rjabceva S, Zalutsky I, Derevyanko M, Kulchitsky V,
Lubitz W, Kudela P, et al: Bacterial ghosts as adjuvants in
syngeneic tumour cell lysate-based anticancer vaccination in a
murine lung carcinoma model. Oncol Rep. 37:171–178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Foged C, Hansen J and Agger EM: License to
kill: Formulation requirements for optimal priming of CD8(+) CTL
responses with particulate vaccine delivery systems. Eur J Pharm
Sci. 45:482–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sandoval F, Terme M, Nizard M, Badoual C,
Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G,
et al: Mucosal imprinting of vaccine-induced CD8(+)T cells is
crucial to inhibit the growth of mucosal tumors. Sci Transl Med.
5:172ra1202013. View Article : Google Scholar
|
|
42
|
Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q,
Chen J, Xia R, Zhou SM, Wu CP, Zhang XG, et al: Higher numbers of
T-bet(+) intratumoral lymphoid cells correlate with better survival
in gastric cancer. Cancer Immunol Immunother. 62:553–561. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Noguchi A, Kaneko T, Naitoh K, Saito M,
Iwai K, Maekawa R, Kamigaki T and Goto S: Impaired and imbalanced
cellular immunological status assessed in advanced cancer patients
and restoration of the T cell immune status by adoptive T-cell
immunotherapy. Int Immunopharmacol. 18:90–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thiery J and Lieberman J: Perforin: A key
pore-forming protein for immune control of viruses and cancer.
Subcell Biochem. 80:197–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pérez O, Batista-Duharte A, González E,
Zayas C, Balboa J, Cuello M, Cabrera O, Lastre M and Schijns VE:
Human prophylactic vaccine adjuvants and their determinant role in
new vaccine formulations. Braz J Med Biol Res. 45:681–692. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schijns V, Tartour E, Michalek J,
Stathopoulos A, Dobrovolskiene NT and Strioga MM: Immune adjuvants
as critical guides directing immunity triggered by therapeutic
cancer vaccines. Cytotherapy. 16:427–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo C, Manjili MH, Subjeck JR, Sarkar D,
Fisher PB and Wang XY: Therapeutic cancer vaccines: Past, present,
and future. Adv Cancer Res. 119:421–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Andersen MH, Junker N, Ellebaek E, Svane
IM and Thor Straten P: Therapeutic cancer vaccines in combination
with conventional therapy. J Biomed Biotechnol. 2010:2376232010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Melero I, Gaudernack G, Gerritsen W, Huber
C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C,
Faulkner I and Mellstedt H: Therapeutic vaccines for cancer: An
overview of clinical trials. Nat Rev Clin Oncol. 11:509–524. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheever MA and Higano CS: PROVENGE
(Sipuleucel-T) in prostate cancer: The first FDA-approved
therapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vesely MD and Schreiber RD: Cancer
immunoediting: Antigens, mechanisms, and implications to cancer
immunotherapy. Ann N Y Acad Sci. 1284:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dang Y, Wagner WM, Gad E, Rastetter L,
Berger CM, Holt GE and Disis ML: Dendritic cell-activating vaccine
adjuvants differ in the ability to elicit antitumor immunity due to
an adjuvant-specific induction of immunosuppressive cells. Clin
Cancer Res. 18:3122–3131. 2012. View Article : Google Scholar : PubMed/NCBI
|