Introduction
Through the immunosurveillance process, the immune
system is the main line of defence against cancer (1). Nevertheless, ~14 million cancer cases
are diagnosed worldwide annually (2),
indicating that, under certain circumstances, malignant cells can
break the protective barrier of spontaneous antitumour immunity and
develop into clinically detectable cancer. Malignant cells exploit
various immune escape mechanisms, creating a microenvironment
favourable for cancer growth and metastasis (3). Therefore, substantial effort has been
initiated to manipulate antitumour immune responses by therapeutic
interventions, known as cancer immunotherapy. Of the various types
of cancer immunotherapy (4),
therapeutic cancer vaccinations are one of the most extensively
studied, with the first clinical trial in melanoma patients dating
back to 1998 (5). Therapeutic cancer
vaccinations exploit dendritic cells in situ and their
unique capacity to induce and orchestrate antigen-specific immune
responses (6). These vaccinations aim
to reprogram imbalanced antitumour immunity by inducing or
re-stimulating robust tumour-associated antigen (TAA)-specific
cytotoxic immune responses (7).
The inherent tolerogenicity/low immunogenicity of
TAAs is an obstacle to effective spontaneous and
vaccination-induced antitumour immunity. Since all TAAs (apart from
oncoviral TAAs) are derived from self-proteins, TAAs possess a
certain degree of tolerance, depending on their type (4,8). Changes
in the structure and/or expression pattern of TAAs should be sensed
as a danger by the immune system and invoke its reactivity.
However, TAAs with minor alterations can resemble self-proteins and
sneak through the ‘detectors’ of the immune system (8). Therefore, a concept of xenogeneic
immunization, using homologous antigens derived from different
species (xenoantigens), was proposed to overcome immune tolerance
to such sham TAAs (9–12). A number of genes are evolutionarily
conserved between different animal species (13,14).
However, interspecies sequence variations also exist (9,15,16). Therefore, homologous xenoantigens
differ sufficiently from self-antigens to render them immunogenic,
but preserve an optimal homology range with self-proteins enabling
them to induce T cell cross-reactivity with self TAAs (9,16,17). Moreover, xenoepitopes can bind host
major histocompatibility complex (MHC) molecules even more strongly
compared with native homologous epitopes (15). Sustained xenogeneic peptide/MHC
complexes induce more robust xenoantigen-specific T-cell responses
that are cross-reactive with self TAAs.
Of the various TAAs, oncofoetal (18) and cancer-testis (CT) (19) antigens are of great interest in the
context of tumour immunotherapy. These antigens are products of
‘silent’ genes whose expression is normally repressed in postnatal
organisms with the exception of the immune-privileged organs,
including the testes and placenta. The expression of these antigens
can be aberrantly reactivated in cancer cells (19). CT antigens possess a high immunogenic
potential since they are ‘unknown’ to the adult immune system
(19). Oncofoetal antigens are
usually not expressed in adult organisms, or are expressed in a
limited quantity in specific organs. However, oncofoetal antigens
can be expressed in cancer cells (20). Notably, antibodies against CT antigens
were detected in patients with gastric and lung cancer, and were
associated with prolonged survival time (21). Similarly, tumour-bearing mice
expressed antibodies against chicken embryo protein
(CEP)-containing vaccine even prior to the administration of the
vaccine, indicating that the CEP vaccine (hereafter referred to as
‘xeno chicken’) contains antigens that were in common with various
types of cancer, including Lewis lung carcinoma (LLC), Ehrlich
carcinoma and Sarcoma 37 (22). This
data supports the credibility of using prenatal tissues as a source
of antigens in therapeutic cancer vaccines, with the aim of
exploiting their strong intrinsic immunogenicity and potential in
facilitating immune recognition. The availability of xenogeneic
foetal tissue would make it an affordable form of treatment among
others, which are very expensive immunotherapies. This potential is
already being investigated in recent trials involving xenogeneic
vaccines (23,24), where patients with stage III melanoma
were treated with xenogeneic polyvalent vaccine, based on murine
B16 and LLC tumours. The 5-year survival rate for patients treated
with the vaccine was significantly better compared with the
controls (55 vs. 18%).
The present study investigated the immunological and
therapeutic (micrometastases-suppressing) efficacy of postoperative
xenovaccination in a murine LLC model. In spite of the reports
raising the question whether LLC and 3LL cell lines are actually
the same cell line, all sources cited in the present study were
using the LLC-labelled cell line for the LLC model, therefore this
variant was used to maintain consistency across the studies. Two
xenogeneic vaccines were investigated, a patented rat embryonic
nervous tissue-based xenovaccine adjuvanated with a
protein-containing metabolite of Bacillus subtilis В-7025
(25) and unadjuvanted whole chicken
embryo xenogeneic vaccine (22).
Materials and methods
Mice and cell lines
A total of 30 C57BL/6 mice (8–12 weeks old; female)
were obtained from the State Research Institute Centre for
Innovative Medicine (Vilnius, Lithuania). The mice were housed in
plastic cages (≤15 mice per cage) under normal daylight conditions
with ad libitum access to water and food. All animal
procedures were performed in accordance with the directive of the
European Parliament and of the Council on the protection of animals
used for scientific purposes (26)
alongside the approval of Lithuania State Food and Veterinary
Service. The mice were sacrificed by cervical dislocation.
The murine metastatic Lewis lung carcinoma LLC1 cell
line was a gift from the RE Kavetsky Institute of Experimental
Pathology, Oncology and Radiobiology (Kiev, Ukraine). The cells
were cultivated in Dulbecco's modified Eagle's medium (Lonza Group,
Ltd., Basel, Switzerland) containing 2 mM L-glutamine, 10% foetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) in a humidified atmosphere containing 5%
CO2 at 37°C.
Vaccine preparation
The rat embryo nervous tissue vaccine (‘xeno rat’
vaccine) used in the present study has been patented by the
Ukrainian Intellectual Property Institute (27). The vaccine contains a protein fraction
of nervous tissue from late gestation stage rat embryo (a source of
TAAs) and an adjuvant, which is a protein-containing metabolite of
B. subtilis В-7025 (molecular weight, 70 kDa) (27). The whole chicken embryo vaccine was
prepared as follows: 7-day-old chicken embryos were rinsed twice
briefly in cold 0.9% NaCl solution, homogenized and then extracted
with 0.9% NaCl solution containing 0.1% EDTA for 60 min at 4°C by
agitation. Following extraction, chicken embryo tissue was removed
by centrifugation at 1,500 × g for 30 min at 4°C. The resulting
supernatant containing chicken embryo protein was collected and
frozen at −20°C (the ‘xeno chicken’ vaccine) (28,29). The
two vaccines were developed at the R. E. Kavetsky Institute of
Experimental Pathology, Oncology and Radiobiology (Kyiv,
Ukraine).
Postoperative (adjuvant) therapeutic
xenovaccination in the LLC model
On day 0, 30 C57BL/6 mice received a subcutaneous
injection of 3×105 LLC cells in the left hind foot. The
foot was chosen as the site of tumour inoculation owing to the ease
of resection, which contributed to the 100% surgical survival rate
in the present study. The hind foot was specifically chosen as mice
recover better with their front legs intact. Although the tumours
were located in the foot, no bleeding or self-harm was noted at the
tumour site. On day 14 following injection, primary tumours were
surgically removed by amputating the foot under general
anaesthesia, using intraperitoneal ketamine (100 mg/kg)-xylazine
(10 mg/kg) injections. The mice were allowed to recover under a
heat lamp and were monitored for 2 h or until they were able to
uphold an upright position, at which point they were returned to
standard cages. The mice with the primary tumour removed were
subsequently treated with either the xeno rat vaccine (n=10) or the
xeno chicken vaccine (n=10). The mice that underwent surgical LLC
removal and were receiving saline injections (n=10) served as the
control group. The mice in each group were vaccinated as follows:
Each vaccine was injected subcutaneously into the nape of the neck
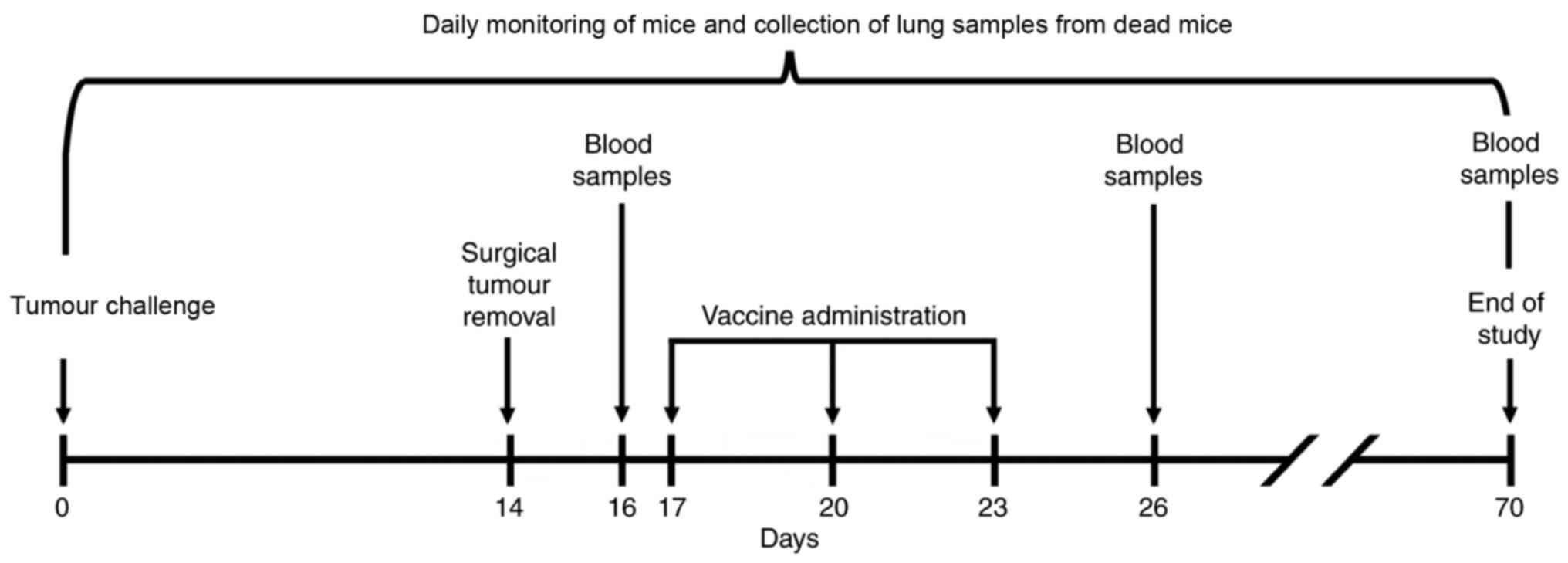
on days 17, 20 and 23. Internal organs were analysed for detection
and characterization of LLC metastases in animals that has
succumbed to disease. Blood samples were collected at various time
points for analysis of cluster of differentiation 8+
(CD8+) T-lymphocyte population. The experimental design
is depicted in Fig. 1.
Sampling
To assess the anticancer effects of the vaccines,
mice lung and blood samples were obtained (Fig. 1). The lungs were analysed
histologically for metastasis, as this is the preferred metastatic
location for the LLC cell line (30).
Other internal organs (liver, kidney, pancreas and brain) were
visually analysed for metastases. Blood samples were collected from
the hip vein prior to the onset of vaccination (on day 16), 3 days
after the completion of therapeutic vaccination (on day 26) and at
the end of the experiment (on day 70) from the surviving mice of
each experimental group. The mice that were found to have succumbed
or sacrificed owing to critical condition during the follow-up
period underwent histological analysis, as did mice sacrificed at
the end of the experiment.
The survival of mice was observed daily throughout
the experiment. Owing to the primary tumour resection, tumour
growth could not be used to establish a humane endpoint of the
study. Therefore, a time limit of 70 days was introduced. The
humane endpoint for individual mice was set subjectively by the
technical staff, which were blinded to the purpose of the
experiment, and euthanized the animal when they appeared to be in a
critical condition. This procedure could only be applied within
working hours (07:00-17:00) and at two time points during the
weekends (mornings and evenings). Owing to these time constraints,
one control mouse was not euthanized, but found to have succumbed
at the beginning of the working day, which was fixed as the date of
mortality. This event contributed to 8% of the total of mice that
succumbed to disease by the end of the experiment.
Histological analysis of LLC
metastases
For histological analysis, lung tissue was fixed in
10% neutral buffered formalin for 24 h at room temperature,
dehydrated in alcohol baths at room temperature (70% for 12 h; 90%
for 12 h; 100% for 24 h) and embedded in paraffin. The paraffinized
samples were serially cut into sections (thickness, 3 µm) using a
microtome. The sections were deparaffinized, rehydrated and stained
with haematoxylin (15 min) and eosin (5 sec) at room temperature.
Each section was examined under a light microscope to identify the
tumour-infiltrated areas. The images were captured using an
automated Leica DM50000 B microscope equipped with a Leica DFC420 C
digital camera (Leica Microsystems, GmbH, Wetzlar, Germany). Images
were processed and analysed using the ImageJ image analysis program
(version 1.48 k; National Institutes of Health, Bethesda, MD, USA),
as described previously (31). The
area of all tumour nodules that were found in lung tissue was
estimated using the ‘Freehand selection tool’ in ImageJ and
measurements were expressed in mm2.
Flow cytometry
For analysis of CD8a+ T cell population,
100 µl blood were collected from the hip vein from each mouse.
Whole blood was stained with anti-CD8-phycoerythrin (PE) (cat no.
552877; BD Biosciences, Franklin Lakes, NJ, USA) and
anti-CD3-allophycocyanin (APC) (cat no. 553066, BD Biosciences)
antibodies (1 µl/100 µl blood, 25 min incubation at room
temperature). Erythrocytes were lysed using ACK Lysing buffer
(Gibco; Thermo Fisher Scientific, Inc.). For FACS, 20,000 events
were collected using BD FACSDiva™ 7.0 flow cytometer (BD
Biosciences) and analyzed with FlowJo™ (version 10.2;
FlowJo LLC, USA) software.
Statistical analysis
P≤0.05 was considered to indicate a statistically
significant difference. For statistical analysis Statistica 12
(Tibco Software, Inc., La Jolla, CA, USA) software was used.
Non-parametric Kruskal-Wallis was used for statistical survival
data analysis of the animals surviving at the end of the
experiment. Once this test was performed, Kaplan-Meier analysis
followed by long-rank analysis was used for group analysis as it is
the most widely used test for survival analysis (32). As it was shown that for smaller
samples (n<50 per group) it is worth using a more powerful
statistical test (33), Cox's F-test
(34) was also used, as groups in
this study were smaller than 50 specimens. Flow cytometry data was
analysed using an unpaired, two-tailed Student's t-test (35). The differences between tumour areas in
lung histological slides were assessed using the Mann-Whitney
U-test (36).
Results
Survival of mice treated with
postoperative therapeutic xenovaccination in the LLC-metastatic
model
A total of 30 C57BL/6 female mice were
subcutaneously injected with 3×105 LLC cells in the left
hind-foot, and all of the mice developed visually detectable
tumours at the injection site. On day 14, the foot with the primary
tumour was surgically resected. No surgery-associated causalities
were recorded. The mice were subsequently treated with either the
xeno rat vaccine (n=10) or the xeno chicken vaccine (n=10) or
injected with saline solution (n=10).
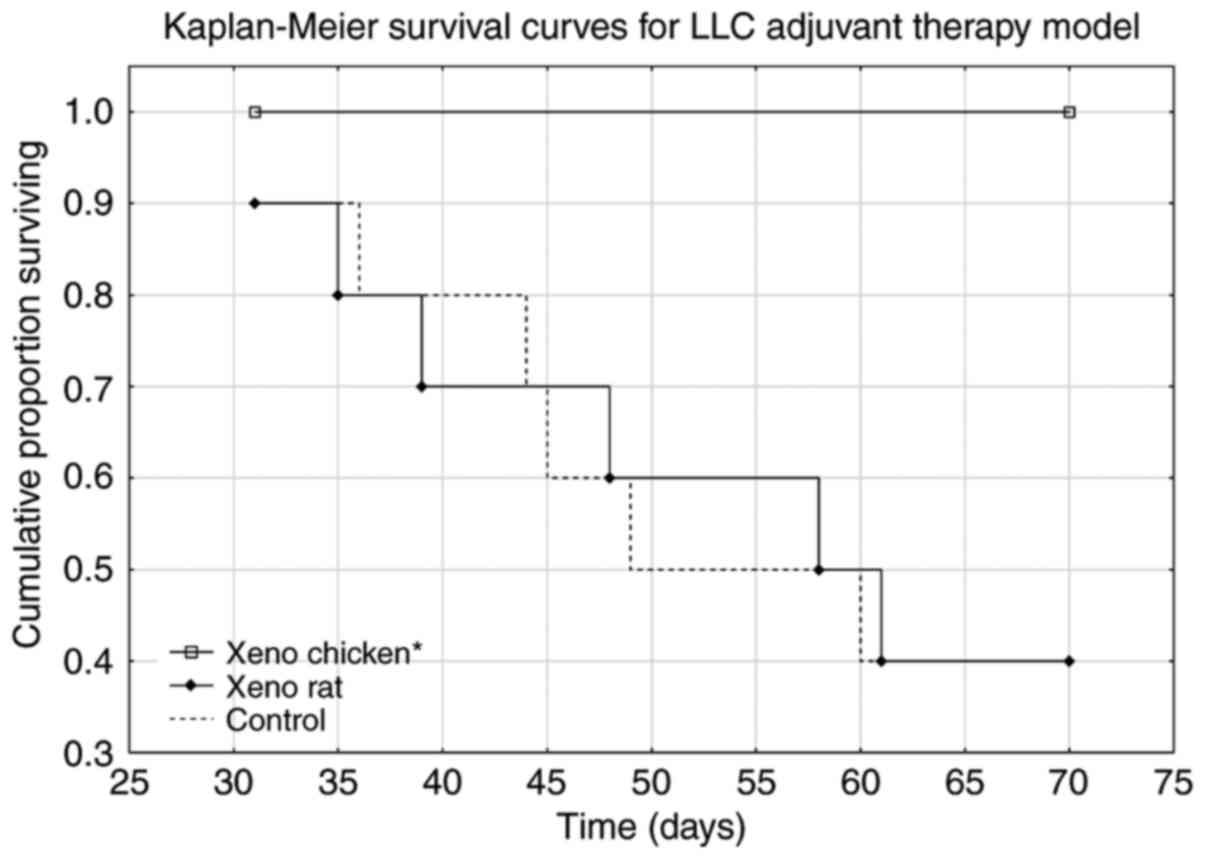
Mice treated with the xeno chicken vaccine
maintained a 100% survival rate over the observation period of 70
days. The xeno chicken vaccine-treated mice survived significantly
longer compared with the mice treated with the xeno rat-vaccine and
control group animals [Kruskal-Wallis analysis, H (2, n=31)=9.644;
P=0.008; Fig. 2].
Xenovaccination-associated changes in
circulating CD8a+ cytotoxic T-cell population
In each experimental group, blood samples were
collected from the hip vein at three time points: i) On day 16
(i.e., two days after tumour removal and one day prior to the start
of therapeutic xenovaccination); ii) on day 26 (two days after
completion of therapeutic xenovaccination), and iii) on day 70 (at
the end of the experiment for the surviving mice).
As shown in Fig. 3, a
significant increase in circulating CD8a+ T-cell level
was observed at the end of the observation period (day 70) only in
mice treated with the xeno chicken vaccine.
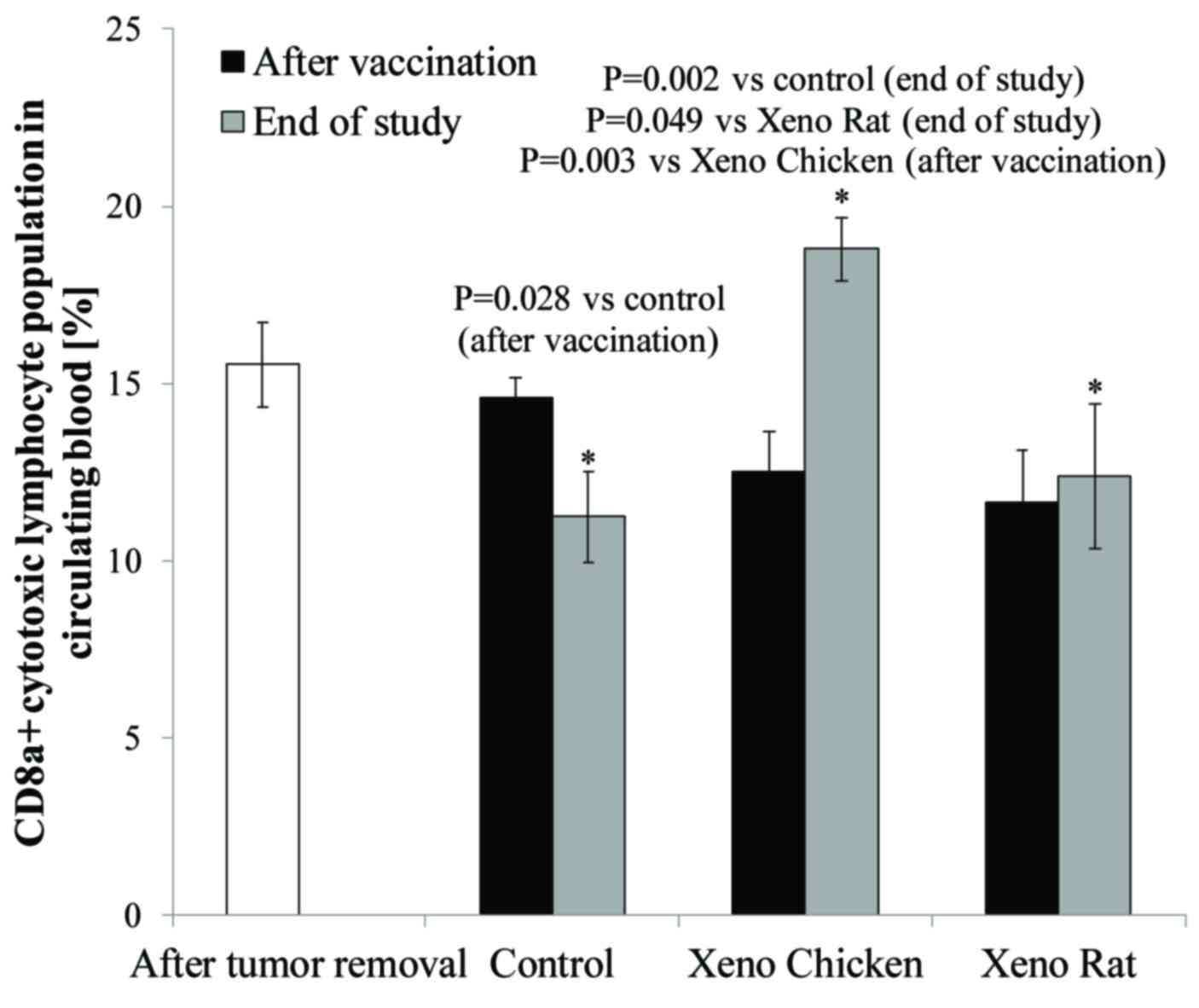 | Figure 3.Percentage of CD8a+ T
cells in circulating peripheral blood monocytes prior to
therapeutic xenovaccination (day 16, white column), 3 days after
the completion of xenovaccination (day 26, black columns) and at
the end of the study (day 70, grey columns). There were no
significant changes in the levels of circulating CD8a+ T
cells 3 days after the completion of xenovaccination, compared with
the levels prior to the start of vaccination (within and between
the experimental arms). However, on day 70 (at the end of the
observation period), the levels of circulating CD8a+ T
cells were significantly higher in the surviving mice vaccinated
with the xeno chicken vaccine (n=10) compared with surviving mice
(n=4) in the control group (P=0.002) or in the Xeno Rat group
(P=0.049). In xeno chicken vaccine-treated mice, the level of
CD8a+ T cells on day 70 was significantly higher
compared with the level on day 16 (following tumor removal, prior
to xenovaccination) (P=0.036) and on day 26 (3 days after the
completion of xenovaccination) (P=0.003). There were no significant
differences in the levels of circulating CD8a+ T cells
in the mice that were treated with xeno rat vaccines evaluated at
different time points or comparing T-cell levels with those in
control group animals. Additionally, there was a significantly
lower CD8a+ T-cell population in the control mice at the
end of the study (day 70) compared with the level following the
completion of vaccination (day 16) (P=0.028). CD8a, cluster of
differentiation 8a. *P≤0.05. |
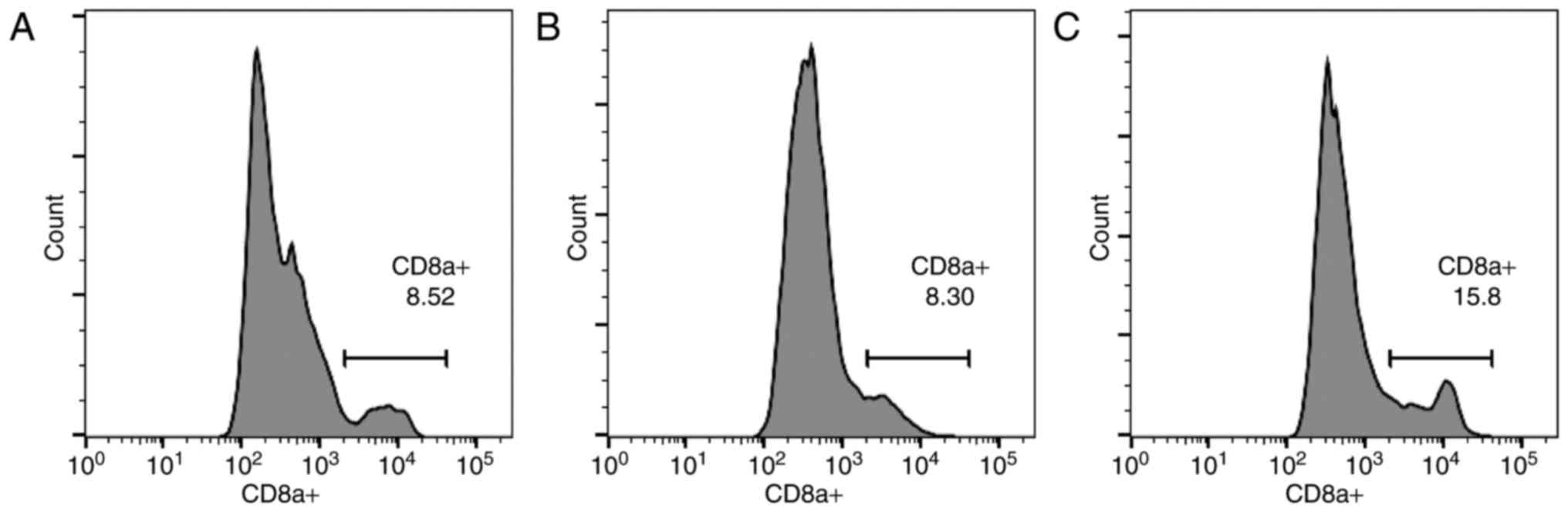
As indicated in Fig.
4, the results from the control (Fig.
4A), xeno rat (Fig. 4B) and xeno
chicken (Fig. 4C) treatment groups
are presented as histograms. Notably, a strong positive correlation
(r=0.985, α=0.05) was established between mean overall survival
(mOS) and circulating CD8a+ T-cell level.
Effect of xenogeneic vaccines on metastatic spread
into the lungs. All mice that succumbed to disease in any
experimental arm during the observation period had metastatic
infiltrates in the lungs. Additionally, all control mice had
metastatic foci in the liver. On day 70 (at the end of the
observation/experiment), metastatic foci in the lungs were detected
in 3 out of 10 of the surviving mice that were treated with the
xeno chicken vaccine and in 1 out of 4 of the surviving mice that
were treated with the xeno rat vaccine. All surviving mice in the
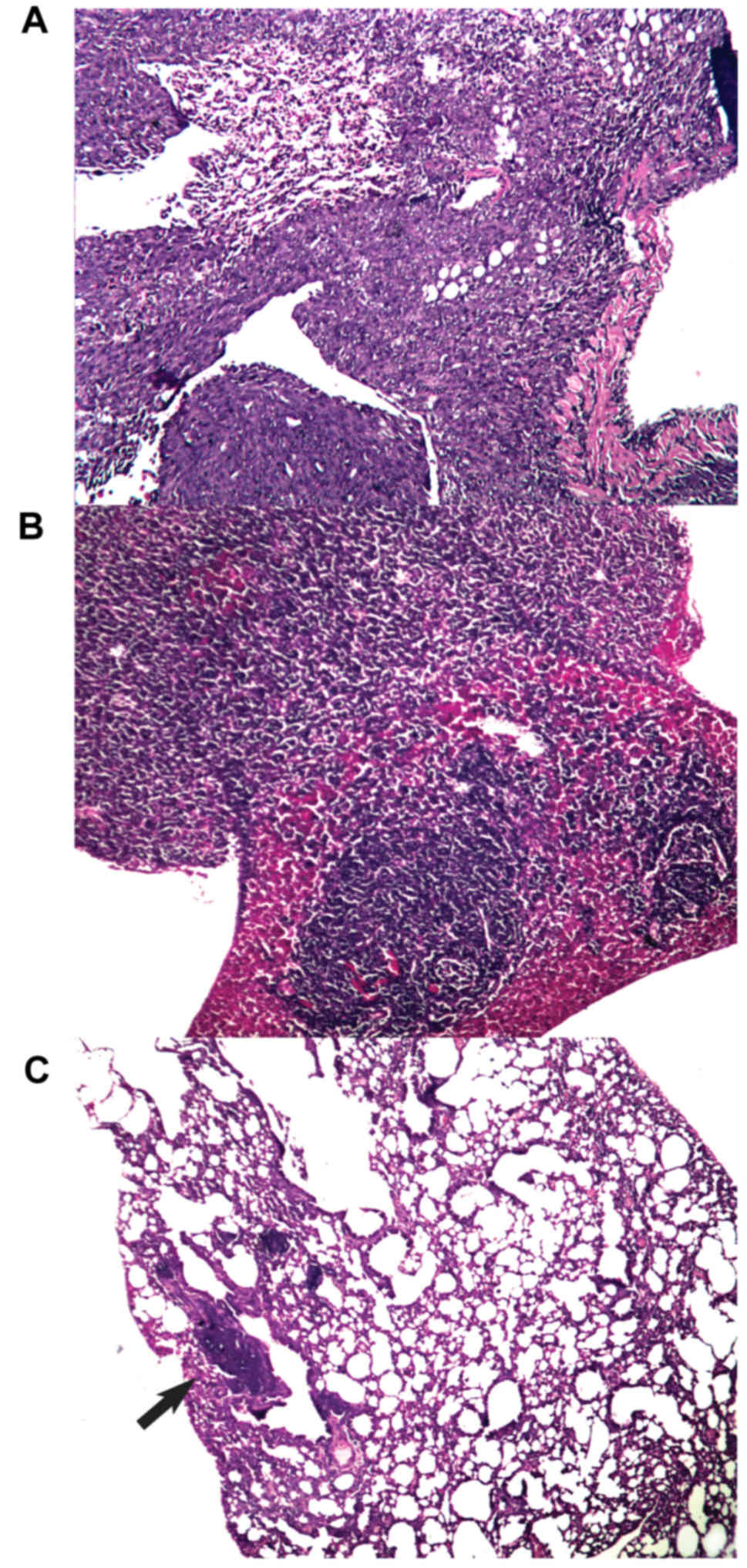
control group (n=4) had metastatic infiltrates in lung tissue (see
Fig. 5 for representative images of
metastatic infiltration). Notably, the analysis of lung metastases
from animals with metastatic spread revealed that the mean area of
metastatic foci in the xeno chicken vaccination group was
significantly smaller compared with mice that were treated with
xeno rat vaccine (0.04±0.001 mm2 vs. 1.812±0.647
mm2, respectively; P=0.034) or the control mice
(1.62±0.2.2 mm2; P=0.027). However, metastatic foci in
mice that were treated with xeno rat vaccine did not differ
significantly from the foci detected in control mice (P=0.753).
Discussion
The metastatic LLC model was introduced in the
present study since the handling of oncological patients following
primary tumour removal remains a challenge in modern oncology
(37,38). Lungs were primarily investigated for
metastatic spread owing to the known characteristic of the LLC cell
line, which spreads preferentially to lung tissue (30).
Survival analysis (Fig.
2) revealed that the xeno rat vaccine (rat brain adjuvanted
with B. subtilis) produced results that were not
statistically significant from the control (P=0.989). The mice in
the xeno chicken vaccine group exhibited a 100% survival rate
throughout the observation period. However, metastasis data
(Fig. 5) revealed that 33% of slides
produced from the lungs of mice that were treated with the xeno
chicken vaccine had metastatic infiltrates in them. However, the
size of these foci was considerably smaller compared with the
control or xeno rat groups.
The long-surviving xeno chicken vaccination group
was characterized by the highest proportion of CD8a+ in
circulating blood from all investigated groups (18.8% of T
lymphocytes vs. 12,4% in Xeno Rat and 11,2% in control groups).
This was a larger percentage compared with the value reported in a
previous study (39), where a value
of 16.6% CD8+ T lymphocytes was reported for an
autologous lysate-based vaccine, which was considered to be
clinically successful. The strong statistical correlation between
the mean survival time and CD8a+ population size in the
present study (r=0.985) indicates that cytotoxic T-lymphocytes may
be a pivotal element in the vaccine-induced antitumour immune
response, which is in agreement with data from the literature
(40–44).
It is already recognized that individual xenogeneic
vaccines do not work equally well against all types of cancer, as
previously demonstrated for xeno chicken vaccine (22). Similarly, it was demonstrated in the
present study that the xeno rat vaccine, which had a significant
anti-metastatic effect following tumour resection on B16-bearing
mice (25), does not succeed in the
metastatic LLC setup. We hypothesize that, for the xeno rat
vaccine, the cross-reactivity between the vaccine proteins and LLC
antigens is inadequate, likely to be due to insufficient protein
homology. In addition, distinct spectrum of TAAs in B16 melanoma
and LCC lung cancer cells may be responsible for the xeno rat
vaccine exhibiting activity in the former, but not the latter,
tumour model, indicating that different xenovaccine formulations
may be required for inducing or expanding immune responses against
different tumours.
Moreover, in a previously investigated metastatic
LLC model, where B. subtilis was used as an adjuvant for an
autologous tumour lysate-based vaccine, B. subtilis failed
to demonstrate satisfactory clinical benefit (39), and this underperformance was repeated
in the present study. Various vaccines (preventive and therapeutic)
are generally used with vaccine adjuvants that shape and potentiate
the induced immune responses, thereby increasing the efficacy of
vaccination (45,46). The present study, however,
demonstrates that a unadjuvanted xenogeneic vaccine (xeno chicken)
can be successfully applied, and the xenogeneic component of the
vaccine can serve as sufficient adjuvant on in its own right.
Therapeutic cancer vaccinations are the source of a
great deal of interest and following various clinical successes and
failures continues to be promising for oncological patients
(47), particularly in combinational
therapy settings (48). Despite the
investigation of various therapeutic vaccines and application
regimes in a plethora of clinical studies (49), only sipuleucel-T (Provenge; Dendreon
Pharmaceuticals LLC, Seal Beach, CA, USA) has been approved to
treat patients with metastatic, asymptomatic, castration-resistant
prostate cancer (50). This indicates
that therapeutic cancer vaccination protocols should be optimized
and standardized in terms of selection of appropriate TAAs,
adjuvants and regimens (route, dose and frequency of treatments) of
vaccine administration, preferably in combination with other cancer
treatment modalities. One of the critical factors determining the
activity of vaccination is the immunogenicity of TAAs used for
vaccination. It is believed that xenogeneic TAAs have increased
immunogenicity due to the lack of intrinsic tolerogenicity, which
is characteristic of self-protein-derived TAAs (8). Polyvalent vaccines based on lysates,
with a rich pool of various antigens, are advantageous as the broad
spectrum of targets provides cover for possible immunoediting
(51) and tumour escape (52). The insight into the clinical outcomes
of various xenovaccination therapies, highlighted in the present
study, may benefit such trials and further enhance the therapeutic
potential of xenogeneic therapeutic cancer vaccination.
Various preventive and therapeutic vaccines are
generally used with vaccine adjuvants that shape and potentiate the
induced immune responses, thereby increasing the efficacy of
vaccination (45,46). In a previously investigated metastatic
LLC model, where B. subtilis was used as an adjuvant for an
autologous tumour lysate-based vaccine, it failed to demonstrate
satisfactory clinical benefits (39);
an underperformance repeated in the present study. It is well
established that the choice of an appropriate vaccine adjuvant is
of critical importance, since inappropriate adjuvants may not only
lack its desired activity, but also trigger tolerogenic immune
response (53). Notably in the
present study, it was revealed that the unadjuvanted xeno chicken
vaccine was effective in terms of immune response and clinical
outcome. The xenogeneic component of the vaccine may serve as a
damage-associated molecular pattern that acts as an adjuvant. The
identification of xenovaccine components that act as the source of
immunogenic TAAs and as immune potentiators would allow for a
reduction in the number of variables responsible for vaccine
activity and the development of a more straightforward and
standardised therapeutic product.
The results of the present study provide a further
insight into the therapeutic potential of xenogeneic therapeutic
cancer vaccinations, and may aid the direction of preclinical
research and clinical trials in this field.
References
|
1
|
Mittal D, Gubin MM, Schreiber RD and Smyth
MJ: New insights into cancer immunoediting and its three component
phases-elimination, equilibrium and escape. Curr Opin Immunol.
27:16–25. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC Cancer Base No. 11 [Internet]. Lyon, France:
2013
|
|
3
|
Zitvogel L, Tesniere A and Kroemer G:
Cancer despite immunosurveillance: Immunoselection and
immunosubversion. Nat Rev Immunol. 6:715–727. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galluzzi L, Vacchelli E, Bravo-San Pedro
JM, et al: Classification of current anticancer immunotherapies.
Oncotarget. 5:12472–12508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nestle FO, Alijagic S, Gilliet M, Sun Y,
Grabbe S, Dummer R, Burg G and Schadendorf D: Vaccination of
melanoma patients with peptide- or tumor lysate-pulsed dendritic
cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Strioga M, Schijns V, Powell DJ Jr,
Pasukoniene V, Dobrovolskiene N and Michalek J: Dendritic cells and
their role in tumor immunosurveillance. Innate Immun. 19:98–111.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Strioga MM, Felzmann T, Powell DJ Jr,
Ostapenko V, Dobrovolskiene NT, Matuskova M, Michalek J and Schijns
VE: Therapeutic dendritic cell-based cancer vaccines: The state of
the art. Crit Rev Immunol. 33:489–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strioga MM, Darinskas A, Pasukoniene V,
Mlynska A, Ostapenko V and Schijns V: Xenogeneic therapeutic cancer
vaccines as breakers of immune tolerance for clinical application:
To use or not to use? Vaccine. 32:4015–4024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Weber LW, Bowne WB, Wolchok JD, Srinivasan
R, Qin J, Moroi Y, Clynes R, Song P, Lewis JJ and Houghton AN:
Tumor immunity and autoimmunity induced by immunization with
homologous DNA. J Clin Invest. 102:1258–1264. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Overwijk WW, Lee DS, Surman DR, Irvine KR,
Touloukian CE, Chan CC, Carroll MW, Moss B, Rosenberg SA and
Restifo NP: Vaccination with a recombinant vaccinia virus encoding
a ‘self’ antigen induces autoimmune vitiligo and tumor cell
destruction in mice: requirement for CD4(+) T
lymphocytes. Proc Natl Acad Sci USA. 96:pp. 2982–2987. 1999;
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei YQ, Wang QR, Zhao X, Yang L, Tian L,
Lu Y, Kang B, Lu CJ, Huang MJ, Lou YY, et al: Immunotherapy of
tumors with xenogeneic endothelial cells as a vaccine. Nat Med.
6:1160–1166. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steitz J, Brück J, Steinbrink K, Enk A,
Knop J and Tüting T: Genetic immunization of mice with human
tyrosinase-related protein 2: Implications for the immunotherapy of
melanoma. Int J Cancer. 86:89–94. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kornberg TB and Krasnow MA: The Drosophila
genome sequence: Implications for biology and medicine. Science.
287:2218–2220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nilsson S, Helou K, Walentinsson A,
Szpirer C, Nerman O and Ståhl F: Rat-mouse and rat-human
comparative maps based on gene homology and high-resolution
zoo-FISH. Genomics. 74:287–298. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Overwijk WW, Tsung A, Irvine KR, Parkhurst
MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA and
Restifo NP: gp100/pmel 17 is a murine tumor rejection antigen:
Induction of ‘self’-reactive, tumoricidal T cells using
high-affinity, altered peptide ligand. J Exp Med. 188:277–286.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soong RS, Trieu J, Lee SY, He L, Tsai YC,
Wu TC and Hung CF: Xenogeneic human p53 DNA vaccination by
electroporation breaks immune tolerance to control murine tumors
expressing mouse p53. PLoS One. 8:e569122013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fong L, Brockstedt D, Benike C, Breen JK,
Strang G, Ruegg CL and Engleman EG: Dendritic cell-based
xenoantigen vaccination for prostate cancer immunotherapy. J
Immunol. 167:7150–7156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wepsic HT: Overview of oncofetal antigens
in cancer. Ann Clin Lab Sci. 13:261–266. 1983.PubMed/NCBI
|
|
19
|
Lim SH, Zhang Y and Zhang J: Cancer-testis
antigens: The current status on antigen regulation and potential
clinical use. Am J Blood Res. 2:29–35. 2012.PubMed/NCBI
|
|
20
|
Malati T: Tumour markers: An overview.
Indian J Clin Biochem. 22:17–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohue Y, Wada H, Oka M and Nakayama E:
Antibody response to cancer/testis (CT) antigens: A prognostic
marker in cancer patients. Oncoimmunology. 3:e9700322014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Symchych TV, Fedosova NI, Karaman OM,
Yevstratieva LM, Lisovenko HS, Voyejkova IM and Potebnia HP: The
anticancer efficiency of the xenogeneic vaccine and the indication
for its use. Exp Oncol. 36:79–84. 2014.PubMed/NCBI
|
|
23
|
Seledtsova GV, Shishkov AA, Kaschenko EA,
Goncharov AG, Gazatova ND and Seledtsov VI: Xenogeneic cell-based
vaccine therapy for stage III melanoma: Safety, immune-mediated
responses and survival benefits. Eur J Dermatol. 26:138–143.
2016.PubMed/NCBI
|
|
24
|
Seledtsova GV, Shishkov AA, Kaschenko EA
and Seledtsov VI: Xenogeneic cell-based vaccine therapy for
colorectal cancer: Safety, association of clinical effects with
vaccine-induced immune responses. Biomed Pharmacother.
83:1247–1252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Voeykova IM, Fedosova NI, Karaman OM,
Yudina OY, Didenko GV, Lisovenko GS, Evstratieva LM and Potebnya
GP: Use of xenogeneic vaccine modified with embryonal nervous
tissue antigens in the treatment of B16-melanoma-bearing mice. Exp
Oncol. 36:24–28. 2014.PubMed/NCBI
|
|
26
|
Directive 2010/63/EU of the European
Parliament and of the Council of 22 September 2010 on the
protection of animals used for scientific purposes. 33–79.
2010.
|
|
27
|
Potebnya GP VI, Yudina OYu, Fedosova NI,
Karaman OM, Didenko GV, Yevstratyeva LM, Lisovenko GS and Chekhun
VF: The way to generate cancer vaccine. UKRPATENT: Ukraine:
2013
|
|
28
|
Isokawa K, Rezaee M, Wunsch A, Markwald RR
and Krug EL: Identification of transferrin as one of multiple
EDTA-extractable extracellular proteins involved in early chick
heart morphogenesis. J Cell Biochem. 54:207–218. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Symchych TV, Fedosova NI, Karaman ОМ,
Yevstratieva LM, Lisovenko HS, Voyeykova IM and Potebnia HP:
Anticancer effectiveness of vaccination based on xenogeneic embryo
proteins applied in different schedules. Exp Oncol. 37:197–202.
2015.PubMed/NCBI
|
|
30
|
Niu PG, Zhang YX, Shi DH, Liu Y, Chen YY
and Deng J: Cardamonin inhibits metastasis of lewis lung carcinoma
cells by decreasing mTOR activity. PLoS One. 10:e01277782015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bland JM and Altman DG: The logrank test.
BMJ. 328:10732004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Judge GD, William EG, Hill RC and Lee TC:
The theory and practise of econometrics. John Wiley & Sons; New
York: pp. 7391980
|
|
34
|
Box GEP and Cox DR: An analysis of
transformations. J R Stat Soc. 26:211–252. 1964.
|
|
35
|
Gosset WS: The probable error of a mean.
Biometrica. 6:1–25. 1908. View Article : Google Scholar
|
|
36
|
Mann HB and Whitney DR: On a test of
whether one of two random variables is stochastically larger than
the other. Ann Math Stat. 18:50–60. 1947. View Article : Google Scholar
|
|
37
|
Schirrmacher V, Fournier P and Schlag P:
Autologous tumor cell vaccines for post-operative active-specific
immunotherapy of colorectal carcinoma: Long-term patient survival
and mechanism of function. Expert Rev Vaccines. 13:117–130. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Laufer I, Iorgulescu JB, Chapman T, Lis E,
Shi W, Zhang Z, Cox BW, Yamada Y and Bilsky MH: Local disease
control for spinal metastases following ‘separation surgery’ and
adjuvant hypofractionated or high-dose single-fraction stereotactic
radiosurgery: Outcome analysis in 186 patients. J Neurosurg Spine.
18:207–214. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kraśko JA, Žilionytė K, Darinskas A,
Strioga M, Rjabceva S, Zalutsky I, Derevyanko M, Kulchitsky V,
Lubitz W, Kudela P, et al: Bacterial ghosts as adjuvants in
syngeneic tumour cell lysate-based anticancer vaccination in a
murine lung carcinoma model. Oncol Rep. 37:171–178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Foged C, Hansen J and Agger EM: License to
kill: Formulation requirements for optimal priming of CD8(+) CTL
responses with particulate vaccine delivery systems. Eur J Pharm
Sci. 45:482–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sandoval F, Terme M, Nizard M, Badoual C,
Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G,
et al: Mucosal imprinting of vaccine-induced CD8(+)T cells is
crucial to inhibit the growth of mucosal tumors. Sci Transl Med.
5:172ra1202013. View Article : Google Scholar
|
|
42
|
Chen LJ, Zheng X, Shen YP, Zhu YB, Li Q,
Chen J, Xia R, Zhou SM, Wu CP, Zhang XG, et al: Higher numbers of
T-bet(+) intratumoral lymphoid cells correlate with better survival
in gastric cancer. Cancer Immunol Immunother. 62:553–561. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Noguchi A, Kaneko T, Naitoh K, Saito M,
Iwai K, Maekawa R, Kamigaki T and Goto S: Impaired and imbalanced
cellular immunological status assessed in advanced cancer patients
and restoration of the T cell immune status by adoptive T-cell
immunotherapy. Int Immunopharmacol. 18:90–97. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thiery J and Lieberman J: Perforin: A key
pore-forming protein for immune control of viruses and cancer.
Subcell Biochem. 80:197–220. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pérez O, Batista-Duharte A, González E,
Zayas C, Balboa J, Cuello M, Cabrera O, Lastre M and Schijns VE:
Human prophylactic vaccine adjuvants and their determinant role in
new vaccine formulations. Braz J Med Biol Res. 45:681–692. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Schijns V, Tartour E, Michalek J,
Stathopoulos A, Dobrovolskiene NT and Strioga MM: Immune adjuvants
as critical guides directing immunity triggered by therapeutic
cancer vaccines. Cytotherapy. 16:427–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo C, Manjili MH, Subjeck JR, Sarkar D,
Fisher PB and Wang XY: Therapeutic cancer vaccines: Past, present,
and future. Adv Cancer Res. 119:421–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Andersen MH, Junker N, Ellebaek E, Svane
IM and Thor Straten P: Therapeutic cancer vaccines in combination
with conventional therapy. J Biomed Biotechnol. 2010:2376232010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Melero I, Gaudernack G, Gerritsen W, Huber
C, Parmiani G, Scholl S, Thatcher N, Wagstaff J, Zielinski C,
Faulkner I and Mellstedt H: Therapeutic vaccines for cancer: An
overview of clinical trials. Nat Rev Clin Oncol. 11:509–524. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cheever MA and Higano CS: PROVENGE
(Sipuleucel-T) in prostate cancer: The first FDA-approved
therapeutic cancer vaccine. Clin Cancer Res. 17:3520–3526. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Vesely MD and Schreiber RD: Cancer
immunoediting: Antigens, mechanisms, and implications to cancer
immunotherapy. Ann N Y Acad Sci. 1284:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dang Y, Wagner WM, Gad E, Rastetter L,
Berger CM, Holt GE and Disis ML: Dendritic cell-activating vaccine
adjuvants differ in the ability to elicit antitumor immunity due to
an adjuvant-specific induction of immunosuppressive cells. Clin
Cancer Res. 18:3122–3131. 2012. View Article : Google Scholar : PubMed/NCBI
|