Introduction
Adoptive cells, including cytokine-induced killer
(CIK) and natural killer (NK) cells, aimed at inducing or boosting
efficient immune responses have exhibited efficacy and safety in
clinical trials (1–6). A typical obstacle to the successful
application of these treatments is the difficulty of generating
clinically relevant numbers of CIK/NK cells (7). A previous study has demonstrated that
immunomodulators may boost the number of immune effector cells and
prime their activity efficiently (8).
Toll-like receptor (TLR) 7 is expressed in T, CIK and NK cells, in
addition to antigen-presenting cells (9). TLR-mediated combination therapies are
becoming attractive approaches due to the low toxic side effects
and high activity in physiological homeostasis that they exhibit. A
phase II clinical study was established in which a TLR9 agonist
(CpG ODN1018 ISS) was used with rituximab to treat follicular
lymphoma (10). Systemic
administration of a synthetic TLR7 agonist (R848) combined with
radiation may prime a cytotoxic T cell response against lymphoma
cells, and prime a memory immune response that may prevent
recurrence of lymphoma (11).
Enhanced antitumor efficacy was also identified when the TLR9
agonist (CpG ODN1826) was combined with radio- and chemotherapy
(12). These previous studies led to
the present study which aimed at determining the role of the TLR7
agonist in the activation of adoptive cells, including CIK and NK
cells.
A protocol has been established previously to
rapidly and reproducibly expand CIK cells in vitro from
human peripheral blood (13–16). In the present study,
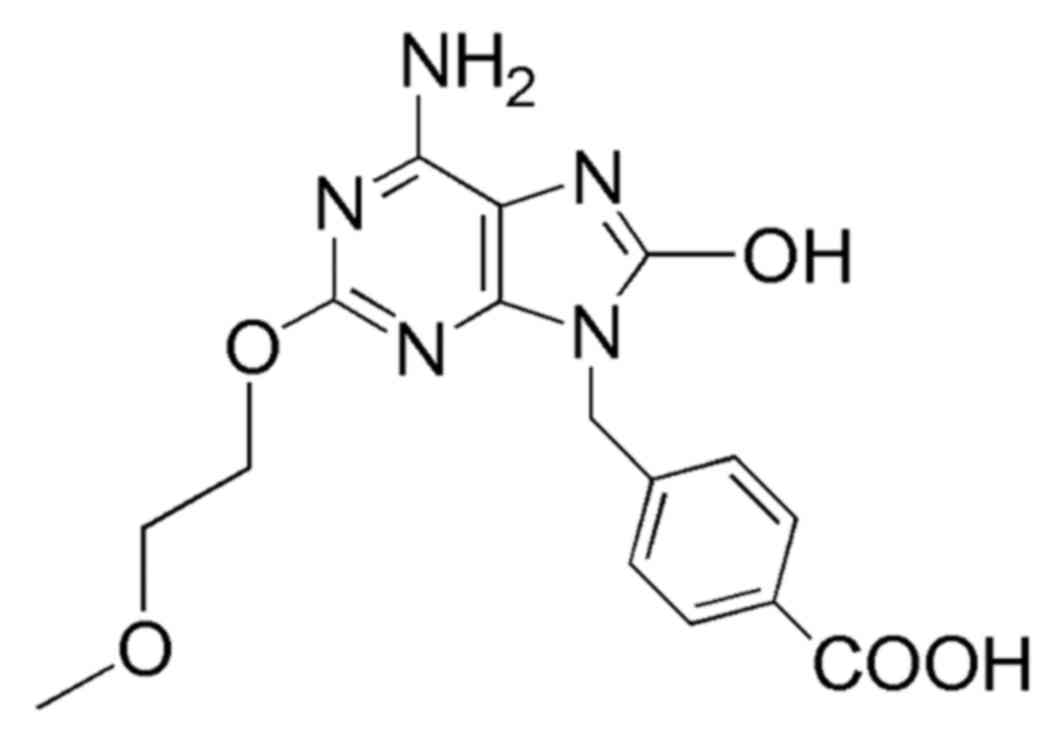
9-(4-carboxyphenyl)-8-hydroxy-2-(2-methoxyethoxy)-adenine (termed
Gao Dong, GD) (17), a novel TLR7
agonist, was combined with the traditional protocol of culturing
CIK cells to determine the role of GD in the activation of CIK/NK
cells. The results of the present study demonstrated that GD may
activate CIK/NK cells. Notably, the combination therapy with CIK/NK
cells, stimulated by GD, markedly suppressed the proliferation of
the chronic myelogenous leukemia K562 cell line. The results of the
present study suggested a novel protocol for CIK/NK cell
proliferation and indicated that GD may serve as a potent innate
and adaptive immunomodulator in immunocyte culture. This novel
combination therapy concept may be a solution to the difficulty of
chemotherapy and adoptive immunotherapy.
Materials and methods
Cell culture media and reagents
RPMI-1640 serum-free medium (Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) was used with 10% fetal bovine
serum (Gibco; Thermo Fisher Scientific, Inc.). CIK/NK cells
(1×106 cells/ml, Pierce; Thermo Fisher Scientific, Inc.)
were generated and cultured with 80 U/ml gentamycin. Human
recombinant interleukin (IL)-2, ≥1,000 U/ml, was purchased from
Beijing ShuangLu Pharmaceutical Co., Ltd. (Beijing, China). GD
(Fig. 1), synthesized in our
laboratory, was added to the cell culture medium at a concentration
of 5 µM.
Cell culture and quantification
The control CIK cells were generated as follows:
Human peripheral blood mononuclear cells (PBMCs) from three donors
were isolated by density gradient centrifugation at 4°C at 700 × g
for 20 min using Ficoll (Takeda Pharmaceutical Company, Ltd.,
Tokyo, Japan). The cells were grown in AIM-V serum-free medium
(Gibco; Thermo Fisher Scientific, Inc.) which consisted of 5%
autologous plasma and 80 U/ml gentamycin. A total of 1,000 U/ml
human recombinant interferon-γ (Gene Tech Co., Ltd., Hong Kong,
China) were added on day 0. After 24 h of incubation at 37°C, 50
ng/ml OKT3 antibody (cat. no. 555339) against cluster of
differentiation (CD)3 (Ortho Biotech, Inc., Raritan, NJ, USA), 100
U/ml IL-1 (Genzyme, Cambridge, MA, USA) and 300 U/ml IL-2 (Genzyme)
were added. Cells were incubated at 37°C in a humidified atmosphere
containing 5% CO2 and were subcultured every 3 days in
fresh AIM-V with the addition of 5% auto serum and 1,000 U/ml IL-2
at 1×106 cells/ml.
The harvested PBMCs from each donor were divided
into three equally and treated as follows: One group was cultured
following the protocol as aforementioned and termed ‘none’ (the
control group); another received the addition of GD with IFN-γ on
day 0 and was termed group ‘+GD’; and the third group received GD
only and on day 0, was termed group ‘+GD-IFN-γ’.
Cell numbers were counted using the Cell Coulter
Technique (Z2 Coulter; Coulter Electronics, Ltd., Luton, UK) on
days 0, 3, 5, 7, 9 and 15. Cell viability was analyzed using the
trypan blue dye exclusion assay at each time point. Trypan blue
dye-exclusion assay was performed by adding 20 µl 0.4% dye
solution, and live (unstained) and dead (stained) cells were
counted under Leica inverted microscope DMi1 (×10 magnification;
Leica Microsystems GmbH, Wetzlar, Germany). Five random microscopic
fields were counted in each sample. A total of 300 cells were
counted per sample. Non-viable cells were detected as those which
took up dye. Written informed consent was obtained from all human
donors of PBMCs, and the present study received ethical approval by
Shenzhen University Health Science Center Medical Ethics Committee
(Shenzhen, China).
Analyses of lymphocyte subsets
Cell cultures were prepared for flow cytometric
phenotypic analysis and four-color fluorescence was performed
according to standard procedures (16). Briefly, 105 CIK cells
derived from PBMCs of donors suspended in 50 µl PBS were stained
with 10 µl fluorochrome-conjugated monoclonal antibodies (1:50; BD
Biosciences, Franklin Lakes, NJ, USA) against CD3 (cat. no.
561809), CD4 (cat. no. 561841), CD8 (cat. no. 566451, all T-cell
antigens) and CD56 (cat. no. 557747, NK-cell antigen). Following
the addition of the primary antibody, cells were incubated for 20
min at room temperature and subsequently washed with PBS. Phenotype
analysis was carried out using an Accuri C6 flow cytometer (BD
Biosciences). The data were analyzed using BD CellQuest Pro
Software (version 5.1, BD Biosciences).
Cell-mediated cytotoxicity
Cells from the three donors were analyzed on day 15
following culture and the K562 cell line was used as the target.
Cell viability of K562 cells was evaluated in vitro using a
Cell Counting Kit-8 (CCK-8). K652 cells were seeded in triplicate
in 96-well plates (2×104 cells/well) and CIK/NK cells
were added at ratios of effector to target of 5:1, 10:1 and 20:1.
Following incubation for 18 h at 37°C in a humidified atmosphere
containing 5% CO2, 20 µl CCK-8 was added to each well
prior to incubation for 4 h at 37°C.
The absorbance of each well was determined with an
ELISA reader (iMark; Bio-Rad Laboratories, Inc., Hercules, CA, USA)
at a wavelength of 450 nm. Cytotoxicity (%) was calculated using
the following equation:
Cytotoxicity(%)=[1–A450(effector+target)–A450(effectorcontrol)A450(targetcontrol)]x100
Statistical analysis
Statistical significance was analyzed using
Student's t-test using Prism software version 4.0c (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Cell proliferation rates
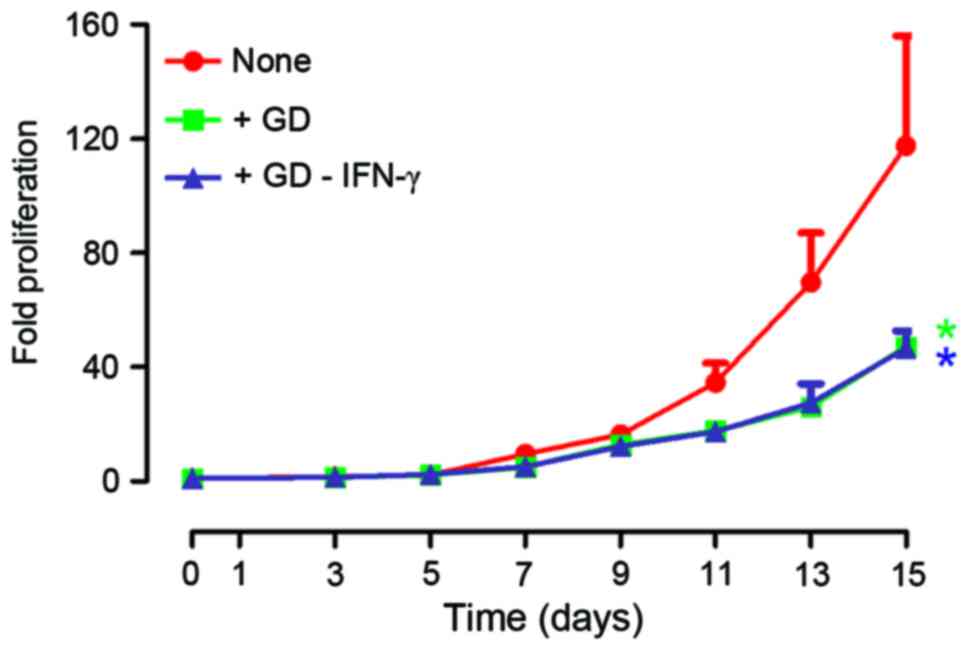
After 15 days, the proliferation rates of cells were
increased 127-fold (median; range, 45–178), 46-fold (median; range,
43–53) and 45-fold (median; range, 37–57) in group ‘none’, ‘+GD’
and ‘+GD-IFN-γ’, respectively (Fig.
2). The results of the present study indicate that GD decreased
the range of proliferation rates from different donors. Thus, the
protocol of the present study may yield relatively uniform CIK/NK
cells that may standardize CIK/NK cells.
Effect of GD on the phenotype of
CIK/NK cells
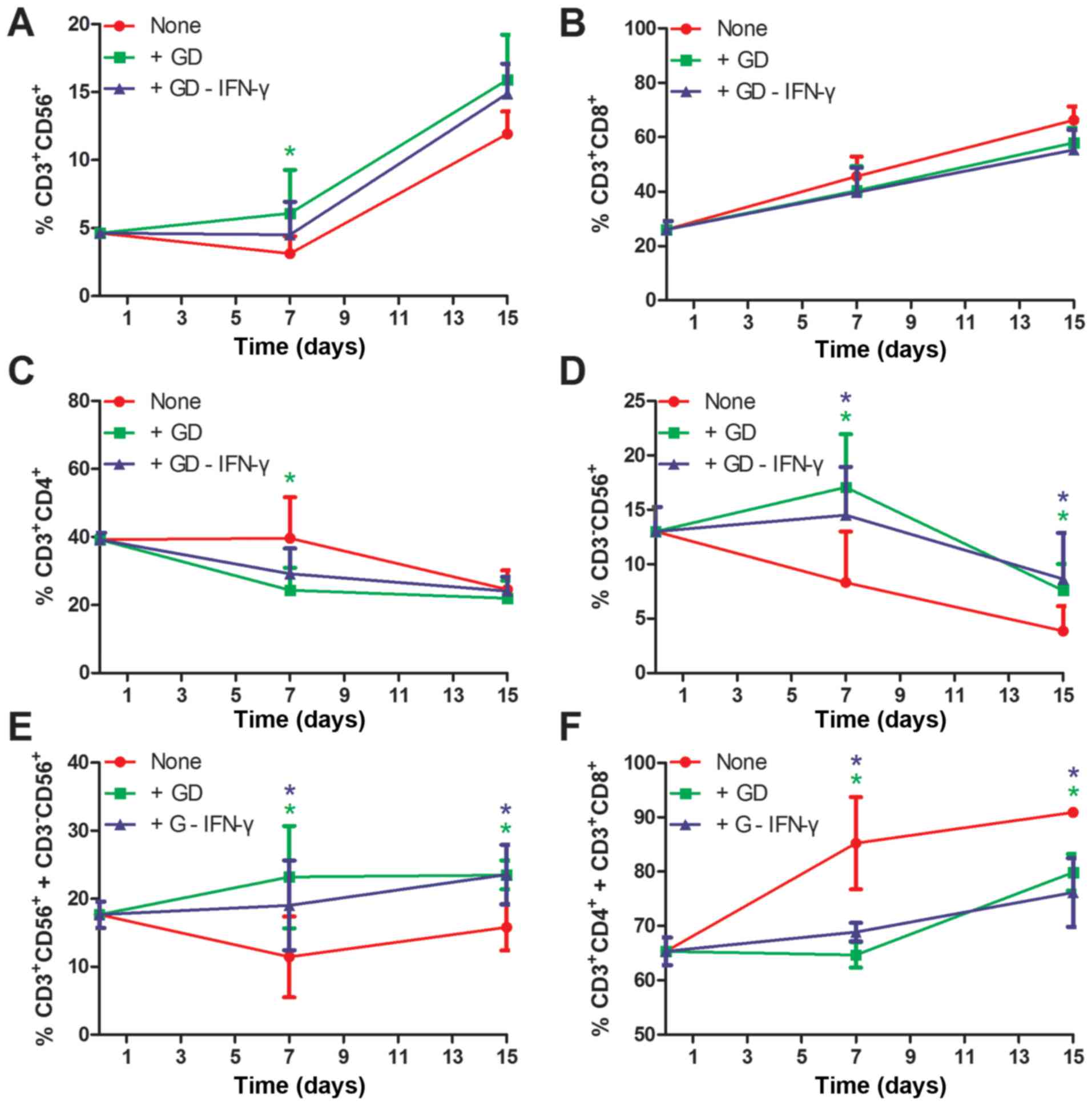
As presented in Figs.
3 and 4, the proportions of
CD3+CD56+ CIK cells (Fig. 3A) and CD3+CD8+ T
cells (Fig. 3B) increased over the
15-day period. However, for the CD3+CD4+ T
cells (Fig. 3C) and
CD3−CD56+ NK cells (Fig. 3D), the proportions decreased over the
15-day period. Furthermore, the proportions of GD-induced CIK cells
and NK cells were ~4% higher compared with the control (15.9±5.8
vs. 11.9±2.9 and 7.6±4.2 vs. 3.8±3.7, respectively; Fig. 3A and D; Fig.
4). Additionally, the proportions of GD-induced
CD3+CD4+ T cells and
CD3+CD8+ cells were ~9% and 3% lower compared
with the control, respectively (Fig. 3B
and C; Fig. 4). Notably, there
were significant differences between GD treatment and none, or
between GD-IFN-γ treatment and none at days 7 and 15, when effector
cells (CIK/NK) and other cells were considered (Fig. 3E and F). Treatment with GD may be able
to counteract the IFN-γ-stimulated function in CIK culture, which
was confirmed by data collected from the ‘+GD-IFN-γ’ group
(Figs. 3 and 4). A notable inter-donor difference was that
the final proportions of CIK and NK cells yield were linearly
dependent on their initial numbers (data not shown).
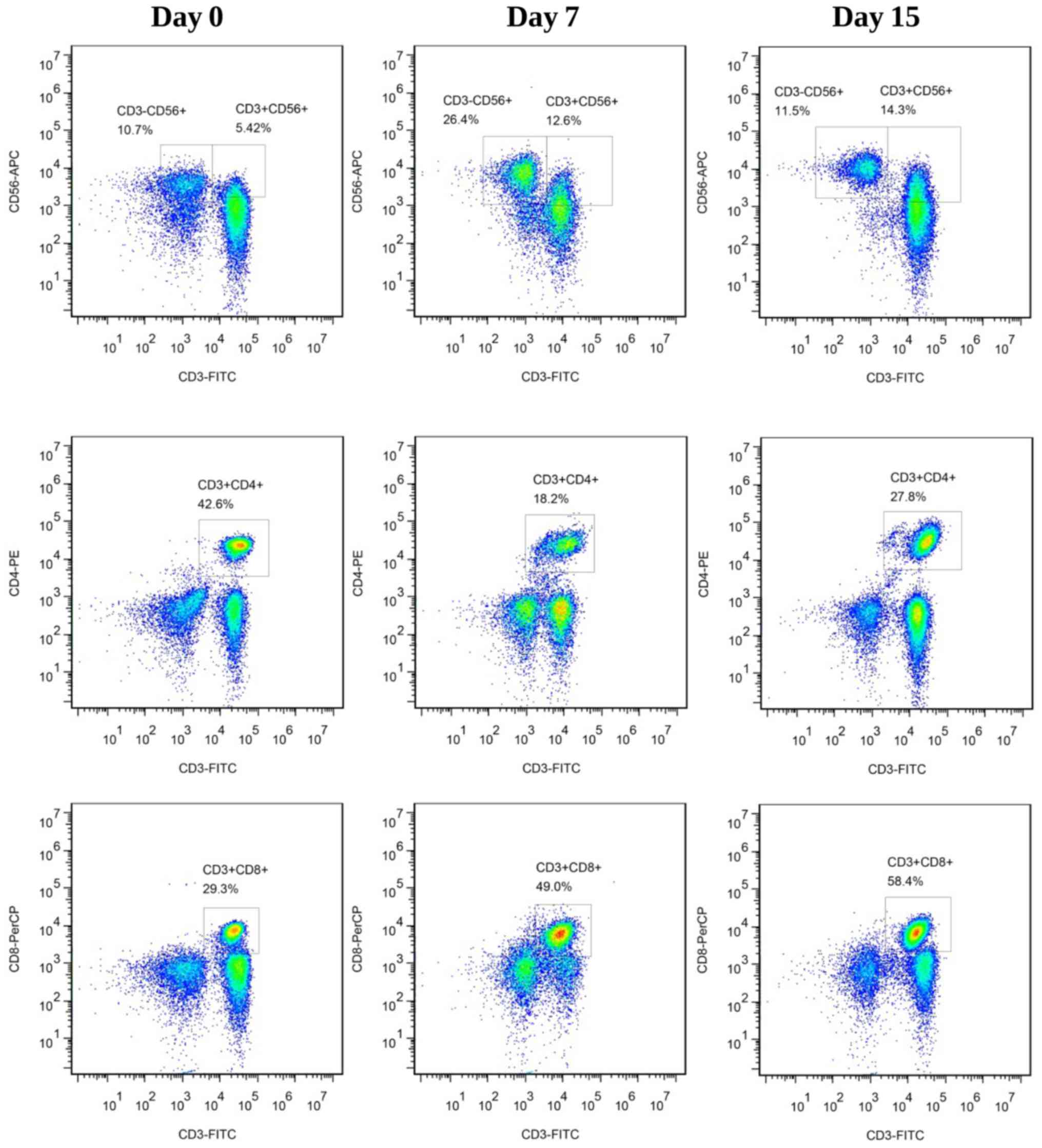 | Figure 4.Phenotype of CIK, NK and T-cells in
group ‘+GD’ on days 0, 7 and 15 of culture. Results are
representative of three experiments. CIK, cytokine-induced killer;
NK, natural killer; GD,
9-(4-carboxyphenyl)-8-hydroxy-2-(2-methoxyethoxy)-adenine; FITC,
fluorescein isothiocyanate; CD, cluster of differentiation; APC,
allophycocyanin; PE, phycoerythrin; PerCP, peridinin chlorophyll
protein complex. |
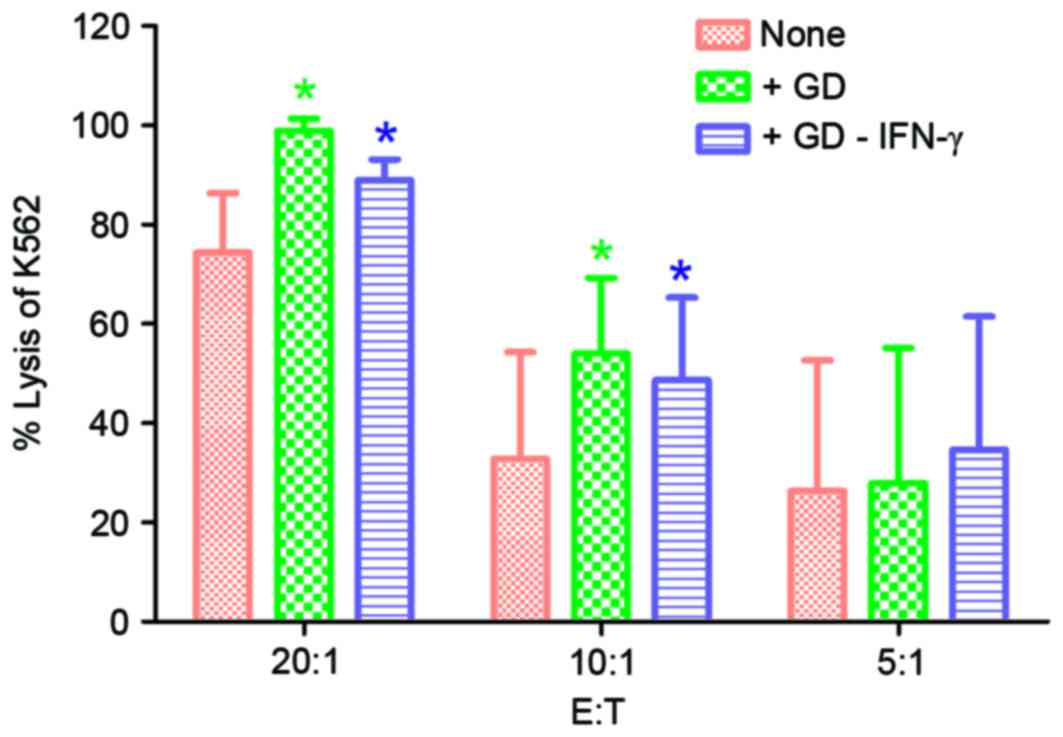
Enhancement of cytotoxic capacity of
CIK/NK cells stimulated by GD
The killing rates were notably increased in the
‘+GD’ and ‘+GD-IFN-γ’ groups compared with the control group
(P<0.05; Fig. 5). The killing
rates for group ‘+GD’ vs. group ‘none’ were 98.9±2.4 vs. 74.4±12.0,
54.0±15.3 vs. 32.8±21.5 and 28.0±27.2 vs. 26.3±26.3%, respectively.
Killing rates of group ‘+GD-IFN-γ’ vs. group ‘none’ were 89.0±4.2
vs. 74.4±12.0, 48.7±16.6 vs. 32.8±21.5 and 34.6±26.8 vs.
26.3±26.3%, at effector/target ratios of 20:1, 10:1 and 5:1,
respectively. The results of the present study indicate that GD
induced a marked cytotoxic effect of CIK/NK cells on K562
cells.
Discussion
CIK cells are a heterogeneous subset of lymphocytes
generated by incubating PBMCs with various cytokines in
vivo. CIK and NK cells in CIK culture systems are important
antitumor effectors in immunotherapy (18). Culture systems used to provide
adequate numbers of immune cells have focused primarily on
stimulation with more effective cytokine and monoclonal antibodies
(19,20). To the best of our knowledge, existing
methods used for expanding CIK cells were almost negative with
regard to expanding NK cells, but provided a high ratio of
CD3+CD8+ T cells (21). Excessive
CD3+CD8+ T cell numbers are not beneficial
for eliminating tumor cells; however, they increase the risk of
graft-versus-host disease (22,23). Thus,
a method in which CIK/NK cells expand synchronously and
CD3+CD8+ T cells proliferation decreases, may
be valuable, economical and practical for clinical application.
TLR7 agonists are effective immunomodulators as they
directly promote the activation of T, CIK and NK cells, induce
tumor cell apoptosis or sensitize tumor cells to be killed by
cytotoxic T lymphocytes and NK cells (24). CIK/NK cells alone and in combination
with other therapies, including TLR7/8 agonists and
imidazoquinoline analogs, are proven to be useful and safe
(10–12). In the present study, the activity of
the TLR7 agonist GD for the proliferation of CIK was determined for
the first time, to the best of our knowledge. The results indicated
that GD significantly promoted the proliferative capacity of CIK/NK
and decreased the proliferative capacity of no-CIK/NK cells,
including CD3+CD4+ and
CD3+CD8+ T cells, compared with the control.
Additionally, GD improved the cytotoxic effect of CIK/NK cells on
K562 cells. Thus, positive T cell co-stimulation by TLR7/8 ligands
is dependent on the cellular environment (25).
TLR7/8 agonists have been demonstrated previously to
markedly upregulate IFN-γ and IL-12 (26), thus it may be possible to decrease the
amount of exogenous IFN-γ used in the cell culture, and
consequently decrease the toxicity exhibited from exogenous IFN-γ.
It is hypothesized that local expression of IFN-γ and IL-12 may be
less harmful to the patient than systemic administration of high
amounts of IFN-γ and IL-12. The results of the ‘+GD-IFN-γ’ group
indicated that the local IFN-γ is a component of exogenous IFN-γ
and, notably, the amount of IFN-γ secreted by the cells appeared to
be sufficient to increase proliferation of CIK/NK cells. IL-12
enhances efficacy and decreases the enrichment time in CIK
immunotherapy (27). Imidazoquinoline
analogs (TLR7/8 agonists) promoted the proliferative capacity of NK
cells to enhance antitumor effects (8). The results of the present study
indicated a marked increase in the cytotoxic activity at a low
ratio of effector to target cells. This appears to be of major
importance, as it is difficult to inject patient effector cells at
a ratio of 40:1 of effector to target cells in a clinical
situation.
The results of the present study have demonstrated
for the first time, to the best of our knowledge, that a TLR7
purine agonist promoted the proliferative capacity of CIK/NK cells
to enhance antitumor effects. Since GD is involved in immune
responses in immunological cell culture in vitro, GD was
separated in culture medium while cells were harvested, therefore
GD-free cells were reinfused in vivo. This strategy may
reduce the risk of toxic cytokine syndrome induced by TLR7 ligands
if injected directly in vivo. Furthermore, the heterogeneous
cultured cell subset rich in CIK/NK cells and a low proportion of
CD3+CD8+ T cells may be valuable in
attenuating side effects and may be economical and practical for
clinical application. This novel combination therapy concept may be
a solution to the problem of chemotherapy and adoptive
immunotherapy.
Acknowledgements
The present study was supported by the Basic
Research Program of Shenzhen (grant nos. JCYJ20130326110139687,
JSGG20160226161357949 and CXZZ20140509144527788) and the China
Postdoctoral Science Foundation (grant no. 2014M552234).
References
|
1
|
Rosenberg SA, Restifo NP, Yang JC, Morgan
RA and Dudley ME: Adoptive cell transfer: A clinical path to
effective cancer immunotherapy. Nat Rev Cancer. 8:299–308. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schmeel FC, Schmeel LC, Gast SM and
Schmidt-Wolf IG: Adoptive immunotherapy strategies with
cytokine-induced killer (CIK) cells in the treatment of
hematological malignancies. Int J Mol Sci. 15:14632–14648. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung MJ, Park JY, Bang S, Park SW and
Song SY: Phase II clinical trial of ex vivo-expanded
cytokine-induced killer cells therapy in advanced pancreatic
cancer. Cancer Immunol Immunother. 63:939–946. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu X, Zhao H, Liu L, Cao S, Ren B, Zhang
N, An X, Yu J, Li H and Ren X: A randomized phase II study of
autologous cytokine-induced killer cells in treatment of
hepatocellular carcinoma. J Clin Immunol. 34:194–203. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pérez-Martínez A, Fernández L, Valentín J,
Martínez-Romera I, Corral MD, Ramírez M, Abad L, Santamaría S,
González-Vicent M, Sirvent S, et al: A phase I/II trial of
interleukin-15-stimulated natural killer cell infusion after
haplo-identical stem cell transplantation for pediatric refractory
solid tumors. Cytotherapy. 17:1594–1603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakamoto N, Ishikawa T, Kokura S, Okayama
T, Oka K, Ideno M, Sakai F, Kato A, Tanabe M, Enoki T, et al: Phase
I clinical trial of autologous NK cell therapy using novel
expansion method in patients with advanced digestive cancer. J
Transl Med. 13:2772015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonanno G, Iudicone P, Mariotti A, Procoli
A, Pandolfi A, Fioravanti D, Corallo M, Perillo A, Scambia G,
Pierelli L and Rutella S: Thymoglobulin, interferon-γ and
interleukin-2 efficiently expand cytokine-induced killer (CIK)
cells in clinical-grade cultures. J Transl Med. 8:1292010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou Z, Yu X, Zhang J, Tian Z and Zhang C:
TLR7/8 agonists promote NK-DC cross-talk to enhance NK cell
anti-tumor effects in hepatocellular carcinoma. Cancer Lett.
369:298–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Smits EL, Ponsaerts P, Berneman ZN and Van
Tendeloo VF: The use of TLR7 and TLR8 ligands for the enhancement
of cancer immunotherapy. Oncologist. 13:859–875. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friedberg JW, Kelly JL, Neuberg D,
Peterson DR, Kutok JL, Salloum R, Brenn T, Fisher DC, Ronan E,
Dalton V, et al: Phase II study of a TLR-9 agonist (1018 ISS) with
rituximab in patients with relapsed or refractory follicular
lymphoma. Br J Haematol. 146:282–291. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dovedi SJ, Melis MH, Wilkinson RW, Adlard
AL, Stratford IJ, Honeychurch J and Illidge TM: Systemic delivery
of a TLR7 agonist in combination with radiation primes durable
antitumor immune responses in mouse models of lymphoma. Blood.
121:251–259. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mason KA, Neal R, Hunter N, Ariga H, Ang K
and Milas L: CpG oligodeoxynucleotides are potent enhancers of
radio- and chemoresponses of murine tumors. Radiother Oncol.
80:192–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schmidt-Wolf IG, Negrin RS, Kiem HP, Blume
KG and Weissman IL: Use of a SCID mouse/human lymphoma model to
evaluate cytokine-induced killer cells with potent antitumor cell
activity. J Exp Med. 174:139–149. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu PH and Negrin RS: A novel population of
expanded human CD3+CD56+ cells derived from T
cells with potent in vivo antitumor activity in mice with severe
combined immunodeficiency. J Immunol. 153:1687–1696.
1994.PubMed/NCBI
|
|
15
|
Hoyle C, Bangs CD, Chang P, Kamel O, Mehta
B and Negrin RS: Expansion of Philadelphia chromosome-negative
CD3(+)CD56(+) cytotoxic cells from chronic myeloid leukemia
patients: In vitro and in vivo efficacy in severe combined
immunodeficiency disease mice. Blood. 92:3318–3327. 1998.PubMed/NCBI
|
|
16
|
Alvarnas JC, Linn YC, Hope EG and Negrin
RS: Expansion of cytotoxic CD3+ CD56+ cells
from peripheral blood progenitor cells of patients undergoing
autologous hematopoietic cell transplantation. Biol Blood Marrow
Transplant. 7:216–222. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao D, Diao Y, Li W, Gao N, Liu Y, Wang Z,
Jiang W and Jin G: Toll-like receptor 7 inactive ligands enhanced
cytokine induction by conjugation to weak antigens. ChemMedChem.
10:977–980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pittari G, Filippini P, Gentilcore G,
Grivel JC and Rutella S: Revving up natural killer cells and
cytokine-induced killer cells against hematological malignancies.
Front Immunol. 6:2302015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li Y, Schmidt-Wolf IG, Wu YF, Huang SL,
Wei J, Fang J, Huang K and Zhou DH: Optimized protocols for
generation of cord blood-derived cytokine-induced killer/natural
killer cells. Anticancer Res. 30:3493–3499. 2010.PubMed/NCBI
|
|
20
|
Deng QI, Bai X, Lv HR, Xiao X, Zhao MF and
Li YM: Anti-CD20 antibody induces the improvement of
cytokine-induced killer cell activity via the STAT and MAPK/ERK
signaling pathways. Exp Ther Med. 9:1215–1222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pievani A, Borleri G, Pende D, Moretta L,
Rambaldi A, Golay J and Introna M: Dual-functional capability of
CD3+CD56+ CIK cells, a T-cell subset that
acquires NK function and retains TCR-mediated specific
cytotoxicity. Blood. 118:3301–3310. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abrahamsen IW, Sømme S, Heldal D, Egeland
T, Kvale D and Tjønnfjord GE: Immune reconstitution after
allogeneic stem cell transplantation: The impact of stem cell
source and graft-versus-host disease. Haematologica. 90:86–93.
2005.PubMed/NCBI
|
|
23
|
Xu K, Li C, Pan X and Du B: Study of
relieving graft-versus-host disease by blocking CD137-CD137 ligand
costimulatory pathway in vitro. Int J Hematol. 86:84–90. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee M, Park CS, Lee YR, Im SA, Song S and
Lee CK: Resiquimod, a TLR7/8 agonist, promotes differentiation of
myeloid-derived suppressor cells into macrophages and dendritic
cells. Arch Pharm Res. 37:1234–1240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Richardt-Pargmann D, Wechsler M, Krieg AM,
Vollmer J and Jurk M: Positive T cell co-stimulation by TLR7/8
ligands is dependent on the cellular environment. Immunobiology.
216:12–23. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ghosh TK, Mickelson DJ, Solberg JC, Lipson
KE, Inglefield JR and Alkan SS: TLR-TLR cross talk in human PBMC
resulting in synergistic and antagonistic regulation of type-1 and
2 interferons, IL-12 and TNF-alpha. Int Immunopharmacol.
7:1111–1121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Helms MW, Prescher JA, Cao YA, Schaffert S
and Contag CH: IL-12 enhances efficacy and shortens enrichment time
in cytokine-induced killer cell immunotherapy. Cancer Immunol
Immunother. 59:1325–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|