Introduction
Pancreatic cancer is a highly malignant disease,
which has the fourth highest mortality rate with a 1-year survival
rate of ~29% globally in 2012, in addition to the fact that the
prognosis of this disease is considerably poor (1). Surgery is a potentially curative option
for pancreatic cancer, however a majority of patients present
symptoms when the tumor has reached an inoperable stage (2). Chemotherapy and/or radiotherapy are the
standard treatments for patients with inoperative pancreatic
cancer. The standard first line drug gemcitabine (GEM), which is a
deoxycytidine analogue, is used to treat patients with advanced
pancreatic cancer; however, its efficacy remains low with 5-year
survival rates remaining at 20–25% in 2005 (3,4).
Therefore, there has been considerable interest in combining GEM
with other systemic agents in order to improve patient
outcomes.
Traditional Chinese herbs may represent a useful
resource for drug development (5).
Among them, baicalein (BAI) is a purified flavonoid extracted from
the root of Scutellaria baicalensis that possesses a wide
variety of biological activities, including anti-inflammatory,
anti-infection, anti-microbial, neuro-protective and anti-oxidant
effects (6,7). BAI has exhibited anti-tumor effects on
multiple different types of cancer by inhibiting proliferation and
inducing cancer cell apoptosis, including in liver cancer, prostate
cancer, lung cancer, breast cancer, bladder cancer and cervical
cancer (8–13). The antitumor activity of BAI is
mediated by activation of Bcl-2-associated X protein (Bax), B-cell
lymphoma 2 (Bcl-2), caspase-3 and poly ADP-ribose polymerase (PARP)
(14). Therefore, it is important to
evaluate the beneficial effect of BAI as a single treatment or when
administered in combination with other conventional anticancer
agents in pancreatic cancer.
In the present study, the effects of BAI on
pancreatic cancer cells were examined. It was revealed that BAI
enhanced the effects of GEM treatment in the inhibition of CFPAC-1
cell viability in vitro by inducing cell apoptosis. In
vivo, combination treatment resulted in synergistically reduced
tumor volume and weight in a xenograft mouse model. Therefore, the
present study identified that combined BAI and GEM treatment may be
a promising potential treatment for pancreatic cancer.
Materials and methods
Cell culture
Human CFPAC-1 pancreatic ductal adenocarcinoma and
PANC-1 pancreatic cancer cell lines were purchased from the
Shanghai Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). The 2 cell lines were cultured in DMEM (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), supplemented with 10%
fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.),
100 U/ml penicillin and 100 µg/ml streptomycin in a 37°C humidified
atmosphere containing 5% CO2/95% air.
MTT assay
CFPAC-1 or PANC-1 cells were cultured in 96-well
plates at 1×105 cells per well. After 24 h, the cells
were treated with multiple concentration of BAI alone (0.1, 0.2,
0.4, 0.8, 1.6, 3.2, 6.25, 12.5, 25, 50 and 100 µM), GEM alone
(0.03, 0.06, 0.125, 0.25, 0.5 and 1 µg/ml) or combination treatment
(0.03, 0.06, 0.125, 0.25, 0.5 and 1 µg/ml GEM with either 1.6 or
6.25 µg BAI) for 48 h, the control group was treated with PBS (pH
7.4). Subsequently, 50 µl MTT (1 µg/ml; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to the cell media and incubated for 4
h at 37°C. MTT was discarded and 150 µl DMSO was loaded in each
well. The spectrophotometric absorbance of the samples was measured
using a microplate reader (Bio-Rad Model 680; Bio-Rad Laboratories,
Inc., Hercules, CA, USA) at 570 nm with a reference wavelength of
655 nm. The percentage of cell survival was calculated using the
following formula: Cell survival=absorbance value of infected
cells/absorbance value of uninfected control cells. Six reduplicate
wells were measured at each concentration and every experiment was
performed at ≥3 times.
Combination index (CI) analysis
CI was used to evaluate the pharmacological
interactions of different combinations of BAI and GEM (15). Briefly, synergism, additivity or
antagonism in the BAI and GEM combinations was calculated on the
basis of the multiple drug effect equation and quantitated by CI,
where CI=1 indicates that the two drugs have additive effects,
CI<1 indicates synergism effects and CI>1 indicates
antagonism effects. The CI was calculated based on:
CI=(D)1/(Dx)1+(D)2/(Dx)2+(D)1(D)2/=(Dx)1(Dx)2, where (Dx) is the
dose of the drug, inhibiting ‘x%’.
Hoechst 33258 staining
Following GEM (0.125 µg/ml), BAI (1.60 or 6.25 µM)
or combination treatment, the control group was treated with PBS,
the cells were fixed and stained using Hoechst 33258 for 10 min at
room temperature and observed using a fluorescence microscope
(Olympus Corporation, Tokyo, Japan) at ×20 magnification. A total
of 6 fields per treatment were assessed. The stained cells were
identified as apoptotic if they were highly condensed with brightly
stained nuclei, or non-apoptotic if they were stained pale
blue.
Apoptosis assay by flow cytometry
CFPAC-1 or PANC-1 cells treated with 0.125 µg/ml GEM
or 1.6/6.25 µM BAI alone or a combination treatment were incubated
for 48 h at 37°C, the control group was treated with vehicle
solution, and then cells were collected and washed twice with cold
PBS and subsequently incubated with Annexin V-FITC/7-ADD double
staining solution at room temperature for 15 min. Then, the stained
cells were analyzed using flow cytometry (BD Biosciences, San Jose,
CA, USA). The cell apoptotic rates were analyzed using ModFIT
software (version 3.2; Verity Software House, Inc., Topsham, ME,
USA). Apoptotic events were recorded as a combination of Annexin
V+/7-AAD− (early apoptotic) and Annexin
V+/7-AAD+ (late apoptotic/dead) events. This
experiment was conducted 3 times.
Western blot analysis
Whole cell lysates from CFPAC-1 cells treated with
vehicle control or drugs for 48 h, then were washed using PBS once
and lysed using RIPA lysis buffer (Beyotime Institute of
Biotechnology, Haimen, China), containing protease inhibitor
cocktail (Roche Diagnostics, Basel, Switzerland) and phosphatase
inhibitor cocktail (Roche Diagnostics), and sonicated for 15 min on
ice. Protein concentration was measured using a BCA protein assay
(Bio-Rad Laboratories, Inc.). A total of 30 µg samples were
separated by SDS-PAGE on a 10% gel and transferred to
polyvinylidene difluoride membranes using the Bio-Rad
electrotransfer system (Bio-Rad Laboratories, Inc.). The membrane
was blocked using 5% fat-free milk for 1 h at room temperature and
then incubated with appropriate primary antibody diluted in 3% BSA
solution at 4°C overnight. For immunodetection, the following
antibodies were used for our analysis: anti-Bax (cat no. 5023),
-Bcl-2 (cat no. 15071), -Caspase-3 (cat no. 9662), -PARP (cat no.
9532) and -Survivin (cat no. 2808) antibodies (1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), followed by
incubation with DyLight dye-conjugated secondary antibodies (cat
nos. 35502 and 35568; 1:10,000; Thermo Fisher Scientific, Inc.) for
1 h at room temperature. Blots were scanned using the Odyssey
Western Detection system (LI-COR Biosciences, Lincoln, NE, USA),
followed by quantification using ImageStudio software (version
5.2.5; LI-COR Biosciences). Anti-β-actin (cat no. sc-47778; Santa
Cruz Biotechnology, Inc.; 1:1,000 dilution) was used as a loading
control.
In vivo model
A total of 24 female BALB/c nude mice (4–5 week-old,
15–16 g) were purchased from the Shanghai Laboratory Animal Center
(Shanghai, China). Mice were allowed free access to sterilized
water and food and were housed in individual ventilated cages at
23±5°C under a 12-h light/dark cycle. CFPAC-1 cells were
resuspended in serum-free DMEM at a concentration of
1×107 cells/ml. A total volume of 0.2 ml cell suspension
(total 2×106 cells) was then injected subcutaneously
into the right anterior armpit of nude mice to establish a
xenograft model. The 24 injected mice were randomly divided into 4
groups: control, BAI, GEM and combination treated groups (n=6 per
group). Control group was treated with vehicle solution. The mice
were sacrificed at day 28 or when the tumor grew to a maximum of
1,500 µM3. The Animal Care and Use Committee of Nanjing
University of Chinese Medicine ethically approved the experimental
protocol.
Immunohistochemical assay
Tumor xenografts were fixed in formalin solution
overnight at room temperature and embedded in paraffin blocks,
which were sliced into 4-µm thick sections for immunohistochemical
staining. Following deparaffinization, the sections were incubated
at 4°C overnight with antibodies of Bcl-2 (cat no. 15071), Bax (cat
no. 5023), caspase-3 (cat no. 9662) and survivin (cat no. 2808)
(1:500; Cell Signaling Technology, Inc.). The sections were washed
3 times with PBS with 0.1% Tween-20 (PBST), and incubated for 1 h
at room temperature with a horseradish peroxidase-conjugated goat
anti-mouse immunoglobulin G antibody (1:200; cat no. sc-2031; Santa
Cruz Biotechnology, Inc.). The slices were washed with PBST 3
times, and incubated in diaminobenzidine (Sangon Biotech Co., Ltd.,
Shanghai, China) for 5 min at room temperature and counterstained
with hematoxylin (Sangon Biotech Co., Ltd.) for 1 min at room
temperature. Images were captured using a light microscope (Olympus
Corporation) at a magnification of ×20. Five sections per tumor
tissue were examined.
Statistical analysis
All experiments were repeated at ≥3 times and all
data are presented as the mean ± the standard error of the mean.
Graph Pad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA) was
use to perform statistical analysis. Comparisons among multiple
groups were determined using a one-way or two-way analysis of
variance test (two-way analysis of variance was used for tumor
volume analysis) followed by Dunnett's or Bonferroni's post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Synergistic cytotoxicity of the
combined treatment of GEM and BAI in pancreatic cancer cell
lines
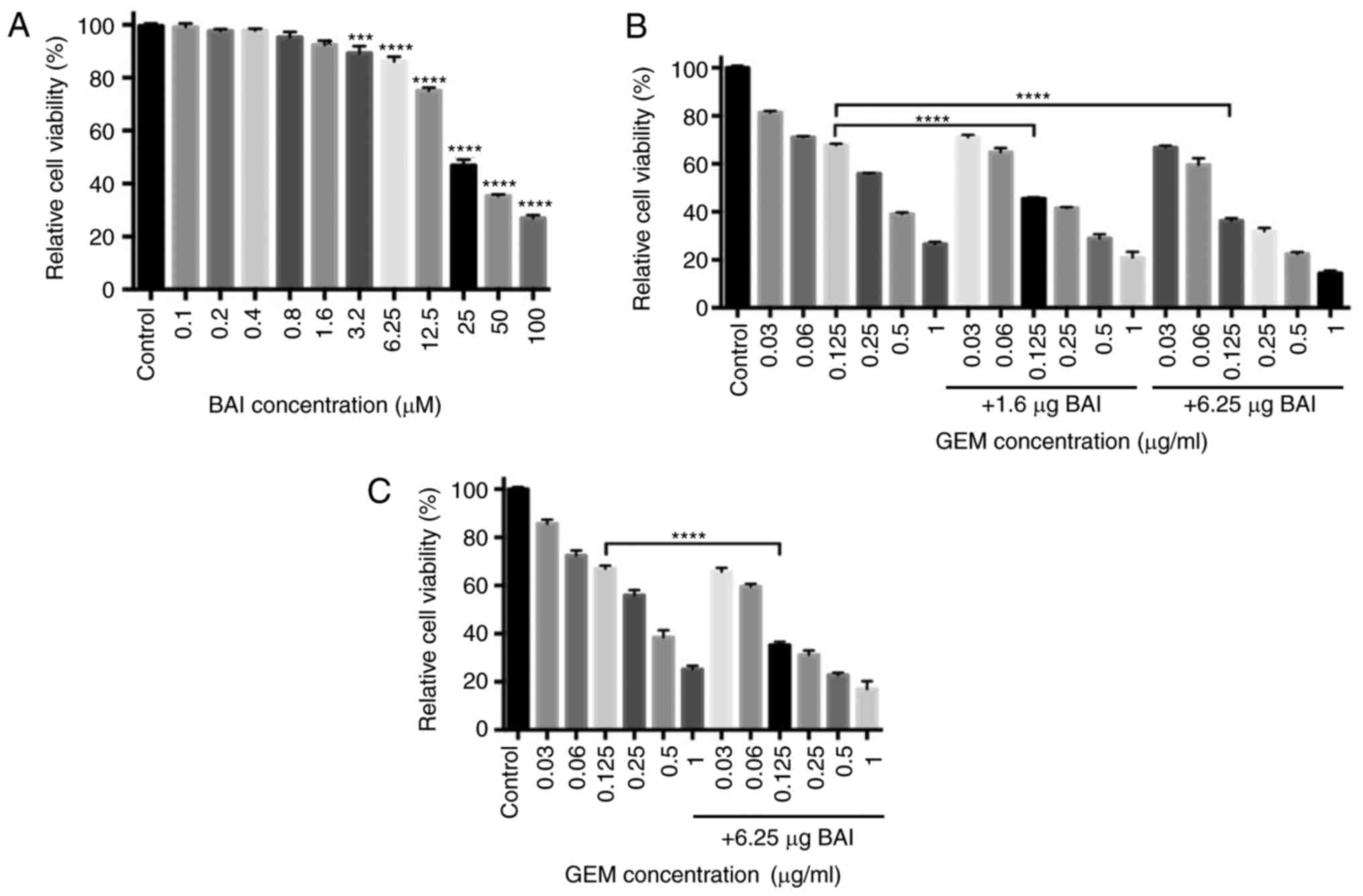
To test the potential of the cytotoxicity of BAI,
the human pancreatic cancer cell line CFPAC-1 was incubated with a
range concentrations (0.1–100 µM) of BAI for 48 h and then the
number of cells in each group was determined. Treatment with higher
concentrations of BAI (3.2–100.0 µM) significantly inhibited the
viability of CFPAC-1 cells compared with the control group
(P<0.001; Fig. 1A). For the cells
treated with 0.125 µg/ml GEM, the number of viable cells decreased
by 32% compared with the control group (Fig. 1B). Treatment with BAI in addition to
GEM or GEM alone revealed that the viability of CFPAC-1 was
inhibited in a concentration-dependent manner. Subsequently, to
assess the synergistic effect of GEM in combination with BAI on
cell proliferation, CFPAC-1 cells were incubated with a range of
concentrations of GEM, in combination with 1.6 or 6.25 µg BAI for
48 h. MTT assays revealed that combined treatment of either
concentration of BAI with 0.125 µg/ml GEM was significantly more
cytotoxic in CFPAC-1 cells compared with the same concentration of
GEM alone (CI (0.125 ug/ml GEM and 1.6 µM BAI)=0.181; CI (0.125
ug/ml GEM and 6.25 µM BAI)=0.465; Fig.
1B). A similar effect was identified in the PANC-1 cell line,
where 0.125 µg/ml GEM in combination with 6.25 µM BAI significantly
decreased the cell viability compared with 0.125 µg/ml GEM
treatment alone (Fig. 1C).
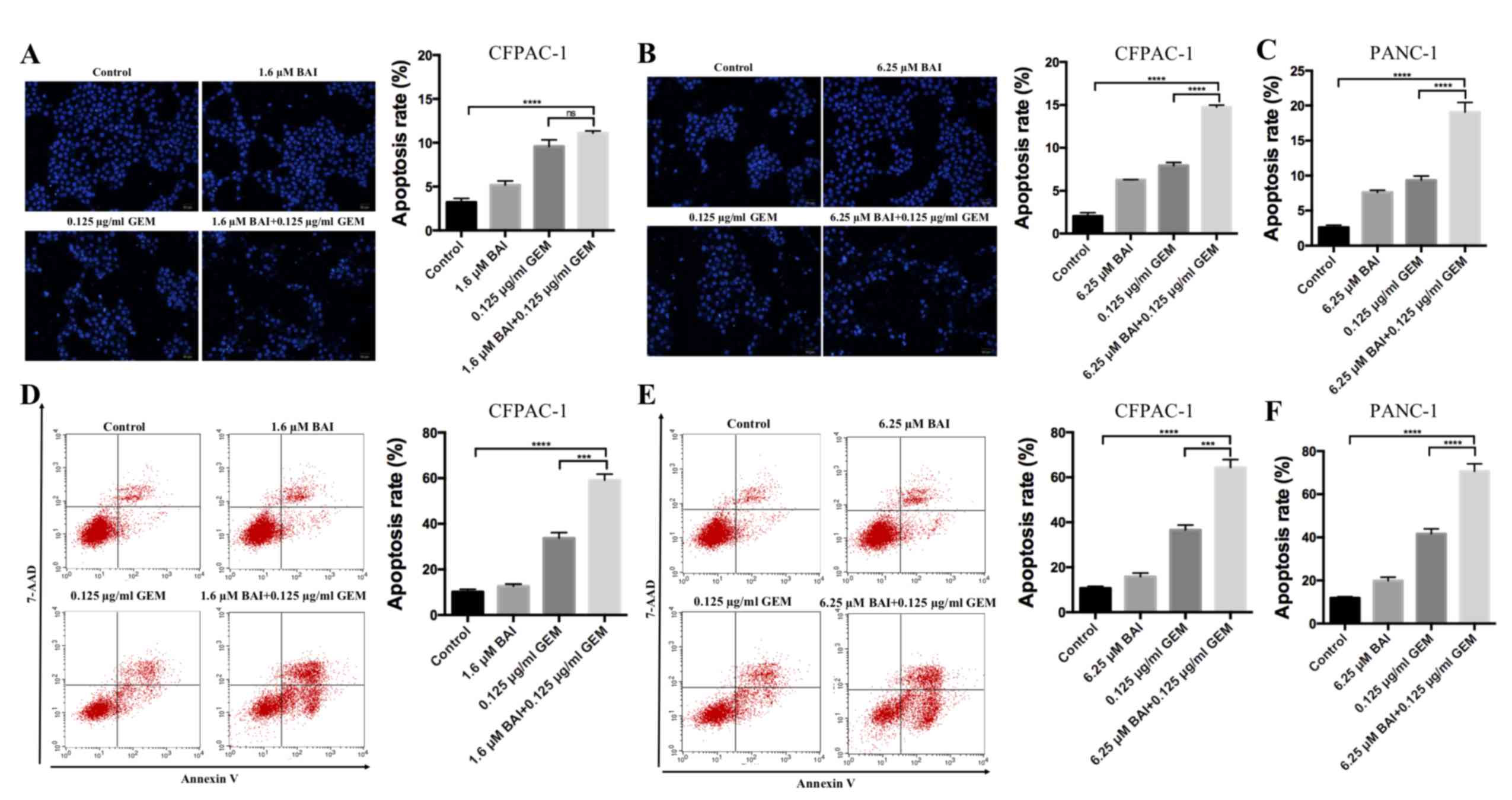
BAI and GEM cooperatively induce
apoptosis in pancreatic cancer cells
To determine if GEM and BAI treatment induces
apoptosis, Hoechst 33258 staining and flow cytometric analysis were
used. As indicated in Fig. 2,
apoptosis analysis revealed that treatment with GEM or BAI alone
induced minimal apoptosis; however, the combined treatment of GEM
with 1.6 or 6.25 µM BAI resulted in a significant increase of the
number of apoptotic cells was observed in CFPAC-1 and PANC-1 cell
lines compared with the control (P<0.0001; Fig. 2). In addition, there was a significant
increase in apoptosis in cells treated with GEM in combination with
6.25 µM BAI compared with GEM alone in CFPAC-1 and PANC-1 cell
lines (P<0.001; Fig. 2).
Furthermore, western blot analysis demonstrated that the expression
levels of Bax, caspase-3 and PARP were significantly increased, and
Bcl-2 and survivin were significantly decreased, in the combination
BAI (1.6 or 6.25 µM) and GEM (0.125 ug/ml) treated group compared
with the control group (P<0.001; Fig.
3), and compared with the BAI-alone treated group (P<0.01;
Fig. 3). These results provide
evidence that the synergistic cytotoxic effects of GEM and BAI
against pancreatic cancer cells may be attributed to the induction
of apoptosis.
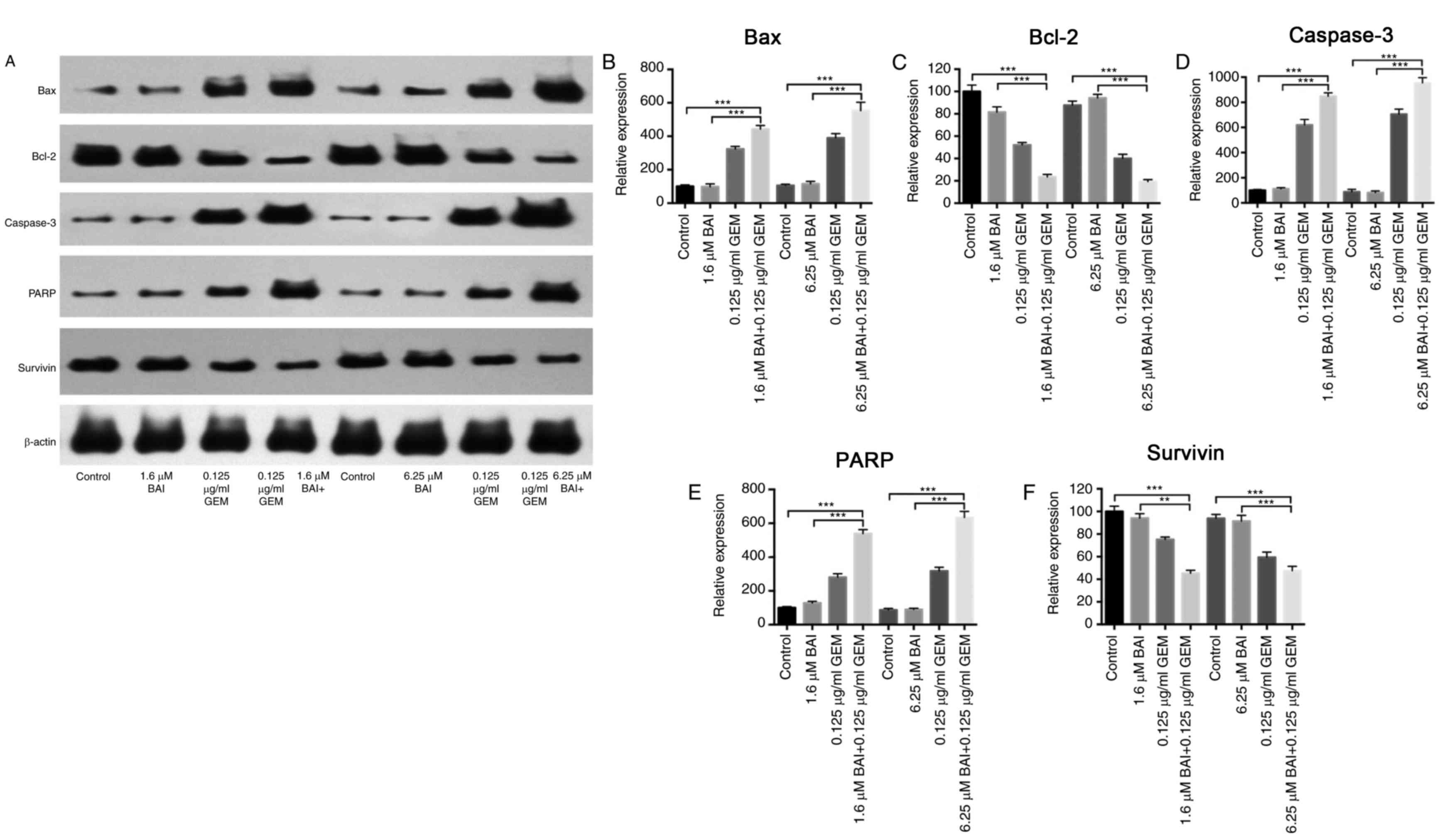 | Figure 3.Effects of BAI alone, GEM alone or
combined treatment on the protein expression levels of Bax, Bcl-2,
caspase-3, PARP and survivin in the human CFPAC-1 pancreatic cancer
cell line. (A) Representative images of Bax, Bcl-2, caspase-3, PARP
and survivin protein expression, generated using a western blot
analysis. β-actin was used as a loading control. Quantification of
the intensity of (B) Bax, (C) Bcl-2, (D) caspase-3, (E) PARP and
(F) and survivin. **P<0.01 and ***P<0.001 with comparisons
indicated by lines. GEM, gemcitabine; BAI, baicalein; Bax, Bcl-2
associated protein X; Bcl-2, B-cell lymphoma 2; PARP, poly ADP
ribose polymerase. |
Growth inhibitory effect of BAI and
GEM in a mouse model
In the CFPAC-1 xenograft nude mouse model, all mice
survived the duration of the experiment with no observable toxic
effect. Mice were sacrificed after 28 days and the weights and
volume of the xenografts were measured. Compared with the control
group, xenografts from the mice treated with BAI or GEM therapy
were decreased in volume and weight (Fig.
4A and B). Furthermore, the combination therapy consisting of
6.25 µM BAI and 0.125 ug/ml GEM induced a significant decrease in
tumor volume and weight compared with the control and with BAI
treatment alone (P<0.0001; Fig. 4C and
D). Immunohistochemical assays additionally revealed that the
protein levels of Bcl-2, survivin, Bax and caspase-3 followed a
similar pattern to that of the in vitro experiment, since
Bcl-2 and survivin were significantly decreased and Bax and
caspase-3 were significantly increased in the combined treatment
group compared with the control group (P<0.0001; Fig. 4E-H). Furthermore, survivin, Bax and
caspase-3 levels were significantly altered in the combination
therapy group compared with the GEM-alone treated group (P<0.01;
Fig. 4E-H).
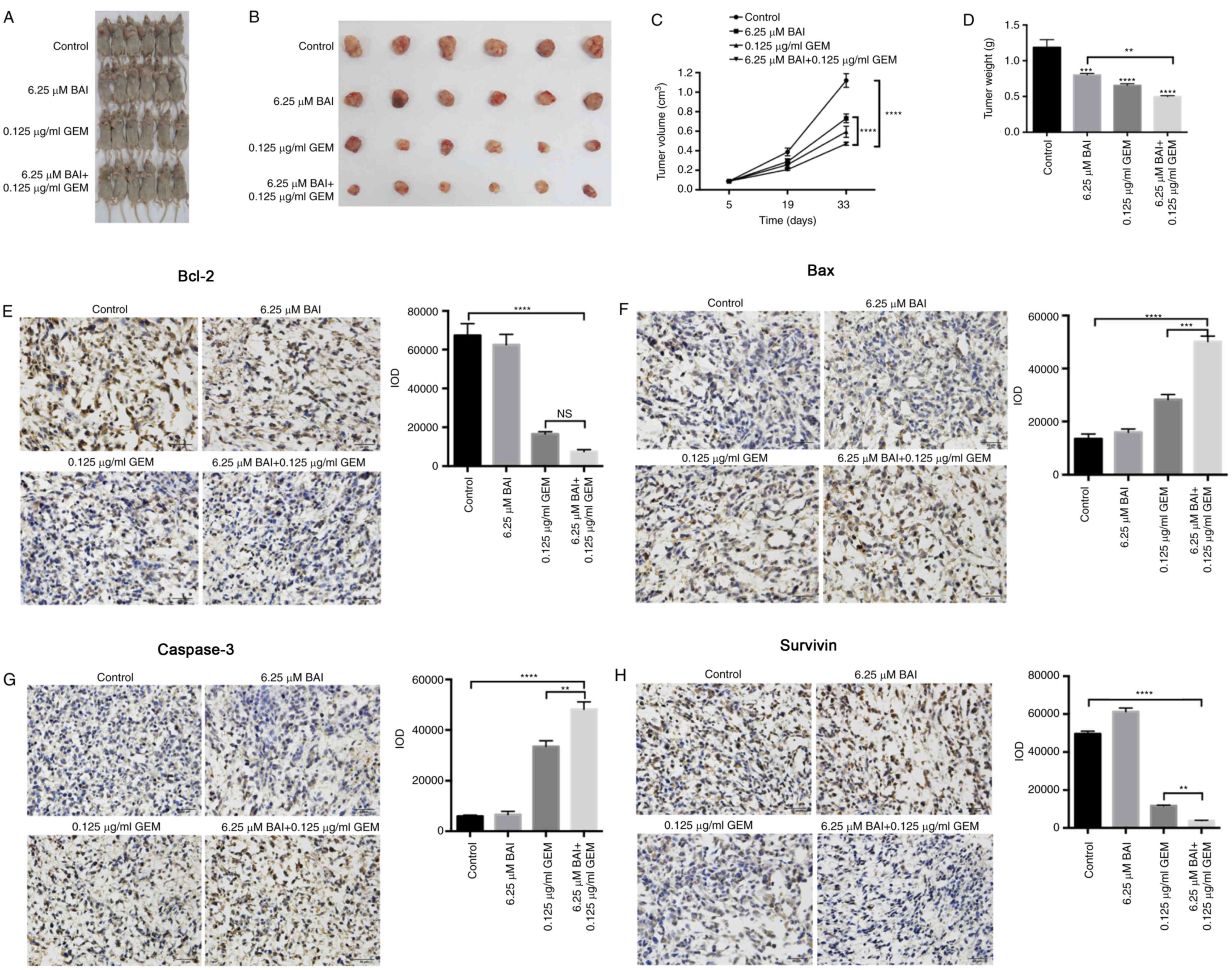 | Figure 4.Inhibitory effect of combined
treatment with BAI and GEM on the proliferation of the human
CFPAC-1 pancreatic cancer cell line in vivo. Representative
images of (A) mice and (B) tumors. (C) Tumor volume changes in the
xenografts. Resected tumors revealed that combination therapy
decreased the tumor volume significantly compared with either the
control group or the BAI-alone treated group. The data are
presented as the mean ± SEM. (D) Following 28 days of treatment,
the tumor weights were significantly decreased in all 3 treatment
groups compared with the control group. The data are presented as
the mean ± SEM. Representative immunohistochemical staining images
and quantification for (E) Bcl-2, (F) Bax, (G) caspase-3 and (H)
survivin from all groups of mice following treatment with BAI
alone, GEM alone or a combination of the 2 treatments. Images were
captured using a light microscope at a magnification of ×20.
**P<0.01, ***P<0.001 and ****P<0.0001 vs. the control
group or with comparisons indicated by lines. GEM, gemcitabine;
BAI, baicalein; Bax, Bcl-2 associated protein X; Bcl-2, B-cell
lymphoma 2; SEM, standard error of the mean. |
Discussion
In the present study, the effect of BAI alone and in
combination with GEM on the apoptosis and cell viability of the
human CFPAC-1 pancreatic ductal adenocarcinoma cell line was
investigated in vitro and in vivo. In addition to
this, the effects of the combination treatment of BAI and GEM in
the PANC-1 cell line in vitro was examined. The mechanism
underlying the induction of apoptosis in cancer cells by BAI may be
associated with the altered expression levels of pro-apoptotic and
anti-apoptotic molecules including Bcl-2, Bax, survivin, PARP and
caspase-3. Furthermore, the combination treatment of BAI with GEM
significantly reduced the tumor weight and volume relative to
either treatment as a monotherapy in the CFPAC-1 xenograft mouse
model. In future studies, the growth inhibitory effect of BAI and
GEM in a PANC-1 xenograft nude mouse model will be examined.
Therefore, the present study investigated for the first time, to
the best of our knowledge, the in vitro and in vivo
effects of BAI in pancreatic cell lines and in a xenograft
model.
BAI, the major bioactive flavone extracted from
S. baicalensis, is more potent than baicalin, which is
hydrolyzed by microflora to BAI (16). So far BAI, at doses that are toxic to
malignant cells, have been demonstrated to exert no or little
toxicity on normal cells (17).
Additionally, BAI has been revealed to inhibit the proliferation of
multiple human cancer cell lines (8,9). The
anticancer effect of BAI is the induction of apoptotic cell death
and the mechanisms include cyclin-dependent kinase modulation, the
activation of PARP, and caspase-3, caspase-7 and cytochrome c
release (18,19). These results indicate that BAI
possesses therapeutic potential against cancer.
Apoptosis is an important process for homeostasis by
maintaining the balance between cell death and cell survival. The
ratio of pro- and anti-apoptotic molecules regulates cell death.
Bax is a member of the Bcl-2 protein family associated with
apoptosis (20). Bax mediates the
permeabilization of the outer mitochondrial membrane and the
release of cytochrome c into the cytoplasm, which activates
caspase-3 to induce chromosome cleavage and apoptosis (21). Survivin belongs to the inhibitor of
apoptosis protein family and is considered a node protein,
inhibiting apoptosis and regulating cell mitosis (22). Promising cancer treatment strategies
that target apoptotic inhibitors, including Bcl-2 family proteins
and inhibitor of apoptosis proteins are presently under
investigation (23).
To conclude, in the present study, BAI was revealed
to inhibit the proliferation of pancreatic cancer cells due to the
induction of apoptosis in vitro and in vivo.
Furthermore, evidence was provided that the efficacy of BAI was
significantly improved when administered in combination with GEM.
Therefore, the present study may provide an alternative strategy
for the treatment of pancreatic cancer.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Boeck S, Ankerst DP and Heinemann V: The
role of adjuvant chemotherapy for patients with resected pancreatic
cancer: Systematic review of randomized controlled trials and
meta-analysis. Oncology. 72:314–321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Normile D: Asian medicine. The new face of
traditional Chinese medicine. Science. 299:188–190. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qi Z, Yin F, Lu L, Shen L, Qi S, Lan L,
Luo L and Yin Z: Baicalein reduces lipopolysaccharide-induced
inflammation via suppressing JAK/STATs activation and ROS
production. Inflamm Res. 62:845–855. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang KA, Zhang R, Piao MJ, Chae S, Kim HS,
Park JH, Jung KS and Hyun JW: Baicalein inhibits oxidative
stress-induced cellular damage via antioxidant effects. Toxicol Ind
Health. 28:412–421. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen K, Zhang S, Ji Y, Li J, An P, Ren H,
Liang R, Yang J and Li Z: Baicalein inhibits the invasion and
metastatic capabilities of hepatocellular carcinoma cells via
down-regulation of the ERK pathway. PLoS One. 8:e729272013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo Z, Hu X, Xing Z, Xing R, Lv R, Cheng
X, Su J, Zhou Z, Xu Z, Nilsson S and Liu Z: Baicalein inhibits
prostate cancer cell growth and metastasis via the
caveolin-1/AKT/mTOR pathway. Mol Cell Biochem. 406:111–119. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marcinkowski EF, Raz D, Shen B, Xing Q,
Yan J, Wen W, et al: Baicalein and meformin decrease small cell
lung cancer growth by inhibiting the mTOR pathway in itro. Cancer
Res. 76:21832016. View Article : Google Scholar
|
|
11
|
Liu Q, Li J, Pu G, Zhang F, Liu H and
Zhang Y: Co-delivery of baicalein and doxorubicin by hyaluronic
acid decorated nanostructured lipid carriers for breast cancer
therapy. Drug Deliv. 23:1364–1368. 2016.PubMed/NCBI
|
|
12
|
Choi EO, Park C, Hwang HJ, Hong SH, Kim
GY, Cho EJ, Kim WJ and Choi YH: Baicalein induces apoptosis via
ROS-dependent activation of caspases in human bladder cancer 5637
cells. Int J Oncol. 49:1009–1018. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peng Y, Guo C, Yang Y, Li F, Zhang Y,
Jiang B and Li Q: Baicalein induces apoptosis of human cervical
cancer HeLa cells in vitro. Mol Med Rep. 11:2129–2134. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Ling Y, Chen Y, Li CL, Feng F, You
QD, Lu N and Guo QL: Flavonoid baicalein suppresses adhesion,
migration and invasion of MDA-MB-231 human breast cancer cells.
Cancer Lett. 297:42–48. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang F, Dai W, Wang Y, Shen M, Chen K,
Cheng P, Zhang Y, Wang C, Li J, Zheng Y, et al: The synergistic in
vitro and in vivo antitumor effect of combination therapy with
salinomycin and 5-fluorouracil against hepatocellular carcinoma.
PLoS One. 9:e974142014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li-Weber M: New therapeutic aspects of
flavones: The anticancer properties of Scutellaria and its main
active constituents Wogonin, Baicalein and Baicalin. Cancer Treat
Rev. 35:57–68. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu C, Zhang Z, Zhang H, Zhen Z, Calway T,
Wang Y, Yuan CS and Wang CZ: Pretreatment of baicalin and
wogonoside with glycoside hydrolase: A promising approach to
enhance anticancer potential. Oncol Rep. 30:2411–2418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takahashi H, Chen MC, Pham H, Angst E,
King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, et
al: Baicalein, a component of Scutellaria baicalensis, induces
apoptosis by Mcl-1 down-regulation in human pancreatic cancer
cells. Biochim Biophys Acta. 1813:1465–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ling Y, Chen Y, Chen P, Hui H, Song X, Lu
Z, Li C, Lu N and Guo Q: Baicalein potently suppresses angiogenesis
induced by vascular endothelial growth factor through the p53/Rb
signaling pathway leading to G1/S cell cycle arrest. Exp Biol Med
(Maywood). 236:851–858. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Knudson CM and Korsmeyer SJ: Bcl-2 and Bax
function independently to regulate cell death. Nat Genet.
16:358–363. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Breckenridge DG and Xue D: Regulation of
mitochondrial membrane permeabilization by BCL-2 family proteins
and caspases. Curr Opin Cell Biol. 16:647–652. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mobahat M, Narendran A and Riabowol K:
Survivin as a preferential target for cancer therapy. Int J Mol
Sci. 15:2494–2516. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hassan M, Watari H, AbuAlmaaty A, Ohba Y
and Sakuragi N: Apoptosis and molecular targeting therapy in
cancer. Biomed Res Int. 2014:1508452014. View Article : Google Scholar : PubMed/NCBI
|