Introduction
Serine/arginine-rich (SR) proteins are a conserved
RNA-binding protein family, which consists of 12 members,
serine/arginine-rich splicing factor (SRSF)1-12, in humans
(1,2).
SR proteins have multiple key roles in the control of gene
expression, including constitutive and alternative pre-mRNA
splicing, transcription, mRNA transport, mRNA stability and
translation (3–5). Changes in the expression of SR proteins
may lead to aberrant alternative splicing and potentially
contribute to various diseases, and in particular, to the
development of cancer. Previous studies have indicated that the
expression of the majority of SR proteins is altered in various
tumor types, and several SR proteins, acting as proto-oncogenes,
are frequently upregulated in cancer (6–8).
SRSF7, previously known as 9G8, is a member of the
SR protein family that was identified in 1994 (9,10). SRSF7
comprises an RNA-recognition motif at the N-terminus that provides
RNA-binding specificity, an arginine/serine domain at the
C-terminus that promotes protein-protein interactions to facilitate
spliceosome assembly, and a zinc-knuckle domain, which is thought
to contact the RNA. SRSF7 regulates the constitutive splicing and
alternative splicing of various pre-mRNAs, including CD44 (11), CD45 (12), BRCA1 (13) and Tau (14). Furthermore, SRSF7 can shuttle
continuously between the nucleus and cytoplasm, with additional
involvement in mRNA transport and translation (15,16).
Several studies have suggested that SRSF7 may have an active role
in cancer cells (13,17,18).
However, the precise effects of SRSF7 on cancer cells remain to be
elucidated.
The Fas protein, also known as CD95, is a widely
expressed cell-surface death receptor, which, upon binding to Fas
ligand, can initiate a cascade that eventually leads to programmed
cell death (19,20). The alternative splicing of Fas
receptor pre-mRNA may be an important strategy used by tumors cells
to evade elimination by the immune system. Fas exon 6 encodes a
transmembrane domain, and skipping of this exon produces an mRNA
encoding a soluble Fas isoform, which prevents cell death (21,22).
Increased levels of the soluble isoform of Fas have been identified
in several types of cancer (23–25).
Tejedor et al (26)
demonstrated that iron homeostasis affects the alternative splicing
of Fas receptor pre-mRNA by SRSF7. However, little is known about
SRSF7 and Fas splicing in cancer cells.
In the present study, it was demonstrated that SRSF7
proteins were expressed at high levels in colon and lung cancer,
both of which have increasing rates of incidence and mortality
worldwide (27,28). In addition, it was found that SRSF7
knockdown inhibited proliferation and enhanced apoptosis of colon
and lung cancer cells. Finally, it was found that SRSF7 targeted
the apoptosis regulator Fas in cancer cells, which may explain a
number of the activities of SRSF7.
Materials and methods
Cell culture
The HCT116 cells were cultured in McCoy's 5A medium
(M&C Gene Technology Ltd., Beijing, China). The A549, H1975,
H1299 and NCM460 cells were cultured in RPMI-1640 (M&C Gene
Technology Ltd.). The BEAS-2B, HCoEpic and SW620 cells were
cultured in DMEM (M&C Gene Technology Ltd.). All culture media
were supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher
Scientific, Inc., Waltham, MA, USA), penicillin and streptomycin.
All the cells were obtained from the Cell Bank of Chinese Academy
of Sciences (Shanghai, China) and incubated in a humidified
atmosphere of 5% CO2 at 37°C.
Transfection and RNA interference
Small interfering RNA (siRNA) transfections were
performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. siRNA
synthesis was performed by Shanghai GenePharma Co., Ltd. (Shanghai,
China) and the siRNA sequences for human SRSF7 were as follows:
SRSF7-1, 5′-AGGAGAGUUAGAAAGGGCU-3′; and SRSF7-2,
5′-GCAUCUCCUCGACGAUCAA-3′. The sequence of the control siRNA was
5′-UUCUCCGAACGUGUCACGUTT-3′.
Western blot, reverse
transcription-polymerase chain reaction (RT-PCR) and MTS cell
proliferation analyses
The methods were performed as described previously
(29). For western blot analysis,
cells were lysed with radioimmunoprecipitation assay cell lysis
buffer (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) containing 1 mM phenylmethylsulfonyl fluoride and
quantified using a bicinchoninic acid assay Kit (Beijing Solarbio
Science & Technology Co., Ltd.). Equal amounts of protein (30
µg) were separated via SDS-PAGE (12% gel) and then transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membrane was blocked for 1 h with 5% skimmed milk at room
temperature and then incubated with primary antibodies overnight at
4°C. The primary antibody against SRSF7 (AP12306a, 1:500) was
purchased from Abgent, Inc. (San Diego, CA, USA). The primary
antibodies against β-tubulin (10068–1-AP, 1:1,000) and β-actin
(60008–1-Ig. 1:3,000) was purchased from Proteintech Group, Inc.
(Rosemont, PA, USA). Following three washes in Tris-buffered saline
with Tween-20, the membrane was incubated with secondary goat
anti-rabbit (SA00001-2) or goat anti-mouse (SA00001-1) antibody
(Proteintech Group, Inc) for 1 h at 37°C with a dilution of
1:3,000. Finally, the proteins were visualized using EasySee
Western Blot Kit (Beijing TransGen Biotech Co., Ltd., Beijing,
China), imaged and quantified using ChemiDoc MP Imaging System
(Image Lab Software, version 4.1; Bio-Rad Laboratories Co., Ltd.,
Hercules, CA, USA).
For RT-PCR, total RNA was extracted using TRIzol
reagent (Life Technologies; Thermo Fisher Scientific, Inc.), and
reverse transcription was performed using a Reverse Transcription
system (Promega Corporation, Fitchburg, WI, USA). Total RNA (4 µg)
was reverse transcribed using TransScript One-Step gDNA Removal and
cDNA Synthesis SuperMix (Beijing TransGen Biotech Co., Ltd.)
according to the manufacturer's protocol. The thermocycling
protocol was listed as follows: Initial denaturation at 95°C for 5
min, followed by 30 repeats of the threestep cycling program
consisting of 30 sec at 95°C (denaturation), 30 sec at 60°C (primer
annealing) and 30 sec at 72°C (elongation), followed by a final
extension step for 5 min at 72°C. The primers used were as follows:
SRSF7, forward 3′-GCGGTACGGAGGAGAAAC-5′ and reverse
3′-TCGGGAGCCACAAATCAC-5′; Fas, forward
3′-GAACATGGAATCATCAAGGAATGCAC-5′ and reverse
3′-AGTTGGAGATTCATGAGAACCTTGG-5′. The primers used to detect the
alternative splicing of Fas were as follows: FAS-L, forward
3′-TGCAAAGAGGAAGGATCCAG-5′; FAS-S, forward
3′-CCAAGTGCAAAGAGGAAGTGA-5′; and FAS-L and FAS-S reverse
3′-GGAGATTCATGAGAACCTTGG-5′ (26).
The housekeeping gene GAPDH was used as the internal control.
Analysis of cell apoptosis
The HCT116 and A549 cells were transfected with
control siRNA or SRSF7 siRNA for 60–72 h. Following transfection,
1×106 cells were harvested, washed twice in PBS and
double-stained with Annexin V-FITC and PI using an Annexin
V-FITC/PI Cell Apoptosis Detection kit (Beijing TransGen Biotech
Co.) according to the manufacturer's protocol. Each sample was then
quantitatively analyzed with an Accuri C6 flow cytometer (BD
Biosciences, San Jose, CA, USA) at 488 nm emission and 570 nm
excitation. Cell apoptosis was also examined using an Apo-ONE
Homogeneous Caspase-3/7 assay (Promega Corporation) according to
the manufacturer's protocol. Caspase substrate and buffer from the
kit were added for cell lysis and incubated at room temperature for
18 h. Fluorescence was measured with an F-7000 spectrofluorometer
(Hitachi, Ltd., Tokyo, Japan) at 521 nm emission and 499 nm
excitation wavelengths.
Generation of stable cell lines
The BEAS-2B cells were infected with a lentivirus
LV5-negative control vector or a LV5-SRSF7 vector, which were
purchased from Shanghai GenePharma Co., Ltd. The A549 cells were
infected with lentivirus LV3-negative control, LV3-SRSF7 short
hairpin shRNA-1 (3′-GATCAAGATCCAGGTCTATTT-5′) or LV3-SRSF7 shRNA-2
(3′-GAACTGTATGGATTGCGAGAA-5′), which were purchased from Shanghai
GenePharma Co., Ltd. At 48 h post-infection, the cells were
cultured in the aforementioned medium containing puromycin (0.5
µg/ml), and the medium was replenished every 2 days. After ~1 week,
stable cell lines were obtained and verified by western blot
analysis, as described above.
Immunohistochemical analysis
Immunohistochemistry was performed by Cybrdi, Inc.
(Xi'an, China) on a multi-organ (colon, pancreas, lung, breast and
prostate) tumor and normal tissue array (cat. no. MC1801, 26 spots
for each tumor type), human colon carcinoma (grades I–IV) tissue
array (cat. no. CO1801, 90 spots for cancer tissues and
paracancerous normal tissues, respectively), and human lung
carcinoma (grades I–III) tissue array (cat. no. LC10012a, 45 spots
for cancer tissues and paracancerous normal tissues, respectively).
The absent or abnormal tissues were not finally counted. Anti-SRSF7
(Abgent, Inc.) antibodies were used at a dilution of 1:50 (for
array MC1801), 1:150 (for array CO1801) and 1:100 (for array
LC10012a) at 4°C for overnight. Semi-quantitative analysis of the
stained sections (H-score) was performed with a light microscope
(Model, CX31; Olympus, Tokyo, Japan) by an independent
pathologist.
Statistical analysis
All statistical analyses were performed by
two-tailed Student's t-test or a one-way analysis of variance
followed by Bonferroni's post-hoc test. Data are presented as the
mean ± standard deviation. P<0.05 was considered to indicate a
statistically significant difference. Computer-based calculations
were conducted using SPSS 19.0 software (IBM Corp., Armonk, NY,
USA).
Results
SRSF7 is upregulated in colon and lung
cancer
To evaluate the role of SRSF7 in human cancer, its
expression was analyzed on cancer arrays with different tissue
origins by immunohistochemical staining. When compared with
corresponding normal tissues, a high frequency of increased
expression of SRSF7 was found in colon adenocarcinoma (21/24) and
lung carcinoma (22/24). This result was confirmed with the specific
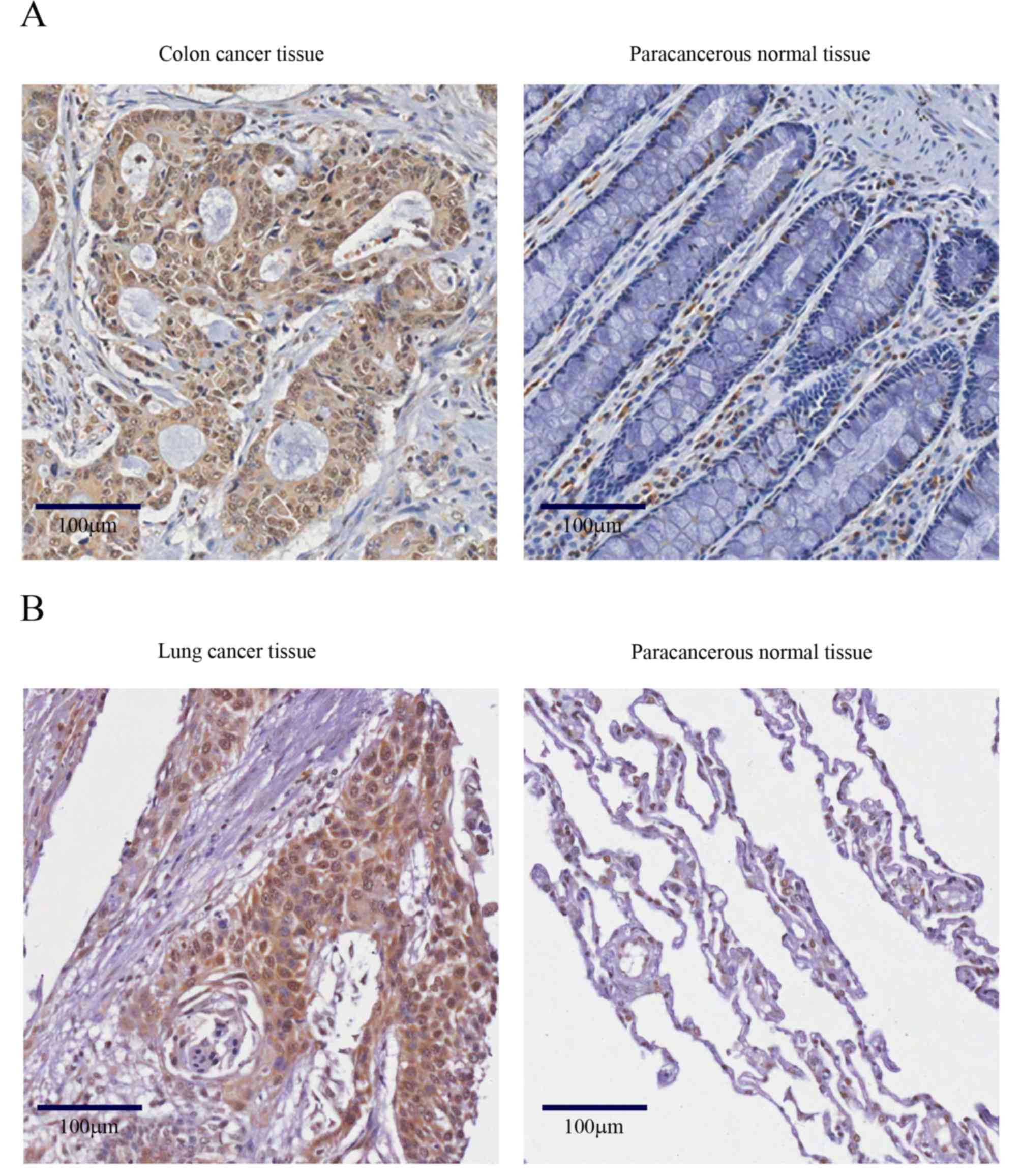
human colon and lung cancer specimens. As shown in Fig. 1A and B, the immunostaining intensity
of SRSF7, compared with paracancerous normal tissues, was more
marked in the colon cancer samples (49/71) and lung cancer samples
(28/39). These results suggested that SRSF7 offers potential as a
marker for colon and lung cancer development, and may be involved
in the development of these types of cancer.
Downregulation of SRSF7 inhibits the
proliferation of HCT116 and A549 cells
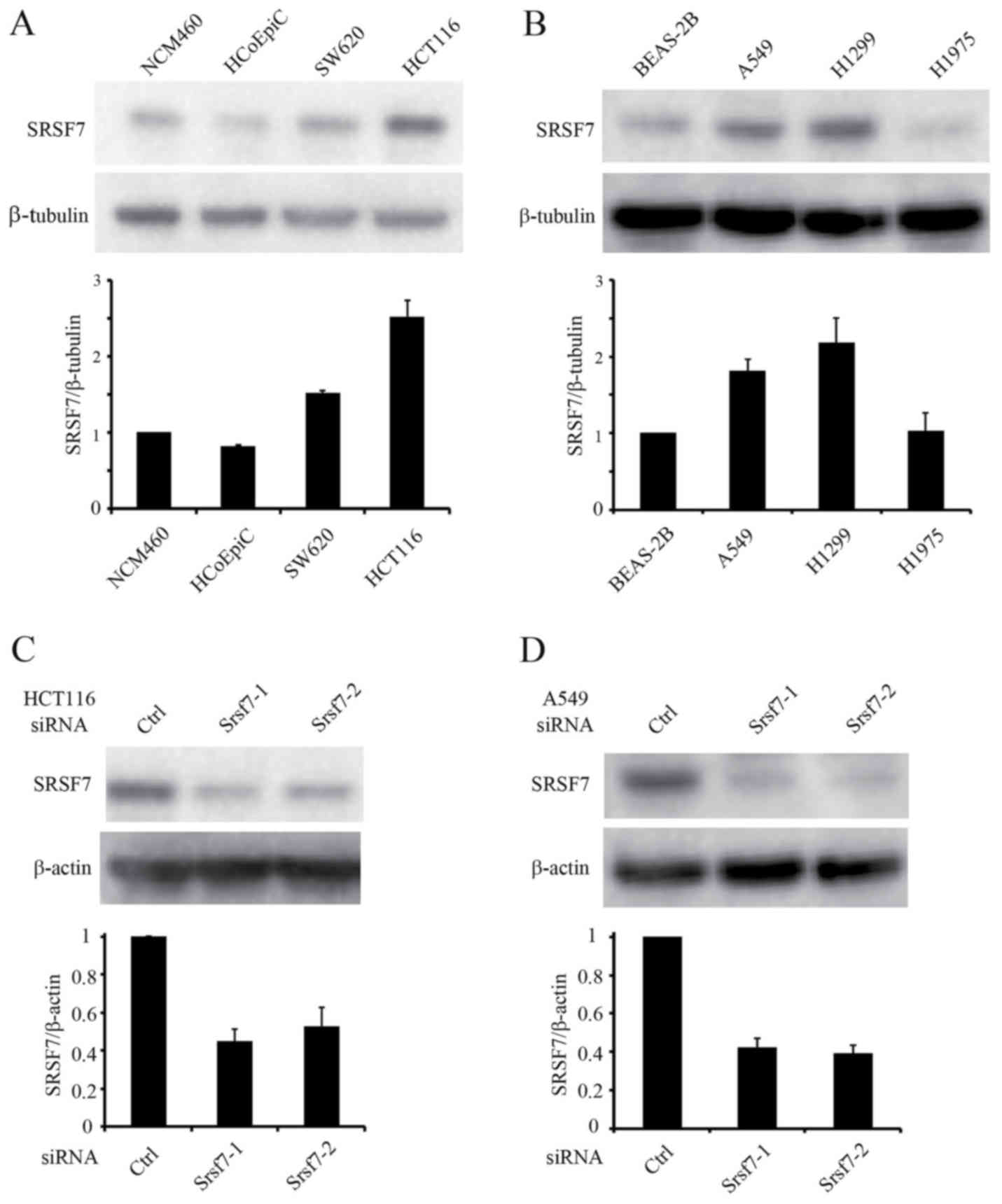
The expression of SRSF7 was analyzed in normal and
cancerous human cell lines of the colon and lung. Western blot
analysis revealed the upregulation of SRSF7 in the colon cancer
cell lines (HCT116 and SW620) and lung cancer cell lines (A549 and
H1299), compared with the normal cells (Fig. 2A and B). Among the colon and lung
cancer cell lines, HCT116 and A549 cells were used in subsequent
assays. To examine the function of SRSF7 in colon and lung cancer
cell lines, downregulation experiments were performed to reduce the
expression of SRSF7. The effect of SRSF7-inhibition on cell
proliferation was then evaluated. The knockdown of SRSF7 was
achieved in HCT116 and A549 cells using two different siRNAs, and
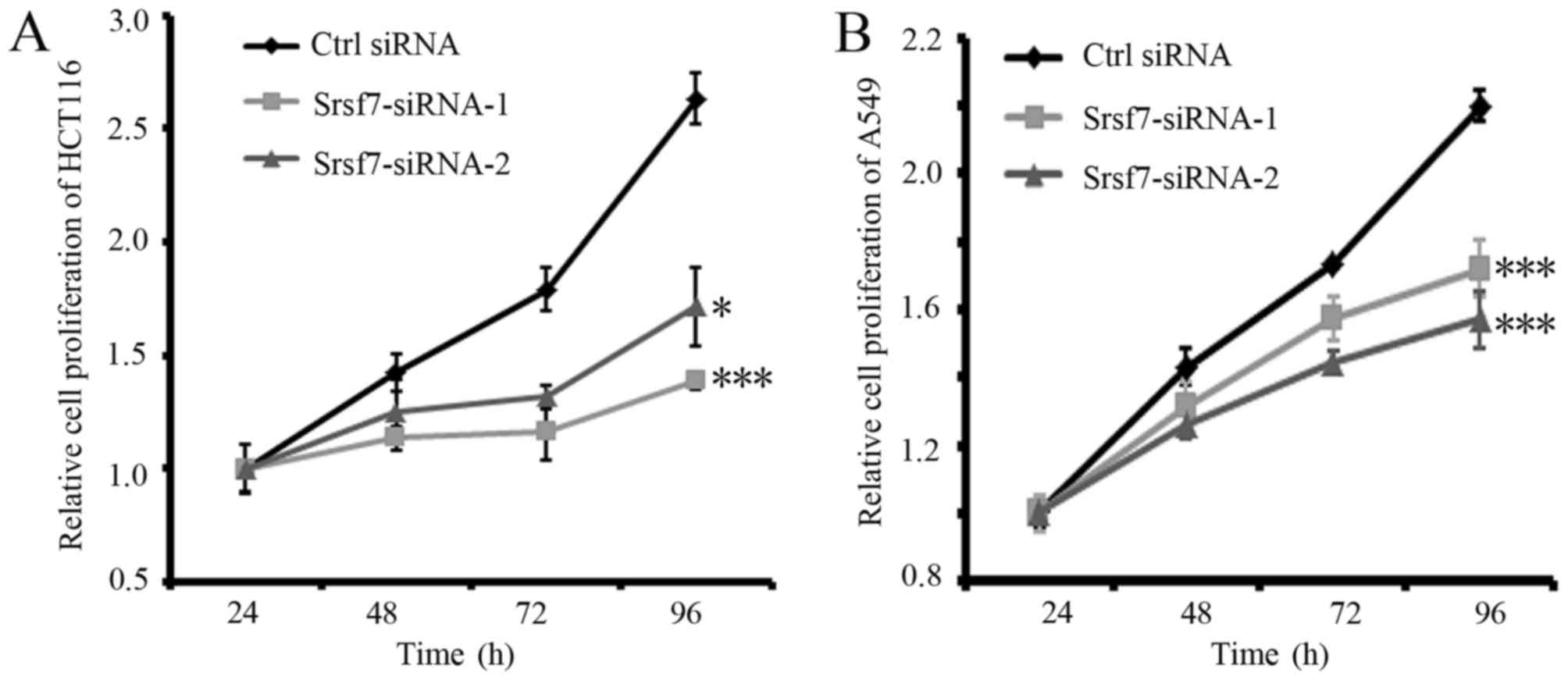
its downregulation was confirmed by western blot analysis (Fig. 2C and D). The results of MTS assays
demonstrated that the inhibition of SRSF7 markedly decreased the
viabilities of the HCT116 and A549 cells, compared with those of
the cells transfected with the control siRNA (Fig. 3A and B). These results suggested that
SRSF7 is required for the proliferation of colon and lung cancer
cells.
Downregulation of SRSF7 promotes
apoptosis of HCT116 and A549 cells
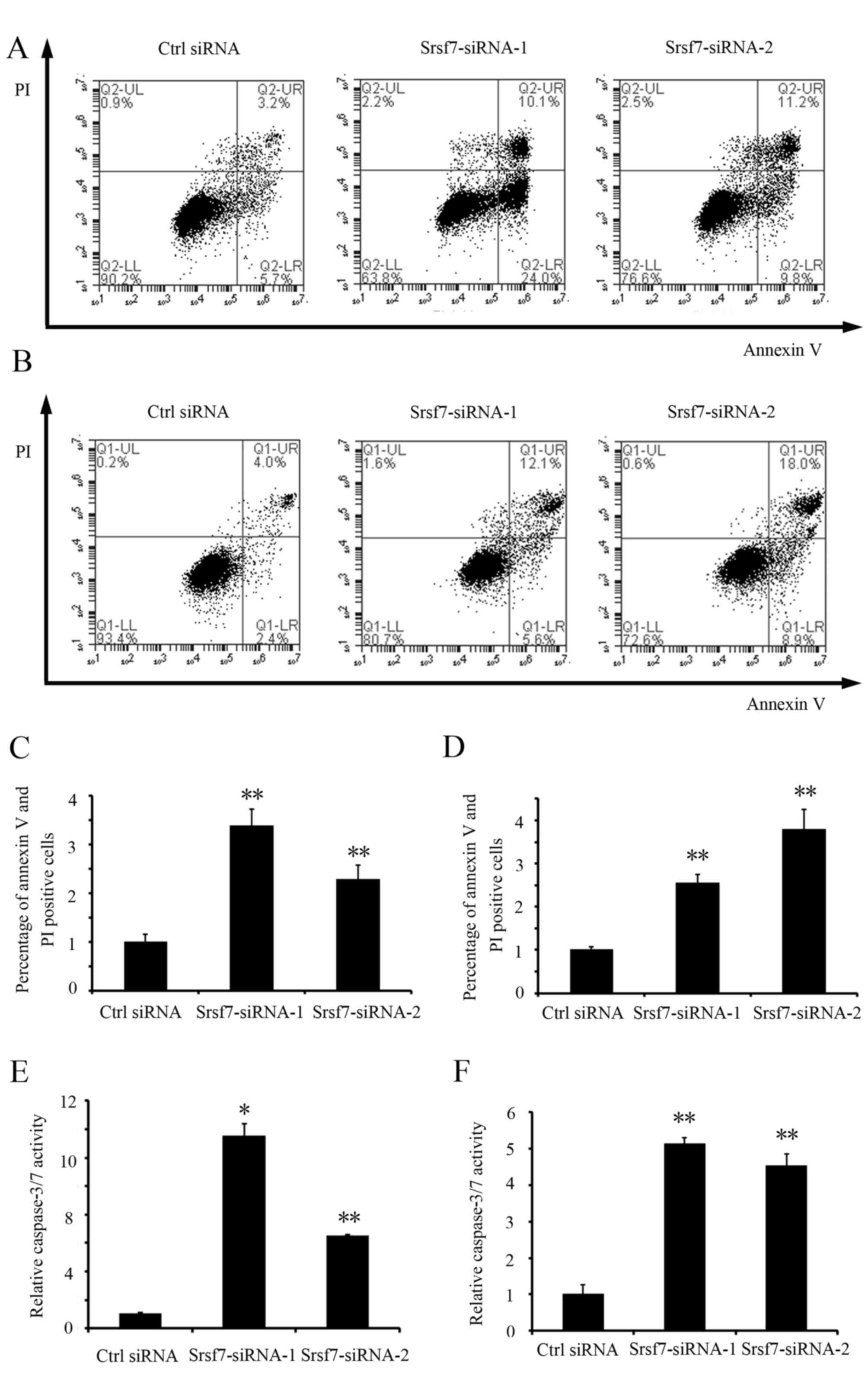
To investigate the effect of the downregulation of
SRSF7 on apoptosis, the rates of total apoptosis in the HCT116 and
A549 cells were detected and quantified by flow cytometric
analysis. The results showed that SRSF7 knockdown significantly
increased the percentage of apoptotic cells (P<0.01) in the
HCT116 and A549 cells, compared with the cells transfected with
control siRNA (Fig. 4A-D). The
induction of apoptosis in these cells was also confirmed by an
increase in caspase 3/7 activity (Fig. 4E
and F). These data indicated that SRSF7 is critical for the
survival of colon and lung cancer cells.
SRSF7 regulates splicing of the
apoptosis regulator Fas
To identify the alternative splicing events
regulated by SRSF7, which may contribute to its effects on
proliferation and apoptosis, the effects of the upregulation or
downregulation of SRSF7 on the altered splicing events in cancer
were examined. Non-malignant BEAS-2B lung epithelial cells were
used to establish a stable SRSF7-overexpression cell line and A549
cells were used to establish a cell line with stable SRSF7
knockdown. In these stable cell lines, it was found that the
splicing of Fas receptor was altered upon upregulation or
downregulation of SRSF7. It has been reported that the alternative
splicing of Fas exon 6 generates either a membrane-bound receptor
that promotes apoptosis, or a soluble isoform that inhibits
apoptosis (21). The results of the
present study revealed that the upregulation of SRSF7 in BEAS-2B
cells increased the skipping of Fas exon 6, whereas SRSF7 knockdown
in A549 cells increased exon 6 inclusion (Fig. 5A and B). These results suggested that
SRSF7 regulated the alternative splicing of Fas and promoted
production of the more soluble, pro-survival variant.
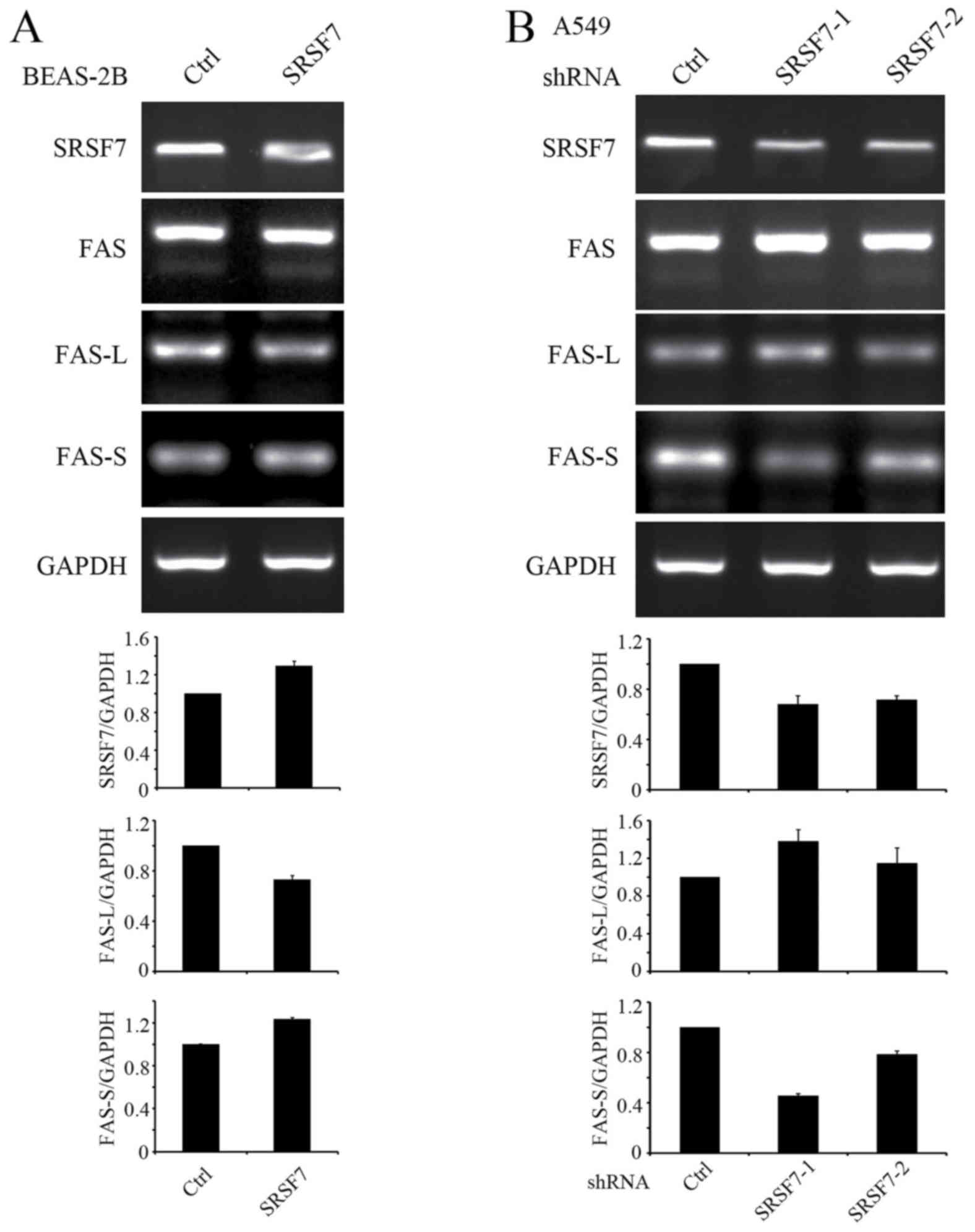 | Figure 5.SRSF7 regulates the alternative
splicing of Fas in human lung cells. (A) Total RNA was extracted
from stable BEAS-2B cell lines expressing empty vector or
SRSF7-overexpression vector. Following reverse transcription, cDNA
was subjected to PCR, using primers to detect the alternative
splicing of Fas. The relative expression levels of SRSF7, FAS-L and
FAS-S against GAPDH were determined from three independent
experiments. (B) Total RNA was extracted from A549 cell lines
stably expressing empty vector or SRSF7-shRNA vectors. Following
reverse transcription, cDNA was subjected to PCR, using primers
that detected the alternative splicing of Fas. SRSF7,
serine/arginine-rich splicing factor 7; FAS-L, Fas long isoform of
exon 6 inclusion; FAS-S, Fas short isoform of exon 6 skipping;
shRNA, short hairpin RNA; PCR, polymerase chain reaction; Ctrl,
control. |
Discussion
Colon and lung cancer are the leading causes of
cancer-associated mortality worldwide (27). In particular, lung cancer has been
associated with the highest rates of incidence in the last two
decades in China (28). In the
present study, the data indicated that SRSF7 was frequently
upregulated in clinical colon and lung samples. It remains to be
elucidated whether this upregulation involves mutational,
transcriptional or epigenetic mechanisms. It was hypothesized that
SRSF7 may act as a proto-oncogene in colon and lung cancer.
However, the overexpression of SRSF7 in BEAS-2B cells cannot induce
tumor growth in nude mice (data not shown). Therefore, the effects
of SRSF7 in tumorigenesis may be dependent on other tumor-related
genes.
To further investigate the function of SRSF7 in
colon and lung cancer, the expression of SRSF7 was measured in
colon and lung cancer cell lines. This indicated that the
expression of SRSF7 was elevated in the HCT116 colon cancer cell
line and A549 lung cancer cell line. The expression of SRSF7 was
subsequently downregulated in these cell lines by siRNA, in order
to examine the effect of SRSF7 on cell proliferation and apoptosis.
It was observed that SRSF7 knockdown inhibited proliferation and
promoted apoptosis of HCT116 and A549 cells. Similarly, Saijo et
al (30) reported that SRSF7
knockdown induced G1 cell cycle arrest in HCT116 cells. These
results indicate that SRSF7 exerts a crucial effect on the growth
of colon and lung cancer cells.
Alterations in the alternative splicing of Fas have
been shown to be an important activity of Fas in regulating cell
apoptosis. Its transmembrane proapoptotic isoform, a soluble
prosurvival isoform of Fas, can be expressed due to the skipping of
exon 6. In the colon and lung cancer cells examined in the present
study, the overexpression of SRSF7 promoted Fas exon 6 skipping and
activated cell proliferation, whereas SRSF7 knockdown promoted Fas
exon 6 inclusion and induced cell apoptosis. Therefore, SRSF7 may
be involved in the growth of cancer cells by regulating the
alternative splicing of Fas receptor pre-mRNA in cancer cells.
Other downstream targets of SRSF7 in cancer cells require
investigation in the future.
In conclusion, the findings of the present study
suggested that SRSF7 knockdown inhibited the growth and promoted
the apoptosis of colon and lung cancer cells by controlling
apoptosis-related splicing events. Therefore, SRSF7 may be a
potential therapeutic target for the treatment of colon and lung
cancer. The specific molecular mechanisms underlying this activity
of SRSF7 in cancer require further investigation.
Acknowledgements
The authors would like to thank Professor Wei Wu
(School of Life Sciences, Tsinghua University, Beijing, China) for
identifying an association between SRSF7 and cancer in his
laboratory and Professor Xingfeng Li (College of Bioscience and
Bioengineering, Hebei University of Science and Technology,
Shijiazhuang, China) for providing the equipment.
Funding
This study was supported by grants awarded to Dr Yu
Fu from the National Natural Science Foundation of China (grant no.
81402305), the Natural Science Foundation of Hebei Province (grant
no. H2015208141), the Science and Technology Research Program for
Colleges and Universities in Hebei Province (grant no. BJ2016024)
and the Doctoral Scientific Research Foundation of Hebei University
of Science and Technology (grant no. QD201411).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF and YW designed the study and performed
experiments, and YF wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Howard JM and Sanford JR: The RNAissance
family: SR proteins as multifaceted regulators of gene expression.
Wiley Interdiscip Rev RNA. 6:93–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Manley JL and Krainer AR: A rational
nomenclature for serine/arginine-rich protein splicing factors (SR
proteins). Genes Dev. 24:1073–1074. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Z and Fu XD: Regulation of splicing
by SR proteins and SR protein-specific kinases. Chromosoma.
122:191–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sahebi M, Hanafi MM, van Wijnen AJ, Azizi
P, Abiri R, Ashkani S and Taheri S: Towards understanding pre-mRNA
splicing mechanisms and the role of SR proteins. Gene. 587:107–119.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Twyffels L, Gueydan C and Kruys V:
Shuttling SR proteins: More than splicing factors. FEBS J.
278:3246–3255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anczukow O and Krainer AR: Splicing-factor
alterations in cancers. RNA. 22:1285–1301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shilo A, Siegfried Z and Karni R: The role
of splicing factors in deregulation of alternative splicing during
oncogenesis and tumor progression. Mol Cell Oncol. 2:e9709552014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Das S and Krainer AR: Emerging functions
of SRSF1, splicing factor and oncoprotein, in RNA metabolism and
cancer. Mol Cancer Res. 12:1195–1204. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cavaloc Y, Popielarz M, Fuchs JP, Gattoni
R and Stevenin J: Characterization and cloning of the human
splicing factor 9G8: A novel 35 kDa factor of the serine/arginine
protein family. EMBO. 13:2639–2649. 1994.
|
|
10
|
Popielarz M, Cavaloc Y, Mattei MG, Gattoni
R and Stevenin J: The gene encoding human splicing factor 9G8.
Structure, chromosomal localization, and expression of
alternatively processed transcripts. J Biol Chem. 270:17830–17835.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Galiana-Arnoux D, Lejeune F, Gesnel MC,
Stevenin J, Breathnach R and Del Gatto-Konczak F: The CD44
alternative v9 exon contains a splicing enhancer responsive to the
SR proteins 9G8, ASF/SF2, and SRp20. J Biol Chem. 278:32943–32953.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
ten Dam GB, Zilch CF, Wallace D, Wieringa
B, Beverley PC, Poels LG and Screaton GR: Regulation of alternative
splicing of CD45 by antagonistic effects of SR protein splicing
factors. J Immunol. 164:5287–5295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raponi M, Kralovicova J, Copson E, Divina
P, Eccles D, Johnson P, Baralle D and Vorechovsky I: Prediction of
single-nucleotide substitutions that result in exon skipping:
Identification of a splicing silencer in BRCA1 exon 6. Hum Mutat.
32:436–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao L, Wang J, Wang Y and Andreadis A: SR
protein 9G8 modulates splicing of tau exon 10 via its proximal
downstream intron, a clustering region for frontotemporal dementia
mutations. Mol Cell Neurosci. 34:48–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang Y and Steitz JA: Splicing factors
SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol
Cell. 7:899–905. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Swartz JE, Bor YC, Misawa Y, Rekosh D and
Hammarskjold ML: The shuttling SR protein 9G8 plays a role in
translation of unspliced mRNA containing a constitutive transport
element. J Biol Chem. 282:19844–19853. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hatakeyama S, Sugihara K, Nakayama J,
Akama TO, Wong SM, Kawashima H, Zhang J, Smith DF, Ohyama C, Fukuda
M and Fukuda MN: Identification of mRNA splicing factors as the
endothelial receptor for carbohydrate-dependent lung colonization
of cancer cells. Proc Natl Acad Sci USA. 106:pp. 3095–3100. 2009;
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HR, Lee GO, Choi KH, Kim DK, Ryu JS,
Hwang KE, Na KJ, Choi C, Kuh JH, Chung MJ, et al: SRSF5: A novel
marker for small-cell lung cancer and pleural metastatic cancer.
Lung Cancer. 99:57–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bouillet P and O'Reilly LA: CD95, BIM and
T cell homeostasis. Nat Rev Immunol. 9:514–519. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Villa-Morales M and Fernandez-Piqueras J:
Targeting the Fas/FasL signaling pathway in cancer therapy. Exp
Opin Ther Targets. 16:85–101. 2012. View Article : Google Scholar
|
|
21
|
David CJ and Manley JL: Alternative
pre-mRNA splicing regulation in cancer: Pathways and programs
unhinged. Genes Dev. 24:2343–2364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng J, Zhou T, Liu C, Shapiro JP, Brauer
MJ, Kiefer MC, Barr PJ and Mountz JD: Protection from Fas-mediated
apoptosis by a soluble form of the Fas molecule. Science.
263:1759–1762. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kondera-Anasz Z, Mielczarek-Palacz A and
Sikora J: Soluble Fas receptor and soluble Fas ligand in the serum
of women with uterine tumors. Apoptosis. 10:1143–1149. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sheen-Chen SM, Chen HS, Eng HL and Chen
WJ: Circulating soluble Fas in patients with breast cancer. World J
Surg. 27:10–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu JH, Wei S, Lamy T, Li Y,
Epling-Burnette PK, Djeu JY and Loughran TP Jr: Blockade of
Fas-dependent apoptosis by soluble Fas in LGL leukemia. Blood.
100:1449–1453. 2002.PubMed/NCBI
|
|
26
|
Tejedor JR, Papasaikas P and Valcarcel J:
Genome-wide identification of Fas/CD95 alternative splicing
regulators reveals links with iron homeostasis. Mol Cell. 57:23–38.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fu Y, Huang B, Shi Z, Han J, Wang Y,
Huangfu J and Wu W: SRSF1 and SRSF9 RNA binding proteins promote
Wnt signalling-mediated tumorigenesis by enhancing beta-catenin
biosynthesis. EMBO Mol Med. 5:737–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saijo S, Kuwano Y, Masuda K, Nishikawa T,
Rokutan K and Nishida K: Serine/arginine-rich splicing factor 7
regulates p21-dependent growth arrest in colon cancer cells. J Med
Invest. 63:219–226. 2016. View Article : Google Scholar : PubMed/NCBI
|