Introduction
Despite advances in surgery, chemoradiotherapy, and
molecular-targeted therapy, the prognosis for patients with
advanced gastric cancer tends to be poor (1–3). The
tumor-node-metastasis (TNM) staging system of the American Joint
Committee on Cancer is a useful model for predicting the prognosis
of patients with gastric cancer (4,5). However,
patients with gastric cancer with the same TNM stage may receive
different prognoses, partly due to heterogeneity at the molecular
level of the disease; an example of this is the different prognosis
of patients with gastric cancer with the same TNM stage in East
Asia and Europe (6,7). The identification of specific and
sensitive markers to supplement the TNM stage is required to treat
patients with gastric cancer more precisely.
Tripartite motif containing 29 (TRIM29), located at
chromosome 11q23, was initially identified in a study researching
the gene responsible for the genetic disorder ataxia telangiectasia
(8). TRIM29 belongs to the TRIM
protein family, which is characterized by a Really Interesting New
Gene (RING) finger domain, a B-box-type zinc finger domain type
(B)1, a B2, and a coiled coil region (CC) (9). Unlike the majority of TRIM proteins,
TRIM29 possesses B1-B2-CC domains but lacks the RING finger domain,
suggesting that TRIM29 exhibits no E3 ubiquitin ligase activity
(9). TRIM29 has been demonstrated to
control important cellular processes, including intracellular
signaling in innate immunity and viral infection, transcriptional
regulation, development, autophagy and carcinogenesis (10). Previous pathological studies have
revealed that TRIM29 may be useful in the diagnosis of multiple
types of cancer, including breast (11), prostate (12), pancreatic (13), lung (14) and bladder cancer (15), and esophageal squamous cell carcinoma
(16). The expression and biological
functions of TRIM29 differ between different types of cancer
(10). A previous study demonstrated
that TRIM29 expression was a marker for lymph node metastasis in
gastric cancer (17). However, the
function of TRIM29 in the prognosis of patients with gastric cancer
remains to be fully understood and requires further study.
The present study assessed the potential function of
TRIM29 expression in the prognosis of patients with resectable
gastric cancer. TRIM29 expression was evaluated using the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) in
gastric cancer tissues. The association between TRIM29 expression
and clinical outcomes in patients with gastric cancer was assessed.
The results of the present study may assist in evaluating the
clinical significance of TRIM29 expression in gastric cancer and
provide means for a more precise prognostic system for evaluating
outcomes in patients with gastric cancer.
Materials and methods
Clinical specimens
A total of 243 fresh gastric adenocarcinoma and
adjacent normal tissues were continuously retrieved from patients
who underwent curative surgery (R0 resection and D2
lymphadenectomy) for gastric cancer at the Cancer Hospital of Henan
Province (Zhengzhou, China) between January 2005 and December 2011.
All surgeries were performed according to the guidelines of the
International Gastric Cancer Association and the Japanese Gastric
Cancer Association (5). The patients
enrolled in the present study provided written informed consent for
publication and were divided into two independent sets (all
clinicopathological data for the patients is provided in Table I). Tissues from the training set
(n=113) were collected between January 2005 and December 2008.
Tissues from the validation set (n=130) were collected between
January 2009 and December 2011. The fresh cancer tissues were
immediately frozen (−180°C) and stored in liquid nitrogen following
resection, until further analysis. Pathological examinations were
performed and the histological evaluation was reassessed
independently by two gastroenterology pathologists of the Cancer
Hospital of Henan Province according to the Japanese General Rules
for Gastric Cancer Study in Surgery and Pathology (5), and the TNM staging system was applied
according to the 2010 International Union Against Cancer TNM
classification system (4). Patients
were excluded if they had previously been exposed to any
radiotherapy, chemotherapy, targeted therapy, or intervention
therapy for gastric cancer. The present study was reviewed and
approved by the Clinical Research Ethics Committee of the Cancer
Hospital of Henan Province.
 | Table I.Association between TRIM29 expression
and clinical characteristics in patients with gastric cancer. |
Table I.
Association between TRIM29 expression
and clinical characteristics in patients with gastric cancer.
|
| Training set | Validation set |
|---|
|
|
|
|
|---|
|
| TRIM29
expression | TRIM29
expression |
|---|
|
|
|
|
|---|
| Factor | Low (n) | High (n) | P-value | Low (n) | High (n) | P-value |
|---|
| All patients | 47 | 66 |
| 61 | 69 |
|
|
Age/yearsa |
|
| 0.272 |
|
| 0.245 |
| ≤60 | 20 | 35 |
| 23 | 33 |
|
|
>60 | 27 | 31 |
| 38 | 36 |
|
| Sex |
|
| 0.104 |
|
| 0.292 |
|
Female | 36 | 41 |
| 38 | 49 |
|
| Male | 11 | 25 |
| 23 | 20 |
|
| Localization |
|
| 0.174 |
|
| 0.593 |
|
Proximal | 3 | 7 |
| 10 | 12 |
|
|
Middle | 23 | 21 |
| 18 | 15 |
|
|
Distal | 21 | 38 |
| 33 | 42 |
|
| Differentiation |
|
| 0.001 |
|
| 0.001 |
| Well | 8 | 1 |
| 15 | 7 |
|
|
Moderately | 25 | 25 |
| 26 | 17 |
|
|
Poorly | 14 | 40 |
| 20 | 45 |
|
| Lauren
classification |
|
| 0.459 |
|
| 0.202 |
|
Intestinal | 31 | 39 |
| 25 | 36 |
|
|
Diffuse | 16 | 27 |
| 36 | 33 |
|
| Tumor stage |
|
| <0.001 |
|
| 0.016 |
| 1+2 | 25 | 13 |
| 16 | 7 |
|
| 3+4 | 22 | 53 |
| 45 | 62 |
|
| Lymph node
metastasis |
|
| 0.016 |
|
| <0.001 |
|
Negative | 19 | 13 |
| 34 | 14 |
|
|
Positive | 28 | 53 |
| 27 | 55 |
|
| TNM stage |
|
| 0.005 |
|
| 0.001 |
| I | 18 | 8 |
| 14 | 6 |
|
| II | 9 | 17 |
| 22 | 13 |
|
|
III | 20 | 41 |
| 35 | 50 |
|
| Tumor
size/cma |
|
| 0.157 |
|
| 0.316 |
|
<4.0 | 23 | 27 |
| 31 | 29 |
|
|
≥4.0 | 24 | 39 |
| 30 | 40 |
|
RT-qPCR
TRIM29 expression was evaluated using RT-qPCR, which
was performed as previously described (11). Briefly, total RNA containing microRNA
(miRNA) was extracted from cultured cells or tissues using the
miRNeasy Mini kit (Qiagen, Inc., Valencia, CA, USA), according to
manufacturer's protocol. cDNA was synthesized using the miScript
Reverse Transcription kit (Qiagen, Inc.) following the
manufacturer's protocol. Reverse transcription was performed using
50 ng total RNA with a primer specific for TRIM29, together with
the SYBR Green microRNA reverse transcription kit (Applied
BioSystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to manufacturer's protocol. miRNAs were quantified using
the SYBR Green miRNA RT-qPCR assay (Applied BioSystems; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
The qPCR reaction was carried out on a 7500 Fast Real-time System
(Applied Biosystems; Thermo Fisher Scientific, Inc.). All RT-qPCRs
were performed in triplicate. The data were analyzed using an
automated baseline. The threshold cycle (Cq) was defined as the
fractional cycle number at which the fluorescence exceeded the
given threshold. The data obtained from the RT-qPCR were analyzed
using the ΔΔCq method (2ΔΔCq) (11). The primer sequences used for RT-qPCR
were as follows: TRIM29 forward, 5′-ACATCATACCAGCCCTCGTC-3′ and
reverse, 5′-AGCCTTTCAGGGAGAAGGAG-3′; RNA, U6 small nuclear 1 (U6)
forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. U6 was used as the internal
control.
Statistical analysis
Statistical analysis was performed using SPSS
Statistics 21.0 software (IBM Corp., Armonk, NY, USA). Pearson's
χ2 tests or Fisher's exact tests were applied for
categorical variables. Survival curves were constructed using the
Kaplan-Meier method and the significance of the difference between
survival curves was assessed using the log-rank test. Numbers at
risk were calculated for the beginning of each time period. The Cox
proportional hazards regression model was used for multivariate
analysis. Receiver operating characteristic (ROC) analysis was used
to compare the sensitivity and specificity for the prediction of
overall survival by the parameters. All P-values were two-sided.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Association between TRIM29 expression
and pathological characteristics
To evaluate whether TRIM29 expression is associated
with the development and progression of gastric cancer, the present
study assessed TRIM29 expression in 243 patients with gastric
cancer using RT-qPCR analysis. Using the results of RT-qPCR,
patients were divided into high or low expression groups according
to the ratio of the mean expression level of TRIM29 in their normal
tissue to that in their cancer tissue (cut-off was 0.5). In the
training and validation sets, 58.41 (66/113) and 53.08% (69/130) of
the tumors were determined to exhibit high TRIM29 expression,
respectively. The characteristics and clinicopathological features
of the patients were provided (Table
I). TRIM29 expression was significantly associated with tumor
cell differentiation (training set, P=0.001; validation set,
P=0.001), tumor stage (training set, P<0.001; validation set,
P=0.016), lymph node metastasis (training set, P=0.016; validation
set, P<0.001), and TNM stage (training set, P=0.005; validation
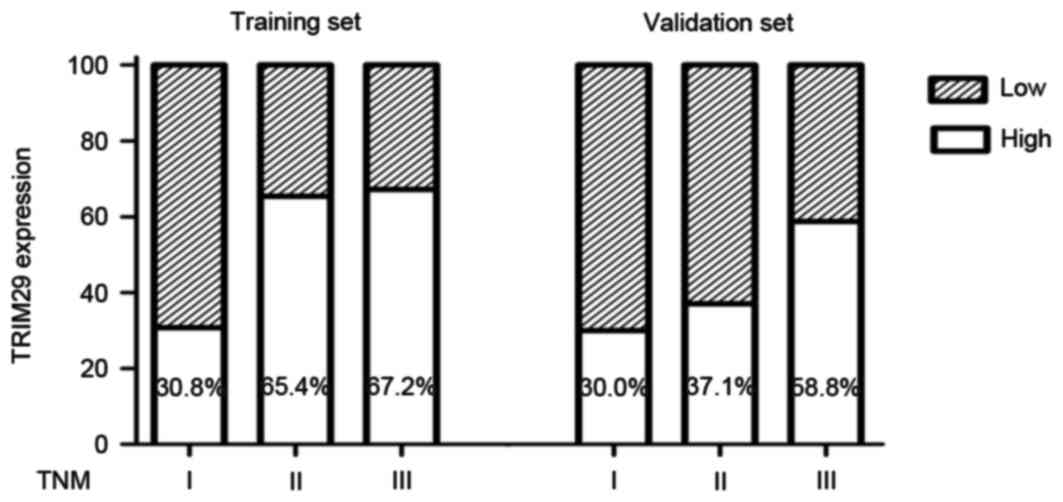
set, P=0.001). Furthermore, the percentage of patients with high
TRIM29 expression increased with cancer progression from TNM stage
I to III in the training and validation sets (Fig. 1).
Survival analysis to evaluate the
prognostic value of TRIM29 in patients with resectable gastric
cancer
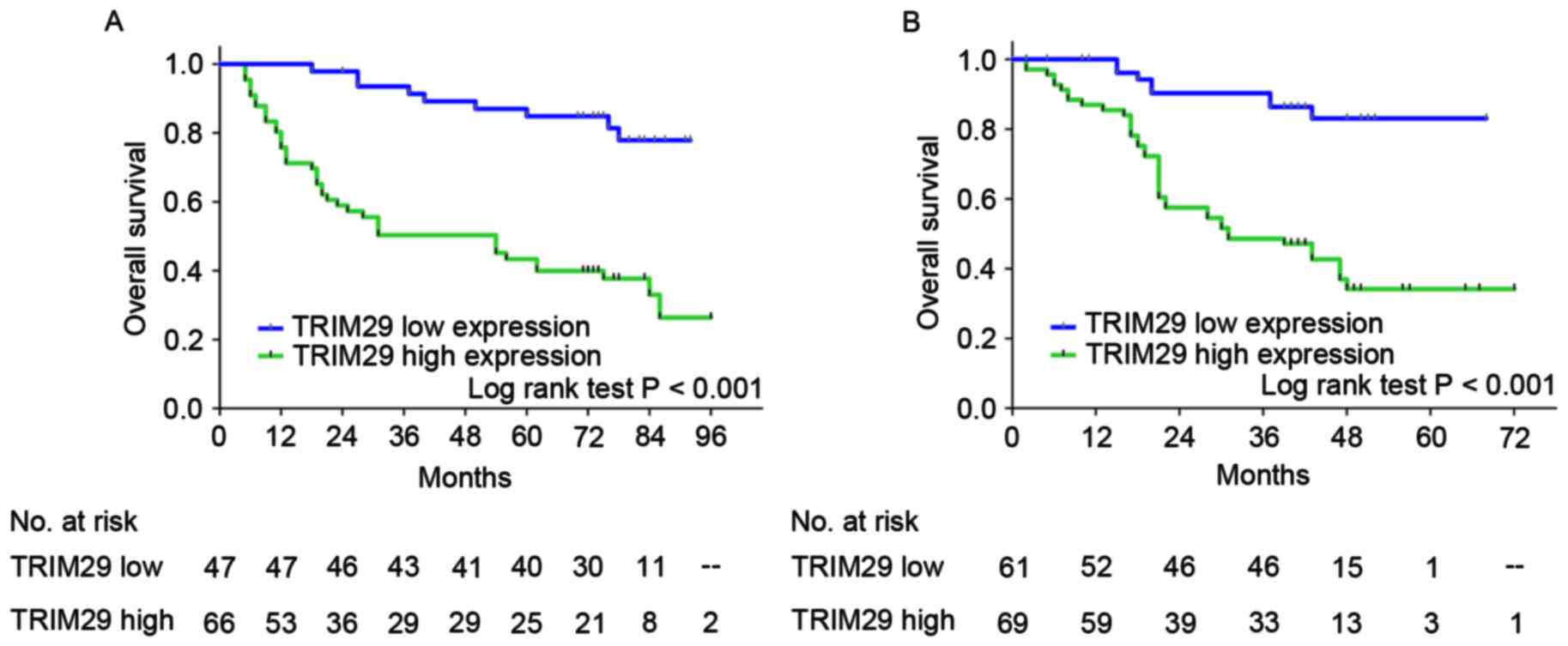
In the training and validation sets, high TRIM29
expression was associated with poorer overall survival than those
with low TRIM29 expression (training set, P<0.001; validation
set, P<0.001; Fig. 2), which
suggested that TRIM29 expression influenced the clinical outcome
for patients with resectable gastric cancer. Furthermore,
univariate analysis demonstrated that high TRIM29 expression is a
significant negative prognostic predictor for patients with gastric
cancer in the training [hazard ratio (HR), 5.14; 95% confidence
interval (CI), 2.49–10.63; P<0.001] and validation sets (HR,
5.22; 95% CI, 2.44–11.14; P<0.001). Furthermore, tumor cell
differentiation (training set, P=0.001; validation set, P=0.010),
Lauren classification (training set, P=0.002; validation set,
P=0.046), tumor stage (training set, P<0.001; validation set,
P=0.007), lymph node metastasis (training set, P=0.001; validation
set, P<0.001), and TNM stage (training set, P<0.001;
validation set, P<0.001) significantly affected the survival of
patients with gastric cancer (Table
II). In addition, using multivariate Cox regression analysis,
Lauren classification, TNM stage, and TRIM29 expression were
identified as independent and significant prognostic parameters in
the training (Lauren classification: HR, 2.81; 95% CI, 1.56–5.09;
P=0.001; TNM stage: HR, 4.93; 95% CI, 2.32–10.49; P<0.001;
TRIM29 expression: HR, 5.68; 95% CI, 2.58–12.51; P<0.001) and
validation sets (Lauren classification: HR, 1.73; 95% CI,
1.46–3.19; P=0.047; TNM stage: HR, 8.28; 95% CI, 3.16–21.70;
P<0.001; TRIM29 expression: HR, 3.32; 95% CI, 1.50–7.41;
P=0.003; Table III).
 | Table II.Univariate Cox regression analysis
for overall survival in patients with gastric cancer. |
Table II.
Univariate Cox regression analysis
for overall survival in patients with gastric cancer.
|
| Overall
survival |
|---|
|
|
|
|---|
|
| Training set | Validation set |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
|
Age/yearsa |
| 0.201 |
| 0.381 |
|
≤60 | 1.00 |
| 1.00 |
|
|
>60 | 1.45
(0.82–2.54) |
| 1.29
(0.73–2.30) |
|
| Sex |
| 0.357 |
| 0.876 |
|
Female | 1.00 |
| 1.00 |
|
|
Male | 1.24
(0.57–1.69) |
| 0.95
(0.53–1.73) |
|
| Localization |
| 0.632 |
| 0.515 |
|
Proximal + middle | 1.00 |
| 1.00 |
|
|
Distal | 1.15
(0.66–2.00) |
| 0.90
(0.65–1.24) |
|
|
Differentiation |
| 0.001 |
| 0.010 |
| Well +
moderately | 1.00 |
| 1.00 |
|
|
Poorly | 1.67
(1.24–2.25) |
| 1.47
(1.10–1.96) |
|
| Lauren
classification |
| 0.002 |
| 0.046 |
|
Intestinal | 1.00 |
| 1.00 |
|
|
Diffuse | 2.41
(1.38–4.21) |
| 1.69
(1.21–3.02) |
|
| Tumor stage |
| <0.001 |
| 0.007 |
|
1+2 | 1.00 |
| 1.00 |
|
|
3+4 | 7.66
(3.02–19.43) |
| 15.25
(2.10–110.62) |
|
| Lymph node
metastasis |
| 0.001 |
| <0.001 |
|
Negative | 1.00 |
| 1.00 |
|
|
Positive | 4.04
(1.72–9.51) |
| 11.42
(4.06–32.13) |
|
| TNM stage |
| <0.001 |
| <0.001 |
|
I+II | 1.00 |
| 1.00 |
|
|
III | 3.82
(2.02–7.23) |
| 12.30
(4.80–31.55) |
|
| Tumor size,
cma |
| 0.205 |
| 0.174 |
|
<4.0 | 1.00 |
| 1.00 |
|
|
≥4.0 | 1.43
(0.82–2.50) |
| 1.56
(0.72–3.50) |
|
| TRIM29
expression |
| <0.001 |
| <0.001 |
|
Low | 1.00 |
| 1.00 |
|
|
High | 5.14
(2.49–10.63) |
| 5.22
(2.44–11.14) |
|
 | Table III.Multivariate Cox regression analysis
for overall survival in patients with gastric cancer. |
Table III.
Multivariate Cox regression analysis
for overall survival in patients with gastric cancer.
| A, Training
set |
|---|
|
|---|
|
| Overall
survival |
|---|
|
|
|
|---|
|
| Patients | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | No. | % | HR (95% CI) | P-value |
|---|
| All training set
patients | 113 | 100 |
|
|
|
Differentiation |
|
|
| 0.446 |
| Well +
moderately | 59 | 52.2 | 1.00 |
|
|
Poorly | 54 | 47.8 | 1.17
(0. 61 to 2.26) |
|
| Lauren
classification |
|
|
| 0.001 |
|
Intestinal | 70 | 61.9 | 1.00 |
|
|
Diffuse | 43 | 38.1 | 2.81 (1.56 to
5.09) |
|
| TNM stage |
|
|
| <0.001 |
|
I+II | 52 | 46.0 | 1.00 |
|
|
III | 61 | 54.0 | 4.93
(2.32 to 10.49) |
|
| TRIM29
expression |
|
|
| <0.001 |
|
Low | 47 | 41.6 | 1.00 |
|
|
High | 66 | 58.4 | 5.68
(2.58 to 12.51) |
|
|
| B, Validation
set |
|
|
| Overall
survival |
|
|
|
|
|
Patients | Multivariate
analysis |
|
|
|
|
| Factor | No. | % | HR (95%
CI) | P-value |
|
| All validation set
patients | 130 | 100 |
|
|
|
Differentiation |
|
|
| 0.264 |
| Well +
moderately | 65 | 50.0 | 1.00 |
|
|
Poorly | 65 | 50.0 | 1.18 (0.88 to
1.60) |
|
| Lauren
classification |
|
|
| 0.047 |
|
Intestinal | 61 | 46.9 | 1.00 |
|
|
Diffuse | 69 | 53.1 | 1.73 (1.46 to
3.19) |
|
| TNM stage |
|
|
| <0.001 |
|
I+II | 45 | 34.6 | 1.00 |
|
|
III | 85 | 65.4 | 8.28
(3.16 to 21.70) |
|
| TRIM29
expression |
|
|
| 0.003 |
|
Low | 61 | 46.9 | 1.00 |
|
|
High | 69 | 53.1 | 3.32 (1.50 to
7.41) |
|
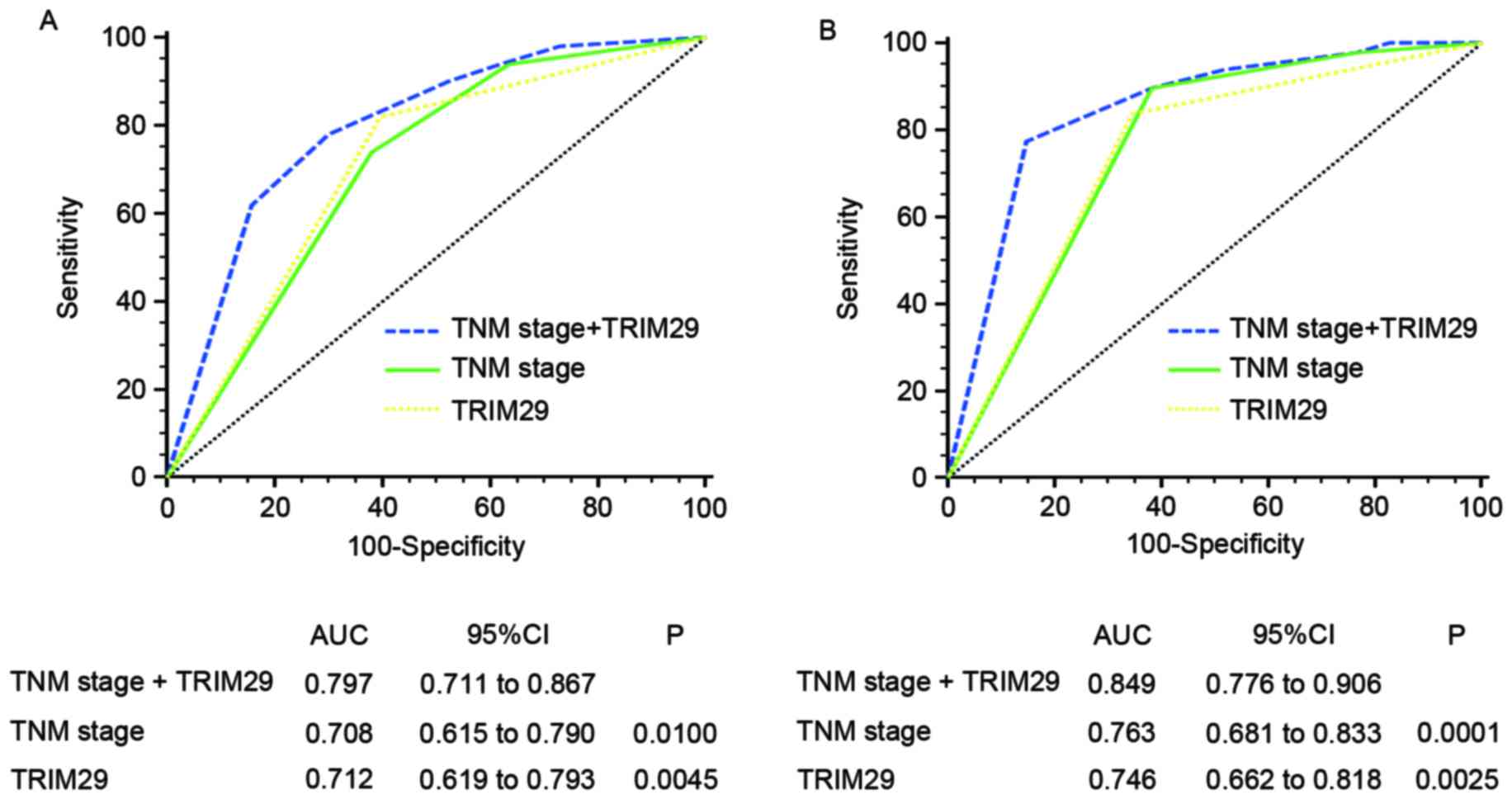
To develop a more sensitive predictive method for
patients with gastric cancer, a novel prognostic model combining
TNM stage and TRIM29 expression was constructed. ROC analysis was
performed to compare its prognostic value against TNM stage or
TRIM29 expression alone. The prognostic model that included TNM
stage and TRIM29 expression [training set, area under the curve
(AUC)=0.797; validation set, AUC=0.849] was associated with
increased prognostic value compared with those that included TNM
stage (training set, AUC=0.708; P=0.0100; validation set,
AUC=0.763; P=0.0001) or TRIM29 expression (training set, AUC=0.712;
P=0.0045; validation set, AUC=0.746; P=0.0025) alone for the
training and validation sets (Fig.
3).
Discussion
Although the incidence of gastric cancer has
decreased during previous decades in numerous industrialized
countries, the incidence in China remains one of the highest
globally, and the disease has already reached an advanced stage in
the majority of patients by the time of diagnosis (1–3,5). The TNM staging system remains an
important factor for predicting the prognosis of patients with
gastric cancer (4). However, patients
with gastric cancer with the same TNM stage may receive different
prognoses, partly due to the heterogeneity of the disease at the
molecular level (6,7). Identifying specific and sensitive
markers to supplement the TNM stage is required to treat patients
with gastric cancer more precisely. The present study demonstrated
that TRIM29 expression in gastric cancer tissues represents a
promising, independent predictor for the survival of patients with
gastric cancer. High TRIM29 expression was associated with a
decreased overall survival time following surgical resection.
Furthermore, incorporating the TRIM29 expression level into the TNM
staging system increased the prognostic value of the latter. The
results of the present study suggested that TRIM29 expression may
possess discriminatory power as a supplementary risk factor in
patients with gastric cancer, and facilitate an increase in
classification accuracy under the TNM staging system. However, the
function of TRIM29 in the prognosis of patients with gastric cancer
remains to be fully understood and requires further study.
A previous study identified that TRIM29 was
associated with increased aggressiveness in multiple types of
cancer (10). TRIM29 antagonizes
multiple processes in a number of different cancer cells by
regulating certain genes and influencing their functions in
numerous cellular signaling pathways (11–16).
Another study reported that TRIM29 expression increased as normal
pancreatic ductal epithelia progressed to infiltrating cancer,
which suggested that upregulating TRIM29 promoted the development
of invasive pancreatic cancer (18).
However, the underexpression of TRIM29 in breast and prostate
cancer has also been reported using serial analysis of gene
expression and DNA microarray analysis (19,20).
TRIM29 may induce a malignant phenotype to revert to a
non-malignant phenotype in osteosarcoma and breast cancer cell
lines (21). Zhou et al
(22) indicated that upregulating
TRIM29 expression promoted proliferation and metastasis in
nasopharyngeal carcinoma via the phosphatase and tensin
homolog/protein kinase B/mechanistic target of rapamycin signaling
pathway. Kosaka et al (17)
reported that TRIM29 expression was positively associated with a
poorer histological grade, increased tumor size and invasion, and
lymph node metastasis in gastric cancer. In addition, Qiu et
al (23) demonstrated that TRIM29
functioned as an oncogene in gastric cancer and was regulated by
microRNA-185. However, the association between TRIM29 expression
and the prognosis of patients with gastric cancer has not yet been
established. The results of the present study revealed that TRIM29
expression was significantly associated with tumor cell
differentiation, tumor stage, lymph node metastasis, and TNM stage.
Furthermore, an increased percentage of patients with high TRIM29
expression was associated with cancer progression from TNM stage I
to III in the training and validation sets. However, elucidating
the mechanism underlying the dysregulation of TRIM29 expression in
gastric cancer tissues requires further study.
In the present study, all samples were collected
from patients at the Cancer Hospital of Henan Province and all
surgeries were performed according to the guidelines of the
International Gastric Cancer Association and the Japanese Gastric
Cancer Association. Immediately following resection of the gastric
cancer specimens from the patients, the fresh cancer tissues were
frozen and stored in liquid nitrogen until analysis. Prior to
RT-qPCR analysis, the specimens were evaluated independently by two
gastroenterology pathologists blind to clinicopathological patient
data. In addition, the experiment was repeated three times with
each gastric cancer tissue. However, the present study is limited
in certain respects. To begin, the present study is retrospective,
and selection bias may not be entirely eliminated. Secondly, data
on disease-free survival were not included in the present study and
should be collected in subsequent studies. Finally, few patients
were enrolled in the present study, confirming the results of which
requires large, randomized controlled trials.
To conclude, the results of the present study
revealed that TRIM29 expression was associated with unfavorable
prognosis and may be adopted as a novel prognostic indicator in
patients with resectable gastric cancer. Furthermore, incorporating
TRIM29 expression level into the TNM staging system increased the
prognostic value of the latter for patients with resectable gastric
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Basic and
Advanced Technology Research Project of Science and Technology
Department of Henan Province (grant no. 142300410276).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
CW and YZ acquired, analyzed and interpreted the
data, performed statistical analysis and drafted the manuscript. BC
and WY offered technical and material support. JH designed the
study, analyzed and interpreted the data, drafted the manuscript,
obtained funding and supervised the study.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Clinical Research Ethics Committee of the Cancer Hospital of Henan
Province. All patients provided written informed consent to
participate.
Consent for publication
All patients provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L: Incidence and mortality of gastric
cancer in China. World J Gastroenterol. 12:17–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen WQ, Zheng RS, Zhang SW, Zeng HM and
Zou XN: The incidences and mortalities of major cancers in China,
2010. Chin J Cancer. 33:402–405. 2014.PubMed/NCBI
|
|
4
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thrumurthy SG, Chaudry MA, Hochhauser D
and Mughal M: The diagnosis and management of gastric cancer. BMJ.
347:f63672013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stadtländer CT and Waterbor JW: Molecular
epidemiology, pathogenesis and prevention of gastric cancer.
Carcinogenesis. 20:2195–2208. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Figueiredo C, Garcia-Gonzalez MA and
Machado JC: Molecular pathogenesis of gastric cancer. Helicobacter.
18 Suppl 1:S28–S33. 2013. View Article : Google Scholar
|
|
8
|
Kapp LN, Painter RB, Yu LC, van Loon N,
Richard CW III, James MR, Cox DR and Murnane JP: Cloning of a
candidate gene for ataxia-telangiectasia group D. Am J Hum Genet.
51:45–54. 1992.PubMed/NCBI
|
|
9
|
Reymond A, Meroni G, Fantozzi A, Merla G,
Cairo S, Luzi L, Riganelli D, Zanaria E, Messali S, Cainarca S, et
al: The tripartite motif family identifies cell compartments. EMBO
J. 20:2140–2151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hatakeyama S: Early evidence for the role
of TRIM29 in multiple cancer models. Expert Opin Ther Targets.
20:767–770. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–428. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanno Y, Watanabe M, Kimura T, Nonomura K,
Tanaka S and Hatakeyama S: TRIM29 as a novel prostate basal cell
marker for diagnosis of prostate cancer. Acta Histochem.
116:708–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun H, Dai X and Han B: TRIM29 as a novel
biomarker in pancreatic adenocarcinoma. Dis Markers.
2014:3178172014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou ZY, Yang GY, Zhou J and Yu MH:
Significance of TRIM29 and β-catenin expression in non-small-cell
lung cancer. J Chin Med Assoc. 75:269–274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fristrup N, Birkenkamp-Demtröder K,
Reinert T, Sanchez-Carbayo M, Segersten U, Malmström PU, Palou J,
Alvarez-Múgica M, Pan CC, Ulhøi BP, et al: Multicenter validation
of cyclin D1, MCM7, TRIM29, and UBE2C as prognostic protein markers
in non-muscle-invasive bladder cancer. Am J Pathol. 182:339–349.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lai W, Zheng X, Huang Q, Wu X and Yang M:
Down-regulating ATDC inhibits the proliferation of esophageal
carcinoma cells. Eur Rev Med Pharmacol Sci. 18:3511–3516.
2014.PubMed/NCBI
|
|
17
|
Kosaka Y, Inoue H, Ohmachi T, Yokoe T,
Matsumoto T, Mimori K, Tanaka F, Watanabe M and Mori M: Tripartite
motif-containing 29 (TRIM29) is a novel marker for lymph node
metastasis in gastric cancer. Ann Surg Oncol. 14:2543–2549. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Heidt DG, Lee CJ, Yang H, Logsdon
CD, Zhang L, Fearon ER, Ljungman M and Simeone DM: Oncogenic
function of ATDC in pancreatic cancer through Wnt pathway
activation and beta-catenin stabilization. Cancer Cell. 15:207–219.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ernst T, Hergenhahn M, Kenzelmann M, Cohen
CD, Bonrouhi M, Weninger A, Klären R, Gröne EF, Wiesel M, Güdemann
C, et al: Decrease and gain of gene expression are equally
discriminatory markers for prostate carcinoma: A gene expression
analysis on total and microdissected prostate tissue. Am J Pathol.
160:2169–2180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nacht M, Ferguson AT, Zhang W, Petroziello
JM, Cook BP, Gao YH, Maguire S, Riley D, Coppola G, Landes GM, et
al: Combining serial analysis of gene expression and array
technologies to identify genes differentially expressed in breast
cancer. Cancer Res. 59:5464–5470. 1999.PubMed/NCBI
|
|
21
|
Hosoi Y, Kapp LN, Murnane JP, Matsumoto Y,
Enomoto A, Ono T and Miyagawa K: Suppression of
anchorage-independent growth by expression of the
ataxia-telangiectasia group D complementing gene, ATDC. Biochem
Biophys Res Commun. 348:728–734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou XM, Sun R, Luo DH, Sun J, Zhang MY,
Wang MH, Yang Y, Wang HY and Mai SJ: Upregulated TRIM29 promotes
proliferation and metastasis of nasopharyngeal carcinoma via
PTEN/AKT/mTOR signal pathway. Oncotarget. 7:13634–13650.
2016.PubMed/NCBI
|
|
23
|
Qiu F, Xiong JP, Deng J and Xiang XJ:
TRIM29 functions as an oncogene in gastric cancer and is regulated
by miR-185. Int J Clin Exp Pathol. 8:5053–5061. 2015.PubMed/NCBI
|