Introduction
Nasopharyngeal carcinoma (NPC) is an endemic disease
particularly prevalent among the people of Southern China (1). As one of the most common malignancies in
this region, the incidence ratio of NPC ranges between 0.015–0.05%,
which is 10- to 20-fold higher than for the population of China as
a whole (2). A number of features
contribute to this high incidence ratio, including ethnic,
geographical and genetic factors (3).
NPC arises from the epithelial lining of nasopharynx. At the early
stage of disease, NPC is always asymptomatic and difficult to be
identified by physical examination. Thus a large number of patients
were diagnosed at advanced stages (1). Due to its relatively high
radiosensitivity and inaccessible anatomical position, radiotherapy
is the primary treatment for patients with NPC diagnosed in the
early stage of disease (4,5). Nevertheless, the prognosis of the
patient worsens significantly when the stage of the tumor increases
or the patient undergoes distant metastasis (6). The overall and relapse-free survival
times of the patients who underwent concurrent chemotherapy are
significantly improved compared with those of the patients who
received radiotherapy alone (7).
However, the undesirable side effects of chemotherapy reduce the
tolerance of patients to treatment. Therefore, investigating the
underlying molecular mechanism of NPC may provide novel therapeutic
targets or prognostic factors for this malignancy.
Chibby (Cby) is a small nuclear protein that
consists of 126 amino acids; it is evolutionarily conserved from
Drosophila to humans, and serves as an antagonist of
β-Catenin, a central component of canonical Wnt signaling pathway
(8). Cby physically interacts with
the C terminus of β-Catenin and inhibits its transcriptional
activation potential by competitive inhibition of the T-cell
factor/lymphoid enhancer factor (TCF/LEF) family of transcription
factors (9). Cby additionally
cooperates with 14-3-3 adaptor proteins to transport β-Catenin out
of the nucleus (10). In previous
studies, overexpression of Cby promoted the differentiation of
mouse cardiomyocytes from embryonic stem cells (11) and mice adipocyte differentiation from
mouse embryo 3T3-L1 cells (12). In
Cby-knockout mice, pulmonary malfunction was observed owing to
reduced proliferation and the abnormal differentiation of lung
epithelial cells (13). Cby is
therefore involved in numerous cellular behaviors, including
proliferation, survival and differentiation through the Wnt
signaling pathway. The Wnt signaling pathway is essential to the
genesis and development of cancer (14,15). The
downregulated expression of Cby was validated in colon carcinoma
cell lines by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) (16). In another
study in pediatric ependymomas, methylation-specific PCR and
bisulphite sequencing revealed the transcriptional inactivation of
chromosome 22 open reading frame 2, where the gene encoding Cby is
located (17). In addition, Cby has
been demonstrated to suppress the growth of human colon
adenocarcinoma SW480 cells through the inhibition of the canonical
Wnt signaling pathway (18). In a
recent study, Cby was found to activate endoplasmic reticulum (ER)
stress and apoptosis in breakpoint cluster protein/Abl kinase
1-positive human myeloid leukemia cells by inducing the cytoplasmic
accumulation of β-catenin (19).
Thus, the downregulation of Cby in cancer cells may provide
information regarding the use of Cby as therapeutic target and
prognostic factor.
The present study first investigated the expression
of Cby in the biopsy specimens of 45 patients with NPC and analyzed
its association with disease development. Next, Cby was
overexpressed in the NPC SUNE1 cell line and demonstrated that Cby
overexpression significantly reduced the proliferation of SUNE1
cells by inducing cell cycle arrest and enhancing the
susceptibility of SUNE1 cells to apoptosis.
Materials and methods
Patients and tissue samples
A total of 45 (32 male, 13 female; age range, 23–68
years; median age, 45 years) patients were diagnosed with NPC by
biopsy between April 2010 to January 2011 in The First Affiliated
Hospital of Xiamen University (Xiamen, China). No patients had
undergone radiotherapy or chemotherapy. All patients were staged
according to the International Union Against Cancer (UICC)
Tumor-Node-Metastasis (TNM) Classification of Malignant Tumors, 7th
edition (20). The pathological
classification was determined according to World Health
Organization Classification of Tumors Pathology and Genetics
(21). Detailed clinical information
of every patient was recorded. Written informed consent was
acquired from every patient enrolled in the present study. The
research program and experimental procedures were approved by the
Ethics Board of The First Affiliated Hospital of Xiamen
University.
Tumor samples and adjacent normal tissue were
obtained by biopsy. Each specimen was divided into two parts: One
was fixed in 10% pH-neutral formalin for 24 h at room temperature,
embedded in paraffin for pathological examination; the other was
snap-frozen and stored in liquid nitrogen for molecular biology
analysis. All sample treatment procedures were finished within 30
min.
Immunohistochemistry
Immunohistochemistry was performed using the
streptavidin-biotin complex technique. From the formalin-fixed,
paraffin-embedded tissue, 4-µm-thick sections were cut and then
dried at room temperature, deparaffinized in xylene for 15 min at
room temperature, and then rehydrated in a descending ethanol
series (100, 90, 80, 70, 50 and 10%) at room temperature. Tissue
sections were submerged in 3% hydrogen peroxide for 10 min at room
temperature to block endogenous peroxidase activity. Antigen
retrieval was performed by immersing sections in 0.01 M sodium
citrate buffer for 20 min at 95°C. Samples were cooled at room
temperature for 1 h and then treated with rabbit serum blocking
solution (Boster Biological Technology, Pleasanton, CA, USA) for a
further 20 min at room temperature. Samples were incubated in
primary rabbit polyclonal anti-Cby antibodies (cat. no., sc-393295;
dilution, 1:500; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
for 1 h at room temperature, followed by biotin-labeled rabbit
anti-mouse secondary antibody (cat. no., BA1005; dilution, 1:300;
Boster Biological Technology) for 20 min. Next,
streptavidin-coupled horseradish peroxidase (Boster Biological
Technology) was added. Complexes were visualized in brown following
treatment with 3,3′-diaminobenzidine (Boster Biological Technology)
at room temperature. The slides were counterstained with
hematoxylin for 1 min at room temperature, dehydrated, mounted and
viewed under a light microscope (Olympus BH-2; Olympus Corporation,
Tokyo, Japan) at a magnification of ×400. For every slide, PBS
instead of primary antibody was used as negative control, and
slides verified as positive prior to the experiment were used as
positive control.
Evaluation of Cby histological
expression
All histological slides were scored independently by
two pathologists blinded for patient characteristics. Cby protein
was present as brown granules in either the nucleus or cytoplasm.
The intensity score of Cby staining was graded as follows: 0, no
staining; 1, mild; 2, moderate; 3, intensive. To evaluate the
percentage of Cby-positive cells, images of the tissue sections
were generated using a ×40 objective lens, five high-magnification
microscope fields (40,000 µm2) were randomly selected
for each section and positive Cby staining cells were counted for
every microscope field. The percentage score of Cby staining was
generated as: 0, ≤10% cells were positively Cby stained; 1, 11–25%
cells stained; 2, 26–50% cells stained; 3, >50% cells stained
positively. The intensity and percentage score were summed to
generate a composite with a maximum score of 6. A score of >2
represented a positive immunohistochemical identification of Cby
(22). The Cby protein expression
level was stratified as: 3, weak; 4/5, moderate; 6, strong.
Cell culture
The NPC SUNE1 cell line and an immortalized
non-malignant nasopharyngeal epithelial NP69 cell line were
purchased from Cell Bank of Xiangya School of Medicine (Central
South University, Hunan, China). The SUNE1 cells were maintained in
Dulbecco's Modified Eagle's Medium (DMEM) with 10% fetal bovine
serum (FBS) and 100 U/ml penicillin-streptomycin at 37°C in 5%
CO2, and the NP69 cells were cultured in defined
keratinocyte-serum-free medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 0.2 ng/ml
growth factors, 5% heat-inactivated FBS, and 100 U/ml
penicillin-streptomycin.
Plasmid construction and
electroporation
Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. cDNA was produced from 1 µg total RNA
using a RT Kit (Takara Bio, Inc., Otsu, Japan). The primer sets
used were as follows: forward,
5′-GCTCTAGAATGGACTACAAAGACGATGACGACAAGATGCCTTTCTTTGGGAATACGG-3′ and
reverse, 5′-CGACGCGTTCATTTTCTCTTCCGGCTGATC-3′, with XbaI and MluI
enzyme digestion site incorporated into the forward and reverse
primers, respectively; a FLAG sequence was inserted into the 5′ end
of forward primer. The PCR products were enzyme digested and
subcloned into plasmid plv-cs2.0 (Invitrogen; Thermo Fisher
Scientific, Inc.), which contains the green fluorescent protein
reporter gene. The recombination plasmid (named plv-cs2.0-Cby) DNA
was identified by 1% agarose gel electrophoresis following cleavage
with different restriction enzymes (XbaI/MluI and XbaI/KpnI) and
DNA sequencing (performed by Shanghai Sangon Biotech Co., Ltd.,
Shanghai, China). A total of 1×106 SUNE1 cells were
electroporated with 1 µg of either plv-cs2.0-Cby or plv-cs2.0
plasmid DNA in 100 µl DMEM media under 250 V square-wave pulse to
reach a total electric capacity of 950 µF using a Gene
PulserXcellEukarotic system (Bio-Rad Laboratories, Inc., Hercules,
CA, USA). Electroporated cells were seeded in 6-well plates. At 48
h after electroporation, cells were further cultured in selection
media containing 1 µg/ml puromycin for 1 week, the plv-cs2.0-Cby
SUNE1 cells and its control plv-cs2.0 SUNE1 cells were collected
for subsequent experiments.
RNA extraction and RT-qPCR
Total RNA was extracted using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions. cDNA was produced from 1 µg total RNA using RT Kit
(Takara Bio, Inc.). qPCR was performed with SYBRGreen Real-Time PCR
Master Mix (TAKARA, TOYOBO, Japan) and gene specific primer pairs
for Cby. The qPCR reaction system was made up as: 10 µl PCR master
mix, 2 µl cDNA, 1.2 µl primer sets (10 µmol) and 6.8 µl ddH2O to
form a total volume of 20 µl. The primer pairs used in this
experiment was as follows: Cby forward, 5′-TTTGGGAATACGTTCAGTCCG-3′
and reverse, 5′-TCAGCCGCAAGAGATTGTTC-3′; 18S RNA forward,
5′-GTCTGTGATGCCCTTAGATG-3′ and reverse, 5′-AGCTTATGACCCGCACTTAC-3′.
The qPCRthermocycling conditions were: Initial denaturation at 95°C
for 30 sec, followed by 45 cycles of denaturation at 90°C for 30
sec, annealing at 60°C for 20 sec, extension at 72°C for 25 sec,
and termination at 72°C for 15 min. Each qPCR reaction was
performed in triplicate. Relative gene expression was analyzed by
using the 2−∆∆Cq method (23). 18S RNA was used as the internal
reference gene. The relative n-fold ratio of tumor samples to the
normal tissue was calculated; a ratio ≤0.66 was considered as
under-expression and ≥1.5 as overexpression, as described
previously (24).
Western blot analysis
Monolayer plv-cs2.0-Cby SUNE1 cells or control
plv-cs2.0 SUNE1 cells in the exponential phase were lysed and total
proteins were extracted using Radioimmunoprecipitation Assay buffer
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), following the
manufacturer's protocol. The protein concentration was determined
using a Bradford assay. A total of 80 µg protein per well were
separated by 12% SDS-PAGE, then transferred onto a
polyvinylidenedifluoride membrane (EMD Millipore, Billerica, MA,
USA). Membranes were blocked in 7% bovine serum albumin (Boster
Biological Technology) in TBS and 0.5% Tween-20 (TBST) for 1 h at
room temperature, and then incubated with rabbit polyclonal
anti-Cby antibody (cat. no., sc-393295; dilution 1:2,000) and
anti-β-actin antibody (cat. no., sc-130300; 1:1,000; Santa Cruz
Biotechnology, Inc.) for 2 h at room temperature and then washed in
0.5% TBST three times for 10 min. Horseradish peroxidase-conjugated
rabbit anti-mouse secondary antibodies (cat. no., BA1005; dilution,
1:5,000; Boster Biological Technology) were then added for 1 h at
room temperature. β-actin was used as an internal control. The
signal was developed using the Western Lightning Plus-ECL reagent
(Pierce; Thermo Fisher Scientific, Inc.). The density of the
protein bands was analyzed using ImageJ 1.48 software (National
Institutes of Health, Bethesda, MD, USA).
Dual-luciferase reporter assay
SUNE1 cells transfected with plv-cs2.0-Cby or
plv-cs2.0 (1×105 cells/well) were seeded in 6-well
plates for 24 h. Cells in each well were cotransfected with 100 ng
pTOPFlash or pFOPFlash reporter plasmid, and 20 ng Renilla
luciferase expression vector (Promega Corporation, Madison, WI,
USA) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) for a further 24 h. Then Wnt3a (30 nM;
Sigma-Aldrich; Merck KGaA), an activator of the Wnt/β-catenin
pathway, was added for 6 h. The luciferase activity was analyzed
using a Dual-Luciferase Reporter Assay system (Promega
Corporation), according to the manufacturer's instructions, with a
Modulus single-tube-type multifunctional detector (Beijing
Yuanpinghao Bio Co., Ltd., Beijing, China). The luciferase activity
was normalized to renilla luciferase activity.
MTT assay
A total of 500 cells per well were transferred to
96-well plate and cultured at 37°C in 5% CO2. The MTT
assay was performed every 24 h from day 0 to day 5. A total of 20
µl MTT reagent (5 mg/ml, ScienCell Research Laboratories, Inc., San
Diego, CA, USA) were added to each well and further incubated in
darkness for 4 h, after which the supernatant was aspirated and 150
µl dimethyl sulfoxide was added to each well. The plate was
incubated at room temperature for 15 min and then the absorbance
(A) was measured at 570 and 630 nm. The A630 value were
subtracted from A570 value and plotted against the time
of culture. Data were collected from three independent experiments
at every time point.
Cell cycle and apoptosis determined by
flow cytometry
A total of 1×106 cells following
thymidine-synchronization at G1 phase prior to cell
cycle analysis were harvested and washed in cold PBS, and fixed by
adding 70% ethanol dropwise to the cell pellet. The cell suspension
was stored at 4°C overnight. Fixed cells were centrifuged for 5 min
at 4°C and 2,000 × g and resuspended in 400 µl cold PBS. Following
this, 10 µl RNaseA (5 mg/ml) was added and incubated at 37°C for 1
h and then 50 µl propidium iodide (1 mg/ml; Sangon Biotech Co.,
Ltd.) was added then incubated in darkness at 37°C for 30 min. Data
was acquired using a flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) and analyzed using ModFit 3.3 (Verity Software
House, Topsham, ME, USA) software.
The apoptosis of plv-cs2.0-Cby SUNE1 cells and
control plv-cs2.0 SUNE1 cells treated with 20 µM 5-fluorouracil
(5-FU) for 24 h (Sigma-Aldrich; Merck KGaA.) were detected by a
FITC Annexin V/Dead Cell Apoptosis kit (Invitrogen, Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Annexin V+, PI−+ cells were regarded as
necrotic cells, whereas Annexin V+ but PI−
cells were regarded as apoptotic cells. Data was acquired using a
flow cytometer (BD Biosciences) and analyzed using ModFit 3.3
(Verity Software House.) software.
Hoechst staining assay
The plv-cs2.0-Cby SUNE1 cells and control plv-cs2.0
SUNE1 cells (5×105 cells/ml) were seeded in 35-mm
culture dishes and cultured at 37°C in 5% CO2. Cells
were fixed in 4% formalin at room temperature for 30 min when
reached 90% confluence, then stained with Hoechst 33342 (5 µg/ml)
for 10 min at room temperature. After washing with PBS, the
apoptotic cells were counted under a fluorescence microscope
(DMi8-M, Leica, Barcelona, Spain) at a magnification of ×400. Five
microscope fields were randomly selected from every culture dish.
Apoptotic rate (%)=apoptotic cells/total cells ×100.
Colony formation
A total of 500 cells of plv-cs2.0-Cby or plv-cs2.0
cells were cultured in six-well plates for 14 days, and then
stained with 0.005% crystal violet for 30 min at room temperature.
The number of foci containing more than 50 cells was counted.
Statistical analysis
All statistical analysis was performed with SPSS
Statistics 17.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed
as the mean ± standard error of the mean. A paired t-test was used
to analyze the difference in mRNA levels between NPC and adjacent
normal tissue. The χ2 test was used to compare Cby
protein expression status between NPC and adjacent normal tissue,
as well as various clinical characteristic groups. Student's t-test
was used to compare the differences of qPCR, western blot, MTT
value, percentage of S phase cells and apoptosis rate in in
vitro experiments. P<0.05 was consider to indicate a
statistically significant difference.
Results
Patient characteristics
Of the 45 patients enrolled in this research, 32
(71.1%) were male while 13 were female. Patients' age ranged from
23–68 years old, with median age of 53. There were 24 (53.3%)
patients with T1-T2 disease and 21 patients with T3-T4 patients. In
total, 27 (60%) patients had regional lymph node metastases,
whereas 18 patients did not; 19 (42.2%) patients had early-stage
(I–II) disease and 26 patients had advanced stage (III–IV) disease.
In total, 9 (20%) patients were diagnosed with keratinizing
squamous cell carcinoma, 20 (44.4%) patients were diagnosed with
undifferentiated non-keratinizing carcinoma, 16 (35.6%) patients
were diagnosed with differentiated non-keratinizing carcinoma and
none had basaloid squamous cell carcinoma.
mRNA expression of Cby is reduced in
NPC tissue
NPC tissue from 45 NPC patients were isolated and
analyzed by RT-qPCR. The 18S RNA was used as internal control. qPCR
revealed that 68.9% (31/45) patients exhibited reduced expression
of Cby in tumor tissue compared with matched non-cancerous tissue
(≤0.66-fold), whereas 15.6% (7/45) patients exhibited
overexpression of Cby (≥1.5-fold), with the other 15.6% (7/45)
exhibiting a similar expression level; the difference had
statistical significance (P<0.05). The relative level of Cby
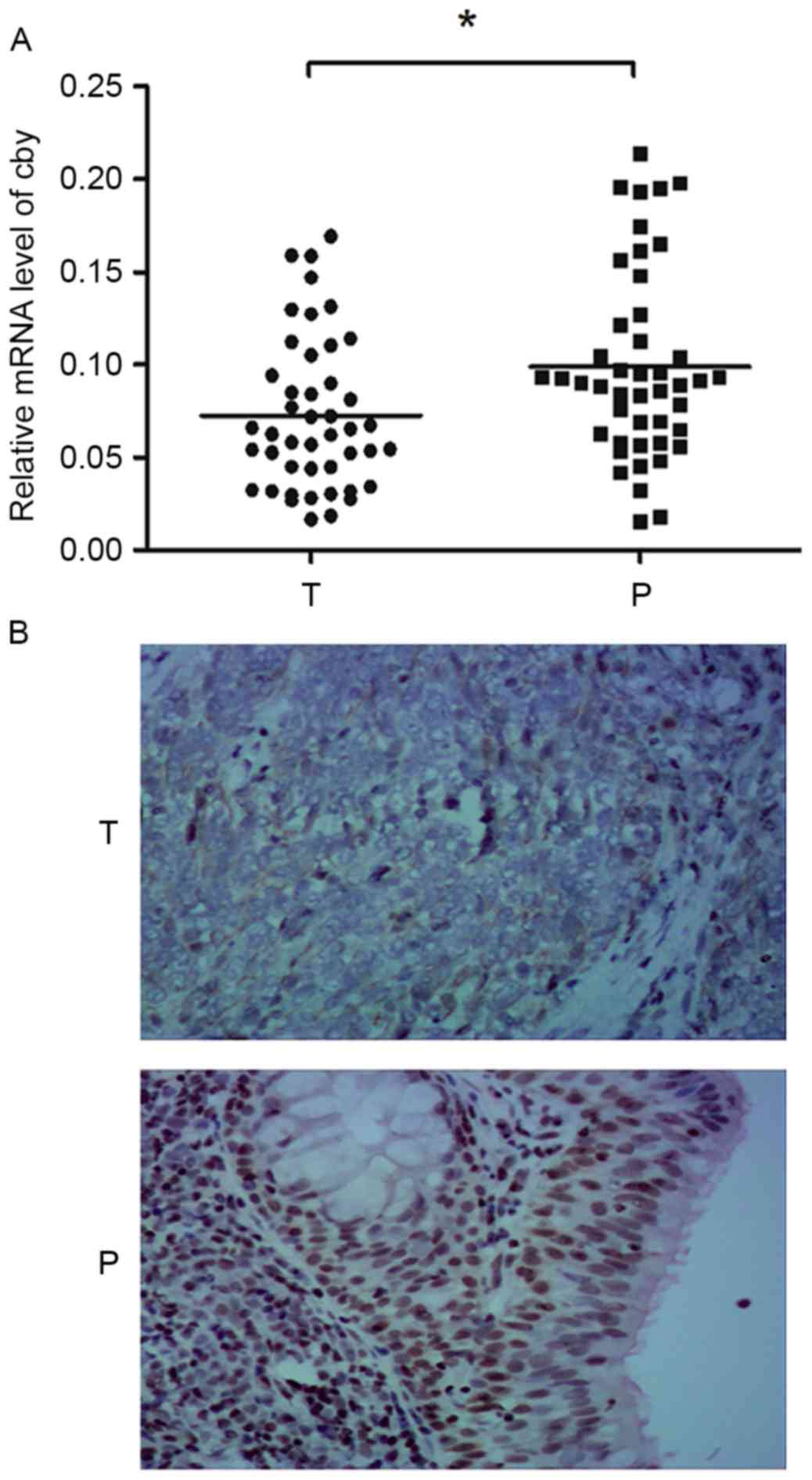
mRNA for each patient is presented in Fig. 1A. This result indicated that the
expression of Cby was reduced in NPC tissue in majority of the NPC
patients.
When the Cby mRNA expression was analyzed based on
different clinical groups, the difference between T1/T2 and T3/T4
groups was statistically significant (P<0.05), as was the
difference between early (I/II) and advanced stage (III/IV) disease
(P<0.05). The Cby mRNA expression level did not differ
statistically between groups separated by sex, age, regional lymph
node metastasis status and pathological classifications (Table I).
 | Table I.The association of Cby mRNA
expression with clinical features. |
Table I.
The association of Cby mRNA
expression with clinical features.
| Clinical
features | Patients, n | RQ of Cby mRNA | 95% CI | P-value |
|---|
| Sex |
|
|
| >0.05 |
|
Male | 32 | 0.0779 | 0.0697–0.0833 |
|
|
Female | 13 | 0.0775 | 0.0770–0.0803 |
|
| Age, years |
|
|
| >0.05 |
|
≤50 | 27 | 0.0777 | 0.0696–0.0825 |
|
|
>50 | 18 | 0.0781 | 0.0752–0.0808 |
|
| T staging |
|
|
| >0.05 |
|
1/2 | 24 | 0.0803 | 0.0770–0.0836 |
|
|
3/4 | 21 | 0.0775 | 0.0685–0.0797 |
|
| Lymph node
metastasis |
|
|
| >0.05 |
| N0 | 18 | 0.0801 | 0.0752–0.0836 |
|
|
N1-3 | 27 | 0.0775 | 0.0696–0.0805 |
|
| Clinical
staging |
|
|
| <0.01 |
|
I/II | 19 | 0.0810 | 0.0775–0.0896 |
|
|
III/IV | 26 | 0.0771 | 0.0687–0.0794 |
|
| Pathological
classification |
|
|
| >0.05 |
| WHO
I | 9 | 0.0803 | 0.0728–0.0836 |
|
| WHO
II | 20 | 0.0773 | 0.0760–0.0821 |
|
| WHO
III | 16 | 0.0776 | 0.0696–0.0805 |
|
Cby protein expression is reduced in
NPC tissue
Cby protein expression in NPC tissue and adjacent
normal tissue were assessed by immunohistochemical staining.
Scarlet-to-brown staining was observed in either the nucleus or the
cytoplasm in Cby-positive cells (Fig.
1B). A total of 73.3% (33/45) patients exhibited a lower Cby
expression score (the staining intensity and positive rate scores
summed) in NPC tissue than normal tissue. The Cby-positive rates
were 42.2% (19/45) and 91.1% (41/45) in NPC and adjacent normal
tissues, respectively. The difference was statistically significant
(χ2=24.2, P<0.01). This result suggested Cby protein
expression was reduced in NPC tissue compared with adjacent normal
tissue.
When Cby protein expression was analyzed based on
different clinical groups, the differences between T1/T2 and T3/T4
groups (χ2=17.3, P<0.01), as well as early-stage
(I/II) and advanced-stage (III/IV) groups (χ2=30.1,
P<0.01) were statistically significant. The difference between
groups separated based on gender, age, regional lymph node
metastasis status and pathological classifications did not differ
statistically (Table II).
 | Table II.The association of Cby protein
expression with clinical features. |
Table II.
The association of Cby protein
expression with clinical features.
| Clinical
features | Patients, n | Cby protein PR, %
(n) | χ2
value | P-value |
|---|
| Sex |
|
| 2.747 | >0.05 |
|
Male | 32 | 50 (16/32) |
|
|
|
Female | 13 | 23.1 (3/13) |
|
|
| Age, years |
|
| 0.137 | >0.05 |
|
≤50 | 27 | 44.4 (12/27) |
|
|
|
>50 | 18 | 38.9 (7/18) |
|
|
| T staging |
|
| 17.257 | <0.01 |
|
1/2 | 24 | 70.8 (17/24) |
|
|
|
3/4 | 21 | 9.5 (2/21) |
|
|
| Lymph node
metastasis |
|
| 2.186 | >0.05 |
| N0 | 18 | 55.6 (10/18) |
|
|
|
N1-3 | 27 | 33.3 (9/27) |
|
|
| Clinical
staging |
|
| 30.097 | <0.01 |
|
I/II | 19 | 89.5 (17/19) |
|
|
|
III/IV | 26 | 7.7 (2/26) |
|
|
| Pathological
classification |
|
| 0.228 | >0.05 |
| WHO
I | 9 | 44.4 (4/9) |
|
|
| WHO
II | 20 | 45.0 (9/20) |
|
|
| WHO
III | 16 | 37.5 (6/16) |
|
|
Cby expression increases following
plasmid electroporation
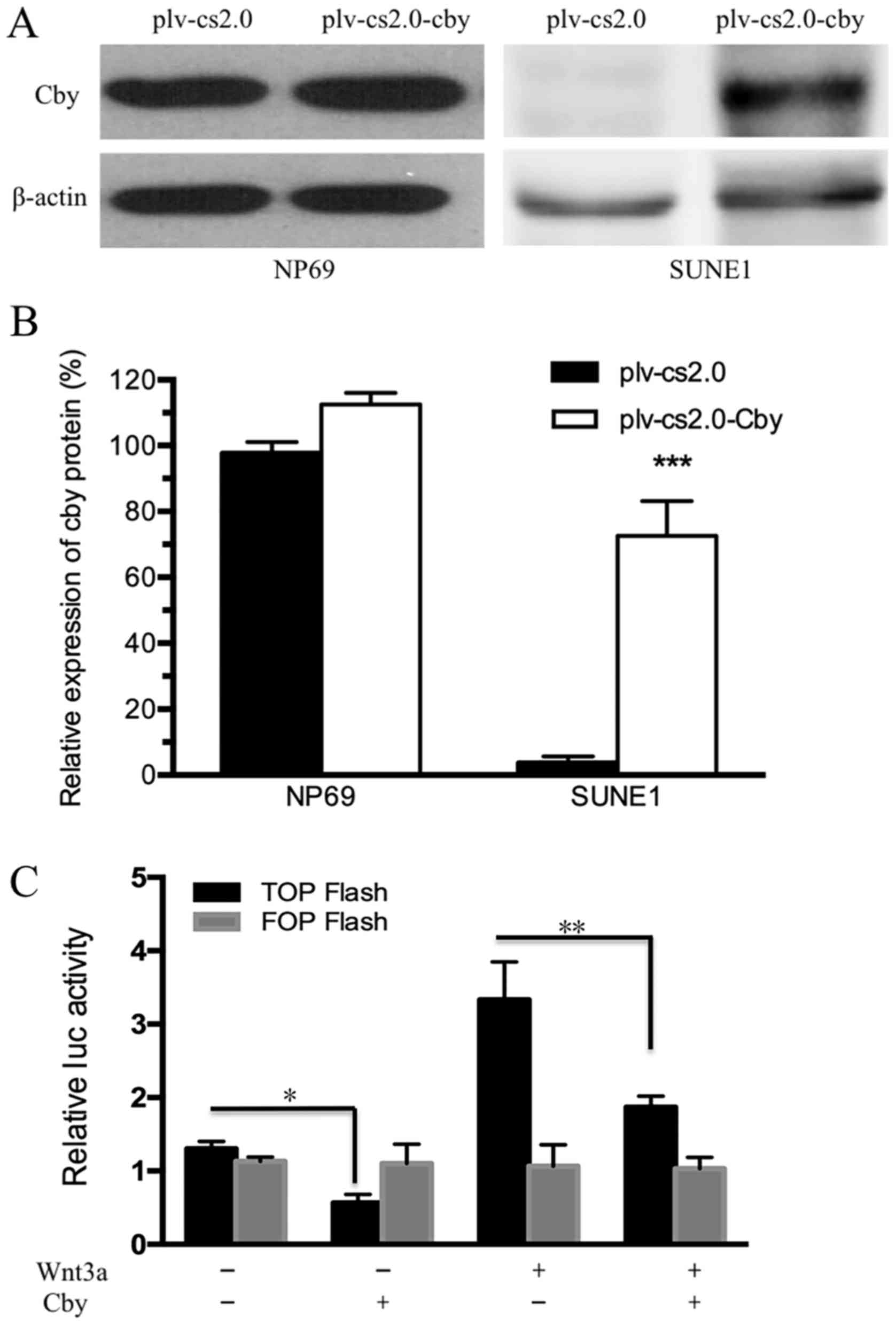
Human Cby DNA was transfected into NP69 and SUNE1
cell lines by electroporation. The results of western blot analysis
indicated that the Cby protein level in SUNE1 cells was extremely
low compared with NP69 cells (Fig.
2A), and was significantly different compared with the
plv-cs2.0 control cells (7.16±0.58-fold of the control group;
P<0.01) following electroporation (Fig. 2B). The results of western blot
analysis indicated that the Cby protein level was increased. The
results of the dual-luciferase reporter assay revealed that
activation of the Wnt/β-catenin pathway was markedly reduced by Cby
overexpression. In addition, activation of the Wnt/β-catenin
pathway induced by Wnt3a was reduced by Cby overexpression
(Fig. 2C). These results indicated
that Cby expression was successfully upregulated and Wnt/β-catenin
pathway was effectively inhibited following transfection with
Cby.
Cby overexpression inhibits the
proliferation of SUNE1 cells
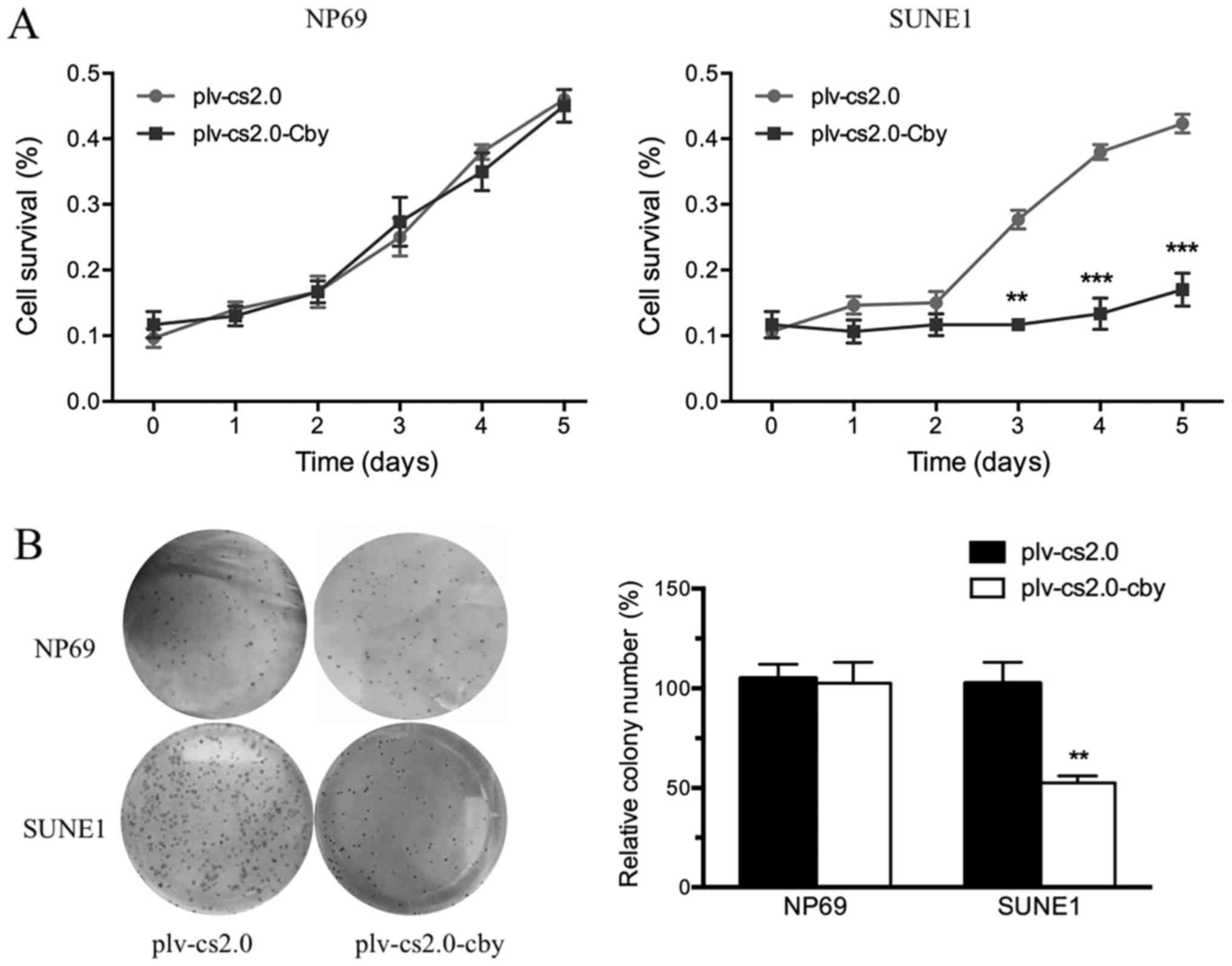
Cell survival was analyzed daily by an MTT assay
between days 1 and 5. The MTT value did not differ significantly
from day 1 to day 5 between the plv-cs2.0 and plv-cs2.0-Cby groups
in NP69 cells; however, the growth rate of SUNE1 cells transfected
with Cby decreased compared with those transfected with the blank
vector from day 3 onwards, a difference that was statistically
significant (P<0.01) following Cby overexpression (Fig. 3A).
To investigate the anti-oncogenic potential of Cby
further, colony formation assays were performed (Fig. 3B). The ability of
plv-cs2.0-Cby-transfected SUNE1 cells to form foci (100±1.6%) was
significantly impaired compared with that of plv-cs2.0 cells
(57±5.2%) (P<0.01); however, no significant difference was
observed in NP69 cells. This result demonstrated that
overexpression of human Cby significantly inhibited the
proliferation of SUNE1 cells.
Cby overexpression induces the cell
cycle arrested and enhances the susceptibility of SUNE1 cells to
apoptosis
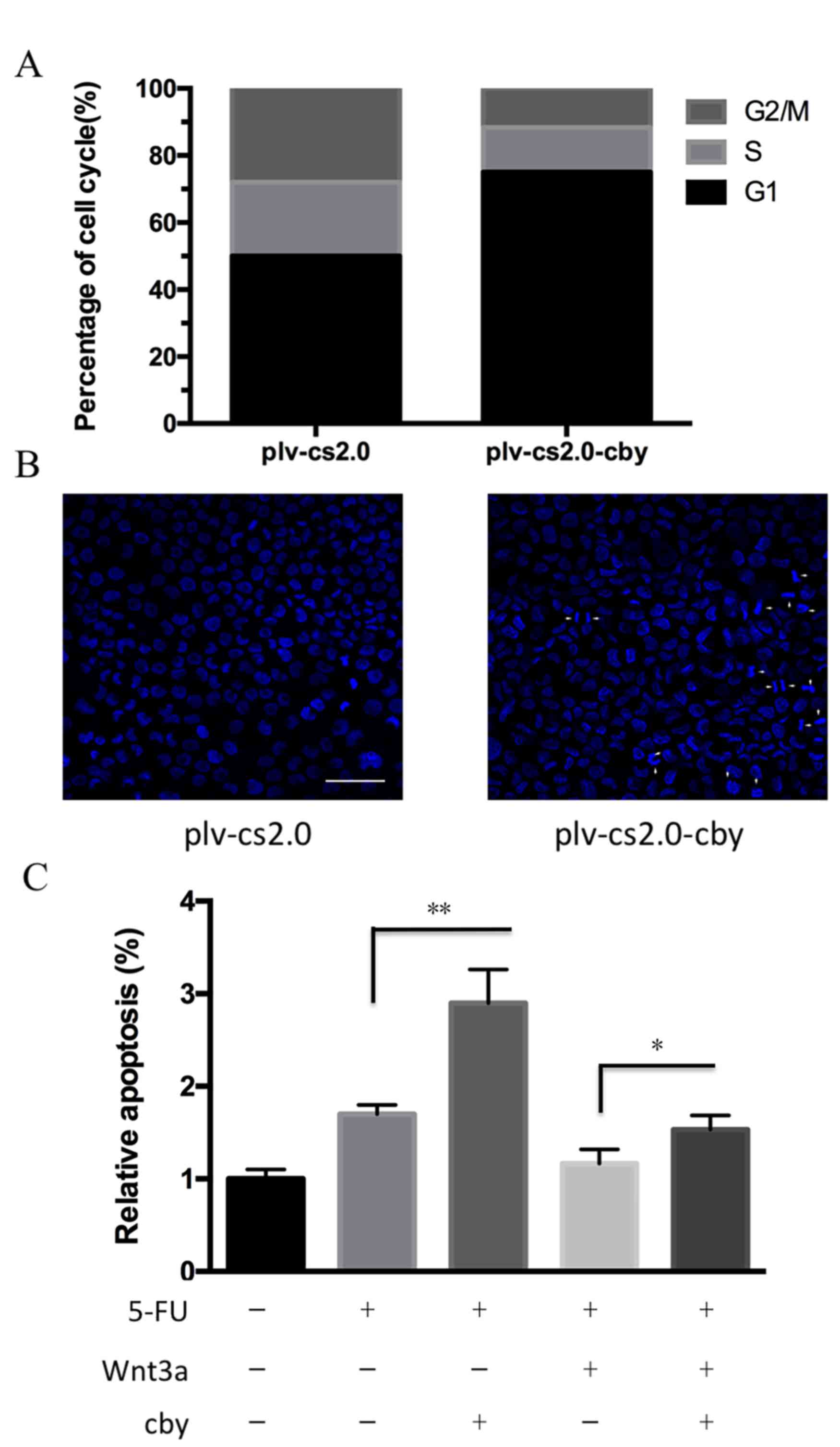
To investigate the potential mechanism by which Cby
overexpression inhibited the proliferation of SUNE1 cells, flow
cytometry was performed to analyze the cell cycle distribution of
cells. As depicted in Fig. 4A and B,
the proportion of G1 phase cells for plv-cs2.0 and
plv-cs2.0-Cby groups was 52.5±2.5 and 73.6±3.2%, respectively,
which differed statistically (P<0.01). This result demonstrated
that overexpression of human Cby significantly inhibited cell cycle
progression in SUNE1 cells.
To detect the susceptibility of SUNE1 cells to
apoptosis following Cby overexpression, the Hoechst staining assay
was performed. Cells exhibiting condensed chromatin or fragmented
nuclei were counted as apoptotic cells (Fig. 4B). The apoptosis rate in plv-cs2.0 and
plv-c.s2.0-Cby was 3.55±0.54 and 9.85±1.46% (P<0.01),
respectively, which differed statistically. Furthermore, the
apoptosis rate induced by 5-FU was significantly increased from
1.82±1.2 to 2.75±1.7% following Cby overexpression (P<0.01).
Down-regulation of 5-FU sensitivity induced by Wnt 3a could be
significantly recued by Cby overexpression from 1.23±1.3 to
1.64±1.4% (P<0.05; Fig. 4C). This
data indicated that overexpression of human Cby significantly
facilitated the apoptosis of SUNE1 cells.
Discussion
The Wnt signaling pathway is an essential and
fundamental signaling pathway that is highly evolutionally
conserved. This pathway participates in a variety of processes,
including development, tissue regeneration following injury and the
maintenance of tissue homeostasis. Wnt signaling has versatile
functions owing to its wide range of downstream target genes
(25). The disordered expression of
any Wnt signaling components will lead to abnormal activation of
Wnt signaling and disruption of cell homeostasis, which in turn
leads to cancer (26). The major
alterations to Wnt signaling in cancer tissue are caused by the
inactivation of negative regulators or the overexpression of
positive regulators for different components of Wnt pathway,
particularly β-catenin, which is a key component of the canonical
Wnt pathway (27). A widely known
example of the inactivation of negative regulators is the human
tumor suppressor, adenomatous polyposis coli (APC). Genetic defects
in APC are the cause of familial adenomatous polyposis, a heritable
syndrome in which affected individuals develop hundreds of polyps
in the large intestine at an early age and ultimately succumb to
colorectal cancer (28). β-catenin
phosphorylation at serines 33 and 37 creates a binding site for the
E3 ubiquitin ligase β-Trcp, leading to β-catenin ubiquitination and
degradation. Mutations of β-catenin at, and surrounding, these
serine residues are frequently identified in cancer, generating
mutant β-catenin that allows it to evade proteolytic degradation
(29). These deficiencies all lead to
the intracellular accumulation of β-catenin, thus enhancing its
transcriptional activities (30).
Evidence demonstrates that the inappropriate stabilization of
β-catenin can cause surplus transcription of Wnt-downstream target
genes, which can lead to cellular proliferation (31); such genes include MYC proto-oncogene,
bHLH transcription factor (c-Myc) (32) and cyclin D1 (33,34).
Cby is a direct antagonist of β-catenin, and has
been demonstrated to suppress colon tumor cell growth in
vitro (18). The present study
revealed that Cby expression was suppressed at the transcriptional
and protein level in >60% of patients; however, these results
were not completely consistent with previous reported studies
(16), which may be due to
differences in the biological characteristics of colon and head and
neck cancer. Nonetheless, ~30% patients exhibited similar or even
higher Cby expression levels in tumor tissue than the adjacent
normal tissue (35). We hypothesize
that there are two possible reasons for this result. First, it is
widely accepted that cancer originates from and is developed under
a series of cellular abnormalities (36). Cby truncation, where it is present,
represents only one link in the chain of tumor development. Second,
there is heterogeneity inside of a tumor, with β-catenin not
uniformly distributed in the tumor (37), which means tumor cells located at the
invasive front are exposed to growth factors and cytokines, which
can further enhance β-catenin function; thus Cby expression in
tumor will be affected by a number of unknown factors. As samples
were acquired from a region of the tumor by biopsy, the Cby
expression level may also vary between samples.
When taking account of clinical characteristics, it
was found that Cby expression was associated with local NPC disease
groups and stages. Cby mRNA and protein expression levels were
declined more significantly in advanced diseases (T3/T4 disease and
stage III/IV) than in preliminary diseases (T1/T2 diseases and
stage I/II). The proliferation of SUNE1 cells was suppressed by Cby
overexpression, which was consistent with the results of a previous
study (18). In cell cycle analyses,
an increased portion of cells arrested in the
G0/G1 phase was observed, indicating that
this effect is most likely mediated by cell cycle arrest caused by
β-catenin dysfunction. Cyclin D1 is a direct target of
β-catenin/TCF/LEF complex and is an important checkpoint regulator
for the G1 to S progression (38). Cby may interfere with the
transcriptional activation effect of β-catenin against cyclin D1.
However, Fischer et al (18)
observed that the major proportion of SW480 cells seemed to shift
from G0/G1 phase to G2/M phase and
then underwent cell cycle arrest. The exact mechanism of Cby
activity remains unclear: One possible interpretation is Cby
suppressed β-catenin's function in centrosomes, allowing mitosis to
proceed by accumulating at G2/M phase induced by
cyclin-dependent kinase 14/cyclin Y phosphorylates low-density
lipoprotein receptor-related protein 6 (39). The difference observed in these two
malignant cell lines may be attributable to the different time
points at which Cby was expressed, thus leads to various dominant
downstream effects. Further research, involving synchronizing the
cell cycle prior to Cby administration, may be required.
The role of the Wnt/β-catenin signaling pathway in
apoptosis is complicated, and the exact mechanism remains unclear.
In the present study, overexpression of Cby increased apoptosis in
SUNE1 cells to a certain extent, which may be caused by the
transcriptional suppression of c-Myc (32), or by another Wnt/β-catenin-independent
mechanism.
Although the association between the expression
level of Cby and clinical prognostic factors has rarely been
reported, β-catenin is considered to affect tumor invasion by
cadherin-mediated cell-cell adhesion through direct binding to
α-catenin (40). Since the extent of
nasopharyngeal carcinoma, as elucidated by the TNM staging system,
is the most import prognostic factor (41), the potential application of Cby for
prognosis prediction is an attractive one. Also as an endogenous
β-catenin antagonist, Cby may be utilized as a Wnt/β-catenin
inhibitor for target therapy. However, the exact role of Cby in
regulating the growth and proliferation of NPC requires elucidation
by gain- and loss-of-function experiments in vitro and in
vivo.
In summary, the data reported in the present study
demonstrated that Cby expression was suppressed at the mRNA and
protein levels in the NPC tissues of >60% patients.
Overexpression of human Cby in SUNE1 cells significantly inhibited
the proliferation and cell-cycle progression of SUNE1 cells, and
significantly facilitated their apoptosis. These findings may serve
as the basis for the use of Cby in future as a β-catenin inhibitor
for targeted therapy, and can be used as the foundation for further
investigation of the exact role of Cby in regulating NPC.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant no. 81670936),
the Project of Young and Middle-aged Backbone Talents Cultivation,
Fujian, China (grant no. 2013-ZQN-JC-32) and the Project from
Science and Technology Bureau of Xiamen, China (grant no.
3502Z20144004).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wu VW and Lam YN: Radiation-induced
temporo-mandibular joint disorder in post-radiotherapy
nasopharyngeal carcinoma patients: Assessment and treatment. J Med
RadiatSci. 63:124–132. 2016. View
Article : Google Scholar
|
|
2
|
He YQ, Xue WQ, Shen GP, Tang LL, Zeng YX
and Jia WH: Household inhalants exposure and nasopharyngeal
carcinoma risk: A large-scale case-control study in Guangdong,
China. BMC Cancer. 15:10222015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ng WT, Choi CW, Lee MC, Chan SH, Yau TK
and Lee AW: Familial nasopharyngeal carcinoma in Hong Kong:
Epidemiology and implication in screening. Fam Cancer. 8:103–108.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mesic JB, Fletcher GH and Goepfert H:
Megavoltage irradiation of epithelial tumors of the nasopharynx.
Int J Radiat Oncol Biol Phys. 7:447–453. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee AW, Poon YF, Foo W, Law SC, Cheung FK,
Chan DK, Tung SY, Thaw M and Ho JH: Retrospective analysis of 5037
patients with nasopharyngeal carcinoma treated during 1976–1985:
Overall survival and patterns of failure. Int J Radiat Oncol Biol
Phys. 23:261–270. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chua DT, Sham JS, Wei WI, Ho WK and Au GK:
The predictive value of the 1997 American joint committee on cancer
stage classification in determining failure patterns in
nasopharyngeal carcinoma. Cancer. 92:2845–2855. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wolden SL, Zelefsky MJ, Kraus DH,
Rosenzweig KE, Chong LM, Shaha AR, Zhang H, Harrison LB, Shah JP
and Pfister DG: Accelerated concomitant boost radiotherapy and
chemotherapy for advanced nasopharyngeal carcinoma. J ClinOncol.
19:1105–1110. 2001. View Article : Google Scholar
|
|
8
|
Takemaru K, Fischer V and Li FQ:
Fine-tuning of nuclear-catenin by Chibby and 14-3-3. Cell cycle.
8:210–213. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Takemaru K, Yamaguchi S, Lee YS, Zhang Y,
Carthew RW and Moon RT: Chibby, a nuclear beta-catenin-associated
antagonist of the Wnt/Wingless pathway. Nature. 422:905–909. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li FQ, Mofunanya A, Harris K and Takemaru
K: Chibby cooperates with 14-3-3 to regulate beta-catenin
subcellular distribution and signaling activity. J Cell Biol.
181:1141–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh AM, Li FQ, Hamazaki T, Kasahara H,
Takemaru K and Terada N: Chibby, an antagonist of the
Wnt/beta-catenin pathway, facilitates cardiomyocyte differentiation
of murine embryonic stem cells. Circulation. 115:617–626. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li FQ, Singh AM, Mofunanya A, Love D,
Terada N, Moon RT and Takemaru K: Chibby promotes adipocyte
differentiation through inhibition of beta-catenin signaling. Mol
Cell Biol. 27:4347–4354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Love D, Li FQ, Burke MC, Cyge B, Ohmitsu
M, Cabello J, Larson JE, Brody SL, Cohen JC and Takemaru K: Altered
lung morphogenesis, epithelial cell differentiation and mechanics
in mice deficient in the Wnt/β-catenin antagonist Chibby. PLoS One.
5:e136002010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Korinek V, Barker N, Morin PJ, van Wichen
D, de Weger R, Kinzler KW, Vogelstein B and Clevers H: Constitutive
transcriptional activation by a beta-catenin-Tcf complex in
APC-/-colon carcinoma. Science. 275:1784–1787. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morin PJ, Sparks AB, Korinek V, Barker N,
Clevers H, Vogelstein B and Kinzler KW: Activation of
beta-catenin-Tcf signaling in colon cancer by mutations in
beta-catenin or APC. Science. 275:1787–1790. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schuierer MM, Graf E, Takemaru K,
Dietmaier W and Bosserhoff AK: Reduced expression of beta-catenin
inhibitor Chibby in colon carcinoma cell lines. World J
Gastroenterol. 12:1529–1535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Karakoula K, Suarez-Merino B, Ward S,
Phipps KP, Harkness W, Hayward R, Thompson D, Jacques TS, Harding
B, Beck J, et al: Real-time quantitative PCR analysis of pediatric
ependymomas identifies novel candidate genes including TPR at 1q25
and CHIBBY at 22q12-q13. Genes Chromosomes Cancer. 47:1005–1022.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fischer V, Brown-Grant DA and Li FQ:
Chibby suppresses growth of human SW480 colon adenocarcinoma cells
through inhibition of β-catenin signaling. J Mol Signal. 7:62012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mancini M, Leo E, Takemaru K, Campi V,
Borsi E, Castagnetti F, Gugliotta G, Santucci MA and Martinelli G:
Chibby drives β catenin cytoplasmic accumulation leading to
activation of the unfolded protein response in BCR-ABL1+ cells.
Cell Signal. 25:1820–1827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J and Zhu XZ: Introduction of WHO
classification of tumours of soft tissue, the fourth edition.
Zhonghua Bing Li XueZaZhi. 42:363–365. 2013.(In Chinese).
|
|
21
|
Jo VY and Fletcher CD: WHO classification
of soft tissue tumours: An update based on the 2013 (4th) edition.
Pathology. 46:95–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mattern J, Koomagi R and Volm M:
Biological characterization of subgroups of squamous cell lung
carcinomas. Clin Cancer Res. 5:1459–1463. 1999.PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gad S, Teboul D, Lièvre A, Goasguen N,
Berger A, Beaune P and Laurent-Puig P: Is the gene encoding Chibby
implicated as a tumour suppressor in colorectal cancer? BMC Cancer.
4:312004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Duchartre Y, Kim YM and Kahn M: The Wnt
signaling pathway in cancer. Crit Rev OncolHematol. 99:141–149.
2016. View Article : Google Scholar
|
|
26
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takahashi-Yanaga F and Kahn M: Targeting
Wnt signaling: Can we safely eradicate cancer stem cells? Clin
Cancer Res. 16:3153–3162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang D, Zhang M and Gold B: Origin of
Somatic mutations in β-catenin versus adenomatous polyposis coli in
colon cancer: Random mutagenesis in animal models versus nonrandom
mutagenesis in humans. Chem Res Toxicol. 30:1369–1375. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Majidinia M, Aghazadeh J,
Jahanban-Esfahlani R and Yousefi B: The roles of Wnt/β-catenin
pathway in tissue development and regenerative medicine. J Cell
Physiol. Nov 18–2017.(Epub ahead of print). View Article : Google Scholar
|
|
31
|
Polakis P: The many ways of Wnt in cancer.
CurrOpin Genet Dev. 17:45–51. 2007. View Article : Google Scholar
|
|
32
|
He TC, Sparks AB, Rago C, Hermeking H,
Zawel L, da Costa LT, Morin PJ, Vogelstein B and Kinzler KW:
Identification of c-MYC as a target of the APC pathway. Science.
281:1509–1512. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tetsu O and McCormick F: Beta-catenin
regulates expression of cyclin D1 in colon carcinoma cells. Nature.
398:422–426. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shtutman M, Zhurinsky J, Simcha I,
Albanese C, D'Amico M, Pestell R and Ben-Ze'ev A: The cyclin D1
gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad
Sci USA. 96:pp. 5522–5527. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ren G, Zhao DA, Xu J and Li BA: Expression
of CBY and methylation of CBY at promoter region in human laryngeal
squamous cell carcinoma. Tumori. 101:215–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brabletz T, Jung A, Hermann K, Günther K,
Hohenberger W and Kirchner T: Nuclear overexpression of the
oncoprotein beta-catenin in colorectal cancer is localized
predominantly at the invasion front. Pathol Res Pract. 194:701–704.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Monin MB, Krause P, Stelling R, Bocuk D,
Niebert S, Klemm F, Pukrop T and Koenig S: The anthelmintic
niclosamide inhibits colorectal cancer cell lines via modulation of
the canonical and noncanonical Wnt signaling pathway. J Surg Res.
203:193–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Davidson G, Shen J, Huang YL, Su Y,
Karaulanov E, Bartscherer K, Hassler C, Stannek P, Boutros M and
Niehrs C: Cell cycle control of wnt receptor activation. Dev Cell.
17:788–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Drees F, Pokutta S, Yamada S, Nelson WJ
and Weis WI: Alpha-catenin is a molecular switch that binds
E-cadherin-beta-catenin and regulates actin-filament assembly.
Cell. 123:903–915. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wei WI and Sham JS: Nasopharyngeal
carcinoma. Lancet. 365:2041–2054. 2005. View Article : Google Scholar : PubMed/NCBI
|