Introduction
Colon cancer is a type of malignant cancer with a
high rate of incidence. Its morbidity is increasing and it is
ranked third among malignant types of cancer in China, with an
incidence rate of 12.81% in 2010 (1).
At present, the morbidity of colon cancer is increasing due to the
advancement of living standards and changes in dietary structure,
including increased consumption of fatty components and decreased
consumption of cellulose (2).
MicroRNA (miR/miRNA)-21 has previously been
demonstrated to be upregulated in various types of tumors and to
control the occurrence and progression of cancer (3). Tumors occur as a result of
downregulation of cancer suppressor genes and overexpression of
oncogenes (3). It has been
demonstrated that miR-21 is a carcinogenic miRNA and it exhibits
high expression levels in solid tumors, including head and neck
neoplasms, esophageal, gastric, pancreatic, lung, liver, breast and
prostatic cancer, and non-solid tumors including B cell lymphoma
and chronic lymphocytic leukemia (4,5).
The occurrence of colon cancer is a complex process
involving multiple genes and multiple steps (6). The mutation and deficiency of tumor
suppressor genes is one of the important factors resulting in cell
malignancy and metastasis (7).
Multiple studies have endeavored to define the use of expression
profiles in determining the occurrence and progression of colon
cancer (7,8). In total, >200 miRNAs have been
identified from 15 paired colon cancer and para-carcinoma tissues
(9). Among the 132 miRNAs which are
expressed in colorectal cancer (CRC) and para-carcinoma tissues, 47
of these have been revealed to be downregulated in colon cancer
(10). A previous study has
demonstrated that the upregulation of miR-21 is associated with CRC
(10).
Studies on berberine (Fig.
1) have revealed that it is able to treat various infectious
diseases, and that it is a potential anti-cancer treatment
(11,12). Berberine is a Chinese herb extract
with a long history. Clinically, it is used to treat
gastrointestinal diseases including enteritis and bacillary
dysentery. In previous years, berberine has been revealed to
possess therapeutic actions against multiple types of cancer,
including osteosarcoma, prostatic and liver cancer (13–15). The
present study sought to define whether berberine inhibited cell
viability and induced apoptosis of the HCT116 colon cancer cell
line via the regulation of miR-21-integrin β4 (ITGβ4)-programmed
cell death 4 (PDCD4).
Materials and methods
Cell lines and cell culture
The human CRC HCT116 cell line was purchased from
Shanghai cell bank (Shanghai, China) maintained in Dulbecco's
Modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), 100 mg/ml
streptomycin (Beyotime Institute of Biotechnology, Haimen, China)
and 100 U/ml penicillin (Beyotime Institute of Biotechnology) at
37°C with 5% CO2 in a humidified atmosphere.
Cellular viability assay
Human CRC HCT116 cells (1×103 cell/well)
were seeded into 96-well plates and cultivated with negative
control (PBS), 1, 10 or 100 µM berberine for 48 h at 37°C. MTT was
added to a final concentration of 0.5 mg/ml 48 h subsequent to
berberine treatment and HCT116 cells were incubated for an
additional 4 h at 37°C. DMSO was then added into each well and
incubated for a further 20 min. The optical density was measured at
570 nm with a microplate spectrophotometer.
Apoptosis assay
HCT116 cells were plated in 6-well plates at a
density of 5×105 cells/well and incubated with 1, 10 and
100 µM berberine for 24 h at 37°C. Flow cytometry (C6; BD
Biosciences, Franklin Lakes, NJ, USA) was used to measure the
apoptosis of HCT116 cells, 24 h following treatment with berberine.
The relative amount of Annexin V-fluorescein
isothiocyanate-positive-propidium iodide-negative cells were
detected using an Apoptosis Detection kit I (BD Biosciences) and
analyzed using Flowjo 7.6.1 (BD Biosciences).
Caspase-3 activity
HCT116 cells were plated in 6-well plates at a
density of 5×105 cells/well and incubated with 1, 10 and
100 µM berberine for 24 h at 37°C. Proteins were extracted from
HCT116 cells or HCT116 cells transfected by miR-21 using a protein
extraction reagent (RIPA; Beyotime Institute of Biotechnology,
Haimen, China). The supernatant was gathered after centrifugation
at 12,000 × g for 10 min at 4°C and the protein concentration was
measured using a BCA Protein Assay kit (Beyotime Institute of
Biotechnology). An equal amount (50 µg) of total protein was
incubated with Ac-IETD-pNA (Caspase-3 activity kit; cat. no. C1116;
Beyotime Institute of Biotechnology) at 37°C for 4 h. Caspase-3
activity was detected using a microplate spectrophotometer, at a
wavelength of 405 nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
HCT116 cells were plated in 6-well plates at a
density of 5×105 cells/well and incubated with 1, 10 and
100 µM berberine for 24 h at 37°C. Total RNA was extracted from
HCT116 cells cultured with berberine using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
protocol. RNA was then reverse transcribed into cDNA using a
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China). QPCR assay was performed on an Applied Biosystems ABI Prism
7000 sequence detection system using QuantiTect SYBR-Green PCR kit
(Qiagen China Co., Ltd., Shanghai, China). The thermocycler
conditions were as follows: 95°C for 5 min, followed by 35 cycles
of 95°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec. The
primers used for stem-loop RT-qPCR for miR-21 and U6 are presented
in Table I. The relative expression
levels of the gene of interest were quantified using the
2−ΔΔCq method, and U6 represented the internal control
(16).
 | Table I.Primers used for reverse
transcription-quantitative polymerase chain reaction analysis. |
Table I.
Primers used for reverse
transcription-quantitative polymerase chain reaction analysis.
| Target | Primer | Sequence |
|---|
| miR-21 | Forward |
5′-GCGGCAACACCAGTCGATG-3′ |
|
| Reverse |
5′-TGCGTGTCGTGGAGTC-3′ |
| U6 | Forward |
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
| Reverse |
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
Cell transfection
miR-21 mimics and negative control mimics sequences
were as follows: 5′-UAGCUUAUCAGACUGAUGUUGA-3′ and
5′-CCCCCCCCCCCCCCCCCCCC-3′, respectively. miR-21 mimics and
negative control mimics were transfected into HCT116 cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol.
Western blot analysis
HCT116 cells were plated in 6-well plates
(5×105 cells/well) and incubated with 1, 10 and 100 µM
berberine for 24 h at 37°C. Proteins were extracted from HCT116
cells or HCT116 cells transfected with miR-21 using a protein
extraction reagent (RIPA; Beyotime Institute of Biotechnology). The
supernatant was gathered after centrifugation at 12,000 × g for 10
min at 4°C to measure protein concentration using a BCA Protein
Assay kit (Beyotime Institute of Biotechnology). A total of 50 µg
protein per lane from each sample was separated using 10–12%
SDS-PAGE (Beyotime Institute of Biotechnology) and transferred onto
a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membrane was then blocked with 5% non-fat
milk for 1 h at 37°C and incubated with ITGβ4 (dilution, 1:3,000;
EPR8559; Abcam, Cambridge, UK), PDCD4 (dilution, 1:3,000;
sc-376430; Santa Cruz Biotechnology, Inc.) and β-actin (dilution,
1:1,000; cat. no. sc-7210; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. The membranes were incubated with horseradish
peroxidase-labeled goat anti-rabbit or anti-mouse immunoglobulin G
(dilution, 1:1,000; sc-2004, sc-2005; Santa Cruz Biotechnology,
Inc.) for 1 h at room temperature, and membranes were developed
using an enhanced chemiluminescence kit according to the
manufacturer's protocol (Beyotime Institute of Biotechnology) and
quantified using sodium Image_Lab_3.0 (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
12.0 software (SPSS, Inc., Chicago, IL, USA). Data are presented as
the mean ± standard deviation. The differences between groups were
analyzed using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
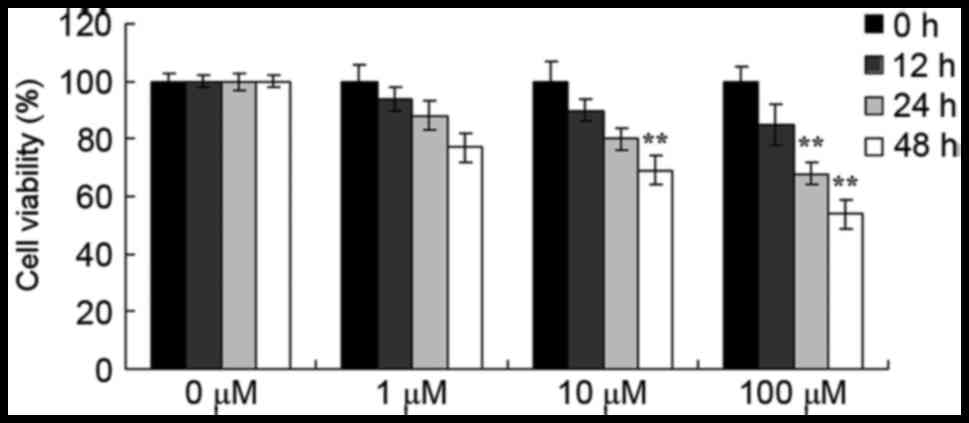
Berberine suppresses the viability of
HCT116 cells
The results of the MTT assay demonstrated the
anti-cancer effects of berberine on HCT116 cell viability.
Berberine reduced the viability of HCT116 cells in a time- and
dose-dependent manner (Fig. 2).
Notably, treatment with 10 µM berberine significantly suppressed
the cellular viability of HCT116 cells at 48 h, and treatment with
100 µM berberine also significantly suppressed the cellular
viability of HCT116 cells at 24 or 48 h (Fig. 2).
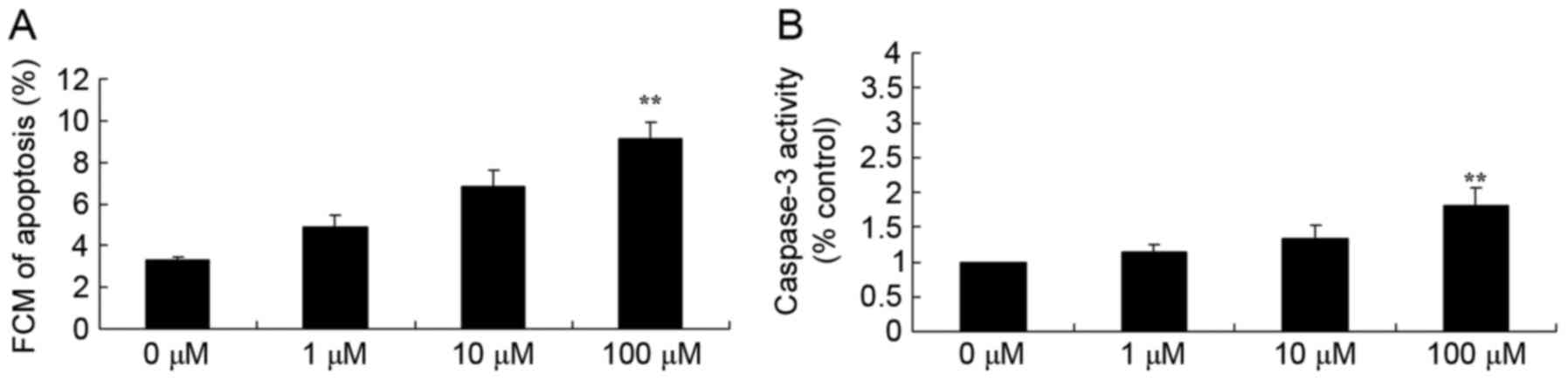
Berberine induces apoptosis and
caspase-3 activity of HCT116 cells
The results from flow cytometry analysis and the
Business kit demonstrated that 100 µM berberine treatment
significantly increased the apoptosis rate and promoted caspase-3
activity of HCT116 cells, (Fig. 3A and
B, respectively), and the effect of berberine was
dose-dependent.
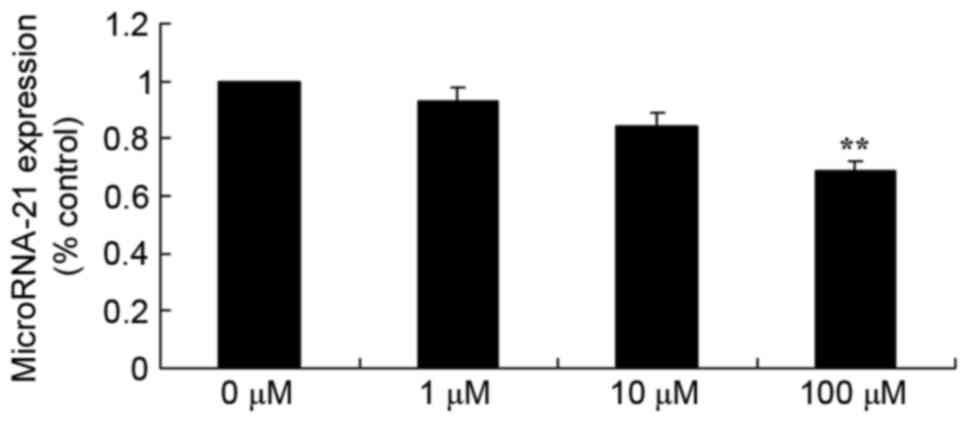
Berberine suppresses miR-21 in HCT116
cells
In addition, the present study examined miR-21
expression in HCT116 cells treated with berberine using RT-qPCR.
Compared with the 0 µM control group, miR-21 expression was
significantly inhibited by treatment with 100 µM berberine
(Fig. 4).
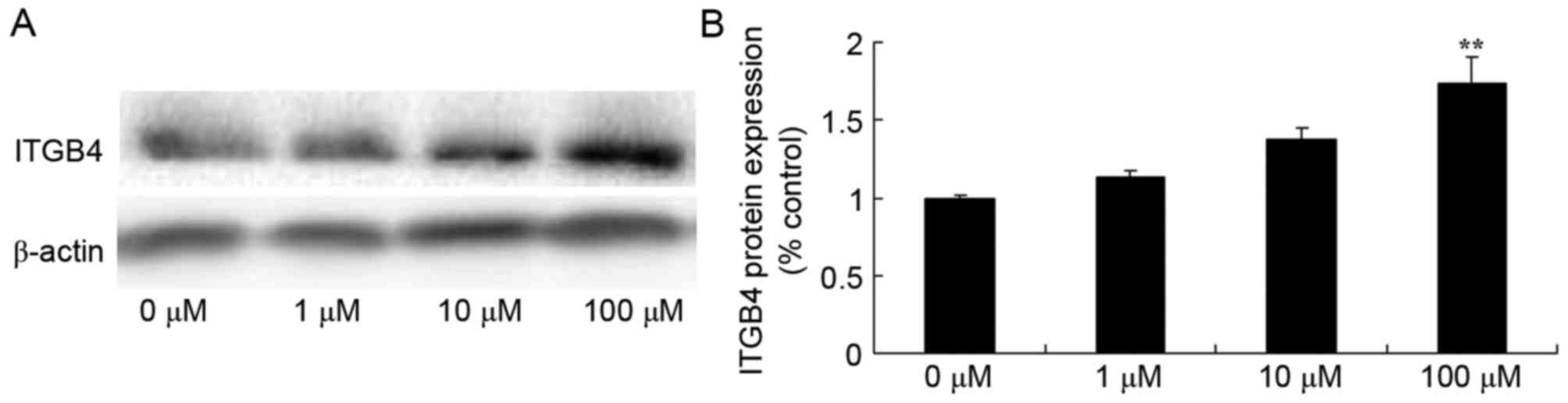
Berberine induces ITGβ4 protein
expression in HCT116 cells
To explore the mechanisms underlying the effect of
berberine on human colon cancer, ITGβ4 protein expression was
detected using western blot analysis. ITGβ4 protein expression was
significantly increased in HCT116 cells treated with 100 µM
berberine compared with the 0 µM berberine group (Fig. 5).
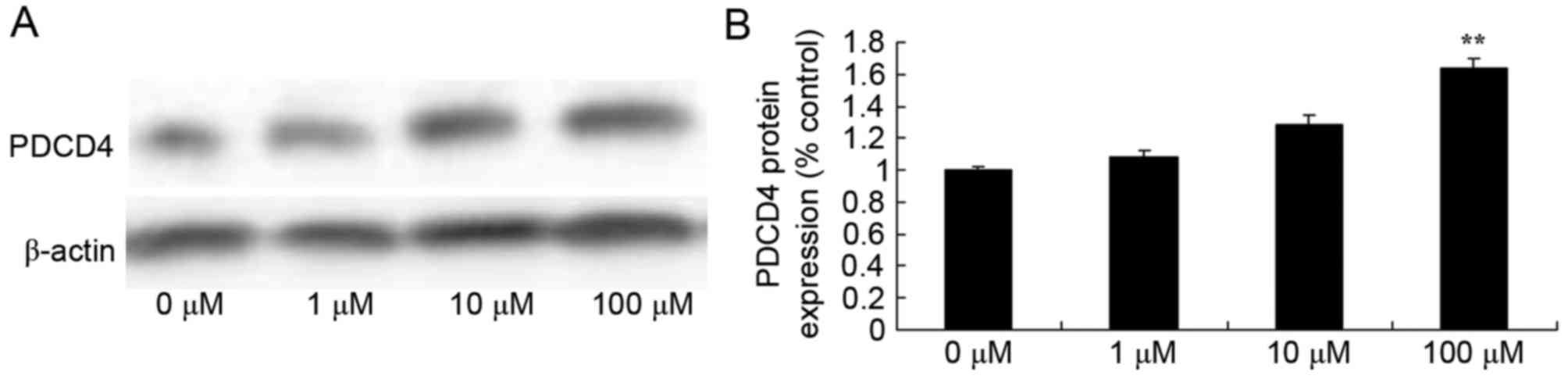
Berberine induces PDCD4 protein
expression in HCT116 cells
In order to detect the mechanisms by which berberine
acts on human colon cancer, western blot analysis was used to
analyze PDCD4 protein expression in HCT116 cell exposed to
berberine. PDCD4 protein expression levels were significantly
increased following treatment with 100 µM berberine compared with
the 0 µM berberine group (Fig.
6).
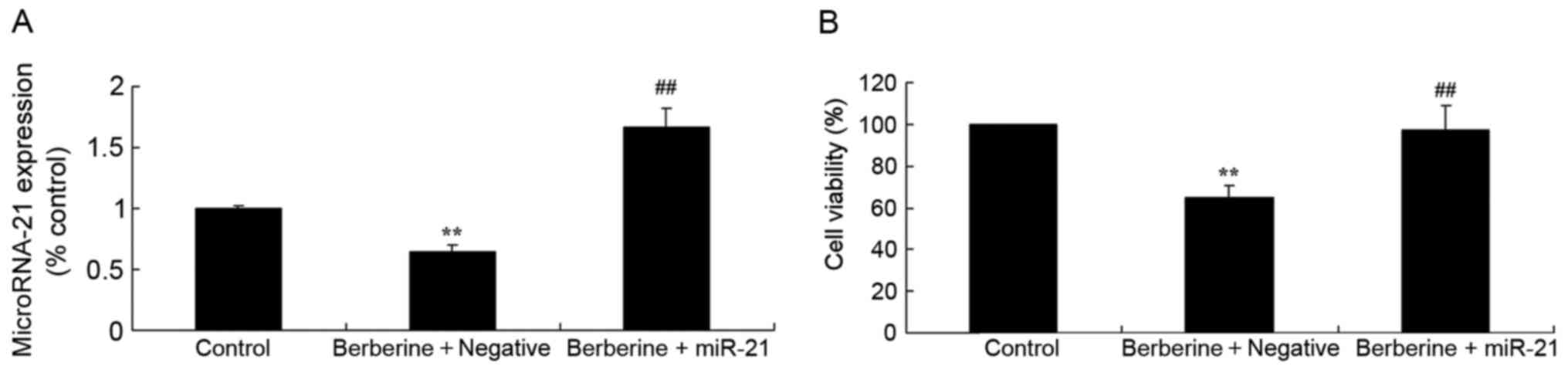
Effect of miR-21 overexpression on the
berberine-induced reduction of HCT116l cell viability
The present study analyzed the association between
miR-21 and the anti-cancer effect of berberine on HCT116 cell
viability. Transfection with miR-21 mimics significantly increased
miR-21 expression levels in HCT116 cells (Fig. 7A) and that HCT116 cell viability was
increased in cells treated with miR-21 mimics and 100 µM berberine
compared with the group treated with 100 µM berberine only
(Fig. 7B).
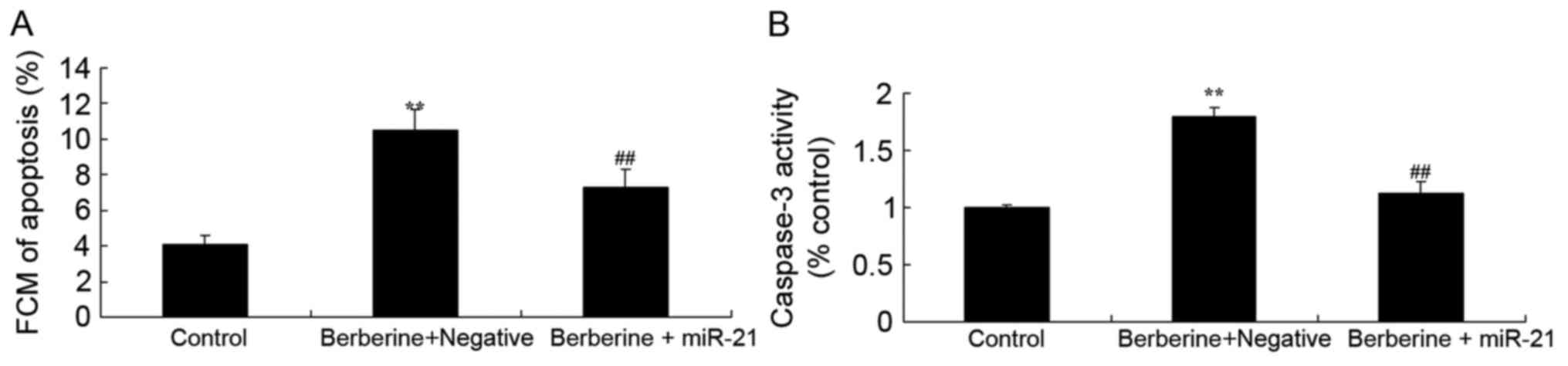
Effect of miR-21 overexpression on the
effect of berberine on apoptosis and caspase-3 activity in HCT116
cells
To investigate the associations between miR-21 and
the anti-cancer effects of berberine on apoptosis in HCT116 cells
and HCT116 cells transfected with miR-21, the apoptosis rate and
caspase-3 activity of HCT116 cells were measured using flow
cytometry and caspase-3 activity kit, respectively. The rate of
apoptosis and caspase-3 activity were significantly decreased in
HCT116 cells treated with 100 µM berberine and miR-21 mimics,
compared with the group treated with 100 µM berberine alone
(Fig. 8A and B, respectively).
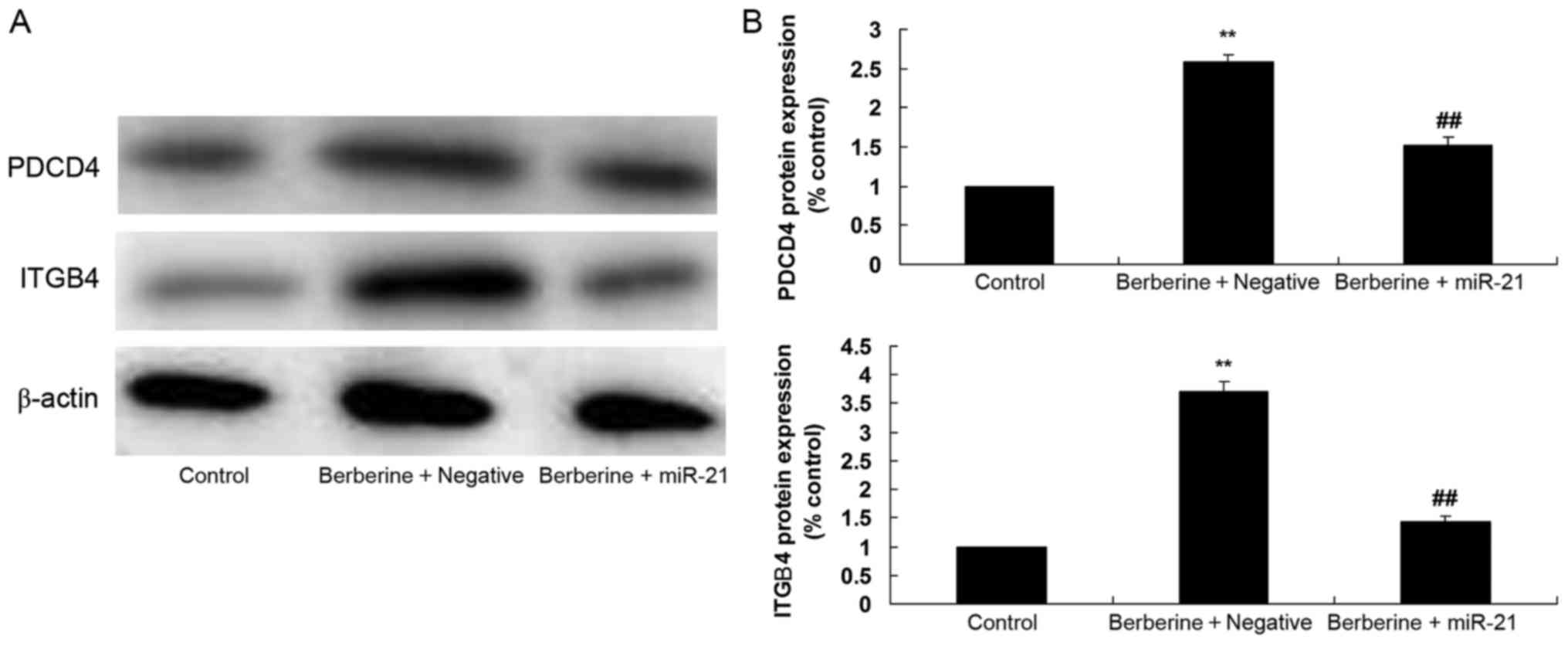
Effect of miR-21 overexpression on the
effect of berberine on ITGβ4 and PDCD4 protein expression levels in
HCT116 cells
To investigate the association between miR-21 and
the anti-cancer effects of berberine on ITGβ4 and PDCD4 protein
expression in HCT116 cells, western blot analysis was performed.
Overexpression of miR-21 significantly suppressed the 100 µM
berberine-induced increase of ITGβ4 and PDCD4 protein expression
levels in HCT116 cells, compared with the group treated with 100 µM
berberine alone (Fig. 9).
Discussion
The morbidity rate of colon cancer is high and is
increasing annually, with a clear increase being observed in
developed countries and developing countries (17). Among malignant tumors of the digestive
tract, colon cancer ranks the fourth in terms of mortality rates in
China (17). The occurrence and
progression of colon cancer involves multiple-genes (18). The present study revealed that
berberine significantly suppressed cell viability, induced
apoptosis and increased caspase-3 activity in human colon cancer
HCT116 cells. Previous studies have demonstrated that berberine
inhibits cell proliferation, induces apoptosis and inhibits the
invasion of human skin squamous cell carcinoma A431 cells (19), human ovarian cancer cells (20) and CRC cells (21).
miRNAs are non-coding molecules which are expressed
in breast, lung, gastric, prostate, liver, colon and pancreatic
cancer, spongioblastoma, pancreatic neuroendocrine tumor and cancer
of the biliary duct (22). Since
miRNAs frequently occur on complementary fragments, it is assumed
that miRNAs have a relatively close association with the occurrence
and progression of various types of cancer (8). It has previously been demonstrated that
miR-21 has clinical and practical values for the diagnosis,
treatment, assessment and prognosis of cancers (23).
miR-21 is involved in the processes of cell
proliferation, invasion, blood vessel invasion and metastasis of
multiple types of cancer (24). It
has been demonstrated that miR-21 is associated with the
sensitivity of anti-cancer drugs in vitro (24). Multiple signal pathways and regulatory
factors are involved in influencing the sensitivity of anti-cancer
drugs (25). Thus, miR-21 is deemed
to be a potential therapeutic target. Antisense
oligodeoxynucleotides combine with mature and carcinogenic miRNAs
to inhibit tumor growth (26). miR-21
inhibitors increase the sensitivity of tumor cells to
chemotherapeutic agents (25).
Following treatment with berberine, miR-21 expression was
significantly inhibited in human colon cancer HCT116 cells. In
addition, overexpression of miR-21 significantly inhibited the
anti-cancer effect of berberine on viability, apoptosis and
caspase-3 activity in HCT116 cells. Hu et al (27) previously reported that berberine
inhibited NF-κB and lead to a decrease in the levels of miR-21 and
B cell lymphoma 2.
Integrin is a heterodimer molecule formed from two
subunits, α and β (28). In total, 20
α and β types have been identified, which combine into various
forms. The subunits of α and β are comprised of an extracellular
region and a transmembrane domain, and the intracellular region
ITGβ4 determines the specificity to physiological and pathological
processes, including the inflammatory response, the immune
response, proliferation, differentiation, vascularization,
fertilization, embryo implantation and growth (29). The present study observed that
berberine induced ITGβ4 protein expression in HCT116 cells.
There have been few previous studies investigating
miR-21 in colon cancer. Studies have identified that increased
expression of miR-21 downregulated expression levels of PDCD4,
which is a cancer suppressor gene (5,9). PDCD4 and
miR-21 have been demonstrated to be associated with gastric
carcinoma, colon and breast cancer (30). The results of the present study
demonstrated that berberine induced PDCD4 protein expression in
HCT116 cells. Overexpression of miR-21 was observed to
significantly inhibit the anti-cancer effect of berberine on
ITGβ4/PDCD4 protein expression levels in HCT116 cells.
In conclusion, the present data provided evidence
that berberine significantly suppressed cell viability, induced
apoptosis and increased caspase-3 activity in human colon cancer
HCT116 cells. The present study also demonstrated an association
between miR-21 and the anti-cancer effect of berberine on the
viability of colon cancer cells, which may be regulated by the
miR-21-ITGβ4-PDCD4 signaling pathway. This is supported by the
results of Liu et al (31),
who also previously suggested that berberine sensitizes
cisplatin-induced ovarian cancer cells through the miR-21/PDCD4
axis. The present study may contribute to the development of
berberine for the treatment and prevention of human colon
cancer.
Acknowledgements
The present study was supported by the Natural
Sciences Foundation of Shandong (grant no. ZR2011HQ049) and the
Jinan Youth Science and Technology Star Plan (grant nos. 20100405
and 20090208).
References
|
1
|
Holt PR, Kozuch P and Mewar S: Colon
cancer and the elderly: From screening to treatment in management
of GI disease in the elderly. Best Pract Res Clin Gastroenterol.
23:889–907. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Radhakrishnan EK, Bava SV, Narayanan SS,
Nath LR, Thulasidasan AK, Soniya EV and Anto RJ: [6]-Gingerol
induces caspase-dependent apoptosis and prevents PMA-induced
proliferation in colon cancer cells by inhibiting MAPK/AP-1
signaling. PLoS One. 9:e1044012014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu W and Xu B: MicroRNA-21 identified as
predictor of cancer outcome: A meta-analysis. PLoS One.
9:e1033732014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ribas J and Lupold SE: The transcriptional
regulation of miR-21, its multiple transcripts, and their
implication in prostate cancer. Cell Cycle. 9:923–929. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Young MR, Santhanam AN, Yoshikawa N and
Colburn NH: Have tumor suppressor PDCD4 and its counteragent
oncogenic miR-21 gone rogue? Mol Interv. 10:76–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang J, Du Y, Liu X, Cho WC and Yang Y:
MicroRNAs as regulator of signaling networks in metastatic colon
cancer. Biomed Res Int. 2015:8236202015.PubMed/NCBI
|
|
7
|
Hollis M, Nair K, Vyas A, Chaturvedi LS,
Gambhir S and Vyas D: MicroRNAs potential utility in colon cancer:
Early detection, prognosis, and chemosensitivity. World J
Gastroenterol. 21:8284–8292. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Melo SA and Esteller M: Dysregulation of
microRNAs in cancer: Playing with fire. FEBS Lett. 585:2087–2099.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu X, Dong S, Qiao F, Lu S, Song Y, Lao
Y, Li Y, Zeng T, Hu J, Zhang L, et al: HBx-mediated miR-21
upregulation represses tumor-suppressor function of PDCD4 in
hepatocellular carcinoma. Oncogene. 32:3296–3305. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mercado-Pimentel ME, Onyeagucha BC, Li Q,
Pimentel AC, Jandova J and Nelson MA: The S100P/RAGE signaling
pathway regulates expression of microRNA-21 in colon cancer cells.
FEBS Lett. 589:2388–2393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shi G, Shao J, Wang T, Wu D and Wang C:
Mechanism of berberine-mediated fluconazole-susceptibility
enhancement in clinical fluconazole-resistant Candida tropicalis
isolates. Biomed Pharmacother. 93:709–712. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Daniel B, Konrad B, Toplak M, Lahham M,
Messenlehner J, Winkler A and Macheroux P: The family of berberine
bridge enzyme-like enzymes: A treasure-trove of oxidative
reactions. Arch Biochem Biophys. 632:88–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Zhang M, Zhang ZL, Liu N, Han XY,
Liu QC, Deng WJ and Liao CX: Induction of apoptosis by berberine in
hepatocellular carcinoma HepG2 cells via downregulation of NF-κB.
Oncol Res. 25:233–239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Zhang A, Sun H, Liu Z, Zhang T, Qiu
S, Liu L and Wang X: Metabolic characterization and pathway
analysis of berberine protects against prostate cancer. Oncotarget.
8:65022–65041. 2017.PubMed/NCBI
|
|
15
|
Zhu Y, Ma N, Li HX, Tian L, Ba YF and Hao
B: Berberine induces apoptosis and DNA damage in MG63 human
osteosarcoma cells. Mol Med Rep. 10:1734–1738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He SY, Jiang RF, Jiang J, Xiang YS and
Wang L: Investigation of methylation and protein expression of the
Runx3 gene in colon carcinogenesis. Biomed Rep. 3:687–690. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee LD, Mafura B, Lauscher JC, Seeliger H,
Kreis ME and Gröne J: Antiproliferative and apoptotic effects of
telmisartan in human colon cancer cells. Oncol Lett. 8:2681–2686.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li DX, Zhang J, Zhang Y, Zhao PW and Yang
LM: Inhibitory effect of berberine on human skin squamous cell
carcinoma A431 cells. Genet Mol Res. 14:10553–10568. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Q, Qin R, Fang Y and Li H: Berberine
sensitizes human ovarian cancer cells to cisplatin through
miR-93/PTEN/Akt signaling pathway. Cell Physiol Biochem.
36:956–965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H,
Fan Z, Cai J and Li Q: Berberine inhibits invasion and metastasis
of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3
signaling pathway. PLoS One. 10:e01234782015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reid G: MicroRNAs in mesothelioma: From
tumour suppressors and biomarkers to therapeutic targets. J Thorac
Dis. 7:1031–1040. 2015.PubMed/NCBI
|
|
23
|
Pink RC, Samuel P, Massa D, Caley DP,
Brooks SA and Carter DR: The passenger strand, miR-21-3p, plays a
role in mediating cisplatin resistance in ovarian cancer cells.
Gynecol Oncol. 137:143–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang W, Li J, Zhu W, Gao C, Jiang R, Li W,
Hu Q and Zhang B: MicroRNA-21 and the clinical outcomes of various
carcinomas: A systematic review and meta-analysis. BMC Cancer.
14:8192014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu G, Zhang Y, Wei J, Jia W, Ge Z, Zhang Z
and Liu X: MicroRNA-21 promotes hepatocellular carcinoma HepG2 cell
proliferation through repression of mitogen-activated protein
kinase-kinase 3. BMC Cancer. 13:4692013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
He C, Dong X, Zhai B, Jiang X, Dong D, Li
B, Jiang H, Xu S and Sun X: MiR-21 mediates sorafenib resistance of
hepatocellular carcinoma cells by inhibiting autophagy via the
PTEN/Akt pathway. Oncotarget. 6:28867–28881. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu HY, Li KP, Wang XJ, Liu Y, Lu ZG, Dong
RH, Guo HB and Zhang MX: Set9, NF-κB, and microRNA-21 mediate
berberine-induced apoptosis of human multiple myeloma cells. Acta
Pharmacol Sin. 34:157–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lo AK, Yuen PW, Liu Y, Wang XH, Cheung AL,
Wong YC and Tsao SW: Downregulation of hemidesmosomal proteins in
nasopharyngeal carcinoma cells. Cancer Lett. 163:117–123. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ferraro A, Kontos CK, Boni T, Bantounas I,
Siakouli D, Kosmidou V, Vlassi M, Spyridakis Y, Tsipras I, Zografos
G and Pintzas A: Epigenetic regulation of miR-21 in colorectal
cancer: ITGβ4 as a novel miR-21 target and a three-gene network
(miR-21-ITGβ4-PDCD4) as predictor of metastatic tumor potential.
Epigenetics. 9:129–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Gao X, Wei F, Zhang X, Yu J, Zhao
H, Sun Q, Yan F, Yan C, Li H and Ren X: Diagnostic and prognostic
value of circulating miR-21 for cancer: A systematic review and
meta-analysis. Gene. 533:389–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu S, Fang Y, Shen H, Xu W and Li H:
Berberine sensitizes ovarian cancer cells to cisplatin through
miR-21/PDCD4 axis. Acta Biochim Biophys Sin (Shanghai). 45:756–762.
2013. View Article : Google Scholar : PubMed/NCBI
|