Introduction
Lung cancer has a substantial mortality rate and the
incidence of lung cancer has been increasing gradually (1–5). Based on
histological type, lung cancer may be divided into two categories:
small cell lung cancer (SCLC) and non-small cell lung cancer
(NSCLC). From all types of lung cancer, 80–85% are classified as
NSCLC (6,7). Additionally, in >70% of newly
diagnosed NSCLC cases, the disease is at an advanced stage, and the
5-year survival rate of patients with NSCLC is only 16% (8). Hence, it is imperative to identify
potential molecular mechanisms of NSCLC tumorigenesis and
progression.
Non-coding RNAs (ncRNAs), including long non-coding
RNAs (lncRNAs) and microRNAs (miRNA/miR), have been demonstrated to
function differently in different types of cancer. Different ncRNAs
in the same cancer may have different underlying functions
(9,10). Previous studies demonstrated that
ncRNAs are extensively involved in the tumorigenesis and
progression of NSCLC (11,12). Various lncRNAs have been confirmed to
have significant functions in epigenetic gene regulation,
transcriptional regulation or disease development (13–16). It is
of note that lncRNAs may regulate the transcription of
corresponding genes by combining with polymerases or transcription
factors due to their pervasive distribution in the nucleus
(15,17). Previous studies have revealed that
lncRNAs may regulate miRNA expression in lung cancer. With regards
to the interplay between lncRNAs and miRNAs during tumorigenesis
and progression, the competing endogenous RNA (ceRNA) hypothesis
has attracted attention (18,19). The ceRNA hypothesis posits that
lncRNAs function as molecular sponges for miRNAs and functionally
liberate the targeted mRNAs regulated by the aforementioned miRNAs
(20–22). For example, the lncRNA nuclear
paraspeckle assembly transcript 1 (NEAT1) may function as a ceRNA
for miR-377-3p, and NEAT1 promotes NSCLC progression by regulating
the expression of miR-377-3p (23).
The lncRNA colon cancer-associated transcript 1 (non-protein
coding) reduced miR-218 levels via the use of BMI1 proto-oncogene,
polycomb ring finger to promote cell cycle transition in cigarette
smoke extract-induced lung carcinogenesis (24). The lncRNA urothelial cancer associated
one performed oncogenic functions in NSCLC by targeting miR-193a-3p
(25). Furthermore, lncRNAs may
co-express with microRNA in diseases. For example, Keniry et
al (26) revealed that lncRNA
H19, imprinted maternally expressed transcript (non-protein coding)
may function as the pre-miRNA of miR-675, and they may interplay to
suppress growth. Furthermore, miRNAs regulate the expression of
their target transcripts by binding to the 3′-untranslated region
(3′-UTR) (27–32). For example, Huang et al
(33) revealed that miRNA-186 may
suppress the cell proliferation and metastasis of NSCLC by
targeting mitogen-activated protein kinase kinase kinase 2. Lu
et al (34) revealed that
miR-541-3p may reverse cancer progression by directly targeting
TGFB induced factor homeobox 2 in NSCLC. Hence, the further
investigation of miRNA profiling associated with lncRNAs in NSCLC
is useful in order to identify novel targeted therapeutic
strategies.
In the present study, a microarray analysis was
performed to verify the differential expression of miRNAs between
the RNA interference (RNAi) and control samples according to the
fold change filtering (fold change ≥1.5 or ≤1) and the P-value
(P<0.05). The five most downregulated miRNAs were selected
(miR-1264, miR-337-3p, miR-302c-5p, miR-642b-3p and miR-3621).
Then, based on original data from The Cancer Genome Atlas (TCGA),
miR-642b-3p was selected for further analysis. Four miRNA target
prediction algorithms were used to identify the potential target
genes of miR-642b-3p. Bioinformatics analysis, including Gene
Ontology (GO), the Kyoto Encyclopaedia of Genes and Genomes (KEGG),
protein-protein interactions (PPIs) and network analysis were
performed in order to investigate the potential functions, pathways
and networks of the target genes (35–38).
Additionally, the expression of miR-642b-3p and its targeted genes
[zinc finger protein 350 (ZNF350), heterogeneous nuclear
ribonucleoprotein U (HNRNPU), high mobility group box 1 (HMGB1),
phosphodiesterase 4D (PDE4D), synaptotagmin binding cytoplasmic RNA
interacting protein (SYNCRIP), basic helix-loop-helix family member
B9 (BHLHB9)] in NSCLC was analysed based on original data from TCGA
database.
Materials and methods
Microarray analysis and the knockdown
of HOXA11 antisense RNA (HOXA11-AS) in the NSCLC cell line
A549
The A549 human NSCLC cell line was purchased from
the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China), and cultivated in a humidified atmosphere of 5%
CO2 at 37°C with 10% heat-inactivated foetal bovine
serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The A549 NSCLC cell line was transfected with HOXA11-AS small
interfering RNA and the subsequent experiments were performed at
least 72 h following transfection. Lipofectamine™ 2000 (cat no.
11668-019; Invitrogen; Thermo Fisher Scientific, Inc.) was used for
transfection, according to the manufacturer's protocol. A
microarray analysis was performed in order to verify the
differential expression of miR-642b-3p between the RNAi and control
samples according to fold-change filtering (fold-change ≥1.5 or ≤1)
and P-value (P<0.05).
miRNA target prediction
Four miRNA target prediction algorithms were
utilized to predict the potential target genes of miR-642b-3p. The
four corresponding prediction algorithms were miRDB (version 4.0,
http://www.mirdb.org/) (39,40),
mirTarBase (http://mirtarbase.mbc.nctu.edu.tw/) (41), TargetScan (version 6.2; http://www.targetscan.org/) (42) and DIANA-microT (version v4.0;
http://www.microrna.gr/microT) (43,44).
Candidate genes were identified and compared with Venn diagrams
(http://bioinformatics.psb.ugent.be/webtools/Venn/).
GO and pathway analysis and
construction of a PPI network
To elucidate the potential functions of the target
genes, GO and KEGG pathway analyses were conducted at the
biological process (BP), cellular component (CC) and molecular
function (MF) levels, as previously described (45). The enrichment of potential target
genes was further analysed using the Database for Annotation,
Visualization and Integrated Discovery (DAVID, version 6.7,
http://david.abcc.ncifcrf.gov/)
(46,47) or Search Tool for the Retrieval of
Interacting Genes (STRING; version 9.0; http://string-db.org) (48). Genes with a false discovery rate of
≤0.05 and P<0.05 were identified as enriched in the target
genes. Then, a functional network of GO analysis was constructed
using Cytoscape (version 2.8; http://cytoscape.org) (49).
The interaction pairs of target genes were
additionally analysed using STRING version 9.0, and the interaction
data was downloaded and analysed as described (50). A combined score >0.4 was selected
as a threshold in order to construct the PPI network.
Additional analysis of miR-642b-3p and
target genes in NSCLC from TCGA
TCGA (http://cancergenome.nih.gov/) is a collection of RNA
sequencing, miRNA sequencing, exome sequencing, single nucleotide
polymorphism array and DNA methylation data (51). TCGA may also be used to analyse
clinical parameters and complicated cancer genomics (52,53). In
the present study, raw data from RNASeq version 2 (54,55) in
lung adenocarcinoma and squamous cell carcinoma for miR-642b and
the 6 target genes (ZNF350, HNRNPU, HMGB1, PDE4D, SYNCRIP and
BHLHB9) were extracted from TCGA. The expression of miR-642b and
the target genes in each case was subsequently calculated according
to the distribution of exon reads.
Statistical analysis
SPSS version 20.0 (IBM Corporation, Armonk, NY, USA)
was used for statistical analysis. An unpaired Student's t-test was
used for comparing the expression of miR-642b-3p and its target
genes in lung adenocarcinoma and squamous cell carcinoma. The
association between HOXA11-AS, miR-642b-3p and target genes were
evaluated using Spearman's test. The association between gene
expression and clinical diagnostic values was analysed using
receiver operating characteristic curves. A Kaplan-Meier survival
analysis was used to produce survival curves. P<0.05 was
considered to indicate a statistically significant difference
(two-tailed).
Results
miR-642b-3p profiling was associated
with lncRNA HOXA11-AS
The transfection efficiency was ~100%, and the
knockdown efficiency of HOXA11-AS in NSCLC cell lines was >75%,
as determined by a reverse transcription-quantitative polymerase
chain reaction (RT-qPCR; data not presented). Following the
knockdown of HOXA11-AS, miR-642b-3p was significantly downregulated
in A549 NSCLC cells (fold-change=0.355 and P=0.008).
miRNA target prediction
In the present study, four miRNA target prediction
algorithms (miRDB, mirTarBase, TargetScan and DIANA-microT) were
used to identify the target genes of miR-642b-3p. Venn diagrams
were generated in order to analyse and compare the candidate genes.
Amongst these target genes, 705 genes (including ZNF350, MAPK8,
ASTN2 and YOD1) that were predicted by >2 algorithms were
selected and these genes were used for GO and pathway analyses.
GO and pathway analysis and
construction of a PPI network
The GO analysis was performed at the BP, CC and MF
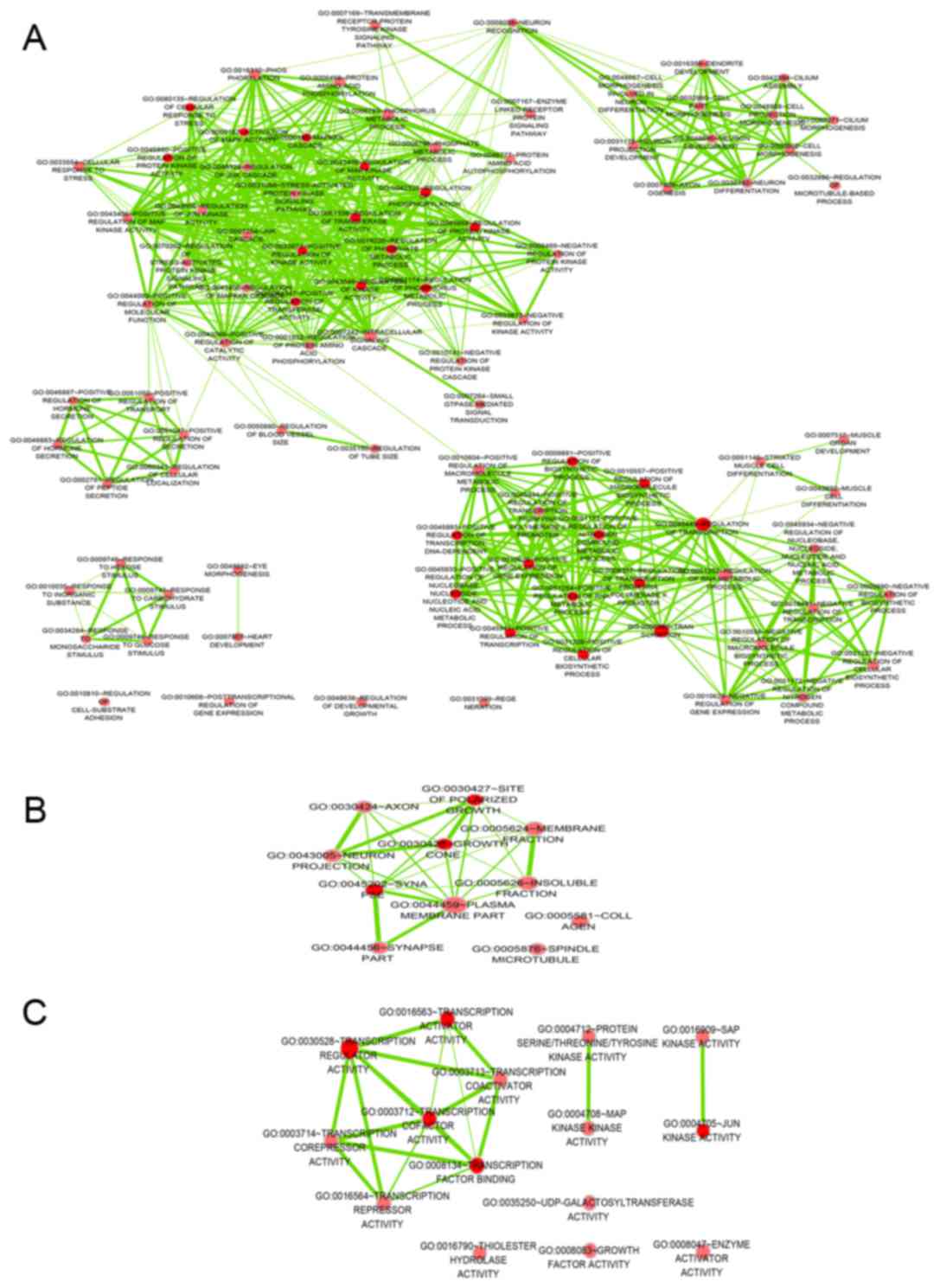
levels. The top 5 GO functional annotations for the target genes of
miR-642b are presented in Table I. GO
analysis indicated the significant functional groups, including
multicellular organism development, intracellular part and
molecular function. To further elucidate the relevant functions of
the target genes, a functional network was constructed based on the
GO analysis using Cytoscape (Fig.
1).
 | Table I.The top 5 GO functional annotation
for the target genes of miR-642b-3p. |
Table I.
The top 5 GO functional annotation
for the target genes of miR-642b-3p.
| A, Biological
processes |
|---|
|
|---|
| GO ID | GO term | Count in
network | FDR | Gene symbol (top 30
genes) |
|---|
| GO.0007275 | Multicellular
organism development | 184 | 0.000122 | ABCA12, ABI1, ADAR,
ADRBK1, AGPAT5, AHR, AK4, AMBN, ANKRD1, ANKRD17, ARL6, ASB2,
ATP8B1, ATXN3, BBS4, BCL11B, BCOR, BHLHB9, BMP3, CACNA1G, CALM1,
CAMSAP1, CASQ1, CCK, CCNA2, CD109, CDH22, CDK5R2, CDKN1A |
| GO.0048731 | System
development | 165 | 0.000122 | ABCA12, ABI1, ADAR,
ADRBK1, AGPAT5, AHR, AK4, AMBN, AMHR2, ANKRD1, ANKRD17, ARL6, ASB2,
ATP8B1, ATXN3, BBS4, BCL11B, BCOR, BHLHB9, BMP3, CACNA1G, CALM1,
CAMSAP1, CASQ1, CCK, CCNA2, CD109, CDH22, CDK5R2, CDKN1A |
| GO.0008152 | Metabolic
process | 353 | 0.000356 | ABCA12, ABHD10,
ABHD2, ABI1, ABRA, ADAM22, ADAR, ADORA2B, ADRBK1, AFF4, AGPAT5,
AHR, AK4, ALDH4A1, AMHR2, ANGPT1, ANKRD1, ARSD, ASB2, ASCL1, ATAD1,
ATG10, ATG12, ATP9A, B4GALT4, BAZ1A, BBS4, BCL11B, BCLAF1,
BCOR |
| GO.0009893 | Positive regulation
of metabolic process | 159 | 0.000356 | ABI1, ACAP2, ADAR,
ADRBK1, AFF1, ANKRD1, ANKRD6, APP, ARHGAP18, ARHGAP31, ARHGAP6,
ARHGEF37, ASB2, ASCL1, ATAD1, ATG10, ATXN3, BCLAF1, CALM1, CAND2,
CARD8, CASP10, CCK, CCNA2, CCPG1, CDC25B, CDK5R2, CDKN1A, CENPE,
CHRNA7 |
| GO.0042325 | Regulation of
phosphorylation | 75 | 0.000356 | ABI1, ADAR,
ADORA2B, ANKRD6, APP, BMP3, CALM1, CCK, CCNA2, CD109, CDC25B,
CDKN1A, CENPE, CHRNA7, CISH, CREBL2, DNAJC27, EIF2AK2, EPGN, EPHA7,
EPHB1, FAM129A, FZD1, FZD4, GAB1, GDF6, GMFB, GRM1, HIPK3,
IBTK |
|
| B, Cellular
components |
|
| GO ID | GO term | Count in
network | FDR | Gene symbol (top
30 genes) |
|
| GO.0005622 | Intracellular | 470 | 3.88E-05 | ABCA12, ABHD10,
ABI1, ABRA, ACAP2, ADAR, ADORA2B, ADRBK1, AFF1, AFF4, AGPAT5, AHR,
AK4, ALDH4A1, ANKRD1, ANKRD17, ANKRD6, ANKS1B, ANO5, ANTXR2, APP,
ARHGAP18, ARHGAP31, ARHGAP6, ARHGEF37, ARSD, ASB2, ASCL1, ASTN2,
ATG10 |
| GO.0043227 | Membrane-bound
organelle | 423 | 0.000126 | ABCA12, ABHD10,
ABI1, ACAP2, ADAR, AFF1, AFF4, AGPAT5, AHR, AK4, AKAP4, ALDH4A1,
AMOTL2, ANGPT1, ANKRD1, ANKRD17, ANKRD6, ANKS1B, ANO5, ANTXR2,
AP1S3, APP, ARL6, ARSD, ASCL1, ASTN2, ATG12, ATP8B1, ATP9A,
ATXN3 |
| GO.0044424 | Intracellular
part | 455 | 0.000126 | ABCA12, ABHD10,
ABI1, ABRA, ACAP2, ADAR, ADRBK1, AFF1, AFF4, AGPAT5, AHR, AK4,
ALDH4A1, ANKRD1, ANKRD17, ANKRD6, ANKS1B, ANO5, ANTXR2, APP,
ARHGAP18, ARHGAP31, ARHGAP6, ARHGEF37, ARSD, ASB2, ASCL1, ASTN2,
ATG10 |
| GO.0043226 | Organelle | 439 | 0.00018 | ABCA12, ABHD10,
ABI1, ABRA, ACAP2, ADAR, ADRBK1, AFF1, AFF4, AGPAT5, AHR, AK4,
ALDH4A1, AMOTL2, ANGPT1, ANKRD1, ANKRD17, ANKRD6, ANKS1B, ANTXR2,
AP1S3, APP, ARHGAP6, ARL6, ARSD, ASCL1, ASTN2, ATG12, ATP8B1,
ATP9A |
| GO.0043229 | Intracellular
organelle | 411 | 0.000197 | ABCA12, ABHD10,
ABI1, ABRA, A CAP2, ADAR, AFF1, AFF4, AGPAT5, AHR, AK4, ALDH4A1,
AMOTL2, ANKRD1, ANKRD17, ANKRD6, ANKS1B, ANO5, ANTXR2, AP1S3, APP,
ARHGAP6, ARL6, ARSD, ASCL1, ASTN2, ATP8B1, ATP9A, ATXN3,
B3GALT1 |
|
| C, Molecular
functions |
|
| GO ID | GO term | Count in
network | FDR | Gene symbol (top
30 genes) |
|
| GO.0005488 | Binding | 392 | 0.00069 | ABCA12, ABI1, ABRA,
ACAP2, ADAR, ADRBK1, AHR, AK4, AKAP4, ALDH4A1, AMBN, AMHR2, ANGPT1,
ANKMY1, ANKRD1, ANKRD17, ANKS1B, ANTXR2, APP, ARHGAP6, ARL6, ARSB,
ARSD, ATAD1, ATP8B1, ATP9A, ATXN3, B4GALT4, BAIAP3, BAZ1A |
| GO.0003674 | Molecular
function | 456 | 0.000857 | ABCA12, ABHD10,
ABHD2, ACAP2, ADAR, ADORA2B, ADRBK1, AFF1, AFF4, AGPAT5, AHR, AK4,
AKAP4, ALDH4A1, AMBN, ANGPT1, ANKMY1, ANKRD1, ANKRD17, ANKS1B,
ANO5, ANTXR2, AP1S3, APP, ARHGAP18, ARHGAP31, ARHGAP6, ARHGEF37,
ARL6, ARSB |
| GO.0005515 | Protein
binding | 200 | 0.000857 | ABCA12, ABI1, ABRA,
ADAM22, ADAMTS8, ADRBK1, AHR, AKAP4, ALDH4A1, AMBN, ANGPT1, ANKRD1,
ANKS1B, APP, ARHGAP6, ASCL1, ATXN3, BAIAP3, BAZ2A, BBS4, BCOR,
BHLHB9, BICD2, BMP3, C18orf42, CACNA1B, CACNA1G, CALM1, CAMSAP1,
CARD8 |
| GO.0004705 | JUN kinase
activity | 3 | 0.0274 | MAPK10, MAPK8,
MAPK9 |
| GO.0061578 | Lys63-specific
deubiquitinase activity | 3 | 0.0274 | ATXN3, CYLD,
YOD1 |
Using the online tools DAVID or STRING, the KEGG
analysis demonstrated that the target genes may be involved in
different pathways, including the avian erythroblastosis oncogene
B2 signalling pathway, RIG-I-like receptor signalling pathway or
mitogen-activated protein kinases (MAPK) signalling pathway (data
not shown), with the false discovery rate value >0.05. Thus,
perform further analyses on these pathways from KEGG were not
performed.
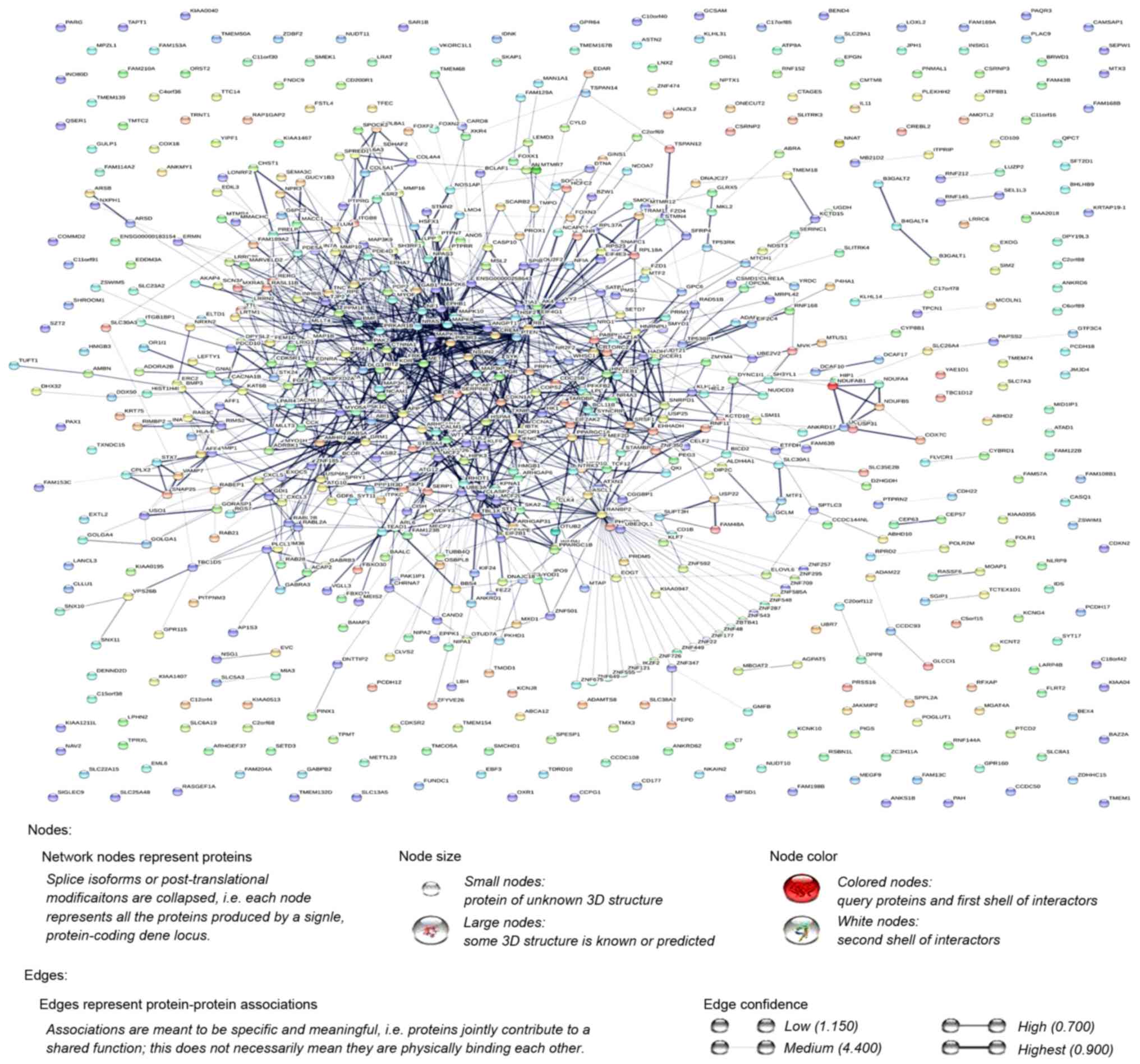
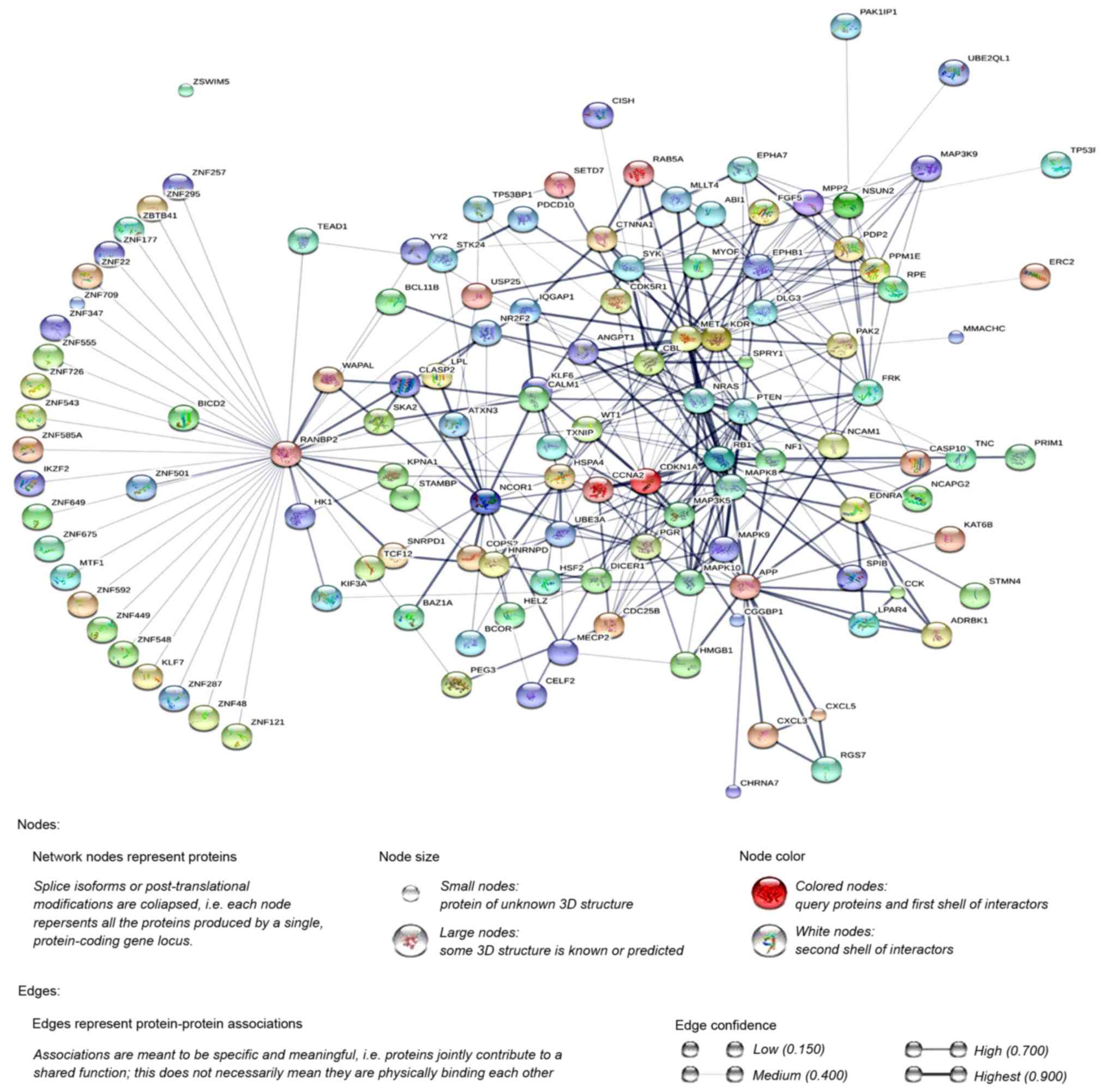
Additionally, the PPI network was constructed using
STRING, and a total of 1,228 PPI pairs with a combined score of
>0.4 were selected. The map of the PPI network is presented in
Fig. 2. The number of nodes was 700,
accounting for 99.30% of all target genes. The clustering
coefficient of the PPI network was 0.626. RAN binding protein 2
(degree=40) had the highest degree of interactions in the PPI
network. A sub-network of 172 PPI pairs (with degree >15) was
selected for further analyses (Fig.
3).
Supplementary information from TCGA
database
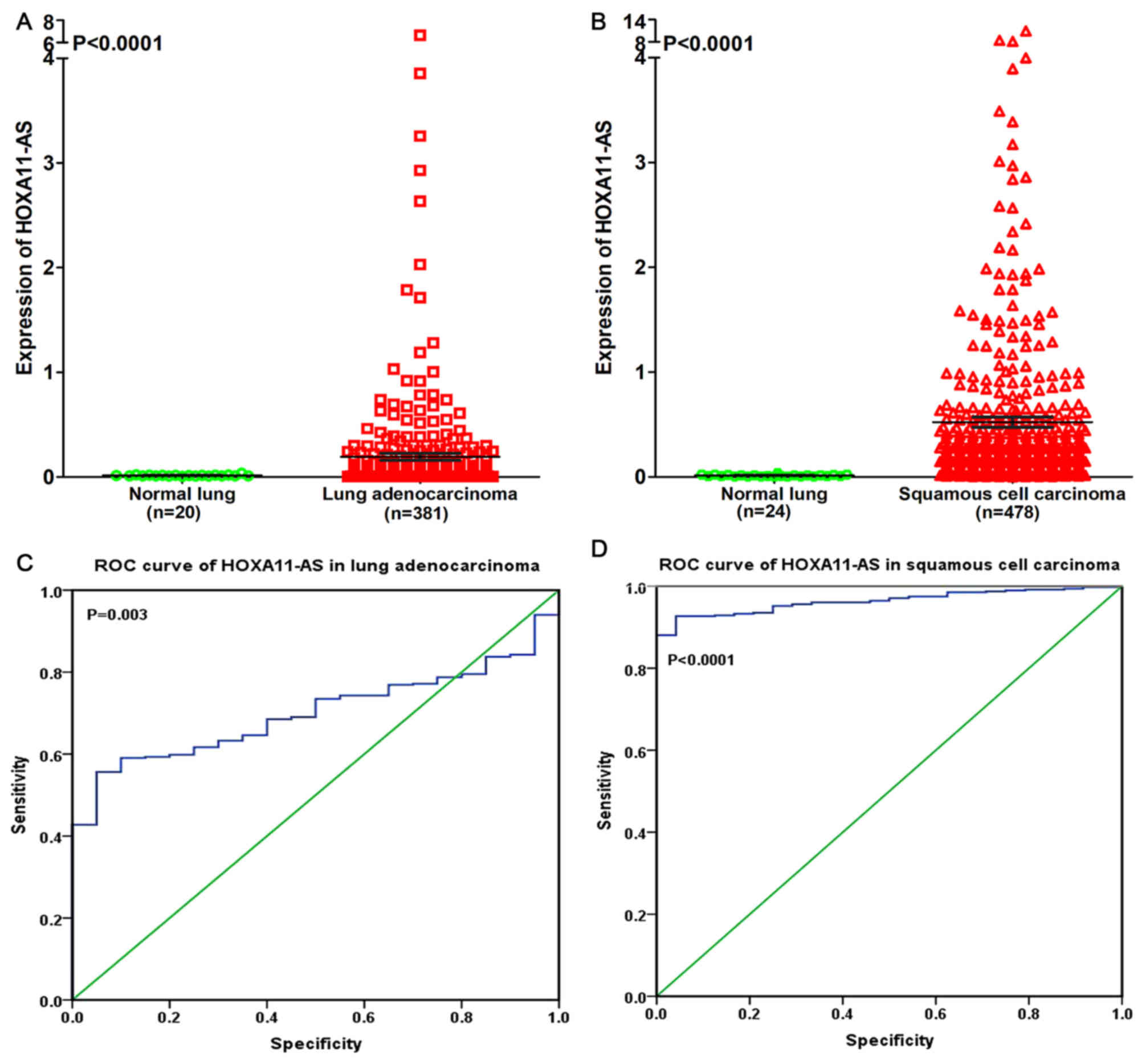
In order to reveal the association between HOXA11-AS
and NSCLC, a clinical study was performed using the raw data in
TCGA. It was revealed that HOXA11-AS was upregulated in lung
adenocarcinoma and squamous cell carcinoma compared with
non-cancerous lung tissues (P<0.0001; Fig. 4A and B). The ROC curve revealed that
the area under curve (AUC) of HOXA11-AS was 0.700 (95% confidence
interval (CI), 0.636~0.764; P=0.003) for patients with lung
adenocarcinoma and 0.964 (95% CI, 0.946~0.981; P<0.0001) for
patients with squamous cell carcinoma, which may gain a moderate or
high diagnostic value of HOXA11-AS level in lung cancer (Fig. 4C and D).
To elucidate the relationships between miR-642b and
NSCLC, a clinical study was performed using the primary data in
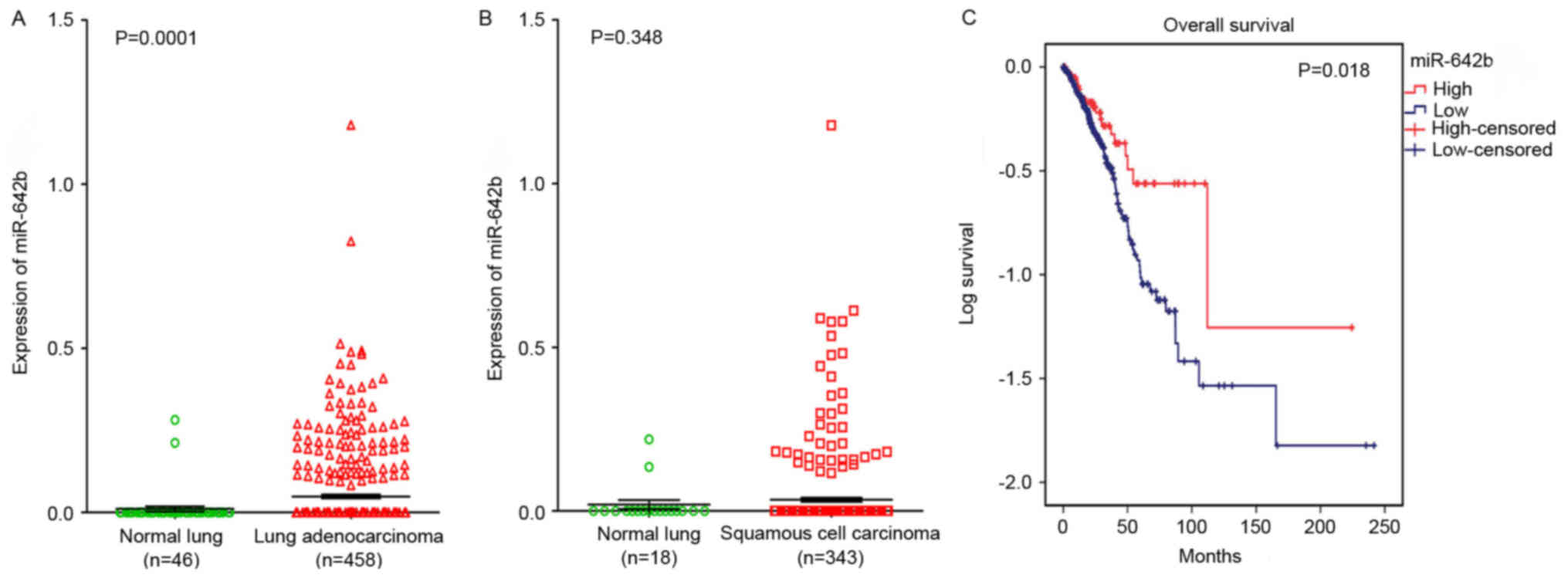
TCGA. It was revealed that miR-642b was significantly upregulated
in lung adenocarcinoma (P=0.0001) and non-significantly upregulated
in squamous cell carcinoma (P=0.348) compared with non-cancerous
lung tissues (Fig. 5A and B). The
relationship between miR-642b and the prognosis of NSCLC was also
investigated, and it was revealed that the high expression of
miR-642b was associated with the overall survival of patients with
adenocarcinoma (108.56±25.23 vs. 79.51±8.75; P=0.018; Fig. 5C), which indicated that miR-642b may
influence the prognosis of adenocarcinoma. The correlation between
HOXA11-AS and miR-642b was also investigated, but no significant
correlation was revealed in lung adenocarcinoma (r=−0.047, P=0.507;
data not shown) and squamous cell carcinoma (r=0.123, P=0.148; data
not shown) partly due to the fact that only 203 lung patients with
adenocarcinoma and 140 patients with squamous cell carcinoma were
included in the data from TCGA.
For target gene prediction, six target genes
(ZNF350, HNRNPU, HMGB1, PDE4D, SYNCRIP and BHLHB9) of miR-642b-3p
were identified by all four prediction algorithms. Then, further
analyses were performed in order to determine whether the
expressions of these genes were associated with NSCLC, based on the
data from TCGA. It was revealed that the expressions of HNRNPU,
SYNCRIP and BHLHB9 were upregulated in lung adenocarcinoma and
squamous cell carcinoma, whereas PDE4D was significantly
downregulated in lung adenocarcinoma and squamous cell carcinoma
(P<0.001). For the remaining two genes (ZNF350 and HMGB1), it
was revealed that ZNF350 was upregulated in lung adenocarcinoma and
significantly downregulated in lung squamous cell carcinoma
(P<0.0001), whereas HMGB1 was significantly downregulated in
lung adenocarcinoma and significantly upregulated in lung squamous
cell carcinoma (P<0.05, Figs. 6
and 7). Subsequently, the
relationship between these genes and overall survival or
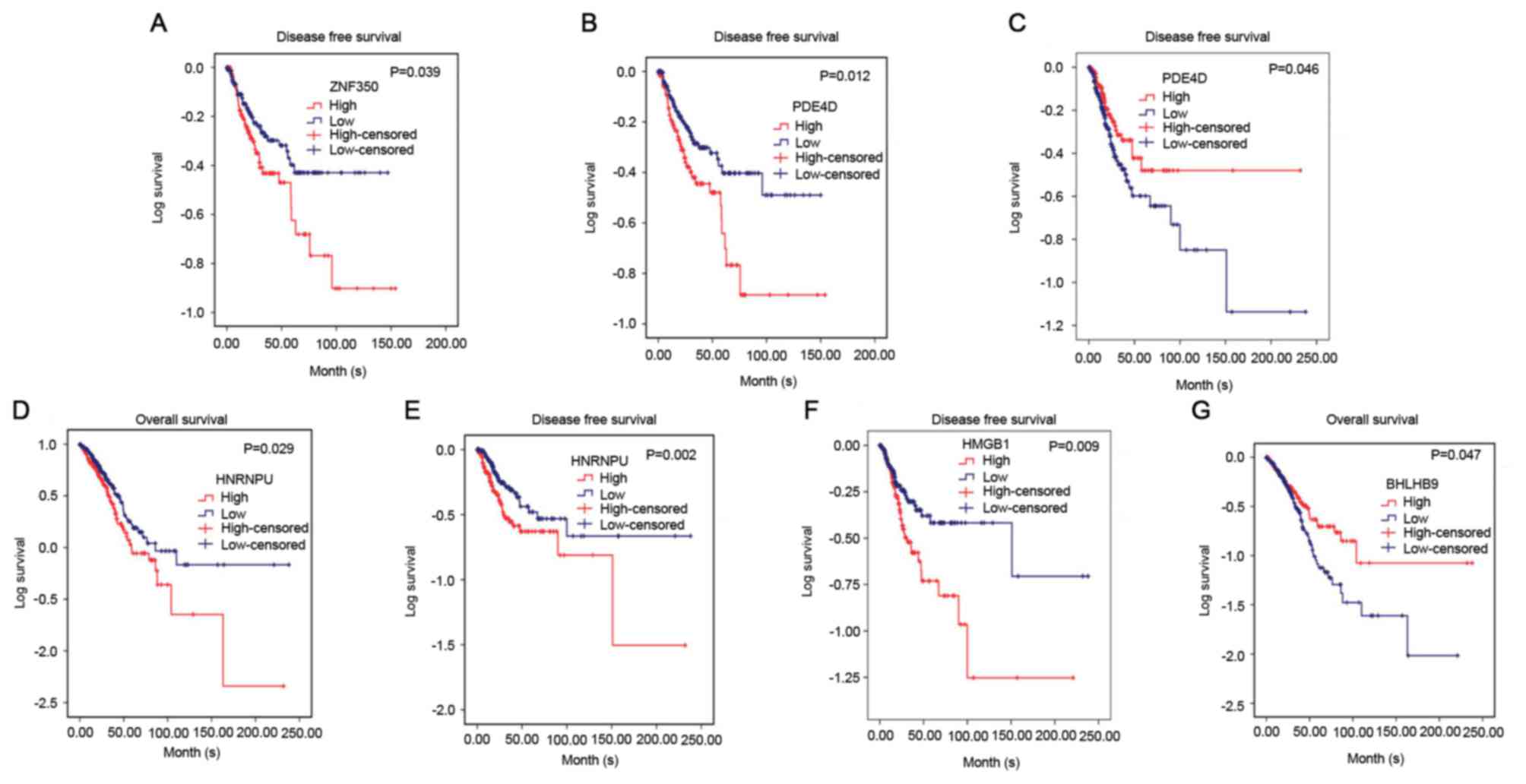
disease-free survival of NSCLC was investigated. It was revealed
that in lung squamous cell carcinoma, ZNF350 and PDE4D were
significantly associated with disease-free survival (P<0.05). In
lung adenocarcinoma, it was discovered that HNRNPU was associated
with overall survival and disease-free survival (P<0.05), and
PDE4D and HMGB1 were related to disease-free survival (P<0.05),
and BHLHB9 was only associated with overall survival (P<0.05,
Fig. 8). Additionally, the
correlation between HOXA11-AS expression and the 6 target genes was
analysed, and a weak negative correlation was revealed between
HOXA11-AS and PDE4D or ZNF350 in lung adenocarcinoma and squamous
cell carcinoma, whereas HNRNPU and SYNCRIP were positively
correlated with HOXA11-AS. BHLHB9 and HMGB1 were revealed to have a
weak positive correlation with HOX11-AS in lung adenocarcinoma, and
a weak negative correlation with HOX11-AS in squamous cell
carcinoma (Table II).
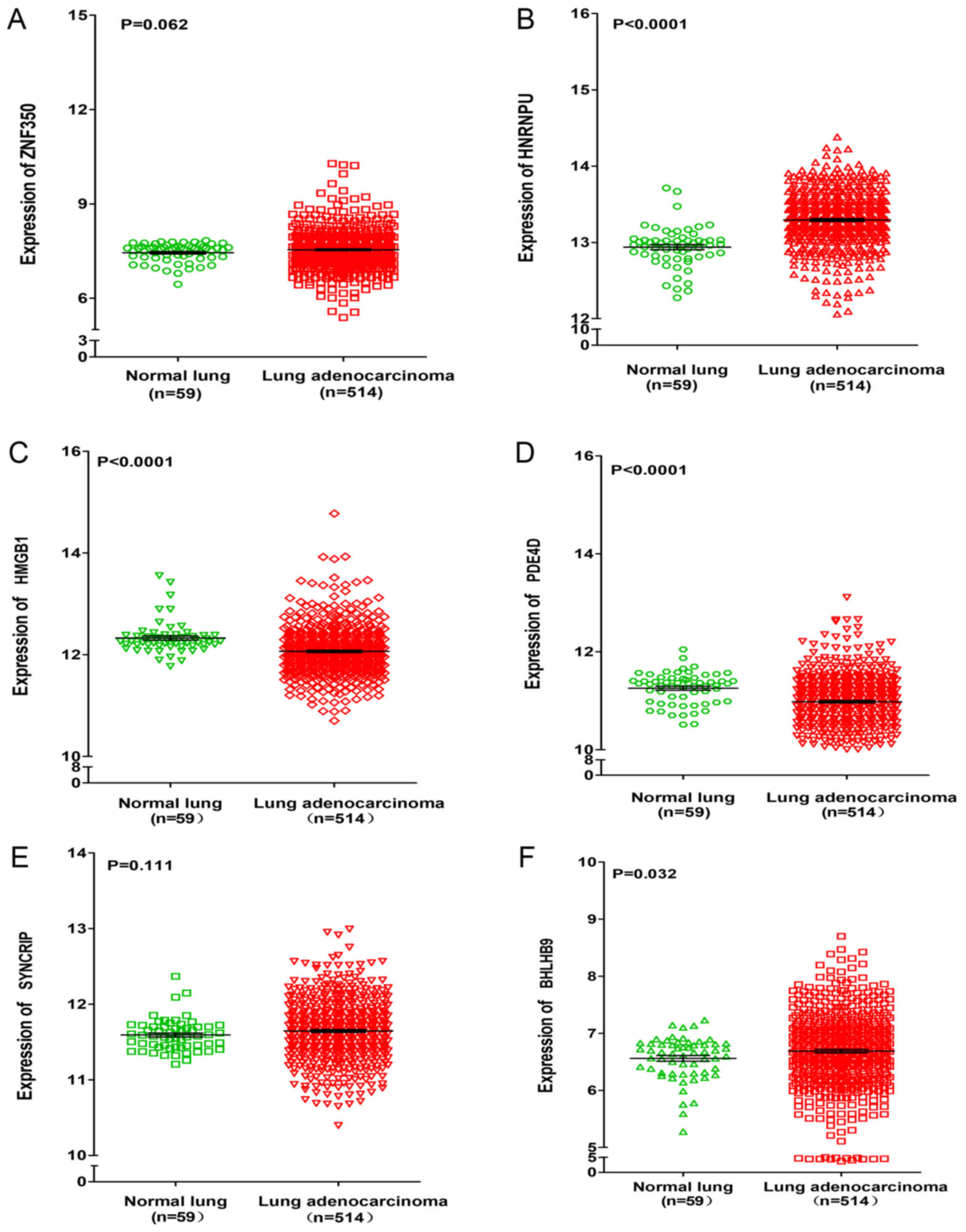 | Figure 6.Differential expression of ZNF350,
HNRNPU, HMGB1, PDE4D, SYNCRIP, and BHLHB9 between lung
adenocarcinoma and normal lung tissues based on the Cancer Genome
Atlas database. Differential expression of (A) ZNF350, (B) HNRNPU,
(C) HMGB1, (D) PDE4D, (E) SYNCRIP and (F) BHLHB9. ZNF350, zinc
finger protein 350; HNRNPU, heterogeneous nuclear ribonucleoprotein
U; HMGB1, high mobility group box 1; PDE4D, phosphodiesterase 4D;
SYNCRIP, synaptotagmin binding cytoplasmic RNA interacting protein;
BHLHB9, basic helix-loop-helix family member B9. |
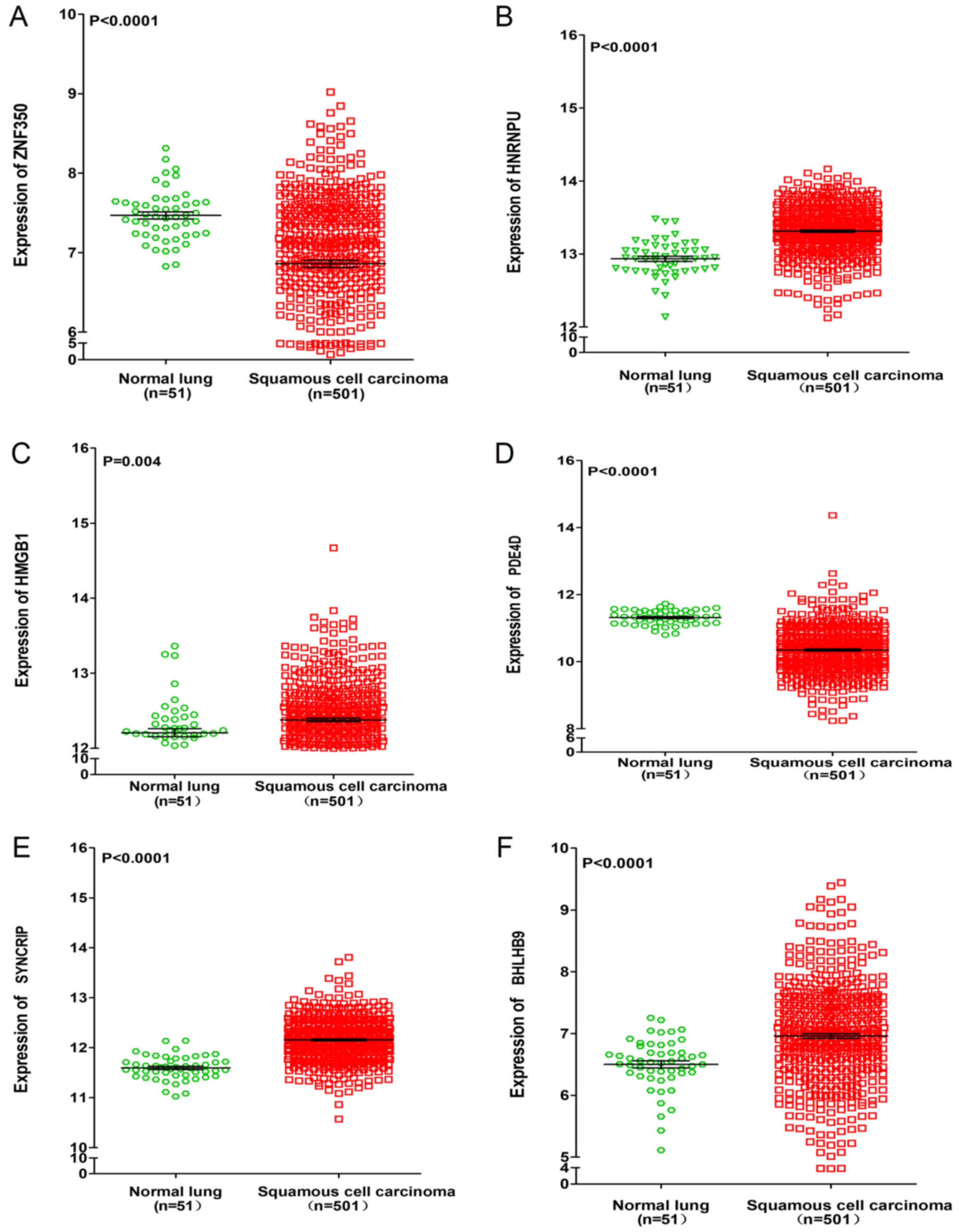 | Figure 7.Differential expression of ZNF350,
HNRNPU, HMGB1, PDE4D, SYNCRIP and BHLHB9 between lung squamous cell
carcinoma and normal lung tissues based on the Cancer Genome Atlas
database. Differential expression of (A) ZNF350, (B) HNRNPU, (C)
HMGB1, (D) PDE4D, (E) SYNCRIP and (F) BHLHB9. ZNF350, zinc finger
protein 350; HNRNPU, heterogeneous nuclear ribonucleoprotein U;
HMGB1, high mobility group box 1; PDE4D, phosphodiesterase 4D;
SYNCRIP, synaptotagmin binding cytoplasmic RNA interacting protein;
BHLHB9, basic helix-loop-helix family member B9. |
 | Table II.Correlations between HOXA11 antisense
RNA expression and the six target genes in lung adenocarcinoma and
squamous cell carcinoma based on The Cancer Genome Atlas via
Spearman's test. |
Table II.
Correlations between HOXA11 antisense
RNA expression and the six target genes in lung adenocarcinoma and
squamous cell carcinoma based on The Cancer Genome Atlas via
Spearman's test.
|
|
| Gene name |
|---|
|
|
|
|
|---|
| Carcinoma type | Value | Zinc finger protein
350 | Heterogeneous
nuclear ribonucleoprotein U | High mobility group
box 1 | Phosphodiesterase
4D |
Synaptotagmin-binding cytoplasmic RNA
interacting protein | Basic
helix-loop-helix family member B9 |
|---|
| Lung
adenocarcinoma | R-value | −0.004 | 0.307 | 0.286 | −0.124 | 0.303 | 0.128 |
|
| P-value | 0.949 | <0.0001 | <0.0001 | 0.049 | <0.0001 | 0.041 |
| Squamous cell
carcinoma | R-value | −0.067 | 0.200 | −0.055 | −0.068 | 0.187 | −0.093 |
|
| P-value | 0.639 | 0.160 | 0.704 | 0.637 | 0.188 | 0.517 |
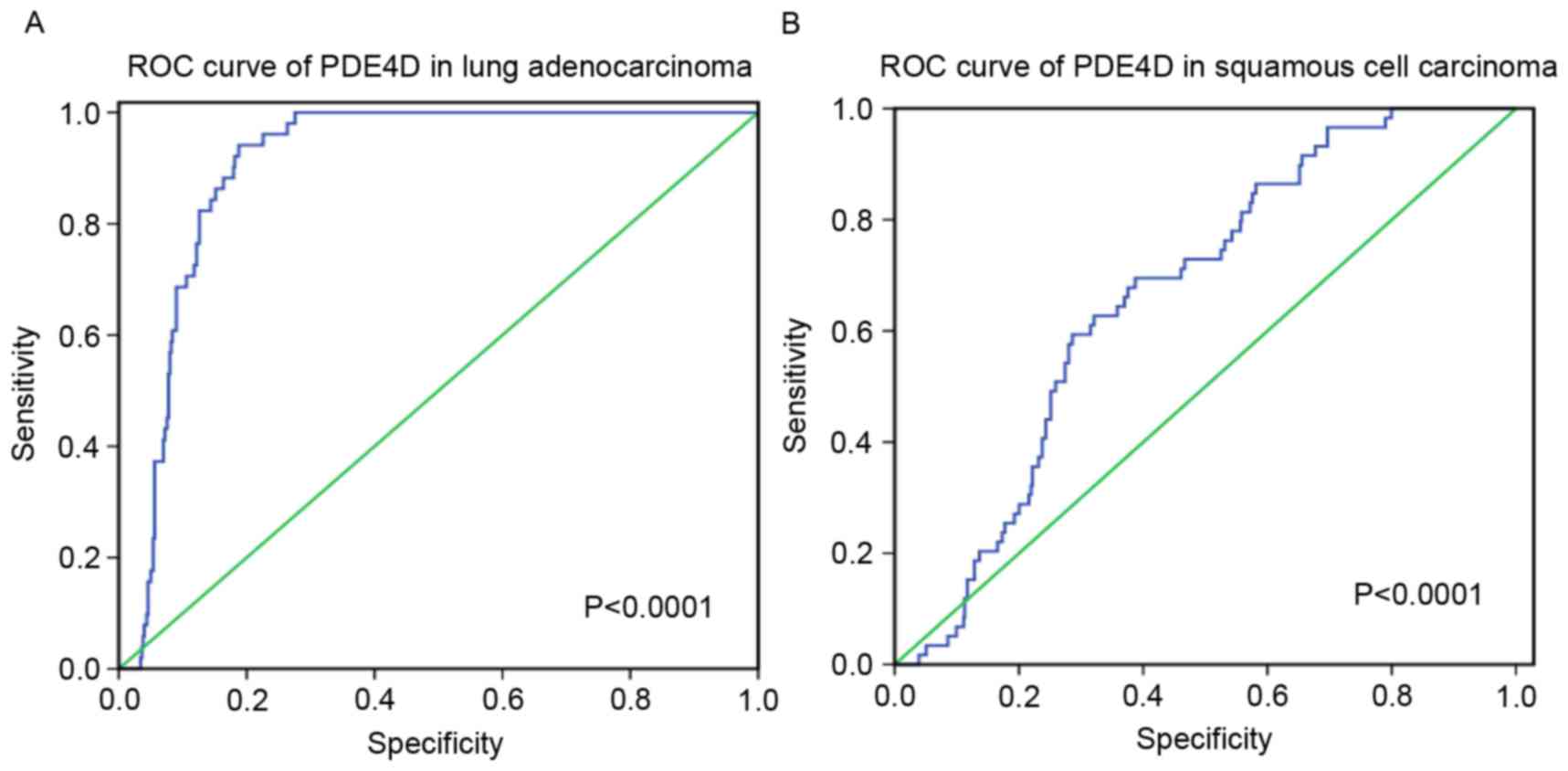
Amongst these results, PDE4D was downregulated in
lung adenocarcinoma and squamous cell carcinoma, and a weak
negative correlation was revealed between HOXA11-AS and PDE4D.
Furthermore, the diagnostic value of PDE4D level in lung cancer was
assessed and it was revealed that the AUC of PDE4D was 0.905 (95%
CI, 0.879–0.931) for patients with lung adenocarcinoma and 0.665
(95% CI, 0.606–0.725) for patients with squamous cell carcinoma
(P<0.0001; Fig. 9).
Discussion
LncRNAs are non-protein-coding RNA molecules and the
length of lncRNAs varies from 200 nucleotides to 100 kb (20). Numerous lncRNAs have been confirmed to
have important functions in transcriptional regulation, epigenetic
gene regulation or disease development (13–15).
Additionally, lncRNAs have been associated with the tumorigenesis
and progression of NSCLCs. To date, different lncRNAs have been
reported to perform different functions in NSCLC, including lncRNA
AK126698, GAS5-AS1 and TUSC7, which may be associated with cell
proliferation, metastasis and a poor prognosis in NSCLC (56–58).
Multiple previous reports have demonstrated that lncRNAs function
in lung cancer by regulating the expression of miRNAs (23–25,59).
HOXA11-AS is a member of the homeobox family of genes. To the best
of our knowledge, only two previous studies have reported the
relationship between HOXA11-AS and cancer. Richards et al
(9) conducted various functional
experiments and analysed genome-wide data, and revealed that
HOXA11-AS may inhibit the oncogenic phenotype of epithelial ovarian
cancer, which may be enhanced by the T allele. Wang et al
(10) used a high-throughput
microarray and gene set enrichment analysis to demonstrate that
HOXA11-AS may have a growth-promoting function in glioma via the
regulation of cell cycle progression. However, the specific
pathogenesis of HOXA11-AS in NSCLC remains unclear. Thus, the
present study was designed using A549 cells to investigate the
expression profile changes of miR-642b-3p following HOXA11-AS
knockdown and the potential molecular mechanisms of HOXA11-AS in
NSCLC.
In the present study, miR-642b-3p and its target
genes (ZNF350, HNRNPU, HMGB1, PDE4D, SYNCRIP, BHLHB9) were
analysed. To the best of our knowledge, only one previous study has
reported on the relationship between miR-642b-3p and cancer. Hamam
et al (60) revealed that
miR-642b-3p was upregulated in breast cancer, and miR-642b-3p in
combination with eight other upregulated miRNAs may be used for the
early detection of breast cancer. Similarly, to this previous
study, miR-642b-3p was upregulated in lung adenocarcinoma and
squamous cell carcinoma, but the specific association between
miR-642b-3p and NSCLC remains to be verified by functional
experiments. Then, six target genes of miR-642b-3p were
investigated and it was revealed that all six target genes were
differentially expressed in lung adenocarcinoma and squamous cell
carcinoma. Additionally, miR-642b-3p, ZNF350, HNRNPU, HMGB1, PDE4D
and BHLHB9 were associated with the overall survival or
disease-free survival of patients with lung adenocarcinoma or
patients with squamous cell carcinoma based on TCGA database.
Numerous previous studies have confirmed the association between
these target genes and NSCLC. Deng et al (61) used a western blot analysis to
determine that miR-193a-3p may inhibit the metastasis of lung
cancer cells by de-regulating the expression of HNRNPU and other
tumour-associated proteins. Numerous studies have assessed HMGB1.
HMGB1 has been associated with cell migration, invasion, apoptosis,
sensitivity to chemotherapy drugs and the prognosis of NSCLC
(62–64). Karachaliou et al (65) revealed that PDE4D was associated with
resistant epidermal growth factor receptor (EGFR)-mutant cancer
cell lines, and the combination of EGFR tyrosine kinase inhibitors
with PDE4D inhibitors may be an effective therapy for patients with
EGFR-mutant NSCLC.
In the findings of the present study, PDE4D was
downregulated in lung adenocarcinoma and squamous cell carcinoma,
and a weak negative association was established between HOXA11-AS
and PDE4D. Additionally, PDE4D was associated with disease-free
survival of lung adenocarcinoma and squamous cell carcinoma, which
indicated that PDE4D may influence the prognosis of adenocarcinoma.
Therefore, the present study hypothesized that miR-642b-3p, which
is regulated by lncRNA HOXA11-AS and targets PDE4D, may have a
function in NSCLC tumorigenesis and deterioration.
Numerous studies have demonstrated that PDE4D may
function in cancer via different pathways, including the
EGFR/phosphoinositide 3-kinase/protein kinase B signalling pathway,
MAPK pathway and Hedgehog pathway (66–68).
However, further exploration is required to clarify the potential
molecular mechanisms of PDE4D in NSCLC.
Additionally, there are limitations in the present
study. The present study was conducted based on several online
tools, including DAVID, STRING, Cytoscape and TCGA, which may only
be used for reference. Furthermore, the specific molecular
mechanisms of miR-642b-3p and genes it is associated with, require
further elucidation through functional experiments. To assess this
hypothesis, a series of experiments may be designed using various
molecule, cell, tissue and animal models (including RT-qPCR,
western blot analysis, proliferation, invasion and metastasis
assays, dual luciferase reporter assay, RNA pull-down, chromatin
immunoprecipitation, chicken embryo chorioallantoic membrane and
nude mouse models). The present study, which focused on the
function of the HOXA11-AS/miR-642b-3p/PDE4D axis, suggests a novel
target for a clinical therapeutic strategy for NSCLC. In
conclusion, HOXA11-AS may influence the expression of miR-642b-3p
in the different biological processes of NSCLC by targeting the
expression of PDE4D or other target genes. Raw data from TCGA
confirmed the oncogenic function of miR-642b in lung adenocarcinoma
and squamous cell carcinoma. The findings of the present study lay
the foundation for future studies on the relationship between
HOXA11-AS and the potential molecular mechanisms in NSCLC
tumorigenesis and progression.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. NSFC81560469 and
NSFC81360327), the Scientific Research Project of Basic Ability
Promoting for Middle Age and Youth Teachers of Guangxi Universities
(grant no. KY2016YB077), the Natural Science Foundation of Guangxi,
China (grant no. 2015GXNSFCA139009) and the Guangxi Provincial
Health Bureau Scientific Research Project (grant nos. Z2013201 and
Z2014055).
References
|
1
|
Xu YJ, Du Y and Fan Y: Long noncoding RNAs
in lung cancer: What we know in 2015. Clin Transl Oncol.
18:660–665. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang CG, Lee HJ, Kim SH and Lee EO:
Zerumbone suppresses osteopontin-induced cell invasion through
inhibiting the FAK/AKT/ROCK pathway in human non-small cell lung
cancer A549 cells. J Nat Prod. 79:156–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Murray T, Ward E, Samuels A,
Tiwari RC, Ghafoor A, Feuer EJ and Thun MJ: Cancer statistics,
2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu G, Chen J, Pan Q, Huang K, Pan J, Zhang
W, Chen J, Yu F, Zhou T and Wang Y: Long noncoding RNA expression
profiles of lung adenocarcinoma ascertained by microarray analysis.
PLoS One. 9:e1040442014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choe C, Shin YS, Kim C, Choi SJ, Lee J,
Kim SY, Cho YB and Kim J: Crosstalk with cancer-associated
fibroblasts induces resistance of non-small cell lung cancer cells
to epidermal growth factor receptor tyrosine kinase inhibition.
Onco Targets Ther. 8:3665–3678. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen G, Umelo IA, Lv S, Teugels E, Fostier
K, Kronenberger P, Dewaele A, Sadones J, Geers C and De Grève J:
miR-146a inhibits cell growth, cell migration and induces apoptosis
in non-small cell lung cancer cells. PLoS One. 8:e603172013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Richards EJ, Permuth-Wey J, Li Y, Chen YA,
Coppola D, Reid BM, Lin HY, Teer JK, Berchuck A, Birrer MJ, et al:
A functional variant in HOXA11-AS, a novel long non-coding RNA,
inhibits the oncogenic phenotype of epithelial ovarian cancer.
Oncotarget. 6:34745–34757. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Q, Zhang J, Liu Y, Zhang W, Zhou J,
Duan R, Pu P, Kang C and Han L: A novel cell cycle-associated
lncRNA, HOXA11-AS, is transcribed from the 5-prime end of the HOXA
transcript and is a biomarker of progression in glioma. Cancer
Lett. 373:251–259. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu B, Zhang H, Wang Z, Zhang F, Wei H and
Li L: LncRNA CCAT1/miR-130a-3p axis increases cisplatin resistance
in non-small-cell lung cancer cell line by targeting SOX4. Cancer
Biol Ther. Oct 11–2017.(Epub ahead of print). View Article : Google Scholar
|
|
12
|
Qu CH, Sun QY, Zhang FM and Jia YM: Long
non-coding RNA ROR is a novel prognosis factor associated with
non-small-cell lung cancer progression. Eur Rev Med Pharmacol Sci.
21:4087–4091. 2017.PubMed/NCBI
|
|
13
|
Wilusz JE: Long noncoding RNAs: Re-writing
dogmas of RNA processing and stability. Biochim Biophys Acta.
1859:128–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan X, Wang J, Tang X, Li Y, Xia P and
Gao X: Berberine ameliorates nonalcoholic fatty liver disease by a
global modulation of hepatic mRNA and lncRNA expression profiles. J
Transl Med. 13:242015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei Y and Niu B: Role of MALAT1 as a
prognostic factor for survival in various cancers: A systematic
review of the literature with meta-analysis. Dis Markers.
2015:1646352015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Song X, Glass CK and Rosenfeld MG:
The long arm of long noncoding RNAs: Roles as sensors regulating
gene transcriptional programs. Cold Spring Harb Perspect Biol.
3:a0037562011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li DS, Ainiwaer JL, Sheyhiding I, Zhang Z
and Zhang LW: Identification of key long non-coding RNAs as
competing endogenous RNAs for miRNA-mRNA in lung adenocarcinoma.
Eur Rev Med Pharmacol Sci. 20:2285–2295. 2016.PubMed/NCBI
|
|
20
|
Xie X, Pan J, Wei L, Wu S, Hou H, Li X and
Chen W: Gene expression profiling of microRNAs associated with UCA1
in bladder cancer cells. Int J Oncol. 48:1617–1627. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM,
Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF and Waye MM: The lncRNA
H19 promotes epithelial to mesenchymal transition by functioning as
miRNA sponges in colorectal cancer. Oncotarget. 6:22513–22525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The rosetta stone of a hidden RNA
language? Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun C, Li S, Zhang F, Xi Y, Wang L, Bi Y
and Li D: Long non-coding RNA NEAT1 promotes non-small cell lung
cancer progression through regulation of miR-377-3p-E2F3 pathway.
Oncotarget. 7:51784–51814. 2016.PubMed/NCBI
|
|
24
|
Lu L, Xu H, Luo F, Liu X, Lu X, Yang Q,
Xue J, Chen C, Shi L and Liu Q: Epigenetic silencing of miR-218 by
the lncRNA CCAT1, acting via BMI1, promotes an altered cell cycle
transition in the malignant transformation of HBE cells induced by
cigarette smoke extract. Toxicol Appl Pharmacol. 304:30–41. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nie W, Ge HJ, Yang XQ, Sun X, Huang H, Tao
X, Chen WS and Li B: LncRNA-UCA1 exerts oncogenic functions in
non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett.
371:99–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Keniry A, Oxley D, Monnier P, Kyba M,
Dandolo L, Smits G and Reik W: The H19 lincRNA is a developmental
reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell
Biol. 14:659–665. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tay Y, Zhang J, Thomson AM, Lim B and
Rigoutsos I: MicroRNAs to Nanog, Oct4 and Sox2 coding regions
modulate embryonic stem cell differentiation. Nature.
455:1124–1128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang H, Tang Y, Guo W, Du Y, Wang Y, Li P,
Zang W, Yin X, Wang H, Chu H, et al: Up-regulation of microRNA-138
induce radiosensitization in lung cancer cells. Tumour Biol.
35:6557–6565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang R, Liang L, Luo D, Feng Z, Huang Q,
He R, Gan T, Yang L and Chen G: Downregulation of MiR-30a is
associated with poor prognosis in lung cancer. Med Sci Monit.
21:2514–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu S, Shen W, Pan Y, Zhu M, Xie K, Geng L,
Wang Y, Liang Y, Xu J, Cao S, et al: Genetic variations in key
MicroRNAs are associated with the survival of nonsmall cell lung
cancer. Medicine (Baltimore). 94:e20842015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang T, She K, Peng G, Wang W, Huang J,
Li J, Wang Z and He J: MicroRNA-186 suppresses cell proliferation
and metastasis through targeting MAP3K2 in non-small cell lung
cancer. Int J Oncol. 49:1437–1444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu YJ, Liu RY, Hu K and Wang Y: MiR-541-3p
reverses cancer progression by directly targeting TGIF2 in
non-small cell lung cancer. Tumour Biol. 37:12685–12695. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xu X, Wang X, Fu B, Meng L and Lang B:
Differentially expressed genes and microRNAs in bladder carcinoma
cell line 5637 and T24 detected by RNA sequencing. Int J Clin Exp
Pathol. 8:12678–12687. 2015.PubMed/NCBI
|
|
36
|
Li Q, Ge X, Xu X, Zhong Y and Qie Z:
Comparison of the gene expression profiles between gallstones and
gallbladder polyps. Int J Clin Exp Pathol. 7:8016–8023.
2014.PubMed/NCBI
|
|
37
|
Jiang CM, Wang XH, Shu J, Yang WX, Fu P,
Zhuang LL and Zhou GP: Analysis of differentially expressed genes
based on microarray data of glioma. Int J Clin Exp Med.
8:17321–17332. 2015.PubMed/NCBI
|
|
38
|
Chen L, Zhuo D, Chen J and Yuan H:
Screening feature genes of lung carcinoma with DNA microarray
analysis. Int J Clin Exp Med. 8:12161–12171. 2015.PubMed/NCBI
|
|
39
|
Wang X: Improving microRNA target
prediction by modeling with unambiguously identified
microRNA-target pairs from CLIP-ligation studies. Bioinformatics.
32:1316–1322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wong N and Wang X: miRDB: An online
resource for microRNA target prediction and functional annotations.
Nucleic Acids Res. 43(Database Issue): D146–D152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chou CH, Chang NW, Shrestha S, Hsu SD, Lin
YL, Lee WH, Yang CD, Hong HC, Wei TY, Tu SJ, et al: miRTarBase
2016: Updates to the experimentally validated miRNA-target
interactions database. Nucleic Acids Res. 44:D239–D247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Paraskevopoulou MD, Georgakilas G,
Kostoulas N, Vlachos IS, Vergoulis T, Reczko M, Filippidis C,
Dalamagas T and Hatzigeorgiou AG: DIANA-microT web server v5.0:
Service integration into miRNA functional analysis workflows.
Nucleic Acids Res. 41(Web Server Issue): W169–W173. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reczko M, Maragkakis M, Alexiou P, Grosse
I and Hatzigeorgiou AG: Functional microRNA targets in protein
coding sequences. Bioinformatics. 28:771–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ashburner M, Ball CA, Blake JA, Botstein
D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT,
et al: Gene ontology: Tool for the unification of biology. The Gene
Ontology Consortium. Nat Genet. 25:25–29. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: Paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Franceschini A, Szklarczyk D, Frankild S,
Kuhn M, Simonovic M, Roth A, Lin J, Minguez P, Bork P, von Mering C
and Jensen LJ: STRING v9.1: Protein-protein interaction networks,
with increased coverage and integration. Nucleic Acids Res.
41(Database Issue): D808–D815. 2013.PubMed/NCBI
|
|
51
|
Bornstein S, Schmidt M, Choonoo G, Levin
T, Gray J, Thomas CR Jr, Wong M and McWeeney S: IL-10 and integrin
signaling pathways are associated with head and neck cancer
progression. BMC Genomics. 17:382016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li B, Ruotti V, Stewart RM, Thomson JA and
Dewey CN: RNA-Seq gene expression estimation with read mapping
uncertainty. Bioinformatics. 26:493–500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang K, Singh D, Zeng Z, Coleman SJ, Huang
Y, Savich GL, He X, Mieczkowski P, Grimm SA, Perou CM, et al:
MapSplice: Accurate mapping of RNA-seq reads for splice junction
discovery. Nucleic Acids Res. 38:e1782010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu Y, Lyu H, Liu H, Shi X, Song Y and Liu
B: Downregulation of the long noncoding RNA GAS5-AS1 contributes to
tumor metastasis in non-small cell lung cancer. Sci Rep.
6:310932016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fu X, Li H, Liu C, Hu B, Li T and Wang Y:
Long noncoding RNA AK126698 inhibits proliferation and migration of
non-small cell lung cancer cells by targeting Frizzled-8 and
suppressing Wnt/β-catenin signaling pathway. Onco Targets Ther.
9:3815–3827. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang Z, Jin Y, Ren H, Ma X, Wang B and
Wang Y: Downregulation of the long non-coding RNA TUSC7 promotes
NSCLC cell proliferation and correlates with poor prognosis. Am J
Transl Res. 8:680–687. 2016.PubMed/NCBI
|
|
59
|
You J, Zhang Y, Liu B, Li Y, Fang N, Zu L,
Li X and Zhou Q: MicroRNA-449a inhibits cell growth in lung cancer
and regulates long noncoding RNA nuclear enriched abundant
transcript 1. Indian J Cancer. 51 Suppl 3:e77–e81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hamam R, Ali AM, Alsaleh KA, Kassem M,
Alfayez M, Aldahmash A and Alajez NM: microRNA expression profiling
on individual breast cancer patients identifies novel panel of
circulating microRNA for early detection. Sci Rep. 6:259972016.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Deng W, Yan M, Yu T, Ge H, Lin H, Li J,
Liu Y, Geng Q, Zhu M, Liu L, et al: Quantitative proteomic analysis
of the metastasis-inhibitory mechanism of miR-193a-3p in non-small
cell lung cancer. Cell Physiol Biochem. 35:1677–1688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang C, Ge S, Hu C, Yang N and Zhang J:
MiRNA-218, a new regulator of HMGB1, suppresses cell migration and
invasion in non-small cell lung cancer. Acta Biochim Biophys Sin
(Shanghai). 45:1055–1061. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang R, Li Y, Wang Z, Chen L, Dong X and
Nie X: Interference with HMGB1 increases the sensitivity to
chemotherapy drugs by inhibiting HMGB1-mediated cell autophagy and
inducing cell apoptosis. Tumour Biol. 36:8585–8592. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Feng A, Tu Z and Yin B: The effect of
HMGB1 on the clinicopathological and prognostic features of
non-small cell lung cancer. Oncotarget. 7:20507–20519. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Karachaliou N, Codony-Servat J, Teixidó C,
Pilotto S, Drozdowskyj A, Codony-Servat C, Giménez-Capitán A,
Molina-Vila MA, Bertrán-Alamillo J, Gervais R, et al: BIM and mTOR
expression levels predict outcome to erlotinib in EGFR-mutant
non-small-cell lung cancer. Sci Rep. 5:174992015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xu T, Wu S, Yuan Y, Yan G and Xiao D:
Knockdown of phosphodiesterase 4D inhibits nasopharyngeal carcinoma
proliferation via the epidermal growth factor receptor signaling
pathway. Oncol Lett. 8:2110–2116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Powers GL, Hammer KD, Domenech M,
Frantskevich K, Malinowski RL, Bushman W, Beebe DJ and Marker PC:
Phosphodiesterase 4D inhibitors limit prostate cancer growth
potential. Mol Cancer Res. 13:149–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ge X, Milenkovic L, Suyama K, Hartl T,
Purzner T, Winans A, Meyer T and Scott MP: Phosphodiesterase 4D
acts downstream of Neuropilin to control Hedgehog signal
transduction and the growth of medulloblastoma. Elife. 4:2015.
View Article : Google Scholar
|