Introduction
Gliomas are considered to be one of the most
aggressive human cancer types, primarily affecting the central
nervous system, and accounting for up to 50% of all primary
malignant tumors of the central nervous system, with high incidence
and mortality rates globally (1).
Gliomas are classified by the World Health Organization according
to the degree of malignancy, into four separate grades with
increasing aggressiveness (2).
Patients with grade 4 gliomas have an average survival period of
≤14 months, despite the availability of multimodal therapies,
including surgery, radiotherapy and finally chemotherapy (3,4). The slow
penetration of gliomas into the brain tissue surrounding the tumor
prevents surgery and radiation therapy. Furthermore, glioma
progression is primarily supported by the microenvironment that
surrounds it. As a result, current treatments become ineffective,
as they are unable to inhibit the supportive effect provided by the
tumor microenvironment. More notably, the cells that constitute the
microenvironment are more genetically stable compared with the
cells that make up the tumor. Malignant glioma appears to
proliferate indefinitely. Therefore, present research attempts to
develop a novel method in order to inhibit the progression of a
tumor and additionally inhibit the supportive effect that the tumor
microenvironment provides to tumor cells. Currently, phytochemicals
are being explored as potential candidates for the treatment of
various cancer types and have gained a substantial amount of
attention due to positive results (5,6).
Polyphenolic phytochemicals are present in abundance in plants and
are known to have antitumor, anti-inflammatory, chemosensitization
and cryo-protective effects (5–10).
Polyphenolic compounds that are plant-derived and have a molecular
weight in the range of 500–3,000 Da are known as tannins, and these
tannins may be categorized as either condensed or hydrolysable
tannins (11). As a hydrolysable
tannin, tannic acid (TA) exists in various forms and is present in
abundance in food plants. In one animal model, TA was demonstrated
to have chemoprotective activity against cancer, including in
hairless mice, and was able to suppress ~70% of the promotion of
skin tumor induced by ultraviolet-B radiations (12) In addition to functioning as a
potential chemopreventive candidate, previously TA has been
revealed to directly inhibit the growth of cancer cells. Various
studies are available where TA has been revealed to suppress
proliferation in various types of cancer cells in vitro and
induce cancer cell death by apoptosis (13–16).
However, the pathway by which TA operates inside a cell has not
been documented yet and requires further study. One previous study
examined proteasome inhibition by TA in cancer cells, which led to
growth arrest or apoptosis of cancer cells (17). Previously, a study also demonstrated
that TA may offer a novel way to treat glioma as it may act within
the tumor microenvironment and lead to inhibition of cluster of
differentiation 38 (18). Therefore,
the present study was designed with the aim of exploring the
effects of TA in vitro on HS 683, a glioma cell line, and to
study the mechanism involved in the induction of cytotoxicity and
apoptosis by TA.
Materials and methods
Chemicals and reagents
RPMI-1640, streptomycin, penicillin G, 3-(4,
5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT),
dimethyl sulfoxide (DMSO), TA, Rhodamine-123 and
2,7-Dichlorodihydrofluorescein diacetate (DCFH-DA) were obtained
from Sigma-Aldrich, Merck KGaA (Darmstadt, Germany). Foetal bovine
serum (FBS) was obtained from Gibco (Thermo Fisher Scientific,
Inc.,Waltham, MA, USA). Pro caspase 3, caspase 9, poly (ADP-ribose)
polymerase (PARP), β actin and Annexin V/propidium iodide (PI) was
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX,
USA).
Cell culture, growth conditions and
treatment
A panel of five cancer cell lines, including
colorectal adenocarcinoma cell line LS-180, human breast
adenocarcinoma cell line MCF-7, human brain glioma cell line Hs
683, mouse neuroblastoma cell line N2a and human promyelocytic
leukemia cell line HL-60 were acquired from the European Collection
of Authenticated Cell Cultures (Public Health England, London
England) were used for initial analysis. RPMI-1640 media
complemented with 10% FBS, streptomycin (100 mg/l), penicillin G
(70 mg/l), and NaHCO3 (3.7 g/l) were used to culture the
cells, maintained in a humidified environment in a CO2
incubator at 37°C with 5% CO2 at 98% humidity. Cells
were treated with a range of concentrations of TA dissolved in
DMSO, and control cells were treated with vehicle only (<0.2%
DMSO).
Viability assay
An MTT assay was performed to assess the effect of
TA on cell viability. Cells were seeded at a density of
0.20×105 cells/well in 96-well plates for 24 h. After 24
h, cells were treated for 48 h with different concentrations of TA
(0, 1, 5 and 10 µM). At 4 h prior to the termination of the
experiment, MTT at a concentration of 2.5 mg/ml was added. Media
was removed, and formazan crystals were dissolved by adding 150 µl
of DMSO and with gentle agitation on an orbital shaker for 3–4 min.
Absorbance was measured at 570 nm using a microplate reader.
Mitochondrial membrane potential (MMP)
assay
Fluorescence of Rhodamine-123 was used to monitor
changes in MMP. Loss of Rhodamine-123 from the mitochondria
decreases the intracellular fluorescence intensity during cell
apoptosis due to the depolarization of MMP. In brief, Hs 683 cells
were treated with TA for 48 h at a range of concentrations (0, 1, 5
and 10 µM). Rhodamin-123 was added 30 min prior to the termination
of the experiment, and incubated at 37°C for 30 min. Cells were
centrifuged at 400 × g for 5 min at 20°C and then washed three
times with phosphate buffered saline (PBS). Fluorescent intensity
was measured at an excitation wavelength of 488 nm and emission
wavelength of 529 nm using a fluorescence microplate reader. The
fluorescence of each TA-treated concentration group was compared
with an untreated group in three independent experiments.
Nuclear morphology by DAPI
Cells were seeded in a 6-well plate at a density of
1×106 cells/well for 24 h. After 24 h, cells were
treated with TA at different concentrations (0, 1, 5 and 10 µM) and
were incubated for 48 h. Cells were harvested using trypsinization
and fixed with acetic acid and methanol (1:3 concentration) for 6
h. Following incubation, cells were centrifuged at 400 × g for 5
min at 20°C, and pellets were resuspended in acetic acid:methanol
solution. Cells were then plated on chilled glass slides. DAPI was
added for 20–30 min in the dark at a concentration of 1 µg/ml at
25°C and images were taken using a fluorescence microscope at ×40
magnification.
Assay for reactive oxygen species
(ROS)
Measurement of ROS in Hs 683 cells was measured
using DCFH-DA. Cells were treated with TA at a range of
concentrations (0, 1, 5 and 10 µM) for 48 h. All cells were
collected using trypsinization, washed three times with PBS, and
then resuspended in 500 µl of PBS containing DCFH-DA at a
concentration of 10 µM for 30 min in the dark at 37°C. Then, all
samples were analysed immediately using a flow cytometer with
CellQuest software version 5.1 BD Biosciences (BD
FACSCalibur™; BD Biosciences, Franklin Lakes, NJ, USA).
A total of 20,000 events for analysis were captured.
Detection of apoptosis via Annexin
V/PI
Hs 683 cells were seeded at a density of
1×106 cells/well in 6-well plates and were treated with
TA at a range of concentrations (0, 1, 5 and 10 µM) for 48 h.
Treated samples were collected and washed twice with PBS and cells
were resuspended in 500 µl of binding buffer with Annexin in the
dark for 20 min according to the manufacturer's protocol. Apoptotic
population was analysed using a flow cytometer with CellQuest
software version 5.1 BD Biosciences (BD FACSCalibur; BD
Biosciences).
Western blot analysis
Hs 683 cells were seeded in a 6-well plate for 24 h
at a density of 1×106 cells/well. After 24 h, cells were
treated with TA at a range of different concentrations (0, 1, 5 and
10 µM) for 48 h. Following treatment, cells were lysed using a RIPA
buffer (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and protein
content was quantified using Bradford reagent. Proteins (60 µg)
were loaded in SDS-PAGE PARP (8% SDS-PAGE), Caspase 3 (15%
SDS-PAGE), Caspase 9 (15% SDS-PAGE), β-actin (10% SDS-PAGE) and
were electro-transferred onto a PVDF membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for 2 h at 100 V.
Non-specific binding of protein membranes was blocked using the
blocking buffer (TBST; 150 mM NaCL, 10 mM Tris-HCL and 0.1% Tween
20) with 5% skimmed milk at room temperature for 1 h. Primary
antibodies PARP (cat no. 9532; dilution 1:1,000); Caspase 3 (cat
no. 9662; dilution 1:1,000); Caspase 9 (cat no. 9508; dilution
1:1,000) β-Actin (cat no. 4970; dilution 1:1,500) were obtained
from Cell Signaling Technology Inc., (Danvers, MA, USA) were
incubated with different protein membranes for 6 h at 20°C and
washed twice with blocking buffer (TBST) for 5 min each. Then,
horseradish peroxidase conjugated anti-rabbit secondary antibody
(cat. no. 93702; dilution 1:2,000) were obtained from Cell
Signaling Technology Inc. were used for incubation for 1 h at 20°C
and washed again three times with blocking buffer (TBST). The bands
of proteins were visualised using an enhanced chemiluminescence kit
(GE Healthcare, Chicago, IL, USA) on an X-ray film.
Statistical analysis
All data is presented as the mean (of three
independent experiments) ± the standard deviation. GraphPad Instat
v3 software (GraphPad software Inc., La Jolla, CA, USA) was used
for statistical analysis. A one-way analysis of variance was used
for statistical analysis with Bonferroni's correction applied.
P<0.05 was considered to indicate a statistically significant
difference.
Results
TA inhibits the cell viability of Hs
683
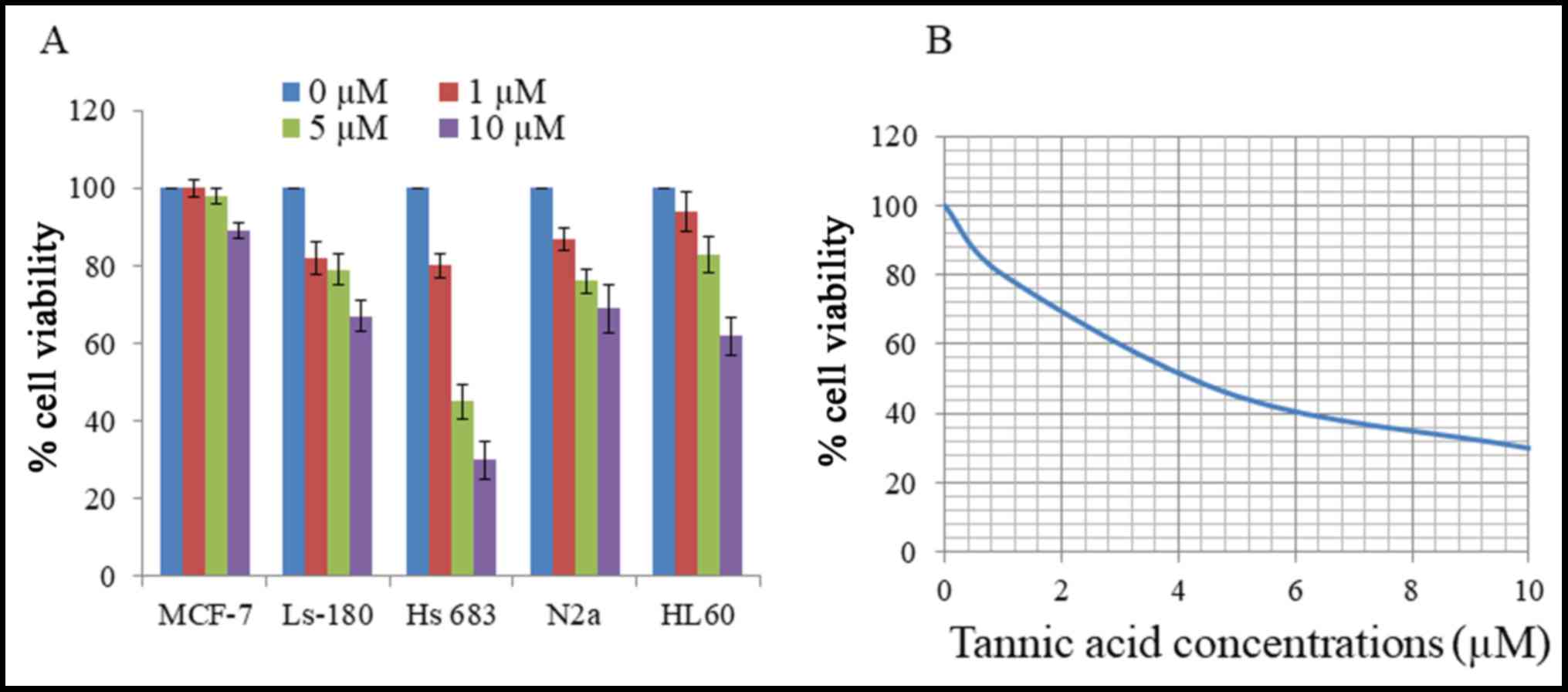
Initial cytotoxicity of TA was assessed using an MTT
assay on various cancer cell lines. Cells were treated with
different concentrations of TA for 48 h, and revealed different
levels of viability inhibition by TA as presented in Fig. 1A. Human brain glioma cell line Hs 683
was the most sensitive to the effect of TA, particularly at the 10
µM concentration, and was selected as the target cell line for
further studies. Viability of Hs 683 cells was reduced by TA in a
concentration-dependent manner for 48 h with an IC50 of
4.2 µM (Fig. 1B). These results
demonstrate that TA had a substantial cytotoxic effect on Hs 683
cells.
TA induces apoptosis in Hs 683
cells
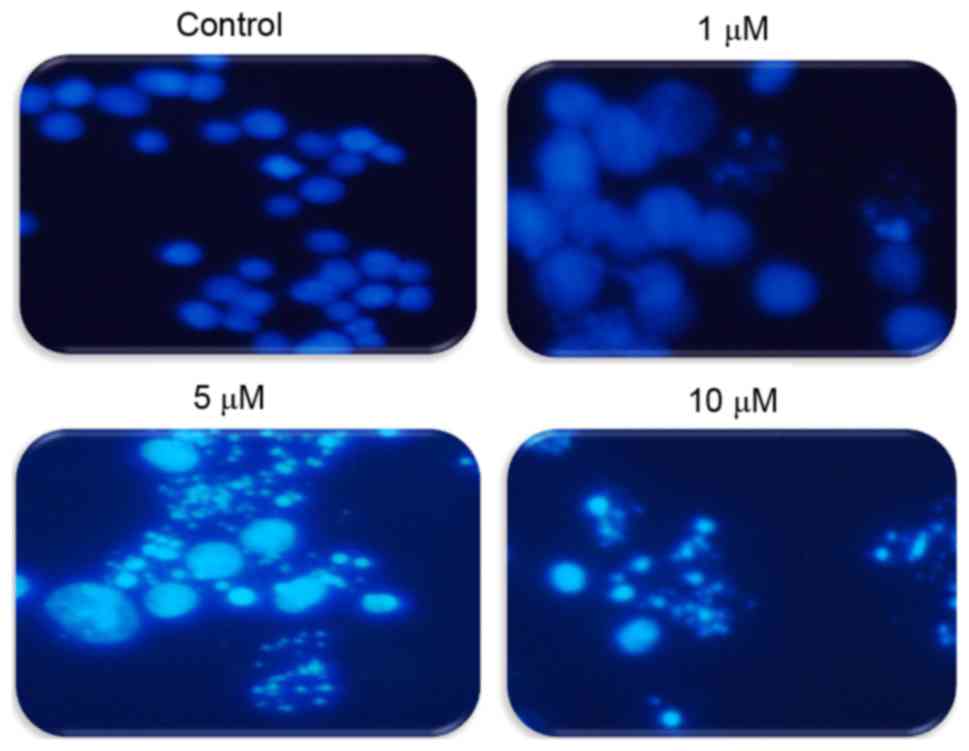
To explore whether TA induces apoptosis in Hs683
cells, cells were treated with differing concentrations of TA (0,
1, 5 and 10 µM) and the induction of apoptosis was observed using
DAPI staining. Under a fluorescence microscope it was observed that
apoptotic bodies increased in a concentration-dependent manner
compared with the untreated cells that have uniformly bright nuclei
(Fig. 2). These results suggest that
TA induced apoptotic cell death in a concentration-dependent
manner.
ROS assay in Hs683 cells
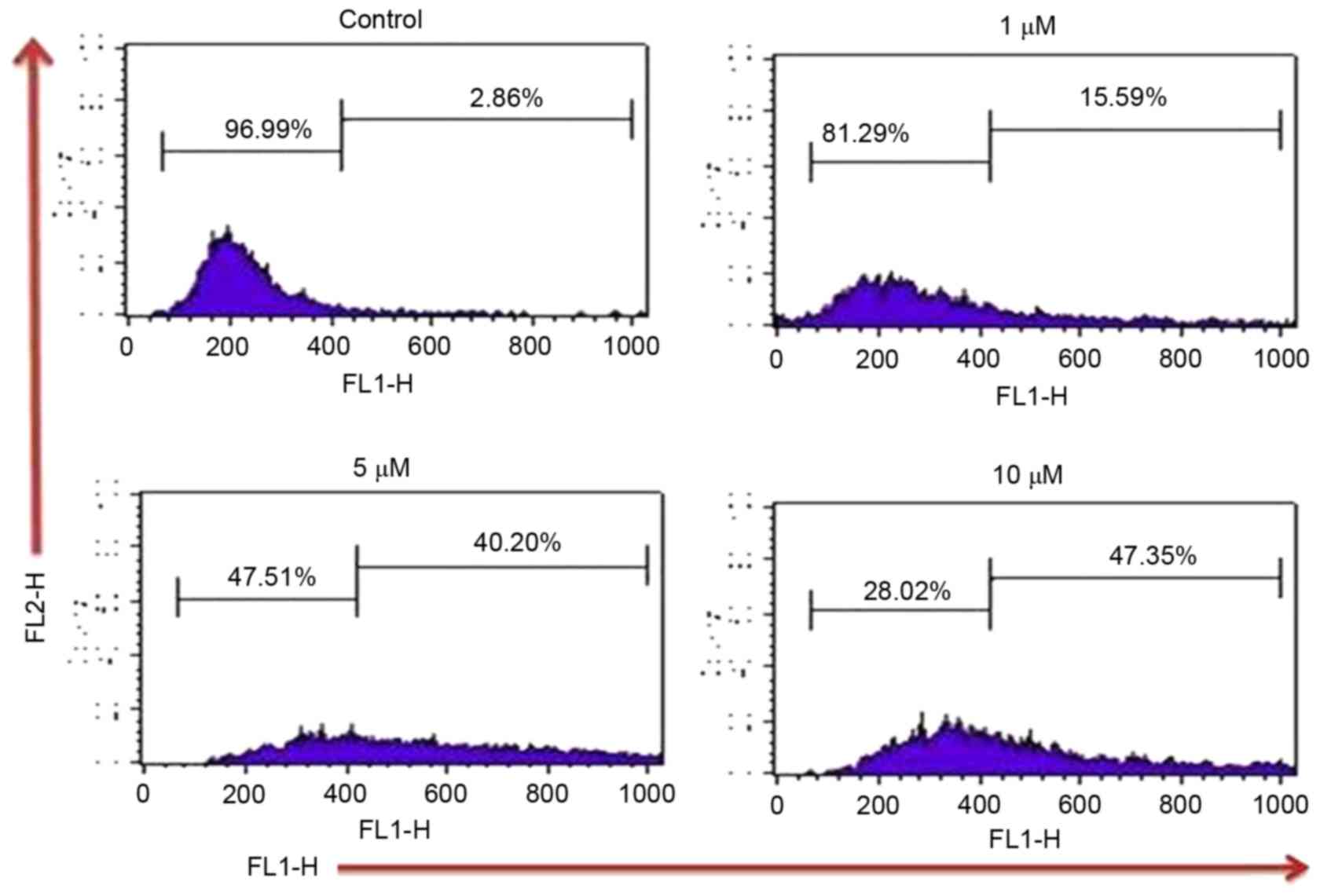
In the present study, the effect of TA on ROS
generation by which the induction of apoptosis takes place in Hs683
cells, was examined by using DCFH2DA dye. Results
indicated that TA increases the intracellular ROS by 2.86, 15.59,
40.20 and 47.35% at TA concentrations of 0, 1, 5 and 10 µM
respectively, compared with the untreated control (Fig. 3). Thus, these results indicate that TA
generated ROS in a concentration-dependent manner which leads Hs
683 cells towards apoptosis.
MMP loss by TA treatment in glioma
Hs683 cells
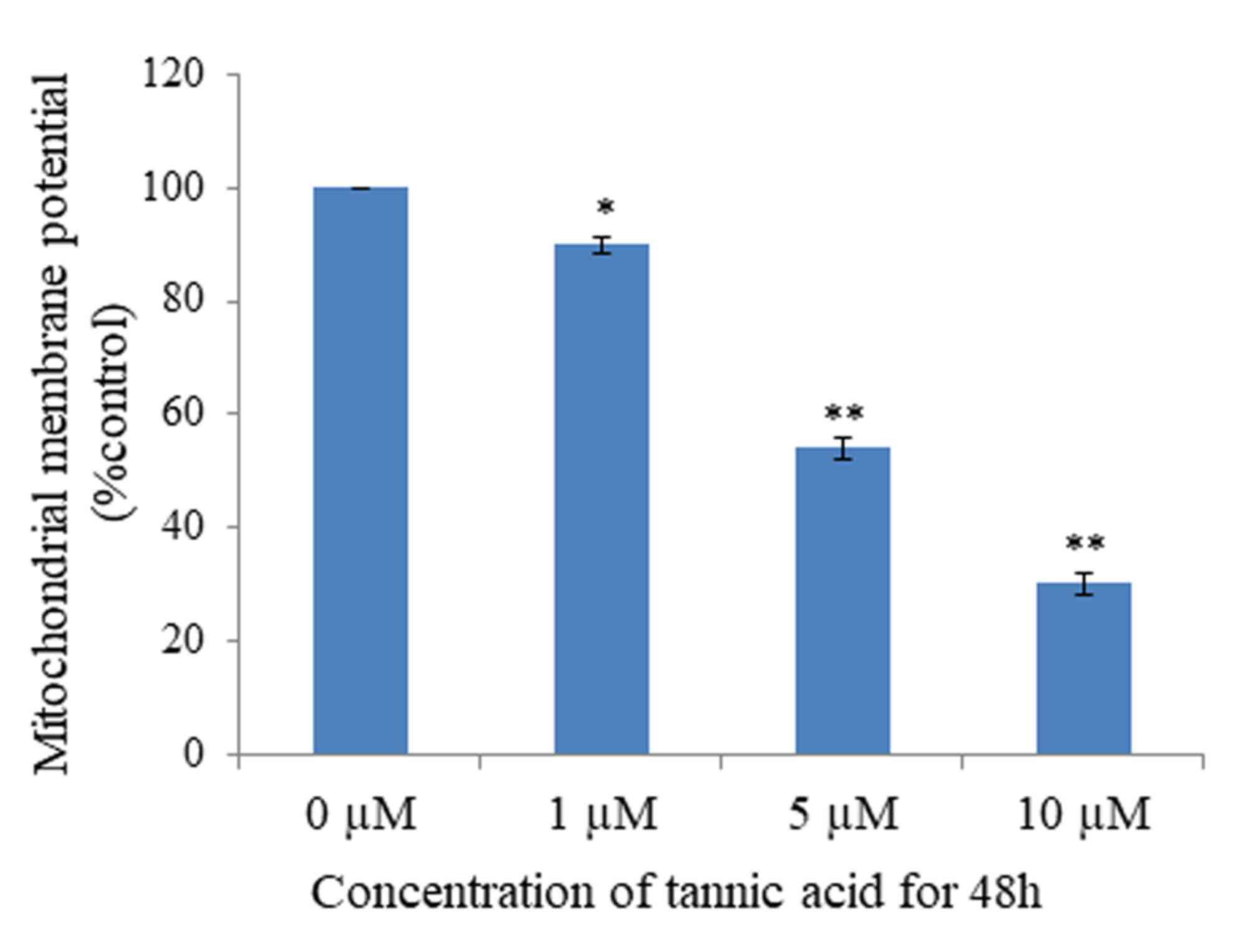
Mitochondria serve an important function in the
progression of apoptosis; loss of MMP is part of the early phase of
apoptosis. To investigate whether TA affects MMP, analysis was
performed using Rhodamine-123 dye, which reveals the loss of MMP.
The results demonstrated that MMP was significantly lost in cells
treated with TA at concentrations of 1 µM (P<0.05), 5 µM
(P<0.01) and 10 µM (P<0.01), compared with untreated cells,
in a concentration-dependent manner (Fig.
4). Untreated Hs 683 cells retained 96% fluorescence. Thus,
these results suggest that TA induced apoptosis by MMP loss.
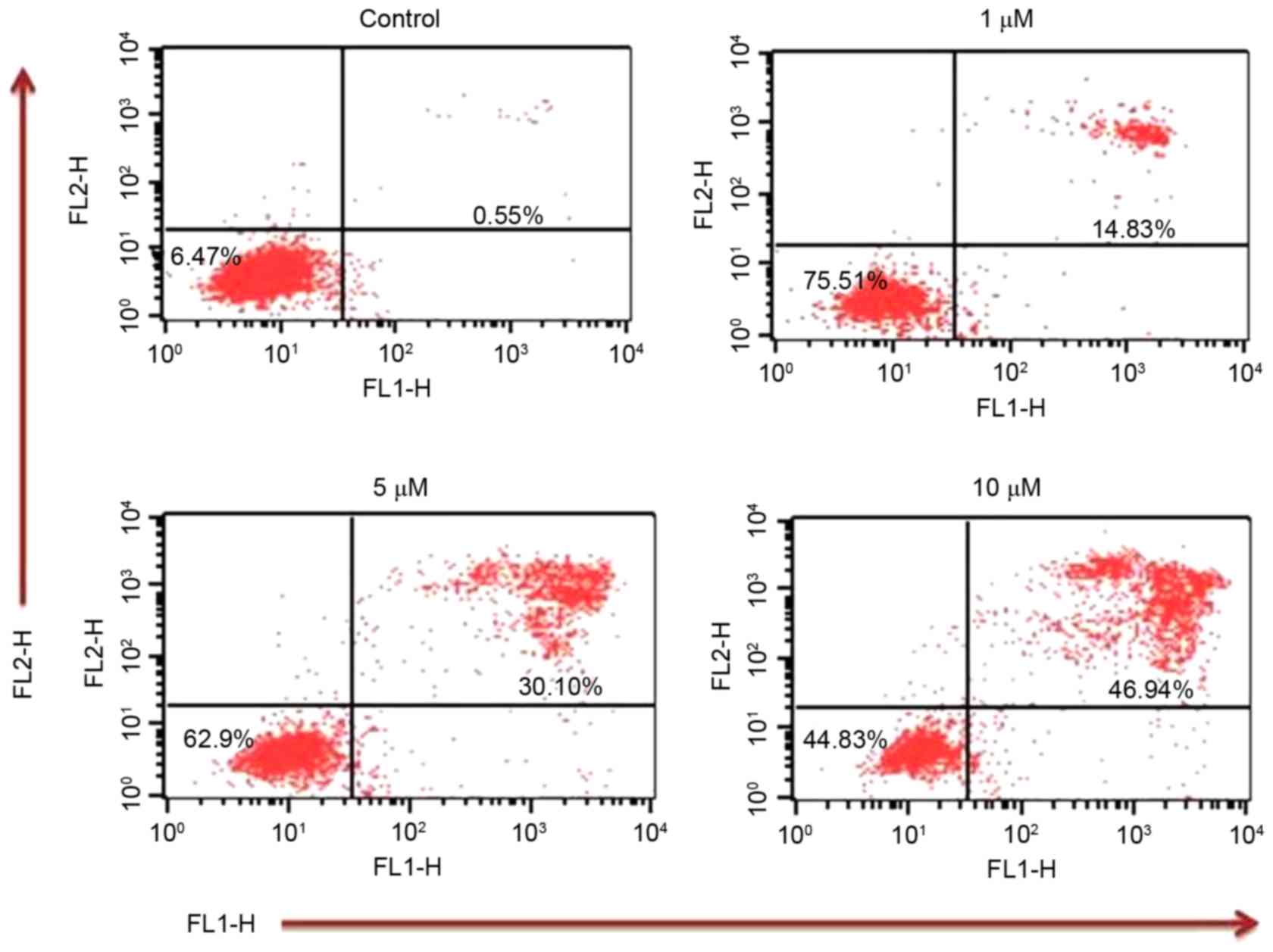
Annexin V/PI assay
In order to confirm that TA induces apoptosis, an
Annexin V/PI assay was performed. Annexin V/PI staining revealed
that the apoptotic population increased to 14.83, 30.10 and 46.94%
at TA concentrations of 1, 5 and 10 µM, respectively, compared with
the untreated control (0.55%) (Fig.
5) These results indicate that TA induced apoptosis in a
concentration-dependent manner.
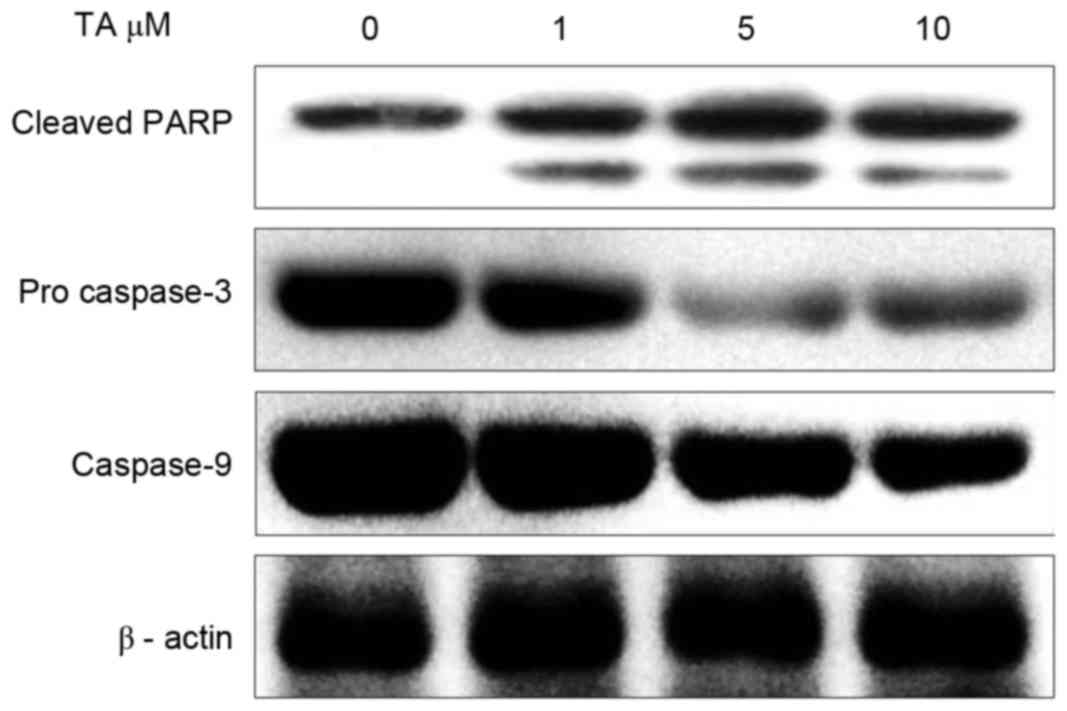
TA induces PARP cleavage and caspase
activation in Hs 683 cells
Regulation of caspases serves an important function
in executing apoptotic cell death (19). Executioner caspases like caspase 3 are
activated by initiator caspases, including caspase 8, caspase 9 and
caspase 10, and cleave PARP once activated (20). In order to investigate the effect of
TA on the activation of caspases, Hs 683 cells were treated with TA
for 48 h and examined using western blot analysis. The results
revealed that, at different concentrations, pro-caspase 3 and
caspase 9 activation was markedly inhibited with increasing
concentrations of TA; however, the expression of cleaved PARP
increased in a concentration-dependent manner compared with the
untreated sample (Fig. 6). Thus,
these results indicate that TA induces apoptosis in a
concentration-dependent manner.
Discussion
In traditional medicine, TA usage has a long
history. Previously, TA was administered in conjugation with either
activated charcoal or magnesium in order to treat toxic substances,
including ptomaine poisoning (21).
Neanderthals used to treat burns with the extracts of plants rich
in TA unknowingly (22). Chinese
traditional medicine also uses TA in a similar manner and it has
been part of this practice for a long time (23). The therapeutic efficacy of herbal
medicine is also known to be enhanced by TA (24). An increasing number of reports have
surfaced over the past few years, examining the chemo-therapeutic
potential of TA against various cancer types, and TA has garnered a
lot of attention (25–27). In the present study, during the
cytotoxic screening of TA against various cancer cell lines, TA
demonstrated a strong cytotoxic effect against glioma Hs 683 cells
with an IC50 of 4.2 µM. However, the exact mechanism by
which TA affects cancer cells is still not fully understood. A
previous study by Nam et al (17), revealed that TA potentially inhibits
the activity of specific proteasomes resulting in a build-up of
proteasome substrates, specifically cyclin-dependent kinase
inhibitor 1B and BCL2 associated X apoptosis regulator. A build-up
of these substrates results in the arrest of the cell growth cycle
in the G1 phase followed by the induction of apoptosis. The present
study revealed that, following the introduction of TA to glioma Hs
683 cells, there was membrane blabbing, chromosome condensation and
fragmentation of glioma Hs 683 cells as confirmed by DAPI staining,
which are all apoptotic hallmarks. Apoptosis caused by TA was
further confirmed by Annexin V/PI staining. It was revealed that
with an increase in the concentration of TA, there was a
significant increase in the production of ROS, which was
accompanied by a significant increase in the loss of MMP in Hs 683
cells. Therefore, it was concluded that TA induces apoptosis in Hs
683 cells by increasing ROS production, which are known to be
responsible for inducing apoptosis in cancer cells by generating
transitional pore opening in mitochondria. Induction of apoptosis
by TA in Hs 683 cells was further validated by western blot
analysis in which the activation of pro-caspase 3, caspase 9 and
cleaved parp were observed. From the results obtained it was
concluded that TA induced ROS generation and loss of MMP in glioma
Hs 683 cells, which may finally drive the cells towards apoptosis.
Therefore, the function of TA as an anticancer agent needs to be
evaluated further. These results validate the requirement for
further studies to explore the function of polyphenols,
particularly TA, in various models of cancer. These studies will
assist in the development of a novel treatment for cancer and lead
to cancer cell growth inhibition.
Acknowledgements
Not applicable.
Funding
This study was supported by Natural Science
Foundation of Shandong Province, China (grant no. ZR2014HM077).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JZ and DC designed and planned the study,
interpreted the results and wrote the manuscript. DH, YC and CD
performed experiments and generated results. XW and FC contributed
the reagents and assisted in designing the study. XH drafted the
manuscript and interpreted the data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Louis DN, Ohgaki H, Wiestler OD, Cavenee
WK, Burger PC, Jouvet A, Scheithauer BW and Kleihues P: The 2007
WHO classification of tumors of the central nervous system. Acta
Neuropathol. 114:97–109. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fuller GN: The WHO classification of
tumours of the central nervous system. 4th edition. Arch Pathol Lab
Med. 132:9062008.PubMed/NCBI
|
|
4
|
Rock K, McArdle O, Forde P, Dunne M,
Fitzpatrick D, O'Neill B and Faul C: A clinical review of treatment
outcomes in glioblastoma multiforme-the validation in a non-trial
population of the results of a randomised Phase III clinical trial:
Has a more radical approach improved survival? Br J Radiol.
85:e729–e733. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Singh S, Sharma B, Kanwar SS and Kumar A:
Lead phytochemicals for anticancer drug development. Front Plant
Sci. 7:16672016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rahman MA, Amin AR and Shin DM:
Chemopreventive potential of natural compounds in head and neck
cancer. Nutr Cancer. 62:973–987. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
D'Alessandro N, Poma P and Montalto G:
Multifactorial nature of hepatocellular carcinoma drug resistance:
Could plant polyphenols be helpful? World J Gastroenterol.
13:2037–2043. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rudolf E, Andelová H and Cervinka M:
Polyphenolic compounds in chemoprevention of colon cancer-targets
and signaling pathways. Anticancer Agents Med Chem. 7:559–575.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stevenson DE and Hurst RD: Polyphenolic
phytochemicals-just antioxidants or much more? Cell Mol Life Sci.
64:2900–2916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fresco P, Borges F, Diniz C and Marques
MP: New insights on the anticancer properties of dietary
polyphenols. Med Res Rev. 26:747–766. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romani A, Ieri F, Turchetti B, Mulinacci
N, Vincieri FF and Buzzini P: Analysis of condensed and
hydrolysable tannins from commercial plant extracts. J Pharm Biomed
Anal. 41:415–420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gali-Muhtasib HU, Yamout SZ and Sidani MM:
Tannins protect against skin tumor promotion induced by
ultraviolet-B radiation in hairless mice. Nutr Cancer. 37:73–77.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sakagami H, Jiang Y, Kusama K, Atsumi T,
Ueha T, Toguchi M, Iwakura I, Satoh K, Ito H, Hatano T and Yoshida
T: Cytotoxic activity of hydrolyzable tannins against human oral
tumor cell lines-a possible mechanism. Phytomedicine. 7:39–47.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan MH, Lin JH, Lin-Shiau SY and Lin JK:
Induction of apoptosis by penta-O-galloyl-beta-D-glucose through
activation of caspase-3 in human leukemia HL-60 cells. Eur J
Pharmacol. 381:171–183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang CC, Chen LG and Yang LL: Cuphiin D1,
the macrocyclic hydrolyzable tannin induced apoptosis in HL-60 cell
line. Cancer Lett. 149:77–83. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang LL, Lee CY and Yen KY: Induction of
apoptosis by hydrolyzable tannins from Eugenia jambos L. on human
leukemia cells. Cancer Lett. 157:65–75. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nam S, Smith DM and Dou QP: Tannic acid
potently inhibits tumor cell proteasome activity, increases p27 and
Bax expression, and induces G1 arrest and apoptosis. Cancer
Epidemiol Biomarkers Prev. 10:1083–8. 2001.PubMed/NCBI
|
|
18
|
Blacher E, Levy A, Baruch BB, Green KD,
Garneau-Tsodikova S, Fridman M and Stein R: Targeting CD38 in the
tumor microenvironment: A novel approach to treat glioma. Cancer
Cell Microenvironment. 2:22015.
|
|
19
|
Lavrik IN, Golks A and Krammer PH:
Caspases: Pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakahira H, Enari M and Nagata S: Cleavage
of CAD inhibitor in CAD activation and DNA degradation during
apoptosis. Nature. 391:96–99. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Daly JS and Cooney DO: Interference by
tannic acid with the effectiveness of activated charcoal in
‘Universal Antidote’. Clin Toxicol. 12:515–522. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hupkens P, Boxma H and Dokter J: Tannic
acid as a topical agent in burns: Historical considerations and
implications for new developments. Burns. 21:57–61. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi-Tsi S: Use of combined traditional
Chinese and Western medicine in the management of burns. Panminerva
Med. 25:197–202. 1983.PubMed/NCBI
|
|
24
|
Mala A and Tulika T: Therapeutic efficacy
of Centella asiatica (L.) and Momordica charantia: As traditional
medicinal plant. J Plant Sci. 3:1–9. 2015.
|
|
25
|
Naus PJ, Henson R, Bleeker G, Wehbe H,
Meng F and Patel T: Tannic acid synergizes the cytotoxicity of
chemotherapeutic drugs in human cholangiocarcinoma by modulating
drug efflux pathways. J Hepatol. 46:222–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ramanathan R, Tan CH and Das NP: Cytotoxic
effect of plant polyphenols and fat-soluble vitamins on malignant
human cultured cells. Cancer Lett. 62:217–224. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Booth BW, Inskeep BD, Shah H, Park JP, Hay
EJ and Burg KJ: Tannic acid preferentially targets estrogen
receptor-positive breast cancer. Int J Breast Cancer.
2013:3696092013. View Article : Google Scholar : PubMed/NCBI
|