Introduction
Breast cancer is the leading cancer in women
worldwide, in terms of incidence and cancer-associated mortality
(1). The brain is one of the most
common sites for breast cancer metastasis, with a rate of brain
metastasis of 10–15% in patients with advanced breast cancer
(2) and a rate of 30–55% in patients
with HER-2 overexpression (3). The
median survival time for patients with breast cancer brain
metastasis (BCBM) ranges between 4 and 14 months (4–6).
In the era of personalized medicine, the use of the
same treatments for all BCBM patients is no longer appropriate. The
choice of treatment for a given patient depends upon numerous
factors, including age, performance status and tumor
characteristics such as breast biological subtypes, tumor site,
number of brain metastases and extracranial metastasis. Considering
that patients with BCBM are a heterogeneous group, it is necessary
to introduce a simple breast cancer-specific prognostic index that
may aid clinicians in selecting the appropriate treatment. Several
prognostic models for patients with cancer have been developed and
widely used in clinical oncology practice (7–9). For
example, the Radiation Therapy Oncology Group established recursive
partitioning analysis (RPA) in 1997 (7). The graded prognostic assessment (GPA)
was constructed in 2008 and has been regarded as more accurate than
RPA (8). Although RPA and GPA are
widely used in the clinic, these were constructed on the basis of
several different histological types of cancer and have limited use
in breast cancer. In 2012, the Breast-GPA was developed based on
analysis of the clinical features of 400 cases of BCBM (9).
Considering the limitations of RPA, GPA and
Breast-GPA, there is a requirement for developing a novel
prognostic model. A nomogram is a visual predictive tool based on
statistical regression models, which measures the impact of various
factors on the possibility of an event (10). This tool may aid clinicians in
assessing patient risk of recurrence and prognosis, and in
selecting appropriate patients for clinical trials. It has been
demonstrated that a nomogram may improve predictive accuracy for
clinical outcomes, compared with traditional prognostic models
(11–17). The present study was designed to
construct a novel prognostic model for BCBM using a nomogram
approach. Furthermore, the present study also compared the novel
model with existing PRA, GPA and Breast-GPA models, with the aim
that the newly developed model would be useful in the treatment of
patients with BCBM.
Materials and methods
Patients and treatment
The medical records of patients with BCBM, who had
been admitted to the Affiliated Hospital of Academy of Military
Medical Sciences (Beijing, China) between January 2002 and December
2014, were retrospectively analyzed. The diagnosis of breast cancer
was pathologically confirmed, and brain metastasis was diagnosed by
imaging or pathology. Patients who had more than one histological
tumor type or missing data on key medical information were excluded
from the present study. A total of 411 female patients with a
median age of 47.6 years (range, 25.3–80.0 years) at brain
metastases were finally included in present study. Based on the
number and dimensions of brain metastases (BMs), these patients
underwent local treatments, including surgical resection, whole
brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS). The
most common regimen of WBRT was 40 Gy in 20 fractions and the most
common regimen of SRS was 17 Gy in 1 fraction. Among the 411
patients with BCBM, 265 (64.5%) were treated with WBRT with or
without SRS, 188 (45.7%) received SRS or surgical resection with or
without WBRT, 154 (37.5%) received WBRT only, and 69 (16.9%) did
not receive local treatment. For patients with fewer than three
BMs, SRS was initially performed and WBRT was administered when the
BM progressed or additional BMs developed.
Variables
In the present study, possible factors that affect
BM prognosis were selected based on review of current literature
(9,18–21),
including age, clinical stage, biological subtype, disease-free
survival (DFS), occurrence time of BM, duration between diagnosis
and BM, Karnofsky performance score (KPS) (22), extracranial metastasis, meningeal
metastasis, symptoms of BM, and the number and size of BM lesions.
The classification of biological subtypes was based on the 2011 St.
Gallen International Expert Consensus (23). The response to treatment was evaluated
using the Response Evaluation Criteria in Solid Tumors (RECIST 1.1)
(24).
Data analysis, model construction and
statistical analysis
Brain metastasis overall survival (BMOS) was defined
as the duration between the diagnosis of BM and mortality or the
end of follow-up. DFS was defined as the duration between surgery
and the first recurrent metastasis. The database was closed on May
15, 2016. Univariate analysis and a multivariate Cox proportional
hazards model were used to analyze the association between risk
factors and survival. Variables that were significant in the
univariate analysis were incorporated into the Cox proportional
hazards model, and variable-independent prognostic factors were
selected through backward stepwise analysis. P<0.05 was
considered to indicate a statistically significant difference.
The nomogram model was constructed on the foundation
of the Cox proportional hazards model, and its performance was
evaluated by internal validation with Bootstrap resampling (1,000
times), in order to minimize biases in the performance of the model
(10,25). Discrimination and calibration were
used to assess nomogram performance. The discrimination ability
(how well a model is able to distinguish between patients who
succumbed to mortality and patients who survived.) of the nomogram
was quantified by using the Harrell C-index (25). The c-index was similar to the area
under the receiver operating characteristic (ROC) curve, with an
index of 0.5 and 1 indicating the lack of concordance and perfect
concordance, respectively (26).
Calibration was obtained by plotting the calibrated curve of the
association between the observed incidence and the predicted
probabilities (27). The c-index was
also used for the comparison of different models. The Kaplan-Meier
method was used to plot the survival curves according to RPA, GPA
and Breast-GPA prognosis models. The RPA model divides the patients
into three different prognostic groups: group I (patients <65
years, KPS ≥70, controlled primary tumor, and no extracranial
metastasis), group II (all other patients not included in group I
or III), and group III (KPS <70). The GPA model divides the
patients into four prognostic groups, according to the sum scores
(GPA score 0–1, 1.5–2.5, 3.0 and 3.5–4) of four factors, including
age, KPS, number of brain metastases, and extracranial metastasis.
Furthermore, the Breast-GPA model also divides the patients into
four prognostic groups, according to the sum scores (Breast-GPA
score 0–1, 1.5–2.0, 2.5–3.0 and 3.5–4.0) of three prognostic
factors: age, KPS and biological subtype. Statistical analyses were
performed using SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) and R
software version 3.2.2 (http://www.r-project.org).
Results
Clinical features and survival
The characteristics of the selected patients are
presented in Table I. The median
follow-up time was 48.2 months. Cases of mortality, survival and
loss to follow-up were 322 (78.3%), 50 (12.2%) and 39 (9.5%),
respectively. The median overall survival (OS) time following
diagnosis of breast cancer was 68.2 months, while the median BMOS
time was 14.1 months (range, 0.1–100.3 months), with 1-, 2- and
3-year survival rates of 55.9, 29.6 and 16.2%, respectively.
Furthermore, the median DFS time was 23.9 months (range, 0–232.3
months), the median duration between diagnosis of breast cancer and
BM was 43.3 months (range, 0–353.2 months), and the median volume
of brain metastases was 4.8 cm3 (range, 0.1–139.7
cm3).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Number (%) | Median survival,
months | P-value |
|---|
| Age at BM,
years |
|
| 0.318 |
|
≤40 | 105 (25.5) | 11.1 |
|
|
40–60 | 259 (63.0) | 14.7 |
|
|
>60 | 47 (11.5) | 16.1 |
|
| Clinical stage |
|
| 0.828 |
| I and
II | 306 (74.5) | 15.0 |
|
| III and
IV | 105 (25.5) | 12.3 |
|
| Biological
subtype |
|
| <0.001 |
| Luminal
A | 140 (34.0) | 14.7 |
|
| Luminal
B | 87 (21.2) | 20.2 |
|
| HER-2
Positive | 86 (20.9) | 14.0 |
|
| Triple
Negative | 94 (22.9) | 8.7 |
|
|
Unknown | 4 (1.0) | – |
|
| DFS, months |
|
| 0.012 |
|
>36 | 125 (30.4) | 17.1 |
|
|
≤36 | 276 (67.2) | 12.1 |
|
|
Unknown | 10 (2.4) | – |
|
| Diagnosis to BM,
months |
|
| 0.072 |
|
>44 | 208 (49.4) | 15.9 |
|
|
≤44 | 203 (50.6) | 11.6 |
|
| Symptoms of BM
present |
|
| <0.001 |
|
Yes | 261 (63.6) | 10.3 |
|
| No | 111 (27.0) | 21.1 |
|
|
Unknown | 39 (9.5) | – |
|
| KPS |
|
| <0.001 |
|
≥90 | 169 (41.1) | 19.3 |
|
|
70–90 | 149 (36.3) | 13.5 |
|
|
<70 | 74 (18.0) | 2.8 |
|
|
Unknown | 19 (4.6) | – |
|
| Extracranial
metastasis control |
|
| <0.001 |
|
Controlled (CR+PR+SD) | 162 (39.4) | 18.5 |
|
|
Uncontrolled (PD) | 227 (55.2) | 9.8 |
|
|
Unknown | 22 (5.4) | – |
|
| Leptomeningeal
metastasis |
|
| <0.001 |
|
Yes | 69 (16.8) | 6.9 |
|
| No | 336 (81.8) | 16.7 |
|
|
Unknown | 6 (1.5) | – |
|
| Number of BM
lesions |
|
| <0.001 |
| ≤3 | 148 (36.0) | 20.9 |
|
|
>3 | 223 (54.3) | 11.8 |
|
|
Unknown | 40 (9.7) | – |
|
| Total tumor volume,
cm3 |
|
| 0.782 |
|
≤4.8 | 113 (27.5) | 16.1 |
|
|
>4.8 | 113 (27.5) | 14.2 |
|
|
Unknown | 185 (45.0) | – |
|
Nomogram model construction and
validation
Model construction
Univariate analysis results indicated that several
factors, including molecular subtype, DFS, KPS, symptoms of BM,
extracranial metastasis control, leptomeningeal metastasis and the
number of BM lesions were associated with the survival of patients
with BCBM (Table I). Furthermore,
multivariate analysis results indicated that molecular type, KPS
score, leptomeningeal metastasis, extracranial metastasis control,
the number of BM lesions and DFS were independent factors that
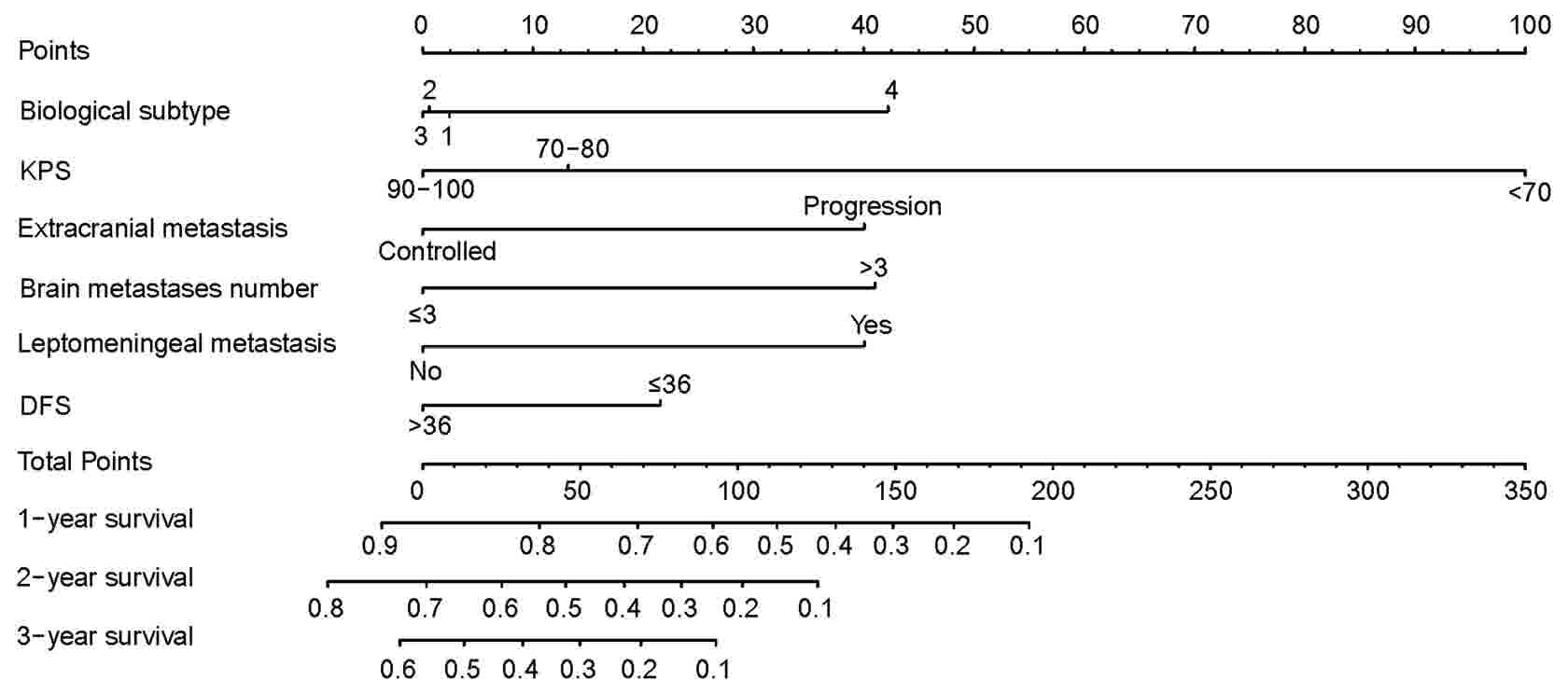
influenced the survival of patients with BCBM (Table II). The nomogram prognostic
evaluation model was constructed based on the multivariate analysis
(Fig. 1).
 | Table II.Multivariate analysis of prognostic
factors. |
Table II.
Multivariate analysis of prognostic
factors.
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|---|
| Factor | b | P-value | HR | Lower | Upper |
|---|
| Biological
subtype |
| <0.001 |
|
|
|
| Luminal B vs.
A | −0.612 | <0.001 | 0.542 | 0.385 | 0.764 |
| HER-2-positive vs.
luminal A | −0.600 | 0.003 | 0.549 | 0.368 | 0.818 |
| Triple negative vs.
luminal A | −0.690 | <0.001 | 0.502 | 0.346 | 0.728 |
| KPS |
|
|
|
|
|
| 70–80
vs. <70 | 1.428 | <0.001 | 4.172 | 2.884 | 6.036 |
| 90–100
vs. <70 | 0.167 | 0.278 | 1.182 | 0.874 | 1.599 |
| Leptomeningeal
metastasis | −0.596 | 0.003 | 0.551 | 0.370 | 0.821 |
| Extracranial
metastasis control | 0.616 | <0.001 | 1.852 | 1.392 | 2.463 |
| Number of brain
metastases (≤3 vs. >3) | 0.571 | <0.001 | 1.770 | 1.338 | 2.343 |
| DFS (>36 vs.
≤36) | −0.312 | 0.039 | 0.732 | 0.544 | 0.985 |
Model validation
The present study employed the bootstrap resampling
method for the internal validation of the model to reduce the
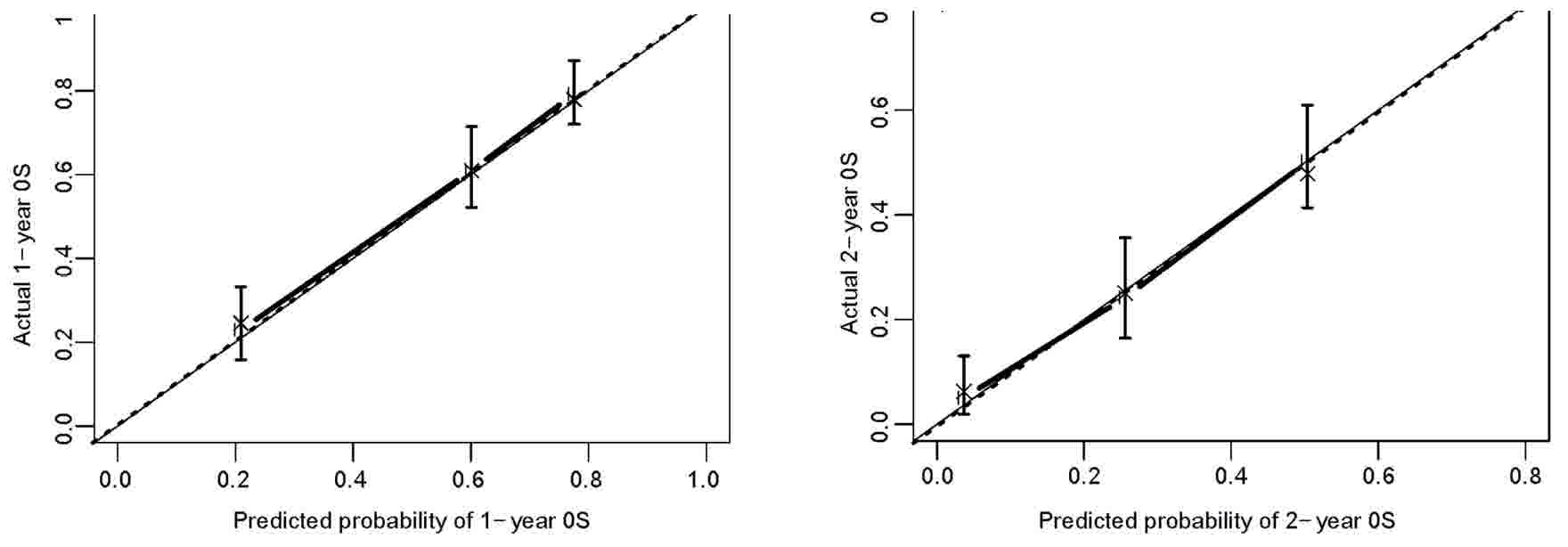
over-fitting of the model. The c-index was 0.73 (95% CI,
0.70–0.77), indicating a good discrimination. The calibration plot
of 1- and 2-year OS rates revealed a good agreement between
observed values and predicted values (Fig. 2).
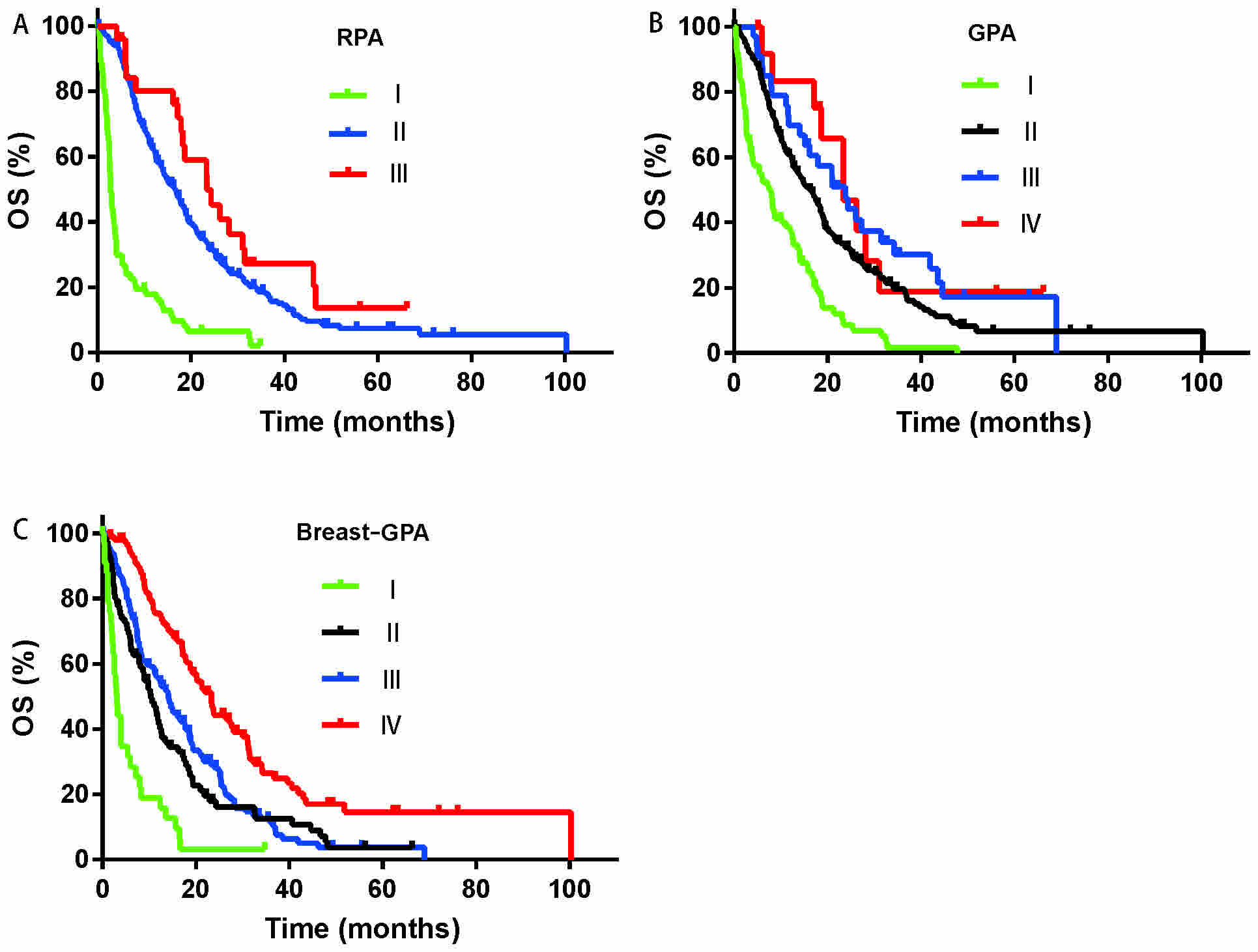
As shown in Fig. 3,
there was overlapping between groups I and II in the RPA model
(Fig. 3A; P=0.091). In addition,
there was overlapping between groups II, III and IV in the using
the GPA model (Fig. 3B; P=0.103).
Furthermore, the discrimination was not satisfactory between groups
II and III in Breast-GPA model (Fig.
3C; P=0.213). Based on the results of the survival curves and
P-values, it was concluded that the three aforementioned prognosis
models were not satisfactory for differentiating patients with
different survival times. The c-indexes were 0.73, 0.64, 0.61 and
0.63 for the nomogram, Breast-GPA, GPA and RPA, respectively
(Table III).
 | Table III.Comparison of the nomogram with
different predictive models. |
Table III.
Comparison of the nomogram with
different predictive models.
|
|
| 95% CI |
|---|
|
|
|
|
|---|
| Method | C-index | Lower | Upper |
|---|
| Nomogram | 0.735 | 0.703 | 0.767 |
| RPA | 0.633 | 0.603 | 0.662 |
| GPA | 0.614 | 0.583 | 0.646 |
| Breast-GPA | 0.640 | 0.609 | 0.671 |
Discussion
Brain metastasis significantly impacts the prognosis
and quality of life of patients with breast cancer. Precise
prognostic predication contributes not only to the selection of a
suitable therapeutic regimen, but also to the selection of
appropriate patients for clinical trials. The present study
developed a novel nomogram model for prognosis prediction through
the evaluation of several prognostic factors in a relatively large
group of patients with BCBM.
The median survival the of the patients with BCBM
enrolled in the present study was 14.1 months, with a 1-year
survival rate of 56.5%, which was slightly higher than that
reported previously (9,28,29). This
may be associated with the recent advances in the diagnosis and
treatment of brain tumors, particularly in the application of
targeted therapies in patients with BCBM. Targeted therapeutic
agents, including trastuzumab, lapatinib and pertuzumab have become
the standard treatment for patients with HER-2 overexpression. In
the present study, up to 85.5% of patients with HER-2
overexpression received anti-HER-2 therapy, with a median survival
time of 17.9 months, which was similar to that reported previously
(range, 11.6–19.5 months) (30,31).
The primary purpose of developing a prognostic model
is to guide clinical treatment. Therefore, it would be better to
exclude therapeutic and subjective factors when selecting
prognostic factors (32,33). Prognostic models used previously or
currently in clinic often lack the evaluation of tumor biology
factors, including tumor volume, meningeal metastases, molecular
types and symptoms of BM (7–9), which are the influencing factors of
prognosis (34,35) Therefore, these models failed to
accurately predict patient survival. In order to overcome the
weaknesses of these models, the present study used univariate and
multivariate analyses to identify factors that influence patient
survival. It was revealed that molecular subtypes, KPS,
extracranial control, leptomeningeal metastasis, number of BM
lesions and DFS were independent factors for the prognosis of
patients with BCBM. Patients with leptomeningeal metastasis were
generally excluded in published studies. However, literature and
clinical experience indicated that meningeal metastasis is one of
the major factors of poor prognosis in patients with BCBM (36). Therefore, the present study included
the clinical conditions of patients in the development of the
model; and multivariate analysis results revealed that meningeal
metastasis was an independent factor for patient prognosis. There
were 69 (16.8%) cases of meningeal metastases among the patients
enrolled in the present study, which was slightly more than the
numbers reported in previous studies (37,38). This
may be associated with the extended survival of patients, as well
as the wide application of magnetic resonance imaging in the
diagnosis of brain tumors.
A nomogram is able to assess patient survival time,
which is beneficial for individualized therapy. The existing RPA,
GPA and Breast-GPA models simply divide these patients into several
subgroups, with great difference existing within the same subgroups
(39). The present study compared
different models using the c-index and survival curves, and
demonstrated that the novel nomogram was superior to existing
prognostic models (RPA, GPA and Breast-GPA). Furthermore, a
crossover of survival curves among different groups in the RPA, GPA
and Breast-GPA models was observed, which may be associated with
the lack of molecular indices of breast cancer, inconsistent
pathological types, differences in patient grouping, the selection
of different prognostic factors, as well as defects in the modeling
methods of RPA and GPA. Although the RPA model was constructed
based on the results of 1,200 cases of BM, there were only 137
(12%) cases of breast cancer (7). The
GPA model was based on the analysis of 1,960 cases, but only 222
(11%) cases of breast cancer were included (8).
Among the 411 patients included in the present
study, 74.5% would be diagnosed with grade II disease based on the
RPA model, and the median survival time of patients with grade II
disease was 16.7 months (range, 0.2–100.3 months). This indicated
the significantly different survival times within the same group.
This discrepancy may result in administering palliative treatment
to patients who should receive active treatment.
Although the nomogram model developed in the present
study exhibited a good predictive ability, certain shortcomings
remained. For example, as is often the case with retrospective
studies, certain patient information was not available and
therefore, bias was inevitable. Although the sample size was
relatively large, the study population was selected from one
hospital. Furthermore, it requires validation in other research
institutions.
In conclusion, the present study developed and
validated a nomogram prognosis evaluation model for patients with
BCBM, which was demonstrated to be improved compared with the
presently used RPA, GPA and Breast-GPA models. This model may be
used to guide individual treatments and in selecting an appropriate
patient population for clinical trials.
Acknowledgements
Not applicable.
Funding
No funding was obtained for the present study.
Availability of data and materials
All data are fully available upon request.
Author's contributions
Conception and design were undertaken by SKW.
Collection of patient information and drafting of the article was
undertaken by ZH. Data interpretation was performed by BS and ZH.
XYM, YC, GS and STS participated in patient treatment, and helped
revising the manuscript. All authors read and approved the final
manuscript.
Ethical approval and consent to
participate
All procedures involving human participants were
performed in accordance with the ethical standards of the
Affiliated Hospital of Academy of Military Medical Sciences and
China Research Committee and with the 1964 Declaration of Helsinki
and its later amendments or comparable ethical standards. Written
informed consent was obtained from all participants included in the
present study.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnholtz-Sloan JS, Sloan AE, Davis FG,
Vigneau FD, Lai P and Sawaya RE: Incidence proportions of brain
metastases in patients diagnosed (1973 to 2001) in the metropolitan
detroit cancer surveillance system. J Clin Oncol. 22:2865–2872.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin NU, Amiri-Kordestani L, Palmieri D,
Liewehr DJ and Steeg PS: CNS metastases in breast cancer: Old
challenge, new frontiers. Clin Cancer Res. 19:6404–6418. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DiStefano A, Yap Yong Y, Hortobagyi GN and
Blumenschein GR: The natural history of breast cancer patients with
brain metastases. Cancer. 44:1913–1918. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tabouret E, Metellus P, Goncalves A,
Esterni B, Charaffe-Jauffret E, Viens P and Tallet A: Assessment of
prognostic scores in brain metastases from breast cancer. Neuro
Oncol. 16:421–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altundag K, Bondy ML, Mirza NQ, Kau SW,
Broglio K, Hortobagyi GN and Rivera E: Clinicopathologic
characteristics and prognostic factors in 420 metastatic breast
cancer patients with central nervous system metastasis. Cancer.
110:2640–2647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gaspar L, Scott C, Rotman M, Asbell S,
Phillips T, Wasserman T, McKenna WG and Byhardt R: Recursive
partitioning analysis (RPA) of prognostic factors in three
radiation therapy oncology group (RTOG) brain metastases trials.
Int J Radiat Oncol Biol Phys. 37:745–751. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sperduto PW, Berkey B, Gaspar LE, Mehta M
and Curran W: A new prognostic index and comparison to three other
indices for patients with brain metastases: An analysis of 1,960
patients in the RTOG database. Int J Radiat Oncol Biol Phys.
70:510–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sperduto PW, Kased N, Roberge D, Xu Z,
Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al: Effect
of tumor subtype on survival and the graded prognostic assessment
for patients with breast cancer and brain metastases. Int J Radiat
Oncol Biol Phys. 82:2111–2117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iasonos A, Schrag D, Raj GV and Panageas
KS: How to build and interpret a nomogram for cancer prognosis. J
Clin Oncol. 26:1364–1370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pietrantonio F, Aprile G, Rimassa L,
Franco P, Lonardi S, Cremolini C, Biondani P, Sbicego EL,
Pasqualetti F, Tomasello G, et al: A new nomogram for estimating
survival in patients with brain metastases secondary to colorectal
cancer. Radiother Oncol. 117:315–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Graesslin O, Abdulkarim BS, Coutant C,
Huguet F, Gabos Z, Hsu L, Marpeau O, Uzan S, Pusztai L, Strom EA,
et al: Nomogram to predict subsequent brain metastasis in patients
with metastatic breast cancer. J Clin Oncol. 28:2032–2037. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahn HK, Lee S, Park YH, Sohn JH, Jo JC,
Ahn JH, Jung KH, Park S, Cho EY, Lee JI, et al: Prediction of
outcomes for patients with brain parenchymal metastases from breast
cancer (BC): A new BC-specific prognostic model and a nomogram.
Neuro Oncol. 14:1105–1113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sternberg CN: Are nomograms better than
currently available stage groupings for bladder cancer? J Clin
Oncol. 24:3819–3820. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marko NF, Xu Z, Gao T, Kattan MW and Weil
RJ: Predicting survival in women with breast cancer and brain
metastasis: A nomogram outperforms current survival prediction
models. Cancer. 118:3749–3757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Zhang YJ, Zhu Y, Cao JZ, Yuan ZY,
Xu LM, Wu JX, Wang W, Wu T, Lu B, et al: Prognostic nomogram for
overall survival in previously untreated patients with extranodal
NK/T-cell lymphoma, nasal-type: A multicenter study. Leukemia.
29:1571–1577. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang LQ, Li CF, Li J, Chen WH, Chen QY,
Yuan LX, Lai XP, He Y, Xu YX, Hu DP, et al: Establishment and
validation of prognostic nomograms for endemic nasopharyngeal
carcinoma. J Natl Cancer Inst. 108:pii: djv291. 2015.
|
|
18
|
Subbiah IM, Lei X, Weinberg JS, Sulman EP,
Chavez-MacGregor M, Tripathy D, Gupta R, Varma A, Chouhan J,
Guevarra RP, et al: Validation and development of a modified breast
graded prognostic assessment as a tool for survival in patients
with breast cancer and brain metastases. J Clin Oncol.
33:2239–2245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dawood S, Gonzalez-Angulo AM, Albarracin
C, Yu TK, Hortobagyi GN, Buchholz TA and Woodward WA: Prognostic
factors of survival in the trastuzumab era among women with breast
cancer and brain metastases who receive whole brain radiotherapy: A
single-institution review. Cancer. 116:3084–3092. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kased N, Binder DK, McDermott MW, Nakamura
JL, Huang K, Berger MS, Wara WM and Sneed PK: Gamma Knife
radiosurgery for brain metastases from primary breast cancer. Int J
Radiat Oncol Biol Phys. 75:1132–1140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Le Scodan R, Massard C, Jouanneau L,
Coussy F, Gutierrez M, Kirova Y, Lerebours F, Labib A and
Mouret-Fourme E: Brain metastases from breast cancer: Proposition
of new prognostic score including molecular subtypes and treatment.
J Neurooncol. 106:169–176. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goldhirsch A, Wood WC, Coates AS, Gelber
RD, Thürlimann B and Senn HJ: Panel members: Strategies for
subtypes-dealing with the diversity of breast cancer: Highlights of
the St Gallen international expert consensus on the primary therapy
of early breast cancer 2011. Ann Oncol. 22:1736–1747. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harrell FE Jr, Lee KL and Mark DB:
Multivariable prognostic models: Issues in developing models,
evaluating assumptions and adequacy, and measuring and reducing
errors. Stat Med. 15:361–387. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Harrell FE Jr, Lee KL, Califf RM, Pryor DB
and Rosati RA: Regression modelling strategies for improved
prognostic prediction. Stat Med. 3:143–152. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coutant C, Olivier C, Lambaudie E,
Fondrinier E, Marchal F, Guillemin F, Seince N, Thomas V, Levêque
J, Barranger E, et al: Comparison of models to predict nonsentinel
lymph node status in breast cancer patients with metastatic
sentinel lymph nodes: A prospective multicenter study. J Clin
Oncol. 27:2800–2808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Niwinska A and Murawska M: New breast
cancer recursive partitioning analysis prognostic index in patients
with newly diagnosed brain metastases. Int J Radiat Oncol Biol
Phys. 82:2065–2071. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HJ, Im SA, Keam B, Kim YJ, Han SW, Kim
TM, Oh DY, Kim JH, Lee SH, Chie EK, et al: Clinical outcome of
central nervous system metastases from breast cancer: Differences
in survival depending on systemic treatment. J Neurooncol.
106:303–313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dawood S, Broglio K, Esteva FJ, Ibrahim
NK, Kau SW, Islam R, Aldape KD, Yu TK, Hortobagyi GN and
Gonzalez-Angulo AM: Defining prognosis for women with breast cancer
and CNS metastases by HER2 status. Ann Oncol. 19:1242–1248. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Le Scodan R, Jouanneau L, Massard C,
Gutierrez M, Kirova Y, Cherel P, Gachet J, Labib A and
Mouret-Fourme E: Brain metastases from breast cancer: Prognostic
significance of HER-2 overexpression, effect of trastuzumab and
cause of death. BMC Cancer. 11:3952011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lagerwaard FJ, Levendag PC, Nowak PJ,
Eijkenboom WM, Hanssens PE and Schmitz PI: Identification of
prognostic factors in patients with brain metastases: A review of
1292 patients. Int J Radiat Oncol Biol Phys. 43:795–803. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Balachandran VP, Gonen M, Smith JJ and
DeMatteo RP: Nomograms in oncology: More than meets the eye. Lancet
Oncol. 16:e173–e180. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chamberlain MC: Leptomeningeal metastases
in the MRI era. Neurology. 76:200author reply 200. –201. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kondziolka D, Kano H, Harrison GL, Yang
HC, Liew DN, Niranjan A, Brufsky AM, Flickinger JC and Lunsford LD:
Stereotactic radiosurgery as primary and salvage treatment for
brain metastases from breast cancer. Clinical article. J Neurosurg.
114:792–800. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song WG, Wang YF, Wang RL, Qu YE, Zhang Z,
Li GZ, Xiao Y, Fang F and Chen H: Therapeutic regimens and
prognostic factors of brain metastatic cancers. Asian Pac J Cancer
Prev. 14:923–927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brower JV, Saha S, Rosenberg SA, Hullett
CR and Robins Ian H: Management of leptomeningeal metastases:
Prognostic factors and associated outcomes. J Clin Neurosci.
27:130–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dayan A, Koca D, Akman T, Oztop I,
Ellidokuz H and Yilmaz U: The factors that have an impact on the
development of brain metastasis in the patients with breast cancer.
J Cancer Res Ther. 8:542–548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Braccini AL, Azria D, Thezenas S, Romieu
G, Ferreri JM and Jacot W: Comparative performances of prognostic
indexes for breast cancer patients presenting with brain
metastases. BMC Cancer. 13:702013. View Article : Google Scholar : PubMed/NCBI
|