Introduction
Patients receiving radical operation of colon
carcinoma need anesthesia during the surgical treatment (1,2). At
present, dexmedetomidine, as a new type of adrenergic receptor
agonist, is increasingly widely used in the field of anesthesia
(3–5).
The drug has been commonly used as the preoperative sedative,
regional anesthesia adjuvant and general anesthesia adjuvant
(6,7).
It is clinically found that the drug also has an anti-inflammatory
effect (8–10). Therefore, 141 patients receiving
radical operation of colon carcinoma in Jining No. 1 People's
Hospital (Jining, China) from January 2014 to April 2017 were
selected in this study, and dexmedetomidine anesthesia was induced,
so as to explore the anti-inflammatory and immune effects of the
sedative, and provide a more scientific clinical basis.
Patients and methods
General data
A total of 141 patients receiving radical operation
of colon carcinoma in Jining No. 1 People's Hospital (Shandong,
China) from January 2014 to April 2017 were collected, including 69
cases in the control group and 72 cases in the treatment group.
There were 33 males and 36 females in the control group, and 36
males and 36 females in the treatment group. In the control group,
they were aged 34–67 years with an average age of 45.3±5.0 years,
and in the treatment group, they were aged 35–69 years with an
average age of 42.5±4.3 years. Patients in the two groups were all
in I–II of American Society of Anesthesiologists (ASA) grade. This
study was approved by the Ethics Committee of Jining No. 1 People's
Hospital. Signed written informed consents were obtained from all
patients or their guardians before the study. Exclusion criteria:
Patients with damage in heart, liver or kidney, or systemic immune
system diseases.
Methods
Before the operation, the patients were fasting for
12 h before anesthesia and general anesthesia was performed before
the operation. The method of anesthesia induction is as follows:
The patients were given intravenous injection of midazolam (0.05
mg/kg), vecuronium (0.1 mg/kg), etomidate (0.3 mg/kg) and fentanyl
(1.0 µg/kg). During ventilation, end-tidal partial pressure of
carbon dioxide (PETCO2) was set at 35–45 mmHg and fentanyl was
administered via veno-micropump target control with the infusion
rate of 0.03–0.2 µg/(kg·min). The tidal volume of the ventilator
was set at 8–10 ml/kg, with a frequency of 12–16 times/min, and
PETCO2 of 35–45 mmHg. The infusion rate of fentanyl was controlled
to adjust the depth of anesthesia. Intravenous injection of
urapidil or ephedrine was selected according to the change in SBP
value. Sevoflurane inhalation was discontinued following the
completion of operation and fentanyl (0.5 µg/kg) was administered
intravenously after operation for systemic analgesia. The patients
in the treatment group were given pump infusion of dexmedetomidine
[1 µg/kg over 10–15 min as a loading dose; then the pump flow rate
was set as 1 µg/(kg·h)] before operation, while the patients in the
control group received pump injection of the same amount of normal
saline.
Evaluation of the efficacy
Ramsay sedation score: Patients were scored before
operation and at 0.5, 12 and 24 h after operation, respectively.
Patients with irritability and restlessness: 1 point; patients who
were cooperative and quiet: 2 points; patients with lethargy and
obeying orders: 3 points, patients who could be awakened during
sleep: 4 points; patients with slow reaction when being called: 5
points; patients who could not be awakened when being called: 6
points.
Observational indexes
The levels of nuclear factor-κB (NF-κB) in
peripheral blood mononuclear cells, serum soluble intercellular
adhesion molecule-1 (sICAM-1), interleukin-8 (IL-8), serum IL-6,
tumor necrosis factor-α (TNF-α) and C-reactive protein (CRP) of
patients in the two groups were observed during perioperative
period. Besides, the levels of T lymphocyte subsets of patients in
two groups were observed by flow cytometry. The dosages of fentanyl
used during operation and after operation were recorded.
Statistical analysis
Data were processed by SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). Measurement data and counting data were
expressed by mean ± standard deviation (SD) and n, respectively,
and t-test and chi-square test were used for intergroup comparison.
P<0.05 suggested that the difference was statistically
significant.
Results
Comparison of general data between two
groups
The general data of patients in the two groups were
compared: In the treatment group, the average age was 42.5±4.3
years, body weight was 52±3.5 kg, ASA grade was I–II, anesthesia
time 109.2±7.4 min, operation time 98.7±4.3 min, and amount of
bleeding was 59.2±3.2 ml; in the control group, the average age was
45.3±5.0 years, body weight 56±4.3 kg, ASA grade I–II, anesthesia
time 110.5±7.4 min, operation time 99.3±4.8 min, and amount of
bleeding was 58.8±3.0 ml. The anesthesia time and the amount of
bleeding in the two groups were similar (Table I).
 | Table I.Comparison of general data between two
groups (mean ± SD). |
Table I.
Comparison of general data between two
groups (mean ± SD).
| Group | Age (years) | Body weight (kg) | ASA grade | Anesthesia time
(min) | Operation time
(min) | Amount of bleeding
(ml) |
|---|
| Treatment group | 42.5±4.3 |
52±3.5 | I–II |
109.2±7.4 |
98.7±4.3 |
59.2±3.2 |
| Control group |
45.3±5.0 |
56±4.3 | I–II |
110.5±7.4 |
99.3±4.8 |
58.8±3.0 |
Effects of NF-κB in peripheral blood
mononuclear cells and serum sICAM-1 and IL-8 levels in the two
groups
There were no significant differences in the
preoperative levels of NF-κB, sICAM-1 and IL-8 in patients between
the two groups. However, they were increased and then decreased at
0.5 and 24 h after operation; the contents of three indexes of
patients in both groups were significantly increased at two
time-points after operation, and the degree of the increase in
control group was significantly higher than that of the treatment
group (P<0.05), with statistical significance (Table II).
 | Table II.Effects of NF-κB in peripheral blood
mononuclear cells and serum sICAM-1 and IL-8 levels in two groups
(mean ± SD). |
Table II.
Effects of NF-κB in peripheral blood
mononuclear cells and serum sICAM-1 and IL-8 levels in two groups
(mean ± SD).
| Group | Time | NF-κB (U/l) | sICAM-1 (mg/l) | IL-8 (pg/ml) |
|---|
| Treatment group | Preoperative |
24.56±1.98 |
320.8±8.6 |
19.2±1.0 |
|
| 0.5 h after
operation |
33.79±2.65a |
507.2±7.5a |
45.7±2.7a |
|
| 24 h after
operation |
28.31±2.51a |
498.8±10.0a |
24.5±1.9a |
| Control group | Preoperative |
25.06±2.48 |
334.3±6.9 |
19.8±2.8 |
|
| 0.5 h after
operation |
42.59±3.65a,b |
588.5±8.6a,b |
59.8±3.0a,b |
|
| 24 h after
operation |
32.32±2.86a,b |
543.1±9.5a,b |
34.3±2.8a,b |
Comparisons of serum IL-6, TNF-α and
CRP levels in patients between two groups
In the treatment group, the levels of IL-6, TNF-α
and CRP before treatment were 3.25±0.98, 16.27±2.21 pg/ml and
4.45±0.32 mg/l, respectively; the levels of IL-6, TNF-α and CRP at
0.5 h after operation were 70.56±3.21, 15.02±2.06 pg/ml and
5.78±0.19 mg/l, respectively; and the levels of the three indexes
at 24 h after operation were 60.72±2.98, 19.72±2.34 pg/ml and
3.21±0.12 mg/l, respectively. In the control group, the levels of
the three indexes before operation were 3.69±0.65, 16.83±3.01 pg/ml
and 4.29±0.18 mg/l, respectively; the levels of the three indexes
at 0.5 h after operation were 90.56±3.65, 16.75±2.10 pg/ml and
8.12±0.21 mg/l, respectively; and the levels of the three indexes
at 24 h after operation were 67.97±3.98, 18.98±2.03 pg/ml and
4.54±0.32 mg/l, respectively. It was found that the levels of IL-6
in two groups were increased at 0.5 and 24 h after operation, and
the levels of IL-6 in the control group were significantly higher
than that in the treatment group. The postoperative level of CRP
was higher than that before operation, and the postoperative level
in the control group was higher than that in the treatment group
(Table III).
 | Table III.Comparison of serum levels of IL-6,
TNF-α and CRP in patients between two groups (mean ± SD). |
Table III.
Comparison of serum levels of IL-6,
TNF-α and CRP in patients between two groups (mean ± SD).
| Group | Time | IL-6 (pg/ml) | TNF-α (pg/ml) | CRP (mg/l) |
|---|
| Treatment group | Preoperative |
3.25±0.98 |
16.27±2.21 |
4.45±0.32 |
|
| 0.5 h after
operation |
70.56±3.21a |
15.02±2.06 |
5.78±0.19a |
|
| 24 h after
operation |
60.72±2.98a |
19.72±2.34 |
4.21±0.12 |
| Control group | Preoperative |
3.69±0.65 |
16.83±3.01 |
4.29±0.18 |
|
| 0.5 h after
operation |
90.56±3.65a,b |
16.75±2.10 |
8.12±0.21a,b |
|
| 24 h after
operation |
67.97±3.98a,b |
18.98±2.03 |
4.54±0.32 |
Comparison of Ramsay scores between
two groups
The preoperative Ramsay scores of patients in the
treatment group and the control group were 1.97±0.21 and 1.98±0.15
points, respectively. The Ramsay scores 0.5 h after operation of
patients in two groups were 4.13±0.43 and 2.59±0.18 points,
respectively. The Ramsay scores at 12 h after operation of patients
in two groups were 2.34±0.17 and 1.99±0.10 points, respectively.
The Ramsay scores at 24 h after operation of patients in two groups
were 2.05±0.19 and 2.06±0.12 points, respectively. The results
showed that the Ramsay scores of patients in the treatment group
were higher than those in the control group at 0.5 and 12 h after
operation, and the differences were significant between the two
groups (Table IV).
 | Table IV.Comparison of Ramsay scores between
two groups (mean ± SD). |
Table IV.
Comparison of Ramsay scores between
two groups (mean ± SD).
| Group | Case | Before operation | 0.5 h after
operation | 12 h after
operation | 24 h after
operation |
|---|
| Treatment group | 72 |
1.97±0.21 |
4.13±0.43 |
2.34±0.17 |
2.05±0.19 |
| Control group | 69 |
1.98±0.15 |
2.59±0.18a |
1.99±0.10a |
2.06±0.12 |
Changes in levels of T lymphocyte
subsets in patients during perioperative period between two
groups
The levels of T lymphocyte subsets had no difference
between the two groups at the first time point. The
CD3+T, CD4+T,
CD4+T/CD8+T, Th1 and Th1/Th2 in two groups
were lower at T2 and T3 than those at T0, and the Treg values at T2
and T3 were significantly higher than those at T0. It was also
found that the indexes of CD3+T, CD4+T,
CD4+T/CD8+T, Th1 and Th1/Th2 in the treatment
group were lower than those in the control group, and the Treg
value was higher than that in the control group (P<0.05)
(Table V).
 | Table V.Changes in levels of T lymphocyte
subsets in patients during perioperative period between two groups
(mean ± SD). |
Table V.
Changes in levels of T lymphocyte
subsets in patients during perioperative period between two groups
(mean ± SD).
| Group | Time | CD3+T
(%) | CD4+T
(%) | CD8+T
(%) |
CD4+T/CD8+T | Th1 (%) | Th2 (%) | Th1/Th2 | Th17 (%) | Treg (%) |
|---|
| Treatment
group | T0 |
61.9±8.6 |
45.7±6.9 |
14.5±2.4 |
2.9±1.0 |
29.9±3.7 |
2.8±1.0 |
16.9±4.6 |
2.5±0.8 |
3.3±1.1 |
|
| T1 (6 hours) |
58.3±8.5 |
45.0±7.2 |
15.6±4.2 |
2.6±0.9 |
28.9±5.3 |
2.7±0.9 |
15.6±4.3 |
2.3±0.9 |
3.0±1.0 |
|
| T2 (1 day) |
57.2±7.5a |
44.3±6.9a |
15.7±3.8 |
2.5±1.0a |
27.9±4.8a |
2.9±0.8 |
14.3±4.9a |
2.4±1.2 |
3.6±1.2a |
|
| T3 (2 days) |
58.4±8.2a |
43.9±7.5a |
15.9±4.0 |
2.7±1.2a |
27.6±4.2a |
2.9±0.9 |
14.3±5.2a |
2.2±1.0 |
3.3±1.2a |
|
| T4 (3 days) |
60.1±7.9 |
44.5±6.9 |
14.5±3.7 |
2.5±0.9 |
27.5±4.0 |
2.8±0.8 |
14.5±5.0 |
2.5±0.9 |
3.5±1.0 |
| Control group | T0 |
61.2±8.5 |
44.2±7.9 |
15.2±3.1 |
2.3±0.9 |
27.1±4.3 |
2.6±0.9 |
14.2±4.9 |
2.3±0.9 |
3.6±1.2 |
|
| T1 (6 hours) |
59.4±7.9 |
42.0±7.4 |
17.3±3.7 |
2.2±1.0 |
25.6±4.8 |
2.7±0.9 |
13.7±3.9 |
2.0±1.0 |
3.5±1.3 |
|
| T2 (1 day) |
56.2±9.6a,b |
40.1±6.4a,b |
17.8±4.1 |
1.9±0.8a,b |
23.0±3.8a,b |
2.2±1.0 |
11.5±2.9a,b |
1.9±0.8 |
4.3±1.3a,b |
|
| T3 (2 days) |
53.9±7.9a,b |
40.2±6.3a,b |
17.9±3.5 |
1.9±0.9a,b |
23.7±3.6a,b |
2.5±0.7 |
11.7±2.7a,b |
2.2±0.9 |
4.2±1.3a,b |
|
| T4 (3 days) |
60.9±8.5 |
44.2±7.5 |
14.3±2.8 |
2.3±1.0 |
25.7±6.0 |
2.7±0.9 |
13.5±3.0 |
2.1±0.9 |
3.9±1.2 |
Comparison of the dosages of fentanyl
in patients between two groups
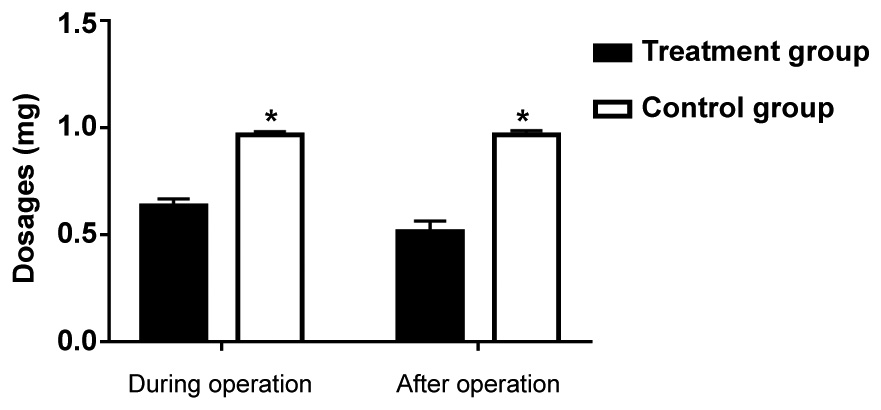
The fentanyl dosages during operation and after
operation in the treatment group were 0.63±0.11 and 0.52±0.09 mg
respectively, and the fentanyl dosages during operation and after
operation in the control group were 0.98±0.21 and 0.99±0.19 mg
respectively, suggesting that the fentanyl dosage whether during
operation or after operation in the treatment group was lower than
that in the control group (P<0.05) (Fig. 1).
Discussion
Inflammation, as a physiological disease, often
occurs in the body. When inflammation occurs in the body, it will
lead to changes in blood vessels, cells and other tissues (11,12).
Clinical studies have found that the use of anesthetics in the body
can act on inflammatory factors, such as mononuclear macrophages,
and also affect inflammatory mediators (13,14). The
research on anti-inflammatory effect of all anesthetics is based on
a variety of inflammatory factors. The most widely used
inflammatory factors are IL-6, TNF-α and IL-8. IL-6 and IL-8 are
produced by mononuclear macrophages in the body, while TNF-α is
secreted by neutrophils. A variety of inflammatory factors can
affect the neutrophil chemotaxis and induce allergic reactions,
leading to chemotaxis of T cells, which can promote the immune
response in the body to a certain extent (15,16).
NF-κB is a key nuclear transcription factor in the
body and plays an important role in the immune and inflammation
processes. When the body is stimulated by inflammatory factors,
IκB, an inhibitor of NF-κB, will be degraded, leading to
over-secretion of NF-κB in the body; the excessive NF-κB will be
transferred into the nucleus, leading to gene expression (17).
ICAM-1 is widely used in antigen production and
leukocyte infiltration process, and it is widely distributed in
lymphocytes. As a kind of multifunctional adhesion molecule, it is
also involved in the process of body immunity. T cell subsets have
been clinically proven to be important indexes of immune disorders
of the body, and Treg is a subset of T cells that can inhibit the
immune response in cancer patients. Clinical studies have found
that dexmedetomidine acts on the blue plaque in brain, which can
reduce norepinephrine secretion, inhibit oxidative stress, and
inhibit the occurrence of inflammation (18–20).
In this study the degree of the increase in the
inflammatory factors at 0.5 and 24 h after operation in the
treatment group was significantly lower than those in the control
group (P<0.05). Patients in two groups had the same factor
levels before operation, indicating that the dexmedetomidine
anesthesia can significantly inhibit the secretion of inflammatory
factors and inhibit the release of inflammatory mediators. Clinical
studies have found that when dexmedetomidine takes effect, the
patient's central nervous system receptors will be affected, and
the norepinephrine will be inhibited, leading to less stress
response in patients and decreased secretion of catecholamine in
adrenergic receptor and glucocorticoid receptor in patients, which
ultimately inhibits the production of inflammatory factors. In this
study, it was also found that the sedation in the treatment group
was better than that in the control group (P<0.05).
Dexmedetomidine can act on the nerve center of the body to reduce
the release of norepinephrine and increase the threshold of
excitement.
In conclusion, the application of dexmedetomidine in
patients receiving radical operation of colon carcinoma has a
better clinical effect, which can reduce the production of
inflammatory factors, decrease the inhibition of T lymphocyte
subsets, and lower the impact on NF-κB in peripheral blood
mononuclear cells, with a higher clinical therapeutic value.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors contributions
KW designed the study and prepared the manuscript.
CL collected and analysed the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Jining No. 1 People's Hospital (Jining, China). Signed written
informed consents were obtained from all patients or their
guardians before the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Walker SM, Grafe M and Yaksh TL:
Intrathecal clonidine in the neonatal rat: Dose-dependent analgesia
and evaluation of spinal apoptosis and toxicity. Anesth Analg.
115:450–460. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ozcengiz D, Unlügenç H, Güneş Y and
Karacaer F: The effect of dexmedetomidine on bispectral index
monitoring in children. Middle East J Anaesthesiol. 21:613–618.
2012.PubMed/NCBI
|
|
3
|
Bannister CF, Brosius KK, Sigl JC, Meyer
BJ and Sebel PS: The effect of bispectral index monitoring on
anesthetic use and recovery in children anesthetized with
sevoflurane in nitrous oxide. Anesth Analg. 92:877–881. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li HL, She SZ, Yan Y and Zhu SM: Effect of
dexmedetomidine on bispectral index and auditory evoked potential
index during anesthesia with target controlled infusion of propofol
and remifentanyl. Zhejiang Da Xue Xue Bao Yi Xue Ban. 39:84–88.
2010.(In Chinese). PubMed/NCBI
|
|
5
|
Ishibashi C, Hayashida M, Sugasawa Y,
Yamaguchi K, Tomita N, Kajiyama Y and Inada E: Effects of
dexmedetomidine on hemodynamics and respiration in intubated,
spontaneously breathing patients after endoscopic submucosal
dissection for cervical esophageal or pharyngeal cancer. J Anesth.
30:628–636. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lin H, Faraklas I, Sampson C, Saffle JR
and Cochran A: Use of dexmedetomidine for sedation in critically
ill mechanically ventilated pediatric burn patients. J Burn Care
Res. 32:98–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao XN, Ran JH, Bajracharya AR and Ma MY:
Effect of different doses of dexmedetomidine on median effective
concentration of propofol for anesthesia induction: A randomized
controlled trial. Eur Rev Med Pharmacol Sci. 20:3134–3143.
2016.PubMed/NCBI
|
|
8
|
Valenza G, Akeju O, Pavone KJ, Citi L,
Hartnack KE, Sampson A, Purdon PL, Brown EN and Barbieri R:
Instantaneous monitoring of heart beat dynamics during anesthesia
and sedation. J Comput Surg. 1:1–18. 2014. View Article : Google Scholar
|
|
9
|
Walker SM, Howard RF, Keay KA and
Fitzgerald M: Developmental age influences the effect of epidural
dexmedetomidine on inflammatory hyperalgesia in rat pups.
Anesthesiology. 102:1226–1234. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong W, Chen MH, Yang YH, Zhang X, Huang
MJ, Yang XJ and Wang HZ: The effect of dexmedetomidine on
expressions of inflammatory factors in patients with radical
resection of gastric cancer. Eur Rev Med Pharmacol Sci.
21:3510–3515. 2017.PubMed/NCBI
|
|
11
|
Chu NJ, Armstrong TD and Jaffee EM:
Nonviral oncogenic antigens and the inflammatory signals driving
early cancer development as targets for cancer immunoprevention.
Clin Cancer Res. 21:1549–1557. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smit MA, Jaffee EM and Lutz ER: Cancer
immunoprevention - the next frontier. Cancer Prev Res (Phila).
7:1072–1080. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kostopanagiotou G, Sidiropoulou T,
Pyrsopoulos N, Pretto EA Jr, Pandazi A, Matsota P, Arkadopoulos N,
Smyrniotis V and Tzakis AG: Anesthetic and perioperative management
of intestinal and multivisceral allograft recipient in
nontransplant surgery. Transpl Int. 21:415–427. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nesković V: Preoperative assesment of the
immunocompromised patient. Acta Chir Iugosl. 58:185–192. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Remotti H, Subramanian S, Martinez M, Kato
T and Magid MS: Small-bowel allograft biopsies in the management of
small-intestinal and multivisceral transplant recipients:
Histopathologic review and clinical correlations. Arch Pathol Lab
Med. 136:761–771. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuki K, Soriano SG and Shimaoka M:
Sedative drug modulates T-cell and lymphocyte function-associated
antigen-1 function. Anesth Analg. 112:830–838. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alonso-Arias R, Moro-García MA,
Vidal-Castiñeira JR, Solano-Jaurrieta JJ, Suárez-García FM, Coto E
and López-Larrea C: IL-15 preferentially enhances functional
properties and antigen-specific responses of
CD4+CD28null compared to
CD4+CD28+ T cells. Aging Cell. 10:844–852.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Can M, Gul S, Bektas S, Hanci V and
Acikgoz S: Effects of dexmedetomidine or methylprednisolone on
inflammatory responses in spinal cord injury. Acta Anaesthesiol
Scand. 53:1068–1072. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fang B, Li XQ, Bi B, Tan WF, Liu G, Zhang
Y and Ma H: Dexmedetomidine attenuates blood-spinal cord barrier
disruption induced by spinal cord ischemia reperfusion injury in
rats. Cell Physiol Biochem. 36:373–383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bicer C, Esmaoglu A, Akin A and Boyaci A:
Dexmedetomidine and meperidine prevent postanaesthetic shivering.
Eur J Anaesthesiol. 23:149–153. 2006. View Article : Google Scholar : PubMed/NCBI
|