Introduction
Chronic viral hepatitis B (CHB) poses a serious
threat to human health. An epidemiological study demonstrated that
the seroprevalence rate of hepatitis B virus surface antigen in the
Chinese population is 7.18% (1).
Hepatitis B may lead to hyperplasia of hepatic fibrous connective
tissues, resulting in liver fibrosis and cirrhosis (2,3). Detection
of hepatitis B virus (HBV) DNA is the most common laboratory method
for the evaluation of virus replication activity. Alanine
aminotransferase (ALT), aspartate aminotransferase (AST) and
γ-glutamyl transferase (γ-GGT) are serum indicators of liver
function in patients with CHB. Hyaluronic acid (HA), type III
procollagen (PCIII), type IV collagen (IV-C) and laminin protein
(LN) are commonly used as indicators of liver fibrosis (4–6). However,
these indicators do not completely meet the clinical requirements
due to their limited sensitivity and specificity (7). Pathological examination by liver biopsy,
which carries a risk of complications, is required for the
diagnosis of liver fibrosis (8–10). Thus,
it is required to identify noninvasive markers of liver fibrosis
with clinical diagnostic or therapeutic significance. Decoy
receptor 3 (DcR3), a novel member of the tumor necrosis factor
receptor (TNFR) superfamily, is a surface receptor that
competitively binds Fas Ligand (FasL), lymphotoxin-related
inducible ligand that competes for glycoprotein D binding to
herpesvirus entry mediator on T cells (LIGHT) and TNF-like ligand
1A (TL1A) ligands to inhibit apoptosis. It has been indicated that
the expression of DcR3 is increased in the inflammatory response to
bacterial infection, rheumatoid arthritis, acute ulcerative colitis
and appendicitis, and in tumors (11). DcR3 is closely associated with cancer
and inflammation (12). The aim of
the present study was to examine the expression of DcR3 in patients
with hepatitis B and hepatic fibrosis and to explore its clinical
diagnostic value.
Materials and methods
Study subjects
A total of 128 patients (male, n=72; female, n=56;
median age, 38 years; range, 20–65 years) diagnosed with CHB were
recruited in The Liver Department of Songjiang Hospital Affiliated
to First People's Hospital (Shanghai, China) between December 2014
and December 2015. All patients with CHB met the criteria for the
diagnosis of CHB published in ‘Chronic HBV Hepatitis Treatment and
Prevention Guide’ (13). Patients
with a history of fatty liver, alcoholic liver disease, obesity,
liver cancer, hepatitis C, hepatitis D, autoimmune liver disease
and other liver diseases, as well as those with a recent history of
infection and allergy were excluded. All patients provided written
informed consent. All procedures were performed in compliance with
the relevant provisions of the Ethics Committee of Songjiang
District Center Hospital (Shanghai, China). The control group
consisted of 50 healthy individuals admitted for physical
examination who had no recent history of infection, autoimmunity or
other diseases, and who had normal indicators in the physical
examination. The healthy control patients also provided written
informed consent and this process was approved by the ethics
committee. Blood samples in the case and control groups were
collected and centrifuged at 1,006.2 × g for 10 min at 4°C. Serum
samples were separated and stored at −80°C for later use.
Serum biomarker detection
Serum level of DcR3 was detected using a Human DcR3
ELISA kit (ELH-DcR3, RayBiotech, Inc., Norcross, GA, USA). Serum
levels of HA, PCIII, IV-C and LN were determined using Human ELISA
kits (CSB-E04805h, 11989h, 17116h, 16273h, CusaBio, Wuhan, China).
The kits were performed according to manufacturer's protocols.
Serum HBV DNA was measured using an ABI7500 system (Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and ALT, AST and γ-GGT were
detected using the Roche automatic biochemical analyzer (Roche
Diagnostics, Indianapolis, IN, USA). Interleukin-6 (IL-6) and PCT
levels were determined using luminscence with a Cobas e601
instrument (Roche, Basle, Switzerland).
Histopathological examination of liver
biopsy tissues and experimental grouping
Liver biopsy specimens were fixed using 10% neutral
formalin for at least 2 h at room temperature, embedded in paraffin
and cut into 3–4 µm sections, which were then subjected to routine
hematoxylin and eosin (H&E) staining using Hematoxylin and
Eosin Staining kit (C0105; Beyotime Institute of Biotechnology,
Nanjing, China). Collagenous connective tissue fibers were stained
using Godon and Sweet reticular fiber stain kit (YDJ4105; Yuduo
Biotech, Shanghai, China) and Trichrome Stain (Masson) kit (HT15;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The staining
procedure followed the manufacturer's protocols. Sections were
examined by two or more physicians and classified as
S0-S4 according to the degree of liver
fibrosis as follows: S0, no fibrosis; S1-3,
gradual increase in the degree of fibrosis; and S4,
liver cirrhosis. The negative control (NC) group consisted of the
healthy individuals. Patients with CHB with ALT>40 IU/l, HBV
DNA>105 copies/ml were classified as active CHB and included in
the present study. The remaining patients were classified as
patients who are CHB carriers.
Bioinformatics and statistical
analysis
Bioinformatics analysis was performed using Oncomine
software (Oncomine™ V4.5 online; https://www.oncomine.com/resource/login.html) to
analyze differences in DcR3 expression in the human hepatocellular
carcinoma (HCC) DNA microarray database (https://www.oncomine.com/resource/login.html).
Differences between two independent samples were analyzed using the
independent samples Student's t-test (Table I) and SPSS 20.0 software (IBM Corp.,
Armonk, NY, USA). The mean differences between numerous samples
were analyzed using Kruskal-Wallis and an analysis of variance,
with Dunn's multiple comparison test as a post-hoc. Levene's Test
was used to analyze the Equality of Variances. Receiver operating
characteristic (ROC) curve analysis was performed. The correlations
between DcR3 and HBV DNA, ALT, AST, GGT, HA, IV-C, PCIII, LN, or
NF-κB were analyzed using Pearson's correlation test. The gender
difference was analyzed using a χ2 test. P<0.05 was
considered to indicate a statistically significant difference. All
the data are presented as median and ranges or mean ± standard
error.
 | Table I.Clinical data of patients with CHB
infection and healthy controls (mean ± standard error). |
Table I.
Clinical data of patients with CHB
infection and healthy controls (mean ± standard error).
|
|
|
| Levene's test for
equality of variances |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristic | Control (n=60) | CHB (n=128) | F-value | Sig. | Student's t-test | P-value |
|---|
| Median age (range),
years | 35 (21–63) | 38 (20–65) |
|
|
|
|
| Male/female (n) | 36/24 | 72/56 |
|
|
| χ2=0.235,
P=0.623 |
| γ-GGT (IU/l) | 13.78±1.00 | 48.02±4.06 | 35.280 | <0.001 | −8.158 | <0.001 |
| AST (IU/l) | 13.18±0.37 | 94.84±14.88 | 25.770 | <0.001 | −5.487 | <0.001 |
| ALT (IU/l) | 13.06±0.70 | 150.65±22.50 | 39.322 | <0.001 | −6.112 | <0.001 |
| PCT (ng/ml) | 0.14±0.01 | 0.14±0.01 | 27.844 | <0.001 | 0.237 | 0.813 |
| IL-6 (pg/ml) | 14.40±1.32 | 18.41±1.43 | 3.797 | 0.053 | −1.773 | 0.078 |
| HA (ng/ml) | 0.57±0.04 | 1.00±0.05 | 90.94 | 0.014 | −6.330 | <0.001 |
| PCIII (ng/ml) | 24.30±1.34 | 35.31±1.39 | 26.863 | 0.916 | −7.495 | <0.001 |
| IV-C (pg/ml) | 475.60±24.84 | 819.55±42.60 | 6.135 | <0.001 | −6.974 | <0.001 |
| LN (ng/ml) | 0.08±0.004 | 0.11±0.005 | 0.011 | 0.0019 | −4.389 | <0.001 |
| DcR3 (ng/ml) | 0.24±0.06 | 213.01±14.50 | 14.519 | <0.001 | −14.676 | <0.001 |
Results
DcR3 expression is significantly
increased in patients with CHB
Serum DcR3 levels (196.88±26.67 ng/ml) were
significantly higher in the CHB group, compared with the healthy
control group (0.047±0.006 ng/ml; P<0.05; Table I). In addition, γ-GGT, AST and ALT
levels were significantly higher in the CHB group, compared with
the healthy control group (P<0.05; Table I). However, there was no significant
difference in procalcitonin and interleukin 6 between the two
groups, which demonstrated similar responses to acute inflammation.
The serum levels of HA, PCIII, IV-C and LN were significantly
higher in the CHB group, compared with the control group
(P<0.05; Table I).
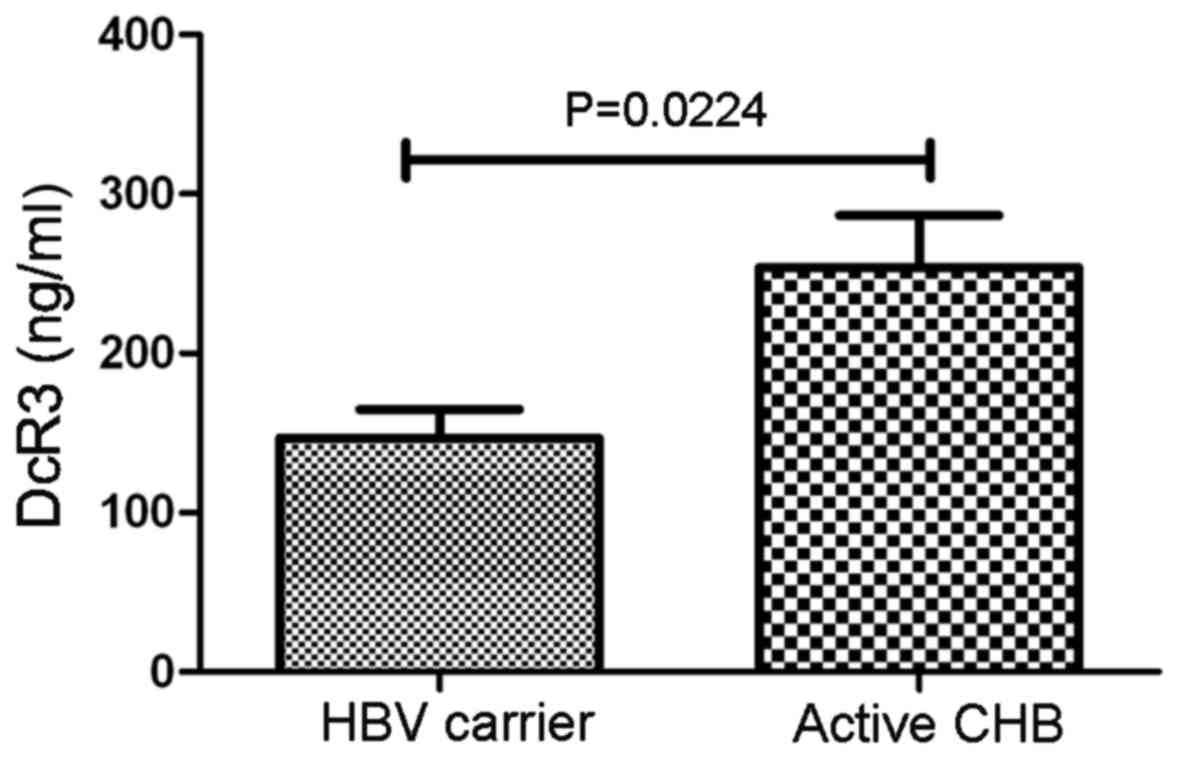
DcR3 expression is significantly
increased in patients with active hepatitis B
Patients with CHB with ALT>40 IU/l, HBV
DNA>105 copies/ml were classified as active hepatitis
B group (active CHB). The remaining patients who did not meet any
of these criteria were classified as hepatitis B carriers (CHB
carrier). Serum DcR3 levels were significantly higher in the active
CHB group (253.82±32.12 ng/ml), compared with the CHB carrier group
(151.35±12.03 ng/ml; P<0.05; Fig.
1).
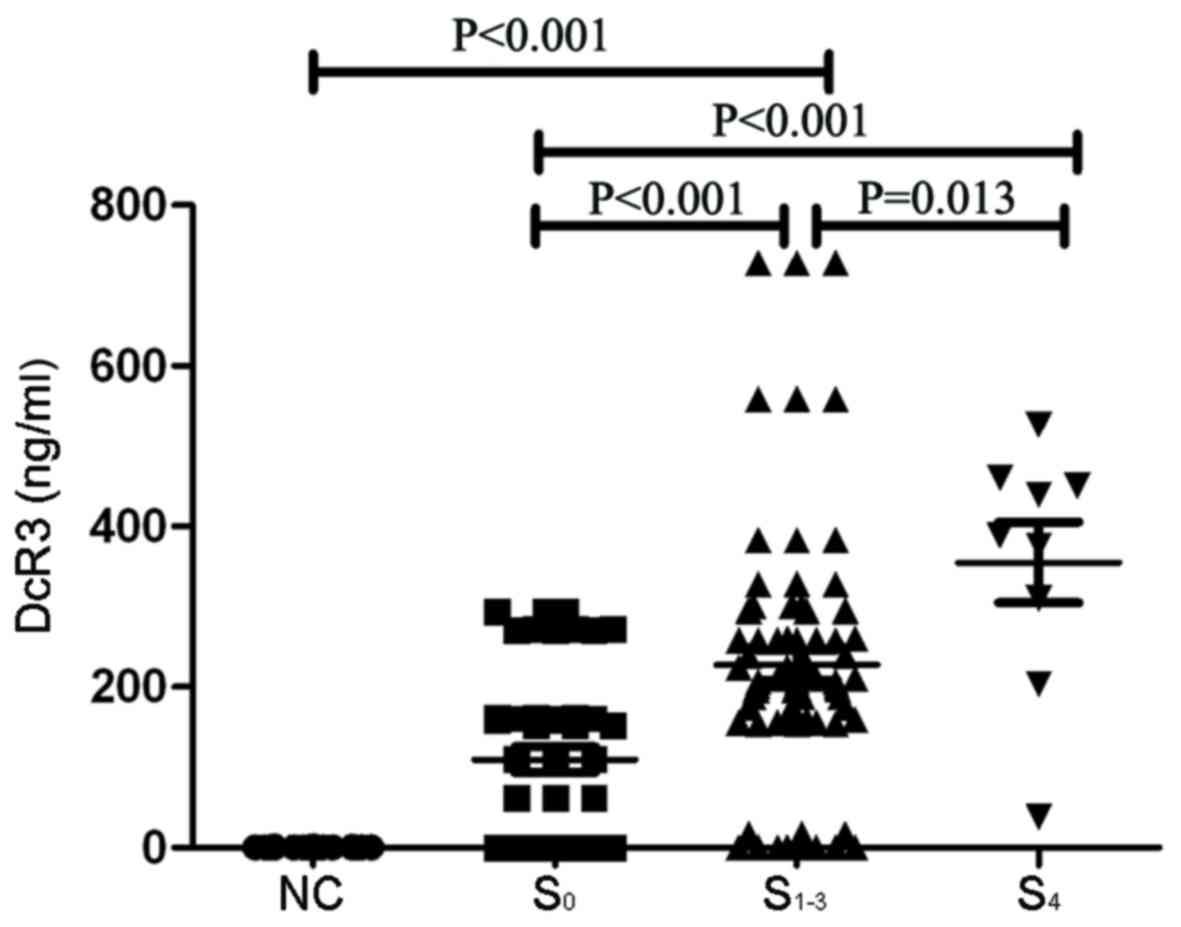
DcR3 levels are increased in patients
with hepatitis B and liver fibrosis
Patients with CHB were divided into three groups
according to pathology results: S0, CHB group without
fibrosis group; S1-3, CHB group complicated with
fibrosis group; and S4, group with cirrhosis. NC was the
healthy control group. The results demonstrated that the DcR3
levels were significantly higher in the S1-3 group
(227.37±18.71 ng/ml) and the S4 group (355.26±5.054
ng/ml), compared with the S0 group (109.66±16.08 ng/ml).
DcR3 levels were significantly higher in the S4 group
(355.26±5.054 ng/ml), compared with the S1-3 group
(227.37±18.71 ng/ml; Fig. 2). The
levels of HA, PCIII, IV-C and LN were significantly higher in the
S1-3 and S4 groups, compared with the
S0 group (P<0.05; Table
II).
 | Table II.Clinical data of patients with CHB
liver fibrosis (mean ± standard deviation). |
Table II.
Clinical data of patients with CHB
liver fibrosis (mean ± standard deviation).
| Characteristic | S0
(n=45) | S1-3
(n=74) | S4
(n=9) | P-value |
|---|
| Male/female
(n) | 20/15 | 41/33 | 6/3 |
|
| DcR3 (ng/ml) | 109.66±16.08 |
227.37±18.71a1 |
355.26±50.54a2,b | a1<0.001,
a2<0.001, b=0.031 |
| HA (ng/ml) | 0.74±0.060 |
1.03±0.05a1 |
1.49±0.39a2,b | a1=0.003,
a2<0.001, b=0.014 |
| PCIII (ng/ml) | 22.88±0.95 |
35.18±1.28a |
49.41±2.41b | a1=0.049,
b=0.037 |
| IV-C (pg/ml) | 548.51±39.33 |
910.52±37.40a1 |
1,266.32±255.32a2,b | a1<0.001,
a2<0.001, b=0.005 |
| LN (ng/ml) | 0.05±0.01 | 0.10±0.003 |
0.15±0.032a, b | a=0.006,
b=0.002 |
| γ-GGT (IU/l) | 23.47±4.00 |
51.35±7.89a | 46.89±11.69 | a=0.008 |
| AST (IU/l) | 35.31±4.50 |
95.78±16.09a1 |
176.80±115.45a2 | a1=0.023,
a2=0.006 |
| ALT (IU/l) | 45.26±5.62 |
198.72±35.09a | 186.93±123.06 | a=0.001 |
| IL-6 (pg/ml) | 15.33±1.81 | 18.01±2.03 | 19.91±5.97 |
|
| PCT (ng/ml) | 0.11±0.071 | 0.16±0.02 | 0.12±0.091 |
|
Significant association between DcR3
and fibrosis indicators
Results demonstrated that DcR3 levels were
significantly increased in the hepatic fibrosis group
(S1-4) and significantly associated with the four
indicators of hepatic fibrosis: HA (r=0.51, P<0.001); IV-C
(r=0.34, P=0.0013); PCIII (r=0.49, P<0.001); and LN (r=0.40,
P<0.001). In addition, serum DcR3 levels in patients with CHB
were positively associated with HBV DNA, ALT, AST and GGT levels
(r=0.27, 0.53, 0.54 and 0.48, respectively; P<0.001; Table III). However, DcR3 levels had no
association with sex and age in patients with CHB.
 | Table III.Association between DcR3 and other
clinical biomarkers. |
Table III.
Association between DcR3 and other
clinical biomarkers.
| Index | Sex | Age | HBV-DNA | γ-GGT | AST | ALT | HA | PCIII | IV-C | LN | NF-κB |
|---|
| DcR3 | r=−0.122 | r=−0.355 | r=0.27 | r=0.53 | r=0.54 | r=0.48 | r=0.51 | r=0.49 | r=0.34 | r=0.40 | r=0.06 |
|
| P=0.434 | P=0.020 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P<0.001 | P=0.001 | P=0.0013 | P<0.001 | P=0.726 |
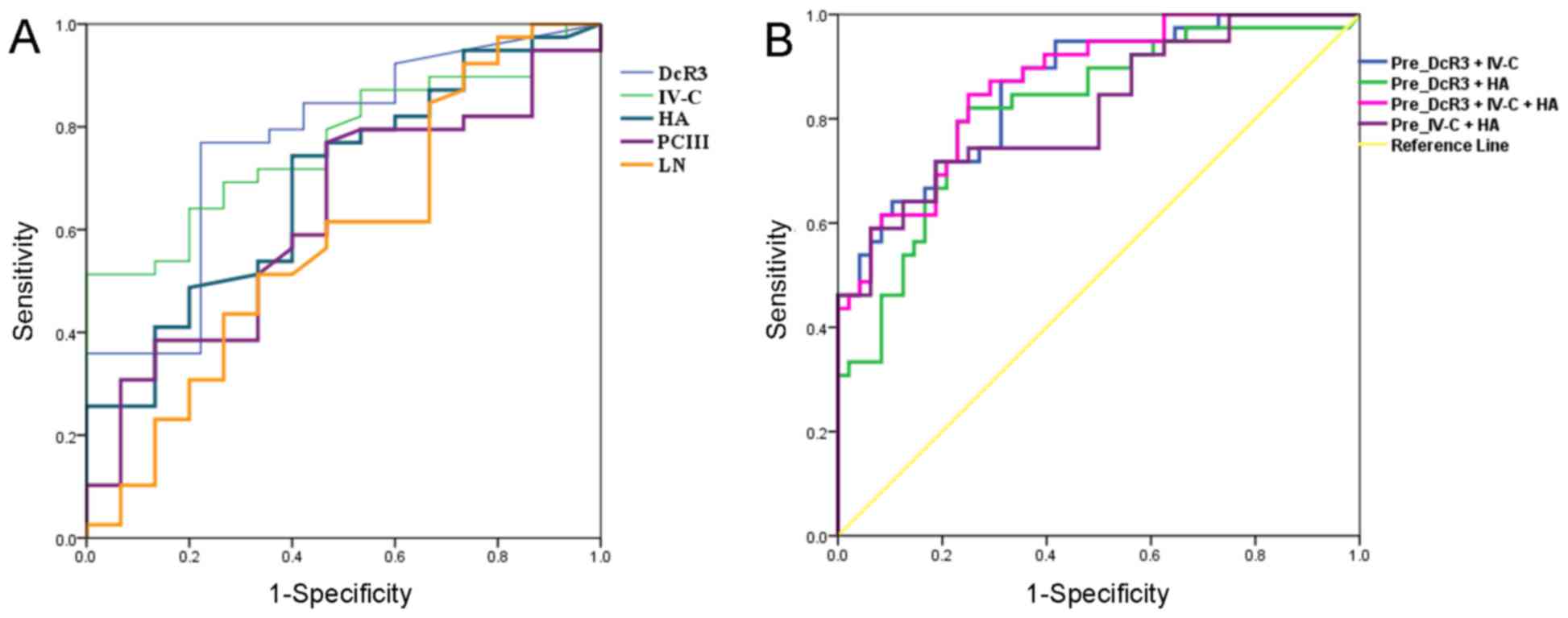
Diagnostic value of DcR3 in CHB and
hepatic fibrosis
The diagnostic ability of DcR3 and liver fibrosis
indicators (IV-C, HA, PCIII and LN) for CHB with hepatic fibrosis
was evaluated using ROC curve analysis (Fig. 3), with area under the curve (AUC)
values of 0.807, 0.770, 0.688, 0.626 and 0.584, respectively. The
95% confidence interval, cut-off value, sensitivity and specificity
are displayed in Table IV. When the
cutoff value for DcR3 was set at 168.67 ng/ml, its diagnostic
sensitivity and specificity were 76.9 and 77.8%, respectively
(Table IV). When liver fibrosis
indicators with improved diagnostic ability, namely IV-C and HA,
were used in combination with DcR3 for diagnosis, the AUC for DcR3
combined with IV-C and HA was 0.869, with sensitivity and
specificity of 84.6 and 81.2%, respectively (Table IV). This indicated that DcR3 may
significantly improve the combined diagnostic effect of IV-C and HA
(AUC of 0.798, with sensitivity and specificity of 71.8 and 75.0%,
respectively) and demonstrated the value of DcR3 as a clinical
diagnostic index of liver fibrosis.
 | Table IV.Receiver operating characteristic
analysis of DcR3 for the diagnosis of chronic hepatitis B liver
fibrosis. |
Table IV.
Receiver operating characteristic
analysis of DcR3 for the diagnosis of chronic hepatitis B liver
fibrosis.
| Parameters | AUC | SD error | P-value | 95% CI lower-upper
limit | Cut-off value | Sensitivity | Specificity |
|---|
| DcR3 (ng/ml) | 0.807 | 0.051 | <0.001 | 0.688–0.887 | 168.67 | 0.769 | 0.778 |
| IV-C (pg/ml) | 0.770 | 0.053 | <0.001 | 0.666–0.875 | 783.48 | 0.641 | 0.800 |
| HA (ng/ml) | 0.688 | 0.058 | 0.003 | 0.575–0.801 | 0.834 | 0.744 | 0.600 |
| PCIII (ng/ml) | 0.626 | 0.062 | 0.046 | 0.504–0.749 | 30.20 | 0.769 | 0.533 |
| LN (ng/ml) | 0.584 | 0.062 | 0.187 | 0.461–0.706 | 0.097 | 0.513 | 0.667 |
| DcR3+IV-C
(ng/ml) | 0.862 | 0.038 | <0.001 | 0.786–0.937 | 0.311 | 0.872 | 0.687 |
| DcR3+HA
(ng/ml) | 0.818 | 0.046 | <0.001 | 0.727–0.908 | 0.388 | 0.821 | 0.750 |
| DcR3+IV-C+HA
(ng/ml) | 0.869 | 0.037 | <0.001 | 0.796–0.941 | 0.387 | 0.846 | 0.812 |
| IV-C+HA
(ng/ml) | 0.798 | 0.046 | <0.001 | 0.725–0.906 | 0.471 | 0.718 | 0.750 |
Nuclear factor (NF)-κB levels are
increased in patients with hepatitis B and liver fibrosis
The serum levels of NF-κB in patients with hepatitis
B and liver fibrosis were detected, which demonstrated that NF-κB
levels were elevated in S0 (2,128.01±552,67 pg/ml),
S1-3 (2,199.50±460.40) and S4
(2,179.56±384.11 pg/ml) groups, compared with the control group
(210.33±53.83 pg/ml) (Fig. 4A). The
association between NF-κB and DcR3 levels was analyzed, which
indicated that there was no association between these two molecules
(r=0.06, P=0.726; Fig. 4B).
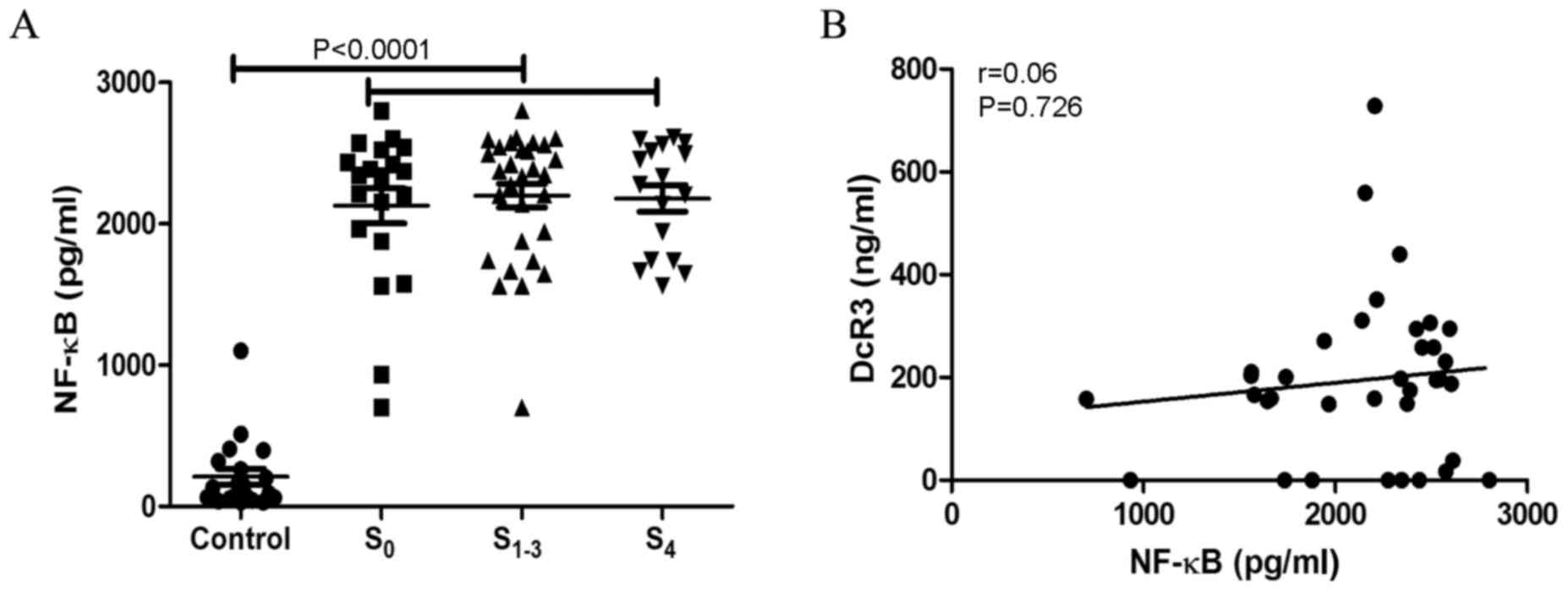 | Figure 4.NF-κB is upregulated in patients with
CHB liver fibrosis and cirrhosis. (A) NF-κB levels were
significantly higher in S0, S1-3 and
S4 groups, compared with the healthy control groups
(P<0.001). (B) However, NF-κB levels had no association with
DcR3 levels (r=0.06, P=0.726). S0, CHB without fibrosis;
S1-3, CHB complicated with fibrosis; S4,
cirrhosis; NF-κB, nuclear factor-κB; CHB, chronic hepatitis B;
DcR3, decoy receptor 3. |
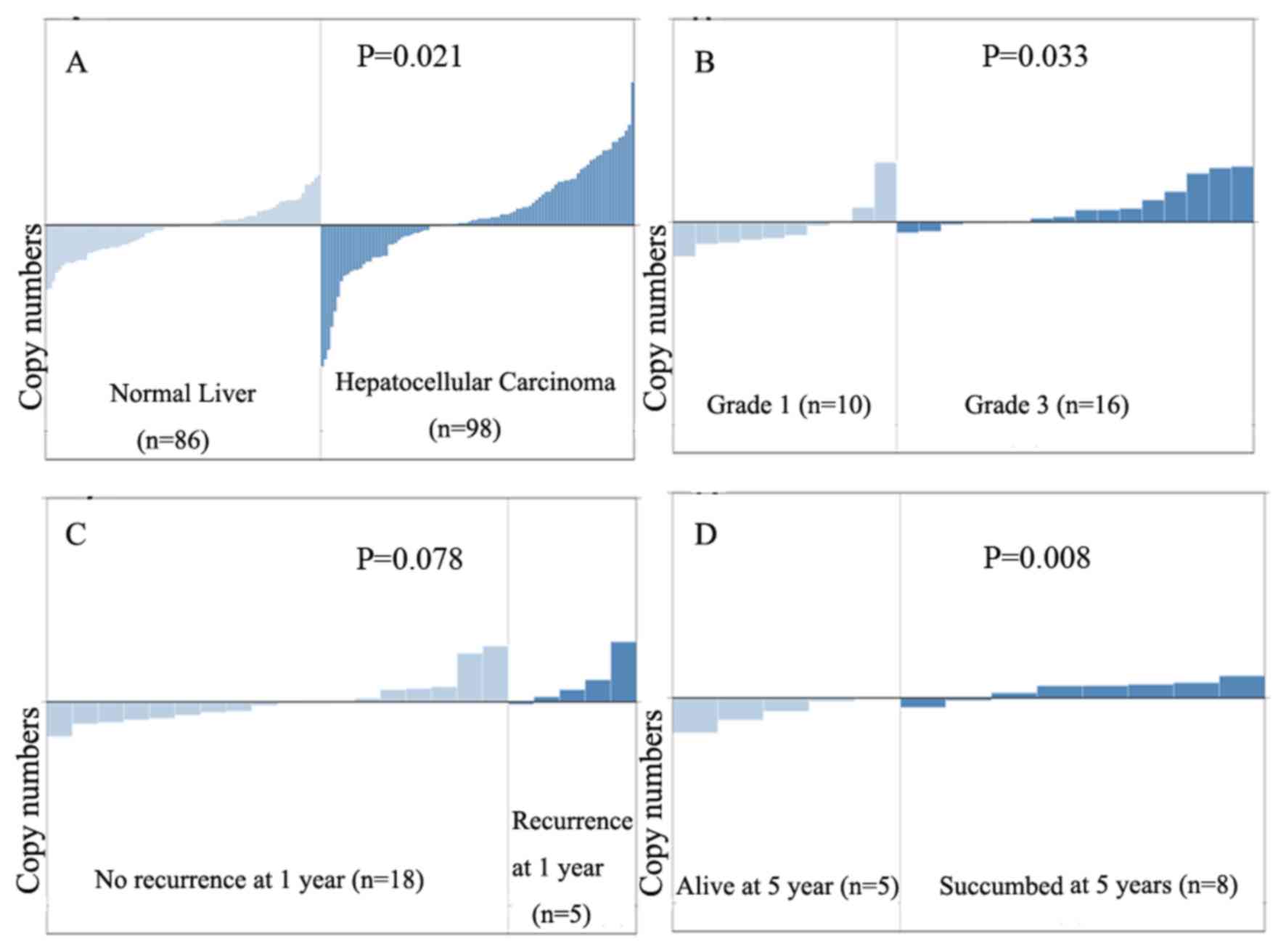
DcR3 mRNA levels are higher in HCC
compared with that in normal liver tissues
Analysis of a publicly available human DNA
microarray database using Oncomine software indicated that DcR3
mRNA levels are higher in liver cancer tissues, compared with
normal liver tissues (P=0.021). DcR3 levels increased with the
grade of hepatic cell carcinoma (P=0.033), and DcR3 mRNA was
significantly higher in patients with HCC who died within 5 years,
compared with those who survived >5 years (P=0.008; Fig. 5). Assessment of DcR3 levels in
patients with CHB associated with liver fibrosis indicated that
DcR3 serves an important role in the CHB-liver fibrosis-liver
cirrhosis-liver cancer progression, although the specific
underlying mechanism of action requires further examination.
Discussion
Accurate assessment of the degree of viral hepatic
fibrosis is important for patient treatment, prognosis and
surveillance. Liver biopsy, which is considered the gold standard
for the diagnosis of liver fibrosis, has certain limitations. Such
invasive procedures are associated with certain complications. The
majority of patients refuse to undergo liver biopsy, and they are
even more reluctant to accept a second liver biopsy, making it
difficult to assess the efficacy of the procedure (14). Additionally, sample errors, and
intraobserver or interobserver differences may affect the accuracy
of the diagnostic results (15–17).
Within the TNFR family, DcR3 is the only member
capable of competitively binding the three ligands TL1A, LIGHT and
FasL (12). In humans, DcR3 is
upregulated in pathological conditions including cancer, and
autoimmune and inflammatory diseases (18,19). The
data from the present study demonstrated that DcR3 is highly
expressed in patients with CHB and its expression is higher in
patients with active CHB, compared with patients who are CHB
carriers. DcR3 expression was significantly associated with ALT,
AST and γ-GGT. Assessment of DcR3 protein levels may provide an
important diagnostic biomarker, for example for inflammatory
diseases or high-grade carcinoma. The present study compared the
performance of DcR3 with that of direct serological markers (HA,
CIV, PCIII and LN) for the diagnosis of liver fibrosis and its
stages.
In the present study, it was indicated that serum
DcR3 levels were significantly increased in patients with hepatic
fibrosis and hepatic cirrhosis (P<0.01) and significantly
associated with the four indicators of hepatic fibrosis, namely HA
(r=0.51, P<0.0001), IV-C (r=0.34, P<0.0013), PCIII (r=0.49,
P<0.0001) and LN (r=0.40, P<0.0001), indicating the clinical
value of DcR3 for the diagnosis of hepatic fibrosis. A study by Kim
et al (20) on the different
stages of chronic hepatitis and fibrosis determined that DcR3
expression levels were elevated in bile duct epithelial cells and
infiltrating lymphocytes, in regenerated and poorly developed bile
ducts, as well as in cultured hepatoma cells, indicating that DcR3
serves an important role in the progression of chronic hepatitis to
hepatic fibrosis, leading to the occurrence of liver cirrhosis and
HCC.
To determine the diagnostic value of DcR3 for liver
fibrosis, ROC analysis was performed. The AUC values for the
diagnosis of hepatitis B and hepatic fibrosis based on DcR3 and the
four indicators of liver fibrosis, including IV-C, HA, PCIII and
LN, were 0.807, 0.770, 0.688, 0.626 and 0.584, respectively. When
IV-C and HA, which have demonstrated superior diagnostic accuracy
among the four indicators, were used in combination with DcR3 as
diagnostic markers, the AUC was 0.869, with sensitivity and
specificity of 84.6 and 81.2%, respectively, indicating that DcR3
significantly improves the combined diagnostic value of IV-C and
HA. These results indicated that the diagnostic value of DcR3 for
CHB may be superior to that of the four conventional liver fibrosis
indicators.
The role of DcR3 in HBV infection remains unclear. A
previous study indicated that DcR3 expression is closely associated
with the degree of inflammation during the pathogenetic process of
acute ulcerative colitis, as increased DcR3 expression levels are
observed in the peripheral blood of patients with a high incidence
of inflammation, and these are significantly reduced following
effective treatment (21). A previous
study indicated that DcR3 is upregulated in patients with hepatitis
B e antigen-negative CHB (22).
However, the present study determined that the DcR3 levels are
increased in patients with active hepatitis and in those with liver
fibrosis. Yang et al (23)
indicated that DcR3 levels were significantly increased in the sera
of patients with HCC and associated with liver cirrhosis, tumor
metastasis and recurrence, which indicated that the highly
expressed and distributed DcR3 may serve as an important role in
occurrence and development of primary HCC.
Bioinformatics analysis of a human HCC DNA
microarray database indicated that DcR3 expression was
significantly higher in HCC tissues compared with that in normal
liver tissues. DcR3 levels increased with the grade of HCC, and the
copy number of DcR3 DNA of patients with HCC who died within 5
years was significantly higher compared with that of patients with
HCC who survived >5 years. These results indicated that DcR3 may
serve as an important role in the occurrence and development of
CHB, liver fibrosis, liver cirrhosis and liver cancer, although the
underlying molecular mechanism requires further exploration.
The expression of DcR3 is high in patients with CHB,
liver fibrosis and liver cirrhosis. However, the molecular
mechanisms underlying liver cancer progression has not yet been
investigated. DcR3 levels are increased in patients with
inflammatory bowel disease, primary Sjogren's syndrome, rheumatoid
arthritis and primary biliary cirrhosis. DcR3 is also upregulated
in Kaposi's sarcoma-associated herpes virus-infected human
umbilical vein endothelial cells (24) and skin lesions of psoriasis patients
(25). In human keratinocytes, DcR3
is transcriptionally regulated by epidermal growth factor via the
NF-κB signaling pathway (26). In
nasopharyngeal carcinoma (NPC) cases with Epstein Barr Virus (EBV)
infection, EBV binds to the promoter of the transcriptional
activation factor RTA and upregulates DcR3 expression (27). Furthermore, its latent membrane
protein-1 activates the NF-κB signaling pathway to promote DcR3
expression, and the upregulated DcR3 enhances the metastatic and
invasive abilities of the NPC cell line HONE-1 (28). HBV protein HBx upregulates gene
expression by binding to pattern recognition receptor at key
sectors of host genes, and indirectly activates host gene promoters
by interacting with the transcription factor NF-κB and regulating
gene transcription (29). In the
present study, the serum levels of NF-κB in patients with CHB were
detected. The results demonstrated that NF-κB levels were elevated
in S0, S1-3 and S4 groups,
compared with the control group. There is no association between
NF-κB and DcR3 levels; however, the molecular mechanism underlying
the role of DcR3 in the occurrence and progression from CHB to HCC
remains to be elucidated.
The present study demonstrated that DcR3 is a novel
indicator for the diagnosis of hepatitis B and liver fibrosis.
Further in-depth study would provide indications to elucidate the
underlying molecular mechanism and assist in designing strategies
for the clinical treatment of liver fibrosis, liver cirrhosis and
liver cancer induced by hepatitis B.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of China (grant no. 81702729), the Scientific
Foundation of Shanghai Municipal Commission of Health and Family
Planning (grant nos. 201540119 and 20164Y0273) and the Science and
Technology Research Project of Songjiang of Shanghai (grant nos.
15SJGG25 and 15SJGG47).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YH conceived the idea and designed the study. YH and
XL contributed to the data analysis and writing of the manuscript.
HC helped to conduct the experiments and data analysis. JZ
collected clinical blood specimens and clinical data. FZ performed
indicator assays. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The human data reported in this manuscript were
collected according to procedures approved by the Institutional
Ethical Review Board of Songjiang District Center Hospital. All
patients provided written informed consent.
Consent for publication
All patients provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lu FM and Zhuang H: Management of
hepatitis B in China. Chin Med J (EngI). 122:3–4. 2009.
|
|
2
|
Liang X, Bi S, Yang W, Wang L, Cui G, Cui
F, Zhang Y, Liu J, Gong X, Chen Y, et al: EpidemioIogicaI
serosurvey of hepatitis B in China-decIining HBV prevaIence due to
hepatitis B vaccination. Vaccine. 27:6550–6557. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seeger C and Mason WS: Molecular biology
of hepatitis B virus infection. Virology. 479–480:672–686. 2015.
View Article : Google Scholar
|
|
4
|
Meng N, Gao X, Yan W, Wang M, Liu P, Lu
XD, Zhang SJ, Lu YQ and Tang WX: Efficacy of telbivudine in the
treatment of chronic hepatitis b and liver cirrhosis and its effect
on immunological responses. J Huazhong Univ Sci Technolog Med Sci.
35:230–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neuman MG, Cohen LB and Nanau RM:
Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin
Biochem. 49:302–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang YF, Ma J, He B, Li NP, Tang W and
Gong GZ: The therapeutic effect of Anluohuaxian capsule combined
with adefovir dipivoxil on patients with chronic hepatitis B and
influence on hepatic histology. Zhonghua Gan Zang Bing Za Zhi.
20:344–347. 2012.(In Chinese). PubMed/NCBI
|
|
7
|
You SP, Zhao J, Ma L, Tudimat M, Zhang SL
and Liu T: Preventive effects of phenylethanol glycosides from
Cistanche tubulosa on bovine serum albumin-induced hepatic fibrosis
in rats. Daru. 23:522015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oztas E, Kuzu UB, Zengin NI, Kalkan IH,
Onder FO, Yildiz H, Celik HT, Akdogan M, Kilic MY, Koksal AS, et
al: Can Serum ST2 levels be used as a marker of fibrosis in chronic
Hepatitis B infection? Medicine (Baltimore). 94:e18892015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Wang G, Kang K, Wu G and Wang P:
The diagnostic accuracy and clinical utility of three noninvasive
models for predicting liver fibrosis in patients with HBV
Infection. PLoS One. 11:e01527572016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lok AS and McMahon BJ: Chronic hepatitis
B. Hepatology. 45:507–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hou YQ, Xu P, Zhang M, Han D, Peng L,
Liang DY, Yang S, Zhang Z, Hong J, Lou XL, et al: Serum decoy
receptor 3, a potential new biomarker for sepsis. Clin Chim Acta.
413:744–748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liang D, Hou Y, Lou X and Chen H: Decoy
receptor 3 improves survival in experimental sepsis by suppressing
the inflammatory response and lymphocyte apoptosis. PLoS One.
10:e01316802015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Easterbrook PJ, Roberts T, Sands A and
Peeling R: Diagnosis of viral hepatitis. Curr Opin HIV AIDS.
12:302–314. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lambrecht J, Verhulst S, Mannaerts I,
Reynaert H and van Grunsven LA: Prospects in non-invasive
assessment of liver fibrosis: Liquid biopsy as the future
goldstandard? Biochim Biophys Acta. 1864:1024–1036. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Ji Y, Ai H, Ning B, Zhao J, Zhang Y
and Dun G: Liver shear-wave velocity and serum fibrosis markers to
diagnosis hepatic fibrosis in patients with chronic vial Hepatitis
B. Korean J Radiol. 17:396–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cho HJ, Kim SS, Ahn SJ, Park JH, Kim DJ,
Kim YB, Cho SW and Cheong JY: Serum transferrin as a liver fibrosis
biomarker in patients with chronic hepatitis B. Clin Mol Hepatol.
20:347–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alempijević T, Štulić M, Popovic D,
Culafic D, Dragasevic S and Milosavljevic T: The role of fecal
calprotectin in assessment of hepatic encephalopathy in patients
with liver cirrhosis. Acta Gastroenterol Belg. 77:302–305.
2014.PubMed/NCBI
|
|
18
|
Lin WW and Hsieh SL: Decoy receptor 3: A
pleiotropic immunomodulator and biomarker for inflammatory
diseases, autoimmube diseases and cancer. Biochem Pharmacol.
81:838–847. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siakavellas SI, Sfikakis PP and Bamias G:
The TL1A/DR3/DcR3 pathway in autoimmune rheumatic diseases. Semin
Arthritis Rheum. 45:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim S, Kotoula V, Hytiroglou P, Zardavas D
and Zhang L: Significance of increased expression of decoy receptor
3 in chronic liver disease. Dig Liver Dis. 41:591–598. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bamias G, Kaltsa G, Siakavellas SI,
Papaxoinis K, Zampeli E, Michopoulos S, Zouboulis-Vafiadis I and
Ladas SD: High intestinal and systemic levels of decoy receptor 3
(DcR3)and its ligand TL1A in active ulcerative colitis. Chin
Immunol. 137:242–249. 2010. View Article : Google Scholar
|
|
22
|
Hou Y, Xu P, Lou X, Liang D, Zhang M,
Zhang Z and Zhang L: Serum decoy receptor 3 is a useful predictor
for the active status of chronic hepatitis B in hepatitis B e
antigen-negative patients. Tohoku J Exp Med. 230:227–232. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang M, Chen G, Dang Y and Luo D:
Significance of decoy receptor 3 in sera of hepatocellular
carcinoma patients. Ups J Med Sci. 115:232–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoo S, Jang J, Kim S, Cho H and Lee MS:
Expression of DcR3 and its effects in kaposi's sarcoma-associated
herpesvirus-infected human endothelial cells. Intervirology.
55:45–52. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bamias G, Evangelou K, Vergou T,
Tsimaratou K, Kaltsa G, Antoniou C, Kotsinas A, Kim S, Gorgoulis V,
Stratigos AJ and Sfikakis PP: Upregulation and nuclear localization
of TNF-like cytokine 1A (TL1A) and its receptors DR3 and DcR3 in
psoriatic skin lesions. Exp Dermatol. 20:725–731. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hsieh SL and Lin WW: Decoy receptor 3: An
endogenous immubomudulator in cancer growth and inflammatory
reactions. J Biomed Sci. 24:392017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ho CH, Hsu CF, Fong PF, Tai SK, Hsieh SL
and Chen CJ: Epstein-Barr virus transcription activator Rta
upregulates decoy receptor 3 expression by binding to its promoter.
J Virol. 81:4837–4847. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ho CH, Chen CL, Li WY and Chen CJ: Decoy
receptor 3, upregulated by Epstein-Barr virus latent membrane
protein 1, enhances nasopharyngeal carcinoma cell migration and
invasion. Carcinogenesis. 30:1443–1451. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xia L, Tian D, Huang W, Zhu H, Wang J,
Zhang Y, Hu H, Nie Y, Fan D and Wu K: Upregulation of IL-23
expression in patients with chronic hepatitis B is mediated by the
HBx/ERK/NF-κB pathway. J Immunol. 188:753–764. 2012. View Article : Google Scholar : PubMed/NCBI
|