Introduction
Pancreatic cancer (PC) is among the most aggressive
types of solid tumor, and has the highest rate of cancer-associated
mortality worldwide (1). Despite
advances in surgical techniques, chemotherapy and radiotherapy, the
long-term survival rate for patients with PC has not significantly
increased since 1977 (2). Therefore,
further research into alternative therapies for PC, which has fewer
associated toxicities, is urgently required.
Trypsin inhibitors (TIs) are widely distributed in
plants and animals, and certain types of TIs have exhibited
antitumor activity in several types of tumor cells (3–5). Although
no approved therapies directly targeting trypsin directly are
currently available, development of novel and more specific TI
therapies is underway (3–5). Sporamin, a sweet potato tuber storage
protein, is a Kunitz-type TI that has been demonstrated to induce
antitumor effects in vitro and in vivo (4,5). Although
the role and mechanism of sporamin as an antitumor agent remain
unclear, the previous research has indicated that sporamin can
mediate the regulation of specific signaling pathways (5). For example, sporamin was demonstrated to
inhibit cell growth and induce apoptosis of human tongue cancer
cells by downregulating RAC serine/threonine-protein
kinase/glycogen synthase kinase-3 signaling (5). However, to the best of our knowledge,
the antitumor effects of sporamin in PC have not been previously
investigated.
Mitogen-activated protein kinases (MAPK) are
critically involved in the signaling cascades that govern various
critical processes, including cell proliferation, differentiation,
apoptosis and survival (6). MAPKs are
conserved enzymes that connect cell-surface receptors to their
regulatory targets within cells (7).
The major MAPKs include the extracellular signal-regulated protein
kinase (ERK), c-Jun N-terminal protein kinase (JNK) and p38 mitogen
activated-protein kinase (p38), which have been identified as
sub-families of MAPK (8). MAPKs are
constitutively activated in PC (9).
Therefore, MAPKs and their associated genes may provide more
detailed insights into the mechanism behind the antitumor activity
of sporamin in PC cells.
The present study investigated the effects of
sporamin on the proliferation, viability and apoptosis of PC cells,
and determined the roles of MAPKs in the antitumor activity of
sporamin in PC cells. The results of the present study demonstrated
that the combined treatment of PC cells with sporamin and MAPK
inhibitors elicited a superior response to treatment with either
sporamin or MAPK inhibitor treatment alone. This result supports
the hypothesis that a sporamin and MAPK inhibitor combination
treatment may be used as a PC therapy. The present study may
provide a novel strategy for the development of targeted antitumor
drugs for PC.
Materials and methods
Cell line and materials
The human PC PANC-1 cell line (American Type Culture
Collection, Manassas, VA, USA), was cultured in Dulbecco's Modified
Eagle Medium (DMEM) (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% heat-inactivated fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 mg/l streptomycin
and 100 kU/l benzylpenicillin at 37°C in an incubator containing
humidified air and 5% CO2. Sporamin was purified from
fresh sweet potato tubers, as previously described (5). The specific phospho-ERK inhibitor
PD98059, phospho-JNK specific inhibitor SP600125 and phospho-p38
specific inhibitor SB203580 were purchased from Calbiochem (Merck
KGaA, Darmstadt, Germany).
Preparation of cell lysates
For western blot analysis, PANC-1 cells were
cultured in 6-well culture plates to 80–90% confluence, then
nutrient-starved for 24 h in serum-free DMEM. Sporamin was added at
0, 25, 50 and 100 µM for 24 h. For MAPK inhibition experiments, the
cells were pretreated for 10 min with 20 µM SP600125, 10 µM
SB203580 or 20 µM PD98059 prior to sporamin treatment. Following 3
washes in PBS at 4°C, the cells were lysed in 60 µl lysis buffer
(50 mmol/NaCl, 0.5 mmol/ml phenylmethylsulfonyl fluoride, 2 mmol/ml
Na3VO4, and 10 mmol/ml HEPES at pH 7.4, with
0.01% Triton X-100 and 10 mg leupeptin) at 4°C for 5 min. The cell
lysates were obtained by centrifugation at 14,000 × g at 4°C for 10
min. The concentrations of proteins were determined using an
Enhanced bicinchoninic acid protein assay kit (Beyotime Institute
of Biotechnology, Haimen, China).
Western blot analysis
SDS sample buffer (0.33 mol/l Tris-HCl, 10% (w/v)
SDS, 40% (v/v) glycerol, and 0.4% bromophenol blue), was added to
the cell lysates (a buffer:lysate ratio of 1:4). Subsequent to
heating for 5 min at 100°C, 20 µg extracted protein was subjected
to 10–12% SDS-PAGE. The protein was transferred into a
nitrocellulose membrane, which was then blocked at 25°C for 1 h
with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA) in 50
mmol/l Tris-HCl, 150 mmol/l NaCl and 0.1% Tween-20 (pH 7.4). The
blots were incubated with primary antibodies against phospho-ERK1/2
(catalog no. 4370), total ERK1/2 (catalog no. 9102), phospho-JNK1/2
(catalog no. 9251), total JNK1/2 (catalog no. 9252), phospho-p38
(catalog no. 9211) and total p38 (catalog no. 9212) (all used at a
dilution of 1:1,000, and acquired from Cell Signaling Technology,
Inc., Danvers, MA, USA) at 4°C overnight. This was followed by a
2-h incubation at room temperature with horseradish
peroxidase-conjugated anti-rabbit (catalog no. 7074) or anti-mouse
(catalog no. 7076) IgG secondary antibodies (acquired from Cell
Signaling Technology, Inc., Danvers, MA, USA; used at a dilution of
1:2,000). Immunoreactive signals were visualized using Pierce™
enhanced chemiluminescence western blotting substrate (Merck KGaA)
and scanned using an LAS-4000 imaging system (Fujifilm Holdings
Corporation, Tokyo, Japan).
Determination of cell proliferation
activity
PANC-1 cells were seeded in 24-well plates in DMEM
supplemented with 10% FBS and grown to 80% confluence. The cultures
were then rinsed in DMEM and incubated with sporamin (50 µM), with
or without inhibitor pre-treatment: 20 µM PD98059, 20 µM SP600125
or 10 µM SB203580, and serum-free DMEM for 12 h. Cells were then
cultured with 1.35×104 Bq/l 3H-thymidine for
another 12 h. The supernatant was aspirated and washed twice with
PBS to remove excess 3H-thymidine. The cells were then
resuspended in 0.2 mol/l NaOH. 3H-Thymidine
incorporation activity was determined with a [3H]
thymidine incorporation assay (Sigma-Aldrich; Merck KGaA) by
scintillation counting (LS6500; Beckman Coulter, Inc., Brea, CA,
USA).
Cell viability assay
To assess the effects of sporamin on the viability
of PANC-1 cells, an MTT assay was performed. PANC-1 cells were
plated in 96-well plates and grown to 80% confluence. The cultures
were then rinsed in DMEM and incubated with sporamin (50 µM), with
or without inhibitor pre-treatment: 20 µM PD98059, 20 µM SP600125
or 10 µM SB203580 in serum-free DMEM for 24 h. MTT was added at a
final concentration of 0.5 g/l. The cultures were removed from the
incubator 1 h later and the formazan crystals were solubilized in a
solution including containing 10% (v/v) Triton X-100 and 0.1 mol/l
HCl in 100% isopropanol equal to the volume of DMEM (100 µl)
culture medium. The absorbance was measured at 570 nm using a
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Flow cytometry analysis for
apoptosis
An Annexin V-Fluorescein Isothiocyanate
(FITC)/Propidium Iodide (PI) Apoptosis Detection kit (BD
Biosciences, San Jose, CA, USA) was used to detect apoptosis
according to the manufacturer's protocol. Briefly, the cells were
incubated with sporamin (50 µM), with or without inhibitor
pre-treatment: 20 µM PD98059, 20 µM SP600125 or 10 µM SB203580 for
24 h. Adherent and non-adherent cells were pooled and harvested by
centrifugation at 100 × g for 5 min, washed twice with 4°C PBS, and
resuspended in 1X binding buffer (140 mM NaOH, 10 mM HEPES and 2.5
mM CaCl2; pH 7.4), at a concentration of
1×106 cells/ml. A total of 1×105 cells were
transferred to a 5-ml culture tube, and stained with 5 µl Annexin
V-FITC and 5 µl PI for 15 min at room temperature in darkness.
Finally, 400 µl 1X binding buffer was added, and the cells were
analyzed using a BD FACSCalibur™ flow cytometer with BD CellQuest™
Pro software version 6.0 (BD Biosciences, San Jose, CA, USA).
Morphological determination and
quantification of apoptosis
Cells were cultured in 12-well plates at a density
of 1×105 cells/well, and incubated with sporamin (50
µM), with or without inhibitor pre-treatment: 20 µM PD98059, 20 µM
SP600125 or 10 µM SB203580 for 24 h. For the nuclear staining
assay, cells were fixed with 4% paraformaldehyde at 4°M for 15 min
(pH 7.4) and stained with 0.5 µg/ml DAPI (Sigma-Aldrich, Merck
KGaA) for 5 min at room temperature. Images were acquired using a
fluorescence microscope (magnification, ×200; DMI4000B; Olympus
Corporation, Tokyo, Japan). Typical apoptotic nuclear morphologic
changes, including condensed and fragmented nuclei, were considered
to be indicative of apoptosis, and were easily distinguishable from
intact nuclei by microscopy. A total of six randomly chosen fields
of view were examined with <500 cells scored in each, and the
percentage of apoptotic cells was calculated.
Statistical analysis
All data are presented as the mean ± standard
deviation. One-way analysis of variance, followed by Dunnett's post
hoc test, was applied to analyze the differences between groups
using SPSS 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of sporamin on the activation
of MAPK in PANC-1 cells
To address the involvement of MAPK in
sporamin-induced cell growth suppression and apoptosis directly,
phospho-ERK1/2, phospho-JNK1/2 and phospho-p38 protein expression
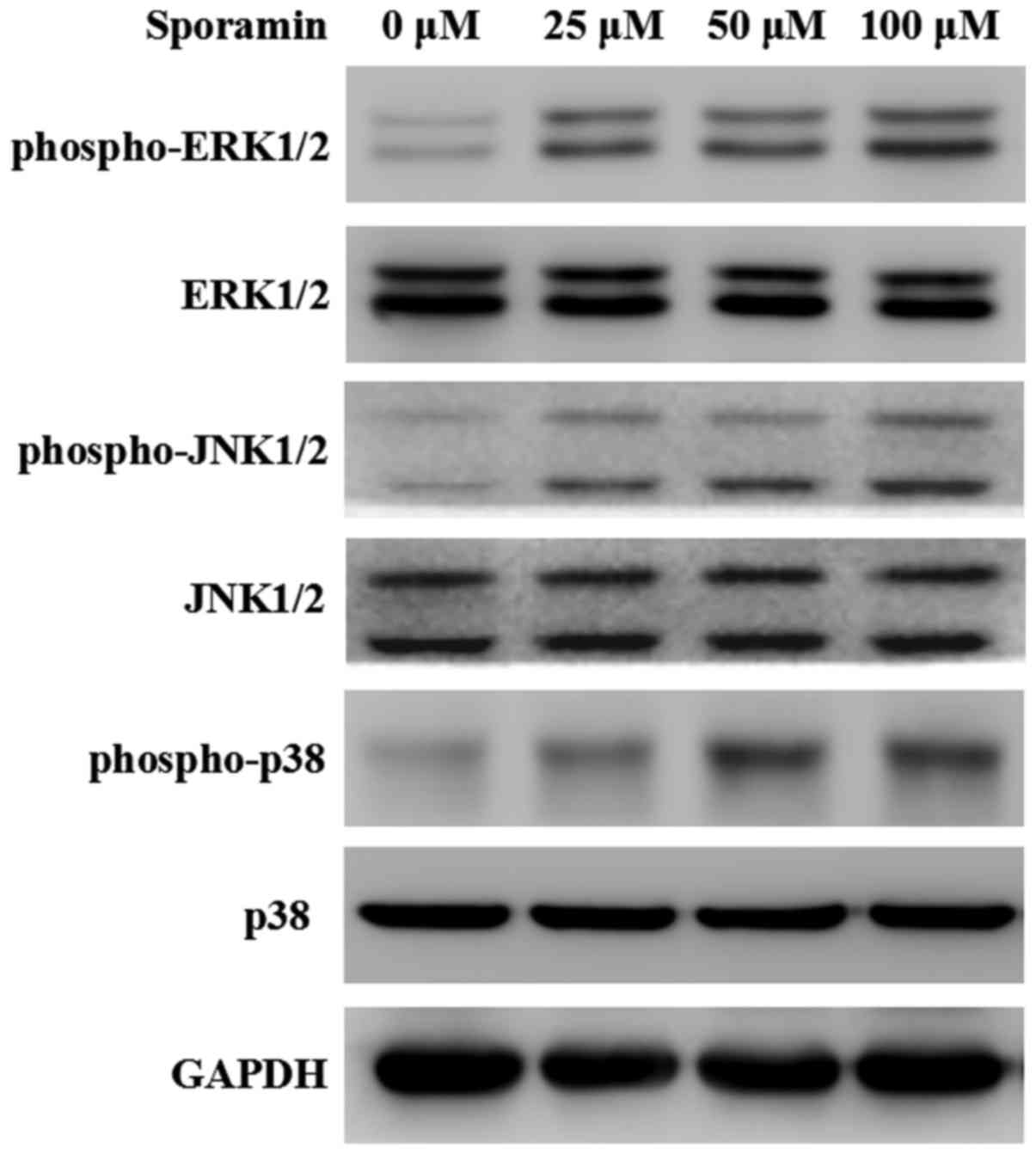
levels were determined by western blotting. As demonstrated in
Fig. 1, sporamin treatment at 25–100
µM for 24 h stimulated an increase in phospho-ERK1/2,
phospho-JNK1/2 and phospho-p38 protein expression in in a
concentration-dependent manner. These results indicated that
sporamin increased the activation of MAPK proteins in PANC-1 cells.
As 50 µM sporamin induced a marked increase in the activation of
MAPK proteins, the following experiments involved cell treatment at
a sporamin concentration of 50 µM.
Effect of MAPK inhibitors on
sporamin-induced activation of MAPKs in PANC-1 cells
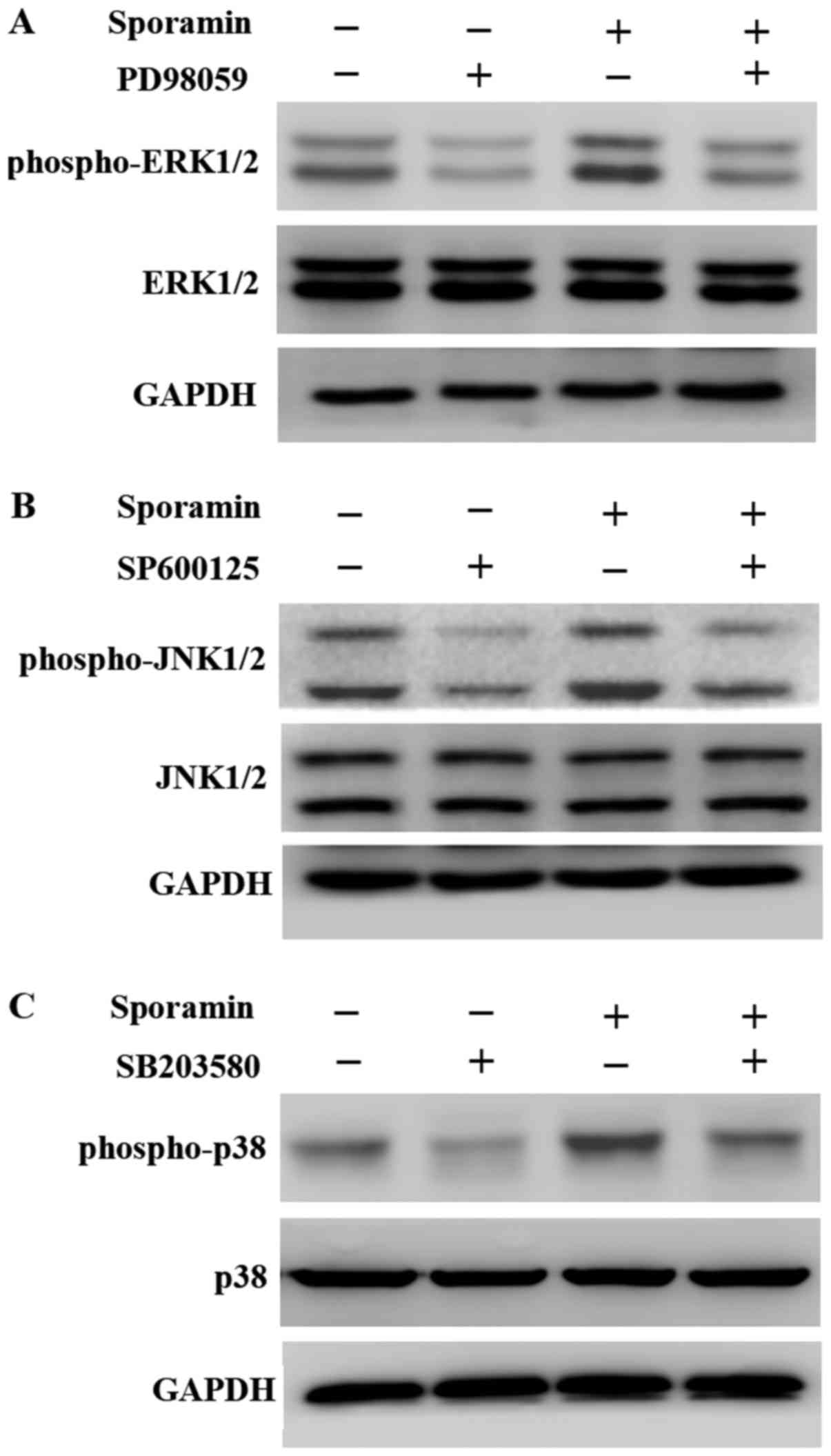
In PANC-1 cells treated with PD98059, SP600125 or
SB203580 for 10 min prior to sporamin-treatment, the
sporamin-induced phosphorylation of ERK1/2 (Fig. 2A), JNK1/2 (Fig. 2B) and p38 (Fig. 2C) was markedly decreased compared with
cells treated with sporamin alone or with untreated cells. These
results demonstrated that the MAPK inhibitors prevented the
sporamin-induced activation of MAPK proteins in PANC-1 cells.
Effect of MAPK inhibitors on
sporamin-induced inhibition of cell proliferation and viability in
PANC-1 cells
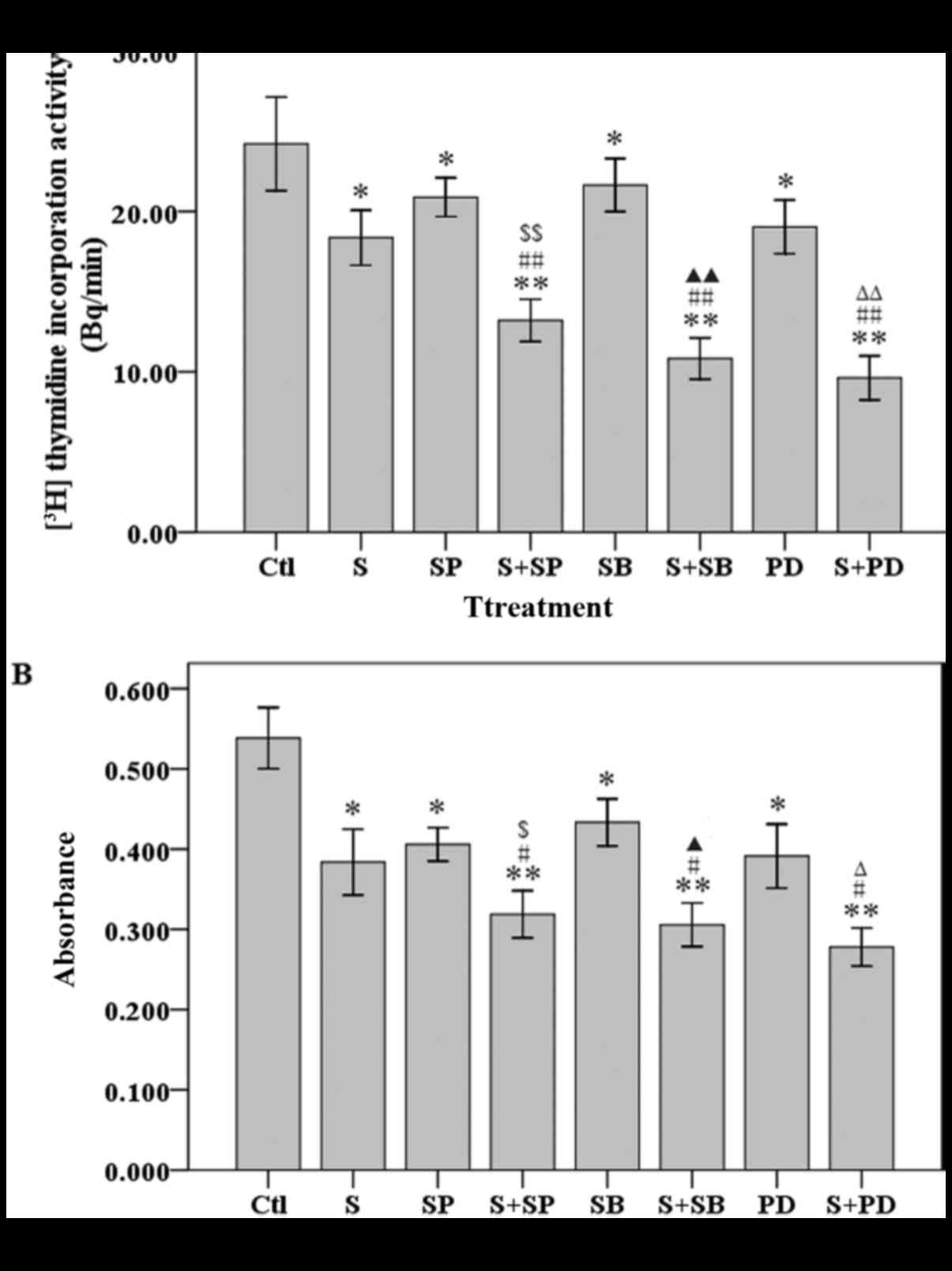
Whether the MAPK inhibitors could achieve a
synergistic suppressive effect on cell growth with sporamin was
investigated. A 3H-thymidine incorporation activity
assay revealed that there was a significant decrease in the
proliferation of PANC-1 cells when they were pre-treated with MAPK
inhibitors, compared with PANC-1 cells treated with sporamin or
with a MAPK inhibitor alone (Fig.
3A). An MTT assay revealed that cell viability was also
significantly reduced in cells pre-treated with MAPK inhibitors,
compared with cells treated with sporamin or a MAPK inhibitor alone
(Fig. 3B). These results demonstrated
that MAPK inhibitors enhanced the sporamin-induced inhibition of
cell proliferation activity and viability in PANC-1 cells.
Effect of MAPK inhibitors on
sporamin-induced apoptosis in PANC-1 cells
The combined treatment with sporamin and MAPK
inhibitors increased the proportion of apoptotic (annexin
V-positive) PANC-1 cells by 21.44±2.44% (sporamin with SP600125
versus sporamin or SP600125 treatment alone; P<0.01),
19.59±1.64% (sporamin with SB203580 versus sporamin or SB203580
treatment alone; P<0.01) and 27.58±3.59% (sporamin with PD98059
versus sporamin or PD98059 treatment alone; P<0.01) (Fig. 4A and B). DAPI nuclear staining.
Consistently, PANC-1 cells exposed to sporamin and MAPKs inhibitors
exhibited an increase in the number of condensed and fragmented
nuclei compared with either single agent alone (Fig. 4C). These results indicated that MAPKs
inhibitors enhanced sporamin-induced apoptosis in PANC-1 cells.
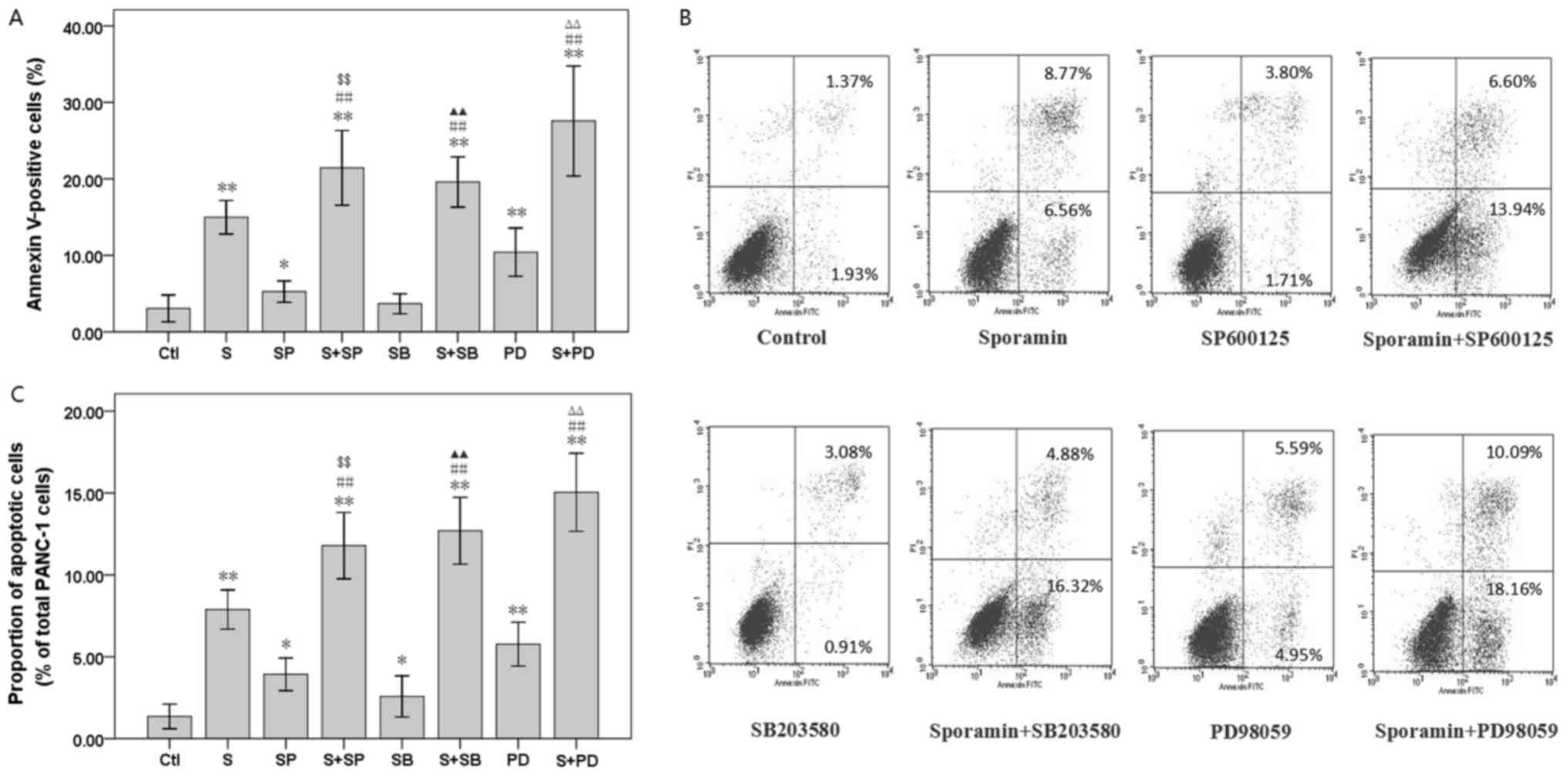 | Figure 4.Effect of SP600125, SB203580 and
PD98059 on the percentage of apoptotic cells in sporamin-treated
PANC-1 cells. Cells were pre-incubated with 20 µM SP600125, 10 µM
SB203580 or 20 µM PD98059 for 10 min prior to treatment with 50 µM
sporamin for 24 h. (A) The percentage of early apoptotic cells
(annexin V-positive, PI-negative) and late apoptotic cells (annexin
V- and PI-positive) were assayed by flow cytometry. (B)
Representative flow cytometry histograms. (C) Apoptotic
morphological changes were used to count apoptotic cells, and
subsequently calculate the percentage rate of apoptosis. A total of
6 randomly chosen fields of view were examined with a minimum
number of 500 cells scored in each treatment. Data are presented as
the mean ± standard deviation; n=4. *P<0.05 and **P<0.01 vs.
untreated control cells; ΔP<0.05 and
ΔΔP<0.01 vs. cells treated with sporamin alone;
&P<0.05 and &&P<0.01 vs.
cells treated with SP600125 alone; ▲P<0.05 and
▲▲P<0.01 vs. cells treated with SB203580 alone;
ΔP<0.05 and ΔΔP<0.01 vs. cells treated
with PD98059 alone. PI, propidium iodide; Ctl, control; S,
sporamin; SP, SP600125; SB, SB203580; PD, PD98059. |
Discussion
The results of the present study demonstrated that
sporamin suppressed the growth of PANC-1 cells by inhibition of
cellular proliferation and viability (Fig. 3). The suppressive effects of sporamin
on cell growth are also partially attributable to the induction of
apoptosis (Fig. 4). Furthermore, it
was demonstrated that MAPK activation may serve a protective role
against the antitumor effects of sporamin, as the combination
treatment of MAPK inhibitors and sporamin resulted in increased
cell growth suppression and apoptosis.
Sporamin inhibits cell growth and induces apoptosis
in colorectal cancer cells (4) and
tongue cancer cells (5). The results
of the present study demonstrated that sporamin suppressed the
growth of PANC-1 cells. Cell growth suppression can be attributable
to PC cell apoptosis (10), and it
was demonstrated in the present study that sporamin induced
apoptosis in PANC-1 cells. The present study also investigated the
potential mechanism underlying the antitumor effects of
sporamin.
MAPKs are involved in the complex signaling
transduction cascades that regulate apoptosis (7). Several previous studies have indicated
that tyrosine kinases, the cellular redox state and phosphatases
are involved in the activation of stress responses, representing
the activation of MAPKs in different cell types subsequent to
paclitaxel (11) or tumor necrosis
factor (12) treatment. Notably, in
the present study, sporamin treatment activated ERK1/2, JNK1/2 and
p38 in a concentration-dependent manner (Fig. 1) during the sporamin-induced apoptosis
of PANC-1 cells (Fig. 4). This result
indicated that MAPK signaling is involved in the regulation of
PANC-1 cell apoptosis due to sporamin treatment.
MAPK proteins may serve an important role in the
complex regulatory mechanisms that underlie resistance to
chemotherapeutics (13,14). In a previous study, the histone
deacetylase inhibitor TSA was demonstrated to induce cell growth
arrest, apoptosis and activation of ERK1/2. ERK1/2 activation
protected gastric carcinoma SGC7901 cells from TSA-induced
apoptosis (15). Similarly, the
results of the present study demonstrated that MAPK proteins are
activated by sporamin treatment, and that MAPK activation could
protect PANC-1 cells from sporamin-induced apoptosis. However,
ERK1/2 inhibition using PD98059 or U0126 markedly blocked
cisplatin-induced apoptosis in human malignant testicular cell
lines (16). By contrast, the present
study demonstrated that MAPK inhibition by PD98059, SP600125 or
SB203580 significantly enhanced the sporamin-induced apoptosis in
PANC-1 cells. The differing effects of MAPK inhibitors on apoptosis
may be due to the different cancer cell types used by different
studies, and their associated genetic/epigenetic statuses. Further
studies using other cancer cell lines are therefore required to
better characterize this phenomenon.
The MAPK signaling pathways are important in cell
growth, drug resistance and malignant transformation in various
tumor types (17–19). Understanding the molecular mechanism
behind the sporamin-induced MAPK activation should benefit the
development of novel therapies for PC. Activation of p38 is
generally associated with the induction of apoptosis, whereas
activation of ERK1/2 is associated with cytoprotective effects
(17). Therefore, the present study
focused on the role of MAPK in sporamin-induced apoptosis. Although
inhibition of MAPK enhanced sporamin-induced apoptosis in PANC-1
cells (Fig. 4), the results also
indicated that the basal levels of endogenous ERK1/2, JNK1/2 and
p38 phosphorylation serve a role in maintaining the survival of
PANC-1 cells. Thus, sporamin may exert a dual role: i) A cytotoxic
role by inducing apoptosis; and ii) a protective role by activating
MAPK signaling proteins, including ERK1/2, JNK1/2 and p38.
A previous study has demonstrated that activation of
ERK1/2 in gastric cancer cells is associated with increased
resistance to chemotherapeutic drugs (20). The activation of ERK1/2, which is
associated with proliferative and anti-apoptotic responses,
resulted in the generation of resistance to chemotherapeutic drugs
in PC cells (21). Increased MAPK
activation following chemotherapeutic drug treatments is indicative
of a potential increase in the abundance of MAPKs (17–21). In
the present study, it was demonstrated that sporamin induced the
activation of ERK1/2, JNK1/2 and p38 in a concentration-dependent
manner, and that MAPK inhibitors attenuated this effect of sporamin
in PANC-1 cells.
MAPK inhibitor treatment not only reduced the
constitutive levels of phospho-ERK1/2, phospho-JNK1/2 and
phospho-p38, but also inhibited the sporamin-induced activation of
MAPK signaling proteins (Fig. 2).
MAPK inhibitor treatment also enhanced the inhibition of cell
proliferation activity and viability (Fig. 3) and induction of apoptosis (Fig. 4) in sporamin-treated PANC-1 cells.
Sporamin and MAPK inhibitor treatments have synergistic effects,
indicating at the therapeutic potential of this combination for PC
patients.
In summary, the use of MAPK inhibitors to enhance
apoptosis via sporamin indicated that MAPK therapies may be
advantageous for the efficacy of TI cancer chemotherapy, and that
combining TIs with MAPKs inhibitors may be an effective treatment
strategy. Therefore, the results of the present study have great
potential in the treatment of PC using MAPK inhibitors with
TIs.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation of Zhejiang Province (grant no.
LY16H160033), the Public Welfare Technical Applied Research Project
of Zhejiang Province (grant no. 2016C33189), the Science and
Technology Plans of Taizhou City (grant no. 1701yw07), and the
National College Students' Innovation and Entrepreneurship Training
Program (grant no. 201710350008).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JY and CQ conceived and directed the project. CQ and
YQ designed the experiments. CQ, YQ, SZ, JZ and XC carried out the
experiments. JY and CQ conducted the data analysis and interpreted
the results. JY and CQ wrote and edited the paper. All the authors
reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
TI
|
trypsin inhibitor
|
|
PC
|
pancreatic cancer
|
|
MAPK
|
mitogen activated protein kinase
|
|
p38
|
p38-mitogen activated protein
kinase
|
|
ERK
|
extracellular signal-regulated protein
kinase
|
|
JNK
|
c-Jun amino-terminal protein
kinase
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
References
|
1
|
Yeo TP: Demographics, epidemiology, and
inheritance of pancreatic ductal adenocarcinoma. Semin Oncol.
42:8–18. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Krška Z, Šváb J, Hoskovec D and Ulrych J:
Pancreatic cancer diagnostics and treatment - current state. Prague
Med Rep. 116:253–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang EF and Ng TB: A trypsin inhibitor
from rambutan seeds with antitumor, anti-HIV-1 reverse
transcriptase, and nitric oxide-inducing properties. Appl Biochem
Biotechnol. 175:3828–3839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li PG, Mu TH and Deng L: Anticancer
effects of sweet potato protein on human colorectal cancer cells.
World J Gastroenterol. 19:3300–3308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao J and Qian C: Sporamin induce
apoptosis in human tongue carcinoma cells by down-regulating
Akt/GSK-3 signaling. Fundam Clin Pharmacol. 25:229–236. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kolch W: Meaningful relationships: The
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J 351 Pt. 2:289–305. 2000. View Article : Google Scholar
|
|
7
|
Peti W and Page R: Molecular basis of MAP
kinase regulation. Protein Sci. 22:1698–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Johnson GL and Lapadat R:
Mitogen-activated protein kinase pathways mediated by ERK, JNK, and
p38 protein kinases. Science. 298:1911–1912. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furukawa T: Impacts of activation of the
mitogen-activated protein kinase pathway in pancreatic cancer.
Front Oncol. 5:232015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Olechowska-Jarząb A, Ptak-Belowska A and
Brzozowski T: Terapeutic importance of apoptosis pathways in
pancreatic cancer. Folia Med Cracov. 56:61–70. 2016.
|
|
11
|
Gong W, Song Q, Lu X, Gong W, Zhao J, Min
P and Yi X: Paclitaxel induced B7-H1 expression in cancer cells via
the MAPK pathway. J Chemother. 23:295–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Tu L, Jiang W, Feng W, Zhao CX and
Wang DW: Bradykinin prevents the apoptosis of NIT-1 cells induced
by TNF-α via the PI3K/Akt and MAPK signaling pathways. Int J Mol
Med. 29:891–898. 2012.PubMed/NCBI
|
|
13
|
Grossi V, Peserico A, Tezil T and Simone
C: P38α MAPK pathway: A key factor in colorectal cancer therapy and
chemoresistance. World J Gastroenterol. 20:9744–9758. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tan W, Yu HG and Luo HS: Inhibition of the
p38 MAPK pathway sensitizes human gastric cells to doxorubicin
treatment in vitro and in vivo. Mol Med Rep. 10:3275–3281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao J, Qian CJ, Ye B, Zhang X and Liang Y:
ERK inhibition enhances TSA-induced gastric cancer cell apoptosis
via NF-κB-dependent and Notch-independent mechanism. Life Sci.
91:186–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schweyer S, Soruri A, Meschter O, Heintze
A, Zschunke F, Miosge N, Thelen P, Schlott T, Radzun HJ and Fayyazi
A: Cisplatin-induced apoptosis in human malignant testicular germ
cell lines depends on MEK/ERK activation. Br J Cancer. 91:589–598.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xia Z, Dickens M, Raingeaud J, Davis RJ
and Greenberg ME: Opposing effects of ERK and JNK-p38 MAP kinases
on apoptosis. Science. 270:1326–1331. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang P, Han J and Hui L: MAPK signaling
in inflammation-associated cancer development. Protein Cell.
1:218–226. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haagenson KK and Wu GS: The role of MAP
kinases and MAP kinase phosphatase-1 in resistance to breast cancer
treatment. Cancer Metastasis Rev. 29:143–149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Zhang H, Sun L, Gao Y, Jin H, Liang
S, Wang Y, Dong M, Shi Y, Li Z and Fan D: ERK/MAPK activation
involves hypoxia-induced MGr1-Ag/37LRP expression and contributes
to apoptosis resistance in gastric cancer. Int J Cancer.
127:820–829. 2010.PubMed/NCBI
|
|
21
|
Zheng C, Jiao X, Jiang Y and Sun S: ERK1/2
activity contributes to gemcitabine resistance in pancreatic cancer
cells. J Int Med Res. 41:300–306. 2013. View Article : Google Scholar : PubMed/NCBI
|