Introduction
Human epidermal growth factor receptor 2 (HER2), is
overexpressed in ~25% of patients with metastatic breast carcinoma
and a number of other human cancer types, including gastric, lung,
ovarian, bladder and kidney carcinomas (1–3). As a therapeutic
target for HER2-overexpressing cancer (4), monoclonal antibodies have been developed
to target HER2-positive tumors (5–7). For example, trastuzumab and
pertuzumab have already been approved clinically for HER2-positive
breast cancer (8,9). Trastuzumab and pertuzumab are able to
directly inhibit HER2 activities and induce antibody-dependent
cell-mediated cytotoxicity. The two antibodies may increase the
survival time when combined with chemotherapy in patients with
HER2-overexpressing breast cancer (10–12). However, for the
majority of patients with metastatic breast cancer, the tumors
eventually resist trastuzumab, and certain patients do not respond
to treatment even with HER2 overexpression (13,14).
In order to improve the therapeutic effect of
antibodies, a number of approaches have been studied, including
antibody conjugate TDM1 (15).
Another approach is to directly engage immune cells to attack tumor
cells. As T cells serve an important function in the killing of
tumor cells (16–20), bispecific antibody that recruits T cells to
kill tumor cells is of interest and has been investigated for
cancer therapy (21–23). For example, blinatumomab, a bispecific T
cell engager antibody (BiTE), has been approved for the treatment
of B-cell leukemia (23). Numerous
bispecific antibodies targeting different tumor biomarkers,
including HER2, have also been reported (24–27).
The present study reports on a T-cell engaging
bispecific antibody, cluster of differentiation (CD)3-S-Fab, which
targets HER2 tumor cells. Distinct from previous studies (28–30),
CD3-S-Fab was designed by linking a camel anti-HER2 single-domain
antibody (VHH) to the C-terminal of a conventional anti-CD3
antigen-binding fragment (Fab). The CD3-S-Fab may be expressed and
purified from Escherichia coli. To improve the purification
process, different expression and purification schemes were tested,
and it was identified that CD3-S-Fab may be secreted and purified
directly from E. coli medium with high efficiency. The
purified CD3-S-Fab is able to recruit T cells to kill HER2-positive
tumor cells specifically. The data gathered in the present study
demonstrate that CD3-S-Fab may present a feasible approach to
produce bispecific antibodies on a large scale.
Materials and methods
Plasmids
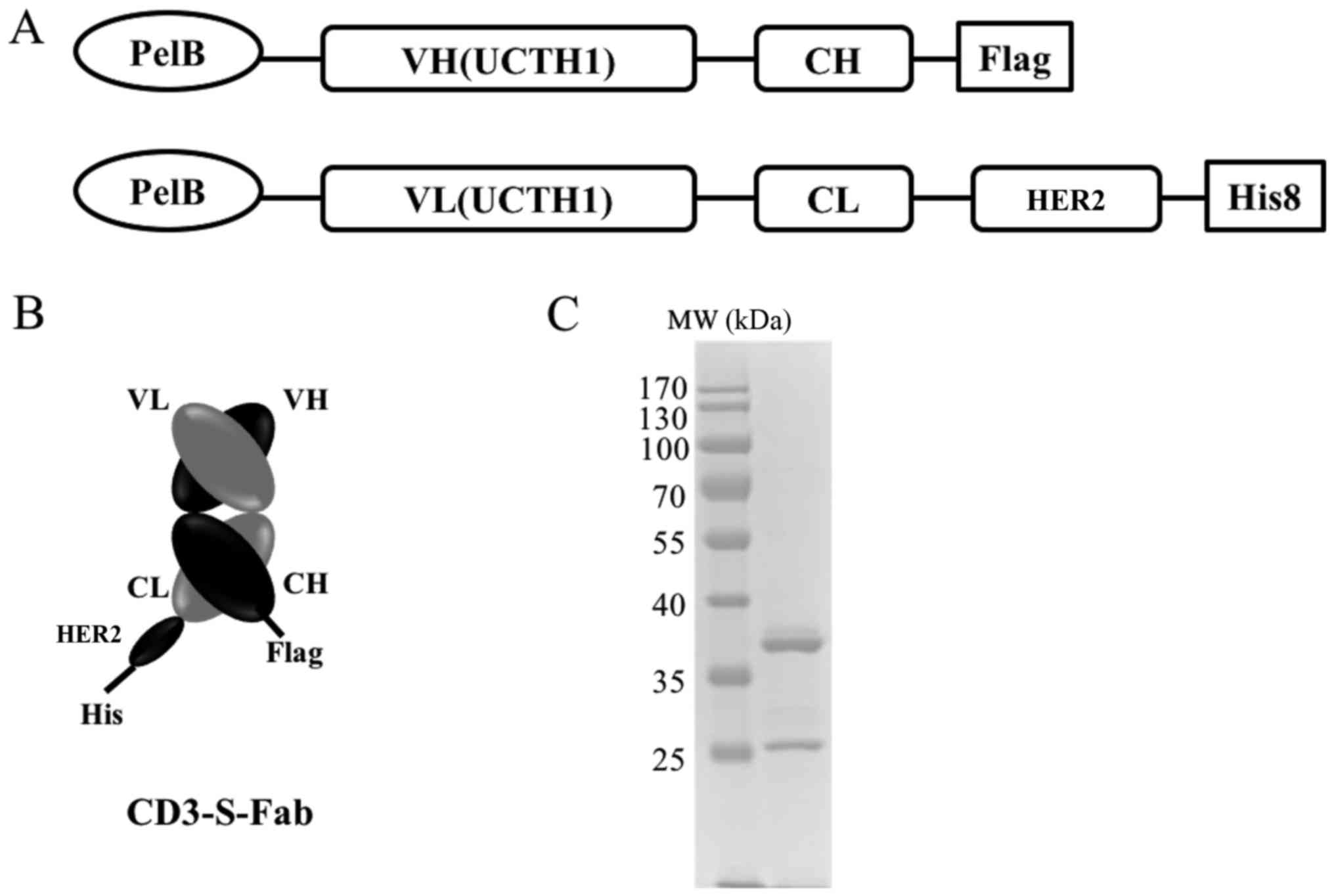
To make the CD3-S-Fab bispecific antibody, the
VH-CH1 and VL-CL of anti-CD3 UCHT1 clone (31) were synthesized (Genscript Biotech.,
Nanjing, China). The VH-CH1 was cloned into the pET26b plasmid
(Addgene, Inc., Cambridge, MA, USA) through restriction enzyme
cutting site NcoI and BamHI (Fig. 1A). The VL-CL of anti-CD3 UCHT1 was
linked with the single domain anti-HER2 VHH (8), and then cloned into the pET21a (Addgene,
Cambridge, MA, USA) through restriction enzyme cutting site
NcoI and XhoI (Fig.
1A). The pelB signal sequence
(5′-ATGAAATACCTGCTGCCGACCGCTGCTGCTGGTCTGCTGCTCCTCGCTGCCCAGCCGGCGATGGCCATGG-3′)
was synthesized (Genscript Biotech., Nanjing, China) and added to
the N-terminals of the two constructs for periplasmic expression
(32,33). A Flag-tag or His-tag (Genscript,
Nanjing, Jiangsu, China) was added to the C-terminals for easy
detection.
Bispecific antibody expression and
purification
In order to purify the CD3-S-Fab protein, E.
coli BL21(DE3) competent cells were transformed with the two
plasmids encoding VH-CH1 and VL-CL-HER2VHH. Briefly, competent
cells and plasmids were mixed and incubated at 42°C for 45 sec,
then cooled on ice for 2 min. After incubating cells for 1 h
(37°C), cells were spread on lysogeny broth (LB) plates and
incubated at 37°C for 12 h. For periplasmic expression, the
bacteria were cultured in (LB) medium (10 g/l tryptone, 5 g/l yeast
extract and 10 g/l NaCl; Sangon Biotech; Shanghai, China) with
antibiotics (0.1 g/l Ampicillin plus 0.05 g/l Kanamycin) at 37°C
until the optical density at a wavelength of 600 nm
(OD600) (measured by NanoDrop2000; Thermo Fisher
Scientific, Inc.). Next, isopropyl β-D-1-thiogalactopyranoside
(IPTG) was added to a final concentration of 0.1 mM to induce
protein expression, and cell growth was continued for an additional
20 h at 16°C or 4 h at 37°C using constant rotary incubator
(Zhicheng Inc; Shanghai, China) at 180 rpm. Periplasmic protein
purification was performed as described previously (34). Briefly, cells were harvested with
centrifugation at 4,000 × g for 30 min at 4°C) and the cell pellet
was resuspended in a chilled sucrose solution (20 mM Tris-HCl pH
8.0; 25% (w/v) sucrose; 1 mM EDTA). Following incubation on ice for
15 min with occasional agitation, the suspension was then
centrifuged at 8,500 × g for 20 min at 4°C. The supernatant was
collected as the sucrose fraction. The cells were resuspended again
and incubated in chilled periplasmic solution (5 mM
MgCl2) for an additional 30 min. Following
centrifugation (20,000 × g, 4°C for 30 min), the supernatant was
collected as the periplasmic fraction.
To test the secreted expression, M9 minimal medium
(Sangon Biotech Co., Ltd., Shanghai, China) was used as described
previously (32,35). Briefly, the bacteria transformed with
the two plasmids were cultured in LB medium with antibiotics at
37°C. The culture was then transferred to M9 minimal medium (12.8
g/l Na2HPO4, 3.0 g/l
KH2PO4, 0.5 g/l NaCl, 2.0 g/l
NH4Cl, 20 g/l glucose, 0.1 mM CaCl2, 1.0 mM
MgSO4 and 10 µM FeCl3), and incubated at 37°C
and 220 rpm in a rotary shaker. When the cell culture reached an
OD600 of 2.7–2.9, IPTG (final concentration, 1 mM) and
Tris-HCl (final concentration, 180 mM) were added to induce protein
expression and secretion. Following culture for another 24 h at
16°C and 220 rpm in a rotary shaker, the cells were removed by
centrifugation (4,000 × g, 4°C, 30 min followed by 20,000 × g, 4°C,
30 min) and the supernatant was recovered and processed for
purification as follows:
CD3-S-Fab was purified from the combined sucrose and
periplasmic fractions or protein containing medium using Ni-NTA
agarose (cat. no., NINTA-300; Molecular Cloning Laboratories, San
Francisco, CA, USA) via a C-terminal His8-Tag. Purified protein was
then further analyzed by SDS-PAGE. Briefly, 10 µg per protein
sample was separated on 12% SDS-PAGE gel under reducing conditions
by adding 2 uM 2-mercaptoethanol, then the gel was stained by
coomassie brilliant blue solution for 1 h at room temperature
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). After destaining,
the gel with water 3 times for 5 min each, the gel was photographed
by ChemiDoc XRS (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The concentration of purified protein was determined by
NanoDrop2000 (Thermo Fisher Scientific, Inc.).
Cell lines and animals
All cell lines, namely CHO, SKBR-3 and LS174T
(HER2+) cells, and Jurkat T cells, were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). SKBR-3 cells were cultured in Dulbeccos modified Eagles
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
with 10% heat-inactivated fetal bovine serum (HI-FBS; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin. LS174T,
CHO and Jurkat cells were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) with 10% HI-FBS (Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin/streptomycin. All cells
were incubated at 37°C in a humidified incubator with 5%
CO2.
A total of 10 of non-obese diabetic-severe combined
immunodeficiency disease (NOD/SCID) mice (female, ~18 g,
6-week-old) were purchased from Beijing Vital River Laboratory
Animal Technology, Co, Ltd. (Beijing, China) and housed in the
Animal Experiment Center of Sun Yat-Sen University (Guangzhou,
China) under sterile and standardized environmental conditions
(20–26°C room temperature, free access to food and water, 40–70%
relative humidity and 12 h light-dark cycle). Animal care and
experimental procedures were approved by the Institutional Animal
Care and Use Committee, Sun Yat-Sen University.
Flow cytometry analysis
Flow cytometry analysis was performed as described
previously (8,29). Briefly, aliquots of 1×106
cells were collected and mixed in ice-cold PBS with 0.2% bovine
serum albumin (BioTeK China, Beijing, China) in the absence or
presence of CD3-S-Fab (final concentration of 50 µg/ml). The
mixture was then incubated on ice for 1 h, followed by washing
twice with ice-cold PBS. The cells were then incubated on ice for 1
h with goat-anti-human immunoglobulin (Ig)G (H+L)-AF488 (1:200,
cat. no. A11013; Invitrogen; Thermo Fisher Scientific, Inc.) as the
secondary antibody. The cells were also incubated with
anti-CD3-FITC antibody (5 µl/test, BioLegend, Inc., San Diego, CA,
USA; cat. no. 317306), anti-HER2-PE antibody (5 µl/test, cat. no.
340552; BD Biosciences, Franklin lakes, NJ, USA) or goat-anti-human
IgG (H+L)-AF488 (1:200, cat. no. A11013; Invitrogen; Thermo Fisher
Scientific, Inc.) on ice for 1 h. After the cells were washed twice
by cold PBS, flow cytometry analysis was performed by the Cytomics
FC500 Flow Cytometer (Beckman Coulter, Inc., Brea, CA USA).
Isolation of T cells from peripheral
blood mononuclear cells (PBMCs)
Human PBMCs were retrospectively obtained from
healthy donors from the Guangzhou Blood Centre (Guangzhou, China),
which provided informed consent (approval no. SYSU-2015-289) using
Ficoll-Paque PLUS (cat. no. 17-1440-03; GE Healthcare, Chicago, IL,
USA) density centrifugation, as described previously (36). The use of the cells was approved by
the Health and Family Planning Commission of Guangdong Province. In
brief, 25 ml two-fold diluted peripheral blood from healthy donors
was layered on 15 ml Ficoll-Paque PREMIUM and centrifuged at 400 ×
g for 30 min at room temperature. PMBCs were collected and washed
three times with PBS. T cells were then enriched from PBMCs using
an EasySep™ Human CD3 Positive Selection kit (Stemcell
Technologies, Inc., Vancouver, BC, Canada) as descried previously
(37). The isolated T cells were
cultured in complete RPMI 1640 with 10% FBS and 1%
penicillin/streptomycin at 37°C in 5% CO2 prior to
usage.
Cytotoxic assays
Cytotoxicity assays were performed as described
previously (29). Briefly, SKBR3,
LS174T and CHO cells were used as target cells. T cells without
prior stimulation were used as effector cells. A total of 5,000
cells/well of target cells (100 µl) was plated in 96-well plates in
triplicate. Following a 6–24 h incubation period, an equal volume
of CD3+ T cells (50,000 cells/well) or complete RPMI
1640 medium were added to each well. The CD3-S-Fab and Trastuzumab
(a gift from Alphamab, Suzhou, China), which is an approved
monoclonal antibody to treat HER2 positive patients with breast
cancer, ranging from 1.56×102 to 1.56×10−5
nM, were then added. After 72 h of incubation, cell viability was
quantified using cell counting kit (cat. no. CK04; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) according to the
manufacturer's protocol. The survival rate (%) of target cells was
calculated using the following formula: [(live target cells
(sample)-medium)/(live target cells (control)-medium)] ×100.
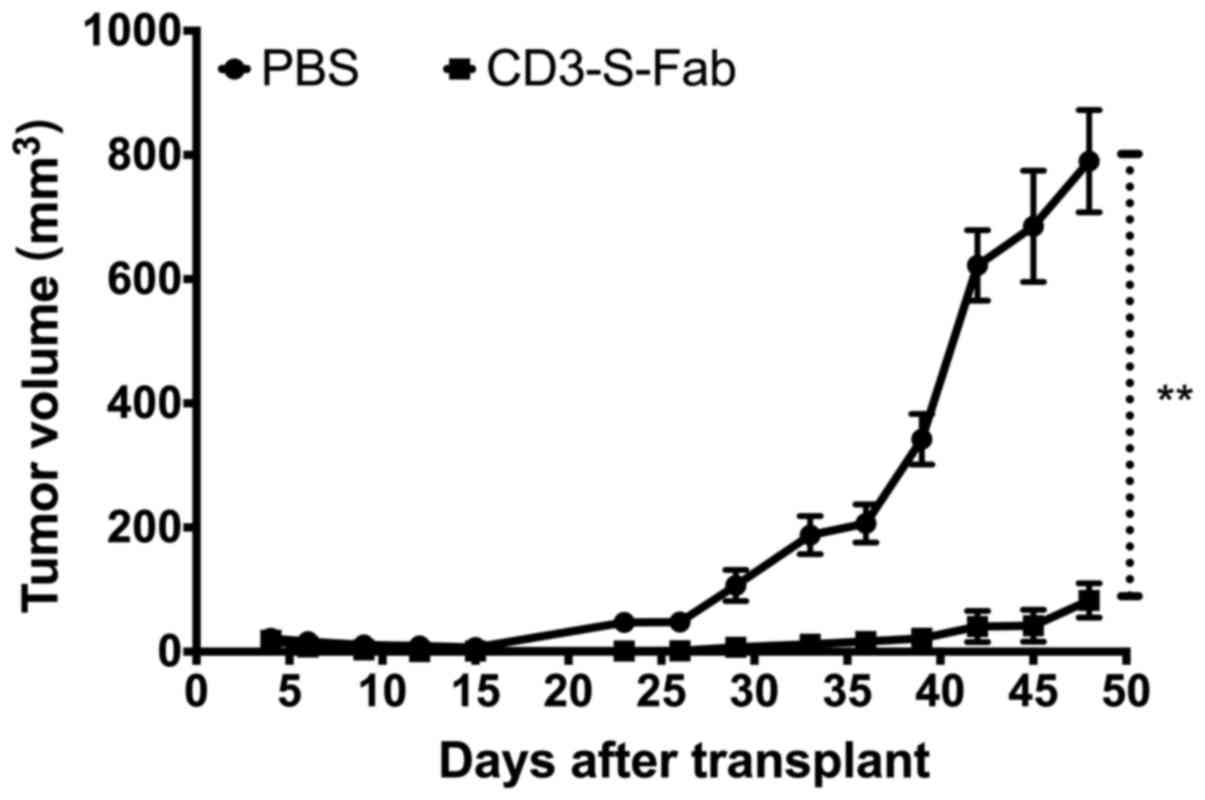
In vivo efficacy studies
In vivo efficacy studies were performed as
described previously, with modifications (18,38).
Briefly, HER2-positive SKOV3 cells were harvested and then mixed
with freshly isolated human PBMCs. Cell suspensions were injected
subcutaneously into the right flank of NOD/SCID mice in a total
volume of 0.2 ml/mouse (mixtures of 2×106 SKOV3 cells
and 1×107 human PBMCs). The mice were grouped into
control group (PBS) and treatment group (CD3-S-Fab) randomly, 5
mice per group. The first antibody treatment (1 mg/kg) was at 2 h
post-transplantation. The animals were then treated daily (1 mg/kg)
over the following 7 days. Tumor volume was measured daily. Mice
were sacrificed when the tumor volume reached 1,500 mm3.
All results are presented as the mean for each group.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 7.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
Statistical analysis was performed using Student's t-test, except
for the T cell-mediated cytotoxicity assay, in which two-way ANOVA
followed by Dunnett's multiple comparisons test was employed. A
non-linear regression analysis was used in Fig. 3B-E. P<0.05 was considered a
statistically significant difference. Data are presented as the
mean ± standard error of the mean unless otherwise noted.
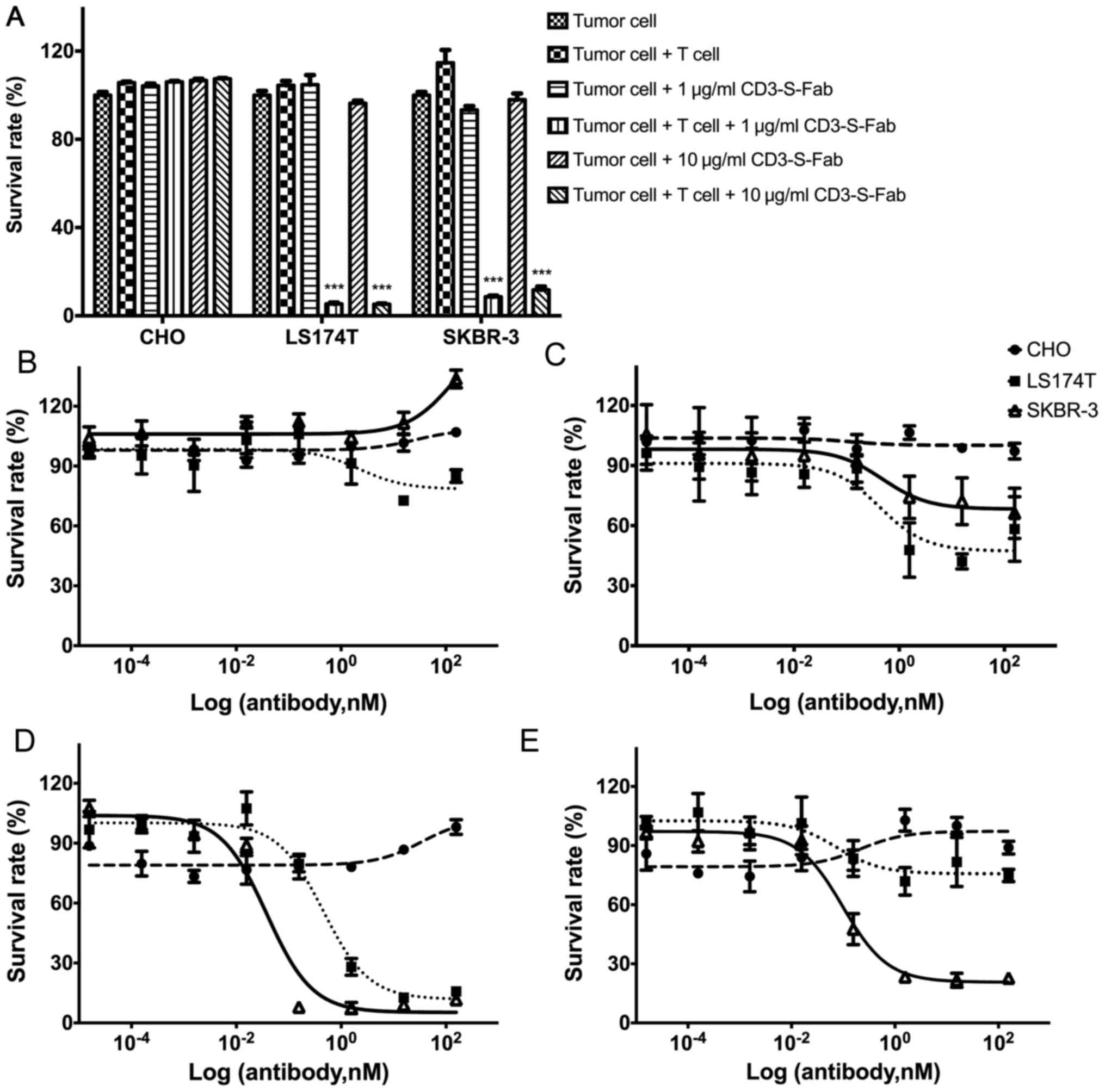 | Figure 3.CD3-S-Fab kills tumor cells in a T
cell-dependent and tumor antigen-dependent manner. (A) Tumor cells
(5,000 cells/well) alone or with T cells (50,000 cells/well) were
incubated together for 72 h in the presence of the indicated
concentrations of CD3-S-Fab (1 or 10 µg/ml). Cytotoxic activity was
measured as described in the Materials and methods section. Data
are presented as the mean ± SEM (n=3, ***P<0.001 vs. tumor cells
+ T cells, two-way analysis of variance followed by Dunnetts
multiple comparisons test). Dose response measurement of CD3-S-Fab
with CHO (circle), LS174T (square) and SKBR-3 (triangle) cells.
Dose-response curves were assessed using a non-linear regression,
log (inhibitor) vs. response using GraphPad Prism software; (B) in
the absence of T cells with different concentrations of CD3-S-Fab,
(C) in the presence of T cells with different concentrations of
CD3-S-Fab, (D) in the absence of PBMCs with different
concentrations of Trastuzumab, and (E) in the presence of PBMCs
with different concentrations of Trastuzumab. All measurements were
normalized against tumor cells only; data points in the figure
represent the mean of three samples and error bars represent the
SEM. SEM, standard error of the mean; PBMC, peripheral blood
mononuclear cell; CD, cluster of differentiation. |
Results
CD3-S-Fab may be secreted and purified
from E. coli culture medium
CD3-S-Fab was designed by genetically linking an
anti-HER2 VHH at the c-terminal of anti-CD3 VL-CL (Fig. 1A). Anti-CD3 VH-CH1 and anti-CD3
VL-CL-VHH were cloned into pET26b and pET21a, respectively. The
pelB signal peptide was added to the N-terminal of the two
constructs for periplasmic expression and secretion in E.
coli. The CD3-S-Fab was formed via the heterodimerization of
VH-CH1/VL-CL-VHH (Fig. 1A).
Periplasmic purification was tested first by
adjusting the IPTG concentrations and culture temperature. The
optimal expression with improved solubility of CD3-S-Fab was
achieved by lowering the induction temperature (0.1 mM IPTG, 16°C
induction for 24 h; data not shown). However, the yield of
CD3-S-Fab remained low with a yield of ~0.4 mg per 6 liters LB
medium following Ni-NTA affinity purification.
To increase the yield of CD3-S-Fab, extracellular
expression of CD3-S-Fab was tested (39,40).
Compared with the periplasmic expression, the yield of CD3-S-Fab
recovered from the M9 medium was ~0.6 mg per 200 ml medium. The
secreted CD3-S-Fab was also able to be purified by Ni-NTA-agarose
affinity chromatography as heterodimers (Fig. 1C). Thus, CD3-S-Fab may be secreted and
purified from E. coli culture medium.
CD3-S-Fab binds CD3- and HER2-positive
cells
In order to confirm whether CD3-S-Fab maintains the
ability to bind CD3-positive T cells, flow cytometry analysis was
conducted using CD3-positive Jurkat cells and CD3-negative CHO
cells. CD3-S-Fab was not able to bind CHO cells based on flow
cytometry analysis (Fig. 2A), but was
able to bind Jurkat cells (Fig. 2B),
suggesting that CD3-S-Fab may bind human T cells.
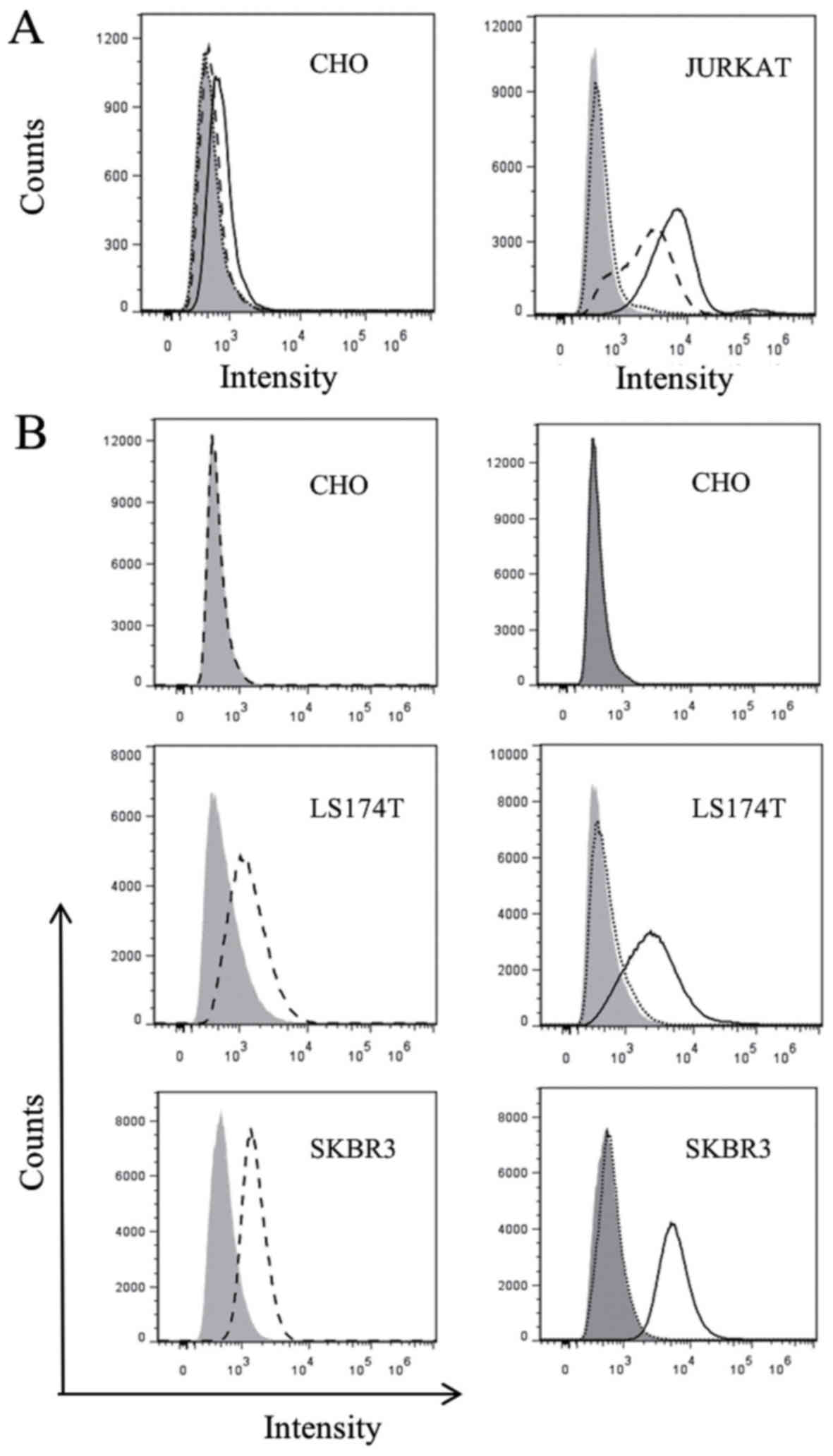 | Figure 2.Purified S-Fab can recognize T cells
and HER2 positive cells. (A) Flow cytometry analysis with CD3-S-Fab
(black line), positive control anti-CD3-FITC (dash line) or
staining with only anti-human IgG-AF488 staining (dotted line), on
CD3-negative CHO cells (left panel) and CD3-positive Jurkat cells
(right panel). (B) Flow cytometry analysis with positive control
anti-HER2-PE (A fluorescent protein) (dash line, left panels),
CD3-S-Fab (black line, right panels) or staining with only
anti-human IgG-AF488 (dotted line), on HER2-negative CHO cells (top
panel) and HER2-positive cell lines, LS174T and SKBR3 (bottom
panels). HER2, human epidermal growth factor receptor 2; Ig,
immunoglobulin; CD, cluster of differentiation. |
To confirm the binding of CD3-S-Fab to HER2-positive
tumor cells, HER2-positive cell lines, SKBR3 and LS174T, and the
HER2-negative cell line CHO, were used for flow cytometry analysis.
Flow cytometry analysis revealed that CD3-S-Fab did not bind to CHO
cells, but that it did bind to SKBR3 and LS174T cells (Fig. 2B, right panels). These data suggest
that CD3-S-Fab is able to specifically bind to HER2-positive
cells.
CD3-S-Fab has T-cell-mediated
cytotoxicity against HER2-positive cells
In order to evaluate whether CD3-S-Fab is able to
mediate HER2 tumor cell killing, HER2-positive and HER2-negative
cell lines were used. CD3-S-Fab did not lead to cytotoxicity in the
HER2-negative cell line CHO (Fig. 3A and
B). For the HER2-positive cell lines LS174T and SKBR3, T cells
alone or CD3-S-Fab alone have no effects on cell viability
(Fig. 3B). However, CD3-S-Fab induced
potent cytotoxicity when the LS174T and SKBR3 cells were incubated
with CD3-S-Fab and T cells (Fig. 3A).
Cell number and morphology observed under microscopy also confirmed
the specific killing in the presence of CD3-S-Fab and T cells.
To further evaluate the cytotoxic activity of
CD3-S-Fab on tumor cells, the dose responses of different cell
lines were measured. No cell killing was observed for CD3-S-Fab in
the absence of T cells (Fig. 3B).
With T cells present, CD3-S-Fab exhibited active cell killing in
HER2-positive LS174T cells and SKBR3 cells (Fig. 3C). This is distinct from Trastuzumab,
which demonstrated partial inhibition in the absence of PBMCs
(Fig. 3D), and higher cell killing in
the presence of PBMCs (Fig. 3E).
These results suggest that CD3-S-Fab exhibits potent cytotoxic
activity against HER2-positive tumor cells in the presence of T
cells.
CD3-S-Fab inhibits tumor growth in
vivo
To analyze the in vivo antitumor effect of
CD3-S-Fab, SKOV3 cells were mixed with freshly isolated human PBMCs
and engrafted subcutaneously into NOD/SCID mice. The mice were then
treated with either PBS or CD3-S-Fab. Compared with animals only
treated with PBMCs, significant tumor growth inhibition was
observed in mice treated with CD3-S-Fab (Fig. 4). Minimal tumor growth was observed in
mice treated with CD3-S-Fab, even 5 weeks after treatment ended.
These data demonstrated that CD3-S-Fab was able to inhibit
HER2-positive tumor growth in xenograft mice.
Discussion
Cancer immunotherapy has demonstrated lasting
clinical benefits in patients with cancer (41). Besides checkpoint antibodies, a
variety of approaches have been actively studied as cancer
immunotherapies. Among them, bispecific antibodies have
demonstrated some promise. For example, blinatumomab, a BiTE, has
already been approved for the treatment of B-cell leukemia, with
excellent efficacy (18–21,23,42).
HER2 is one of the most studied oncogenes.
Antibodies or small molecule inhibitors have exhibited clinical
efficacy by inhibiting HER2 activity. Besides functioning as an
oncogene, HER2 also presents as an excellent tumor antigen, as it
is overexpressed in numerous tumors and is rarely expressed in
normal tissues (4,8,43–46). Vaccines, bispecific antibodies and
other approaches have been studied to further improve the clinical
outcomes of current HER2 therapeutics.
Different formats of anti-HER2 bispecific antibodies
have been studied previously (15,25,28,45,47,48),
including recruitment of T cells in various bispecific antibody
formats (17,19,49,50).
However, those bispecific antibodies present a number of
challenges, including a mixed population during purification, a low
yield of production, a tendency to aggregate and a short half-life.
Previously, it was demonstrated that an S-Fab bispecific antibody
against carcinoembryonic antigens demonstrated several advantages,
including excellent efficacy, and reasonable expression and
solubility in E. coli (29).
In the present study, CD3-S-Fab, the bispecific antibody targeting
HER2, is described. The purified bispecific antibody CD3-S-Fab can
be used for the redirection of T cells toward HER2-positive tumor
cells, and was demonstrated to be efficient in vitro and
in vivo at killing HER2-positive cancer cells.
Although CD3-S-Fab may be produced in E.
coli, the yield of CD3-S-Fab was very low based on purification
from periplasmic fractions. Recombinant antibodies are commonly
produced by eukaryotic cells or periplasmic expression of
gram-negative bacteria (8,18,21,35,50,51).
Due to the easy culture and low cost, E. coli has been
widely used as an expression host for recombinant proteins.
However, the high yield of correctly folded proteins is a frequent
problem, and the purification process of the recombinant proteins
is also complicated. In the present study, the defined M9 medium
was used to facilitate the secretion of CD3-S-Fab complex into the
medium, and then the recombinant antibody was purified directly
from this medium. This purification schedule greatly increased the
yields of CD3-S-Fab.
Compared with the intracellular expression of
recombinant proteins, extracellular expression exhibits several
advantages, including the following: i) It is efficient at
obtaining correctly folded proteins; ii) the secreted proteins are
less likely to be degraded by various proteases in the periplasm;
iii) secretion reduces the cellular burden for cell growth when a
large amount of recombinant protein is produced; and iv) The
process of purification is easier due to the elimination of
cellular component contamination (33,40,52,53).
In summary, the novel bispecific antibody CD3-S-Fab
can be used for the redirection of T cells toward HER2-positive
tumor cells and is efficient at killing HER2-positive cancer cells
in vitro and in vivo. The easy purification and high
yield of CD3-S-Fab suggests this format may be applied to other
bispecific antibodies.
Acknowledgements
The authors would like to thank Dr. Jiang Li at the
Sun Yat-Sen University School of Medicine and Dr. Wei Xie at the
Sun Yat-Sen University School of Life Sciences, for their technical
support.
Funding
This study was financially supported by the R&D
Plan of Guangdong Province (China) (grant no. 20160503).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
LL, LLi, CZ, JL, JLiu, RS, and BD, performed the
experiments. LL, QL and ZW designed the experiments, and wrote the
manuscript.
Ethics approval and consent to
participate
The use of animals was approved by the Institutional
Animal Care and Use Committee, Sun Yat-Sen University (Guangzhou,
China). (Approve No. IACUC-DD-18-02-01). The use of human blood was
approved by Health and Family Planning Commission of Guangdong
Province (approval no. SYSU 2015-289).
Consent for publication
The PBMCs were from provided from Health and Family
Planning Commission of Guangdong Province (SYSU 2015-289) with
consent from healthy donors.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Slamon DJ, Clark GM, Wong SG, Levin WJ,
Ullrich A and McGuire WL: Human breast cancer: Correlation of
relapse and survival with amplification of the HER-2/neu oncogene.
Science. 235:177–182. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Press MF, Pike MC, Hung G, Zhou JY, Ma Y,
George J, Dietz-Band J, James W, Slamon DJ, Batsakis JG, et al:
Amplification and overexpression of HER-2/neu in carcinomas of the
salivary gland: Correlation with poor prognosis. Cancer Res.
54:5675–5682. 1994.PubMed/NCBI
|
|
3
|
Daniele L and Sapino A: Anti-HER2
treatment and breast cancer: State of the art, recent patents, and
new strategies. Recent Pat Anticancer Drug Discov. 4:9–18. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yarden Y: Biology of HER2 and its
importance in breast cancer. Oncology. 61 Suppl 2:S1–S13. 2001.
View Article : Google Scholar
|
|
5
|
Ben-Kasus T, Schechter B, Lavi S, Yarden Y
and Sela M: Persistent elimination of ErbB-2/HER2-overexpressing
tumors using combinations of monoclonal antibodies: Relevance of
receptor endocytosis. Proc Natl Acad Sci USA. 106:3294–3299. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Keler T, Graziano RF, Mandal A, Wallace
PK, Fisher J, Guyre PM, Fanger MW and Deo YM: Bispecific
antibody-dependent cellular cytotoxicity of HER2/neu-overexpressing
tumor cells by Fc gamma receptor type I-expressing effector cells.
Cancer Res. 57:4008–4014. 1997.PubMed/NCBI
|
|
7
|
Vasconcellos FA, Aleixo PB, Stone SC,
Conceicao FR, Dellagostin OA and Aleixo JA: Generation and
characterization of new HER2 monoclonal antibodies. Acta Histochem.
115:240–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vaneycken I, Devoogdt N, Van Gassen N,
Vincke C, Xavier C, Wernery U, Muyldermans S, Lahoutte T and
Caveliers V: Preclinical screening of anti-HER2 nanobodies for
molecular imaging of breast cancer. FASEB J. 25:2433–2446. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hicks DG and Kulkarni S: HER2+ breast
cancer: Review of biologic relevance and optimal use of diagnostic
tools. Am J Clin Pathol. 129:263–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ranson M and Sliwkowski MX: Perspectives
on anti-HER monoclonal antibodies. Oncology. 63 Suppl 1:S17–S24.
2002. View Article : Google Scholar
|
|
11
|
Hudis CA: Trastuzumab-mechanism of action
and use in clinical practice. N Engl J Med. 357:39–51. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spector NL and Blackwell KL: Understanding
the mechanisms behind trastuzumab therapy for human epidermal
growth factor receptor 2-positive breast cancer. J Clin Oncol.
27:5838–5847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valabrega G, Montemurro F and Aglietta M:
Trastuzumab: Mechanism of action, resistance and future
perspectives in HER2-overexpressing breast cancer. Ann Oncol.
18:977–984. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Junttila TT, Parsons K, Olsson C, Lu Y,
Xin Y, Theriault J, Crocker L, Pabonan O, Baginski T, Meng G, et
al: Superior in vivo efficacy of afucosylated trastuzumab in the
treatment of HER2-amplified breast cancer. Cancer Res.
70:4481–4489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arteaga CL, Sliwkowski MX, Osborne CK,
Perez EA, Puglisi F and Gianni L: Treatment of HER2-positive breast
cancer: Current status and future perspectives. Nat Rev Clin Oncol.
9:16–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benchetrit F, Gazagne A, Adotevi O,
Haicheur N, Godard B, Badoual C, Fridman WH and Tartour E:
Cytotoxic T lymphocytes: Role in immunosurveillance and in
immunotherapy. Bull Cancer. 90:677–685. 2003.PubMed/NCBI
|
|
17
|
Nagorsen D, Bargou R, Ruttinger D, Kufer
P, Baeuerle PA and Zugmaier G: Immunotherapy of lymphoma and
leukemia with T-cell engaging BiTE antibody blinatumomab. Leuk
Lymphoma. 50:886–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Junttila TT, Li J, Johnston J,
Hristopoulos M, Clark R, Ellerman D, Wang BE, Li Y, Mathieu M, Li
G, et al: Antitumor efficacy of a bispecific antibody that targets
HER2 and activates T cells. Cancer Res. 74:5561–5571. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Baeuerle PA and Reinhardt C: Bispecific
T-cell engaging antibodies for cancer therapy. Cancer Res.
69:4941–4944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schlereth B, Fichtner I, Lorenczewski G,
Kleindienst P, Brischwein K, da Silva A, Kufer P, Lutterbuese R,
Junghahn I, Kasimir-Bauer S, et al: Eradication of tumors from a
human colon cancer cell line and from ovarian cancer metastases in
immunodeficient mice by a single-chain Ep-CAM-/CD3-bispecific
antibody construct. Cancer Res. 65:2882–2889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Taki S, Kamada H, Inoue M, Nagano K, Mukai
Y, Higashisaka K, Yoshioka Y, Tsutsumi Y and Tsunoda S: A novel
bispecific antibody against human CD3 and ephrin receptor A10 for
breast cancer therapy. PLoS One. 10:e01447122015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dreier T, Lorenczewski G, Brandl C,
Hoffmann P, Syring U, Hanakam F, Kufer P, Riethmuller G, Bargou R
and Baeuerle PA: Extremely potent, rapid and
costimulation-independent cytotoxic T-cell response against
lymphoma cells catalyzed by a single-chain bispecific antibody. Int
J Cancer. 100:690–697. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oak E and Bartlett NL: Blinatumomab for
the treatment of B-cell lymphoma. Expert Opin Investig Drugs.
24:715–724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Haense N, Atmaca A, Pauligk C, Steinmetz
K, Marmé F, Haag GM, Rieger M, Ottmann OG, Ruf P, Lindhofer H and
Al-Batran SE: A phase I trial of the trifunctional anti HER2 × anti
CD3 antibody ertumaxomab in patients with advanced solid tumors.
BMC Cancer. 16:4202016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vaishampayan U, Thakur A, Rathore R,
Kouttab N and Lum LG: Phase I study of Anti-CD3 × Anti-HER2
bispecific antibody in metastatic castrate resistant prostate
cancer patients. Prostate Cancer. 2015:2851932015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cao Y, Axup JY, Ma JS, Wang RE, Choi S,
Tardif V, Lim RK, Pugh HM, Lawson BR, Welzel G, et al: Multiformat
T-cell-engaging bispecific antibodies targeting human breast
cancers. Angew Chem Int Ed Engl. 54:7022–7027. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Gou LT, Guo ZH, Liu HR, Wang JM,
Zhou SX, Yang JL and Li XA: Fully human HER2/cluster of
differentiation 3 bispecific antibody triggers potent and specific
cytotoxicity of T lymphocytes against breast cancer. Mol Med Rep.
12:147–154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li A, Xing J, Li L, Zhou C, Dong B, He P,
Li Q and Wang Z: A single-domain antibody-linked Fab bispecific
antibody HER2-S-Fab has potent cytotoxicity against HER2-expressing
tumor cells. AMB Express. 6:322016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li L, He P, Zhou C, Jing L, Dong B, Chen
S, Zhang N, Liu Y, Miao J, Wang Z and Li Q: A novel bispecific
antibody, S-Fab, induces potent cancer cell killing. J Immunother.
38:350–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vincke C, Loris R, Saerens D,
Martinez-Rodriguez S, Muyldermans S and Conrath K: General strategy
to humanize a camelid single-domain antibody and identification of
a universal humanized nanobody scaffold. J Biol Chem.
284:3273–3284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shalaby MR, Shepard HM, Presta L,
Rodrigues ML, Beverley PC, Feldmann M and Carter P: Development of
humanized bispecific antibodies reactive with cytotoxic lymphocytes
and tumor cells overexpressing the HER2 protooncogene. J Exp Med.
175:217–225. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
von Roman Freiherr M, Koller A, von Rüden
D and Berensmeier S: Improved extracellular expression and
purification of recombinant Staphylococcus aureus protein A.
Protein Expr Purif. 93:87–92. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yoon SH, Kim SK and Kim JF: Secretory
production of recombinant proteins in Escherichia coli. Recent Pat
Biotechnol. 4:23–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kwong KY and Rader C: E. coli expression
and purification of Fab antibody fragments. Curr Protoc Protein Sci
Chapter 6. Unit 6.10. 2009. View Article : Google Scholar
|
|
35
|
Skrlj N, Serbec VC and Dolinar M:
Single-chain Fv antibody fragments retain binding properties of the
monoclonal antibody raised against peptide P1 of the human prion
protein. Appl Biochem Biotechnol. 160:1808–1821. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
So EC, Sallin MA, Zhang X, Chan SL, Sahni
L, Schulze DH, Davila E, Strome SE and Jain A: A high throughput
method for enrichment of natural killer cells and lymphocytes and
assessment of in vitro cytotoxicity. J Immunol Methods. 394:40–48.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Busch R, Cesar D, Higuera-Alhino D, Gee T,
Hellerstein MK and McCune JM: Isolation of peripheral blood CD4(+)
T cells using RosetteSep and MACS for studies of DNA turnover by
deuterium labeling. J Immunol Methods. 286:97–109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rozan C, Cornillon A, Petiard C, Chartier
M, Behar G, Boix C, Kerfelec B, Robert B, Pèlegrin A, Chames P, et
al: Single-domain antibody-based and linker-free bispecific
antibodies targeting FcγRIII induce potent antitumor activity
without recruiting regulatory T cells. Mol Cancer Ther.
12:1481–1491. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Choi JH and Lee SY: Secretory and
extracellular production of recombinant proteins using Escherichia
coli. Appl Microbiol Biotechnol. 64:625–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fu XY: Extracellular accumulation of
recombinant protein by Escherichia coli in a defined medium. Appl
Microbiol Biotechnol. 88:75–86. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Scott AM, Wolchok JD and Old LJ: Antibody
therapy of cancer. Nat Rev Cancer. 12:278–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Osada T, Patel SP, Hammond SA, Osada K,
Morse MA and Lyerly HK: CEA/CD3-bispecific T cell-engaging (BiTE)
antibody-mediated T lymphocyte cytotoxicity maximized by inhibition
of both PD1 and PD-L1. Cancer Immunol Immunother. 64:677–688. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Karagiannis P, Singer J, Hunt J, Gan SK,
Rudman SM, Mechtcheriakova D, Knittelfelder R, Daniels TR, Hobson
PS, Beavil AJ, et al: Characterisation of an engineered trastuzumab
IgE antibody and effector cell mechanisms targeting
HER2/neu-positive tumour cells. Cancer Immunol Immunother.
58:915–930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lambertini M, Ponde NF, Solinas C and de
Azambuja E: Adjuvant trastuzumab: A 10-year overview of its
benefit. Expert Rev Anticancer Ther. 17:61–74. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xin Y, Guo WW, Huang Q, Zhang P, Zhang LZ,
Jiang G and Tian Y: Effects of lapatinib or trastuzumab, alone and
in combination, in human epidermal growth factor receptor
2-positive breast cancer: A meta-analysis of randomized controlled
trials. Cancer Med. 5:3454–3463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Malenfant SJ, Eckmann KR and Barnett CM:
Pertuzumab: A new targeted therapy for HER2-positive metastatic
breast cancer. Pharmacotherapy. 34:60–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zazo S, Gonzalez-Alonso P, Martin-Aparicio
E, Chamizo C, Cristóbal I, Arpí O, Rovira A, Albanell J, Eroles P,
Lluch A, et al: Generation, characterization, and maintenance of
trastuzumab-resistant HER2+ breast cancer cell lines. Am J Cancer
Res. 6:2661–2678. 2016.PubMed/NCBI
|
|
48
|
James ND, Atherton PJ, Jones J, Howie AJ,
Tchekmedyian S and Curnow RT: A phase II study of the bispecific
antibody MDX-H210 (anti-HER2 × CD64) with GM-CSF in HER2+ advanced
prostate cancer. Br J Cancer. 85:152–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu Z and Carter P: Identification of
heavy chain residues in a humanized anti-CD3 antibody important for
efficient antigen binding and T cell activation. J Immunol.
155:1903–1910. 1995.PubMed/NCBI
|
|
50
|
Loffler A, Kufer P, Lutterbüse R, Zettl F,
Daniel PT, Schwenkenbecher JM, Riethmuller G, Dörken B and Bargou
RC: A recombinant bispecific single-chain antibody, CD19 × CD3,
induces rapid and high lymphoma-directed cytotoxicity by
unstimulated T lymphocytes. Blood. 95:2098–2103. 2000.PubMed/NCBI
|
|
51
|
Qasemi M, Behdani M, Shokrgozar MA,
Molla-Kazemiha V, Mohseni-Kuchesfahani H and Habibi-Anbouhi M:
Construction and expression of an anti-VEGFR2 Nanobody-Fc
fusionbody in NS0 host cell. Protein Expr Purif. 123:19–25. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mergulhao FJ, Summers DK and Monteiro GA:
Recombinant protein secretion in Escherichia coli. Biotechnol Adv.
23:177–202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Khushoo A, Pal Y, Singh BN and Mukherjee
KJ: Extracellular expression and single step purification of
recombinant Escherichia coli L-asparaginase II. Protein Expr Purif.
38:29–36. 2004. View Article : Google Scholar : PubMed/NCBI
|