Introduction
In 1989, Rosenberg first advocated the term
‘sarcopenia’ for reductions in muscle mass due to aging (1). Sarcopenia is a syndrome characterized by
progressive and generalized loss of skeletal muscle mass (SMM) and
strength, and is associated with increased risks of adverse
outcomes such as physical disability, frailty, poor quality of
life, and mortality. Sarcopenia has been reported to relate to poor
prognosis in many malignant cancers, including hepatocellular
carcinoma (2), lung cancer (3), bladder cancer (4), pancreatic cancer (5), melanoma (6), and colorectal cancer (7,8). By
clarifying the mechanisms of sarcopenia, it may be possible to
improve the prognosis of patients with sarcopenia. In addition,
understanding the mechanisms of sarcopenia may also help elucidate
the mechanisms of cachexia.
In gastric cancer, preoperative sarcopenia has been
shown to be a predictive marker of postoperative complications and
poor prognosis (9). In two previous
studies, a 6.2% loss of skeletal muscle was observed after total
gastrectomy (10), and adjuvant
chemotherapy with S-1 was identified as an independent risk factor
for significant loss of skeletal muscle (11). Moreover, reductions of lean body
muscle mass after gastrectomy has been reported to prevent the
continuation of adjuvant chemotherapy (12). Despite this prior evidence, however,
the effect of postoperative SMM loss on long-term survival remains
unclear.
With this in mind, the aim of the present study was
to clarify: i) the factors that cause loss of SMM at 6 months
post-gastrectomy; and ii) the impact of postoperative SMM loss on
prognosis.
Patients and methods
Patients
The present study enrolled patients with gastric
cancer who underwent curative surgery with nodal dissection at
Yamaguchi University Hospital (Yamaguchi, Japan) between January
2009 and April 2016. Computed tomography (CT) was performed within
1 month before surgery and at 6 months postoperatively. Patients
who relapsed within 6 months or did not undergo CT examination at
these fixed times were excluded.
Data collection
All relevant data, including the clinical, surgical,
and pathological records, were collected retrospectively from the
database of our hospital. All cancers were staged in accordance
with the tumor node metastasis (TNM) classification system used in
the 14th edition of the Japanese Gastric Cancer Classification.
Measurement of SMM
A skeletal muscle area was measured retrospectively
on CT scans performed before gastrectomy and 6 months after
surgery. The muscle area was evaluated at the level of the third
lumbar vertebra (L3) in the inferior direction, with the patient in
the supine position. Cross-sectional areas (cm2) of
skeletal muscles in the L3 region were measured using AZE Virtual
Place image analysis software (AZE, Tokyo, Japan). SMM was
identified by Hounsfield unit thresholds of −30 to +150 on CT. A
SMM index was defined such that the cross-sectional areas were
normalized for the patient height
(cm2/m2).
Statistical analysis
Statistical analyses were performed using SPSS
version 22 for Windows (IBM Corp., Armonk, NY, USA). The percentage
loss of skeletal body muscle mass was defined as follows:
Percentage loss of SMM=(skeletal body muscle mass 6 months after
surgery/preoperative skeletal body muscle mass) ×100. Subsequently,
the patients were divided into two groups based on the percentage
loss of SMM: <5 vs. ≥5%. Each of the remaining cutoff values,
including the operation time, surgical blood loss, and SMM, was set
at the median point of the recorded values. Surgical complications
were classified according to the Clavien-Dindo classification.
Differences between groups were analyzed using the
t-test or χ2 test, as appropriate. Univariate and
multivariate logistic regression analyses were performed to
identify risk factors for loss of SMM. Cox proportional hazards
regression models were used to identify variables associated with
relapse-free survival (RFS) or overall survival (OS). Backward
stepwise elimination with a threshold of P=0.10 was used to select
the variables to be included. Survival curves were generated using
the Kaplan-Meier method and compared using the log-rank test. For
all analyses, P<0.05 was considered to indicate a statistically
significant difference.
Results
Patients
From January 2009 to April 2016, a total of 119
patients met our inclusion criteria and were included in the
analysis. The median follow-up time for these patients was 30.9
months (range; 7.3–94.0 months).
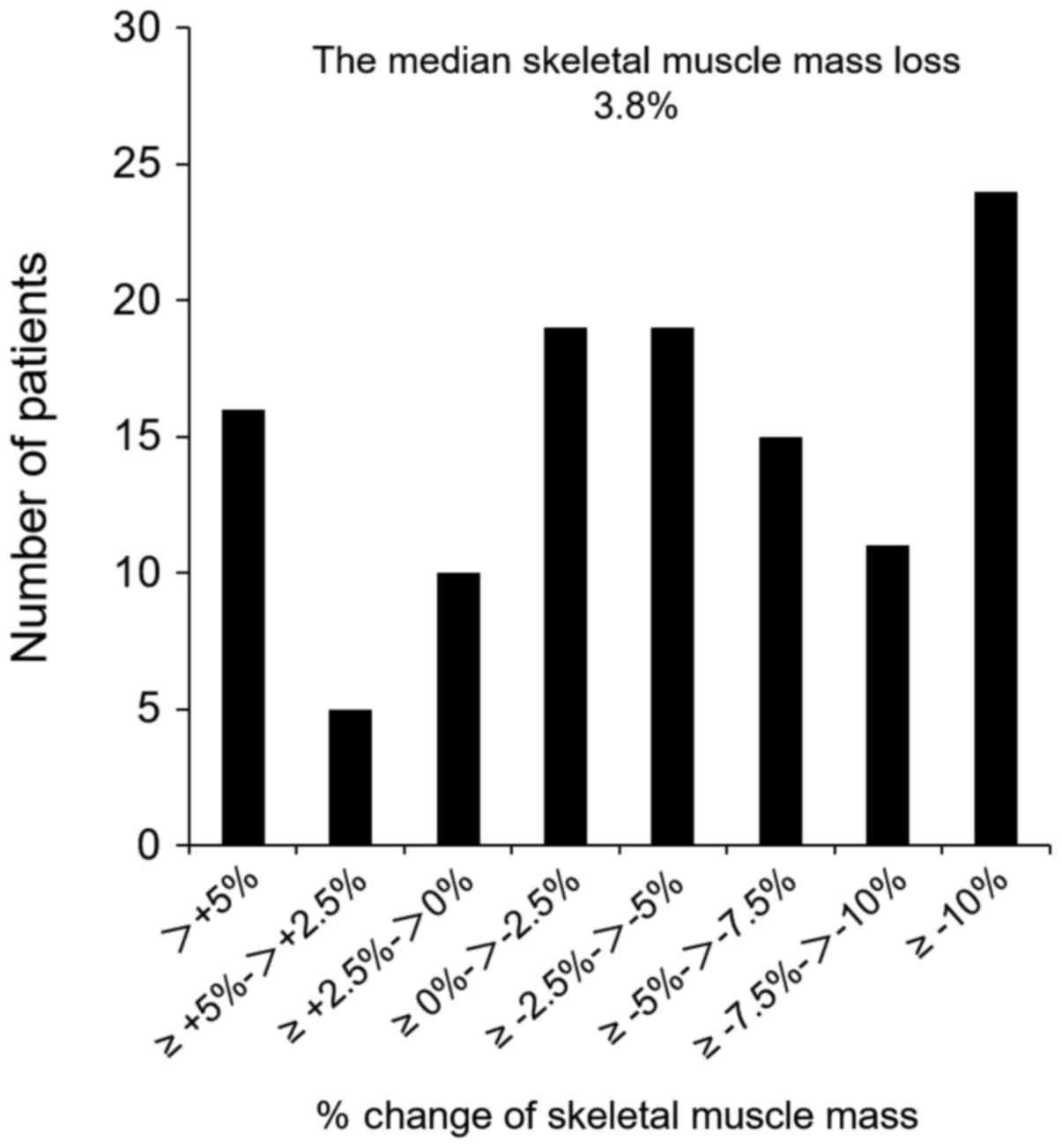
The median loss of SMM was 3.8%. Histograms showing
the loss of SMM in this study are presented in Fig. 1. A SMM loss of ≥5% was noted in 51
patients (42.9%). Patients with a SMM loss of ≥5% showed a
significantly higher preoperative SMM index than those with a SMM
loss <5% (P=0.005), and showed a significantly higher frequency
of total gastrectomy (P=0.01; Table
I).
 | Table I.Comparison of the clinicopathological
and surgical factors between patients with a loss of SMM <5% vs.
≥5% at 6 months after gastrectomy. |
Table I.
Comparison of the clinicopathological
and surgical factors between patients with a loss of SMM <5% vs.
≥5% at 6 months after gastrectomy.
| Factors | Loss of skeletal
muscle mass <5% (N=68) N (%) | Loss of skeletal
muscle mass ≥5% (N=51) N (%) | P-value |
|---|
| Age | 70.8±10.4 | 69.7±10.3 | 0.56 |
| Sex |
|
| 0.14 |
| Male | 42 (61.8) | 38 (74.5) |
|
|
Female | 26 (38.2) | 13 (25.5) |
|
| BMI:
kg/m2 | 22.9±3.2 | 23.0±3.7 | 0.86 |
| SMM Index:
cm2/m2 | 42.8±7.4 | 52.2±8.9 | 0.005 |
| Albumin: g/dl | 4.1±0.4 | 4.1±0.5 | 0.67 |
| Hemoglobin: g/dl | 12.6±2.3 | 12.7±2.4 | 0.78 |
| PNI | 49.2±5.3 | 49.2±7.0 | 0.99 |
| CEA: ng/ml | 7.4±21.2 | 5.5±8.2 | 0.50 |
| Comorbidity |
|
|
|
| Diabetes
mellitus | 11 (16.2) | 8 (15.7) | 0.94 |
| Cardiac
disease | 3 (0.4) | 2 (3.9) | 0.90 |
| Type of
gastrectomy |
|
| 0.01 |
| Total
gastrectomy | 19 (27.9) | 26 (51.0) |
|
| Distal
gastrectomy | 49 (72.1) | 25 (49.0) |
|
| Type of approach |
|
| 0.26 |
|
Conventional | 3 (4.4) | 5 (9.8) |
|
|
Laparoscopy | 65 (95.6) | 46 (90.2) |
|
| Lymphadenectomy |
|
| 0.36 |
| D1+ | 31 (45.6) | 19 (37.3) |
|
| D2 | 37 (54.4) | 32 (62.7) |
|
| Blood loss: ml | 383.6±660.8 | 415.4±459.2 | 0.76 |
| Operation time:
min | 312.5±91.4 | 329.9±82.3 | 0.28 |
| Complication |
|
|
|
| ≥grade
2 | 14 (20.6) | 14 (27.5) | 0.38 |
| T stage |
|
| 0.43 |
| T1 | 36 (52.9) | 19 (37.3) |
|
| T2 | 9 (13.2) | 12 (23.5) |
|
| T3 | 15 (22.1) | 14 (27.5) |
|
| T4 | 8 (11.8) | 6 (11.8) |
|
| N stage |
|
| 0.93 |
| N0 | 40 (58.8) | 28 (54.9) |
|
| N1 | 12 (17.6) | 9 (17.6) |
|
| N2 | 8 (11.8) | 8 (15.7) |
|
| N3 | 8 (11.8) | 6 (11.8) |
|
| TNM stage |
|
| 0.60 |
| IA,
IB | 37 (54.4) | 23 (45.1) |
|
| IIA,
IIB | 18 (26.5) | 16 (31.4) |
|
| IIIA,
IIIB, IIIC | 13 (19.1) | 12 (23.5) |
|
| Adjuvant
chemotherapy |
|
| 0.30 |
|
Absent | 45 (66.2) | 29 (56.9) |
|
|
Present | 23 (33.8) | 22 (43.1) |
|
Risk factors for loss of SMM
The risk factors for SMM loss ≥5% were investigated
using univariate and multivariate analyses including clinical,
surgical, and pathological factors. The results are shown in
Table II. In the multivariate
analysis, total gastrectomy was the only independent predictor of
the loss of SMM (P=0.02).
 | Table II.Univariate and multivariate analyses
of clinicopathological factors associated with a loss of SMM
<5%. |
Table II.
Univariate and multivariate analyses
of clinicopathological factors associated with a loss of SMM
<5%.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | N (%) | OR (95% CI) | P-value | OR (95% CI) | P-value |
|---|
| Age | >70 47
(34.4) | 0.85
(0.40–1.79) | 0.67 |
|
|
| Gender | Male 80 (67.2) | 1.81
(0.82–4.02) | 0.15 |
|
|
| BMI | >25 31
(26.1) | 1.14
(0.50–2.59) | 0.76 |
|
|
|
| <18 9 (7.6) | 2.89
(0.69–12.2) | 0.15 |
|
|
| SMM Index | Male: >50.7,
Female: >44.6 58 (48.7) | 0.59
(0.28–1.22) | 0.15 |
|
|
| Type of
gastrectomy | Total gastrectomy
45 (37.8) | 2.68
(1.25–5.75) | 0.01 | 2.58
(1.19–5.58) | 0.02 |
| Approach | Conventional 8
(6.7) | 2.36
(0.54–10.3) | 0.25 |
|
|
|
Lymphadenectomy | D1+ 50 (42.0) | 1.41
(0.67–2.96) | 0.36 |
|
|
| Blood loss | >230 60
(50.4) | 1.19
(0.58–2.47) | 0.63 |
|
|
| Operation time | >309 60
(50.4) | 1.37
(0.66–2.84) | 0.40 |
|
|
| Complication | ≥Grade 2 28
(23.5) | 1.46
(0.62–3.42) | 0.38 |
|
|
| T stage | ≥2 64 (53.8) | 1.90
(0.90–3.98) | 0.09 | 1.78
(0.83–3.82) | 0.14 |
| N stage | ≥1 51 (42.9) | 1.17
(0.56–2.44) | 0.67 |
|
|
| TNM stage | ≥II 59 (49.6) | 1.45
(0.70–3.01) | 0.31 |
|
|
| Adjuvant
chemotherapy | Present 45
(37.8) | 1.48
(0.70–3.14) | 0.30 |
|
|
Loss of SMM and RFS
In the univariate Cox proportional hazards
regression analyses, the following variables were significantly
associated with worse RFS: Lymphadenectomy (D1+; P=0.04), operation
time (>309 min; P=0.01), T stage (≥2; P=0.005), N stage (≥1;
P=0.001), TNM stage (≥2; P=0.002), adjuvant chemotherapy (present;
P=0.001), and loss of SMM (≥5%; P=0.01; Table III). Furthermore, in the
multivariate analysis using a stepwise Cox model, adjuvant
chemotherapy (present; P=0.001) remained significantly associated
with worse RFS (Table III).
 | Table III.Univariate and multivariate analyses
of clinicopathological factors associated with relapse-free
survival. |
Table III.
Univariate and multivariate analyses
of clinicopathological factors associated with relapse-free
survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | N (%) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | >70 47
(34.4) | 0.53
(0.20–1.46) | 0.22 |
|
|
| Gender | Male 80 (67.2) | 1.57
(0.57–4.28) | 0.38 |
|
|
| BMI | >25 31
(26.1) | 0.95
(0.37–2.48) | 0.92 |
|
|
|
| <18 9 (7.6) | 0.86
(0.12–6.51) | 0.89 |
|
|
| SMM Index | Male: >50.7,
Female: >44.6 | 0.72
(0.30–1.74) | 0.47 |
|
|
|
| 58 (48.7) |
| Type of
gastrectomy | Total gastrectomy
45 (37.8) | 1.21
(0.51–2.89) | 0.66 |
|
|
| Approach | Conventional 8
(6.7) | 0.43
(0.34–5.38) | 0.39 |
|
|
|
Lymphadenectomy | D1+ 50 (42.0) | 3.21
(1.08–9.56) | 0.04 | 1.52
(0.48–4.77) | 0.48 |
| Blood loss | >230 60
(50.4) | 2.12
(0.82–5.48) | 0.12 |
|
|
| Operation time | >309 60
(50.4) | 3.78
(1.38–10.3) | 0.01 | 2.42
(0.85–6.84) | 0.10 |
| Complication | ≥Grade 2 28
(23.5) | 1.36
(0.53–3.52) | 0.52 |
|
|
| Histological
grade | Undiff 62
(52.1) | 1.56
(0.65–3.77) | 0.32 |
|
|
| T stage | ≥2 64 (53.8) | 5.78
(1.70–19.6) | 0.005 | 1.46
(0.24–8.88) | 0.68 |
| N stage | ≥1 51 (42.9) | 6.59
(2.21–19.6) | 0.001 | 2.35
(0.51–10.9) | 0.28 |
| TNM stage | ≥II 59 (49.6) | 7.18
(2.11–24.4) | 0.002 | 0.80
(0.06–11.8) | 0.87 |
| Adjuvant
chemotherapy | Present 45
(37.8) | 7.76
(2.61–23.1) | 0.001 | 6.15
(2.03–5.53) | 0.001 |
| Loss of SMM | ≥5% 51 (42.9) | 3.15
(1.29–7.72) | 0.01 | 2.23
(0.90–5.53) | 0.08 |
Loss of SMM and OS
In the univariate Cox regression analyses, the
following variables were significantly associated with worse OS: T
stage (≥2; P=0.03), TNM stage (≥2; P=0.03), and loss of SMM (≥5%;
P=0.04; Table IV). In the
multivariate analysis using a stepwise Cox model, age (>70
years; P=0.04), TNM stage (≥2; P=0.04), and loss of SMM (≥5%;
P=0.03) were found to be significantly associated with poor
prognosis after curative gastrectomy (Table IV).
 | Table IV.Univariate and multivariate analyses
of clinicopathological associated with overall survival. |
Table IV.
Univariate and multivariate analyses
of clinicopathological associated with overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Factors | N (%) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | >70 47
(34.4) | 2.17
(0.93–5.02) | 0.07 | 2.46
(1.07–6.13) | 0.04 |
| Gender | Male 80 (67.2) | 0.84
(0.35–2.00) | 0.70 |
|
|
| BMI | >25 31
(26.1) | 0.85
(0.33–2.18) | 0.73 |
|
|
|
| <18 9 (7.6) | 2.00
(0.46–8.66) | 0.36 |
|
|
| SMM Index | Male: >50.7,
Female: >44.6 | 0.86
(0.37–2.01) | 0.73 |
|
|
|
| 58 (48.7) |
|
|
|
|
| Type of
gastrectomy | Total gastrectomy
45 (37.8) | 0.85
(0.36–2.03) | 0.72 |
|
|
| Approach | Conventional 8
(6.7) | 0.61
(0.08–4.57) | 0.63 |
|
|
|
Lymphadenectomy | D1+ 50 (42.0) | 1.68
(0.68–4.12) | 0.26 |
|
|
| Blood loss | >230 60
(50.4) | 1.27
(0.53–3.05) | 0.60 |
|
|
| Operation time | >309 60
(50.4) | 1.82
(0.76–4.34) | 0.18 |
|
|
| Complication | ≥Grade 2 28
(23.5) | 1.60
(0.65–3.92) | 0.31 |
|
|
| Histological
grade | Undiff 62
(52.1) | 1.34
(0.57–3.14) | 0.50 |
|
|
| T stage | ≥2 64 (53.8) | 3.08
(1.14–9.37) | 0.03 | 1.47
(0.28–7.77) | 0.65 |
| N stage | ≥1 51 (42.9) | 2.07
(0.88–4.87) | 0.09 | 1.12
(0.37–3.43) | 0.84 |
| TNM stage | ≥2 59 (49.6) | 2.94
(1.15–7.53) | 0.03 | 2.65
(1.03–6.84) | 0.04 |
| Adjuvant
chemotherapy | Present 45
(37.8) | 1.90
(0.82–4.42) | 0.14 |
|
|
| Loss of SMM | ≥5% 51 (42.9) | 2.40
(1.02–5.62) | 0.04 | 2.57
(1.07–6.13) | 0.03 |
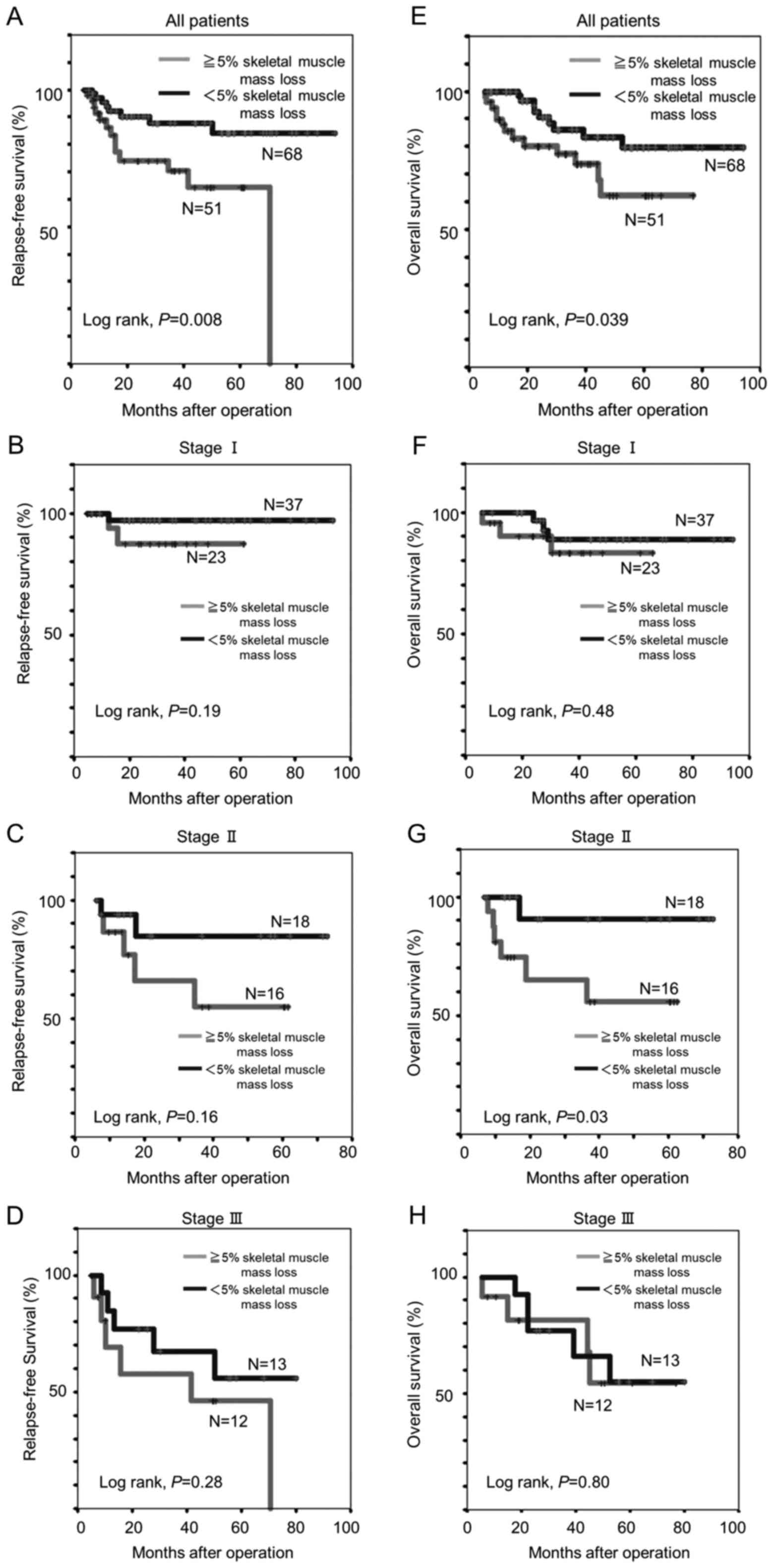
In the Kaplan-Meier analysis, patients with a SMM
loss of ≥5% experienced significantly shorter 5-year RFS (62.2 vs.
79.9%, log-rank P=0.008) and OS (64.5 vs. 84.2%, log-rank P=0.039)
than those with a SMM loss <5% (Fig.
2A and E). Among patients with TNM stage II disease, the 5-year
OS was remarkably shorter for patients with SMM loss of ≥5% than
for those SMM loss of <5% (55.8 vs. 90.9%, log-rank P=0.03;
Fig. 2G).
Discussion
This study was designed to evaluate the relation
between postoperative SMM loss and 5-year survival in gastric
cancer patients after gastrectomy. First, we investigated the risk
factors for loss of SMM at 6 months after gastrectomy. In this
study, the median loss of SMM was 3.8% at 6 months after
gastrectomy. Other authors have reported that the body weight loss
at 1 month postoperatively was 6% (13) and that the loss of lean body mass was
4.7% (10). In these studies, the
risk factors for reductions in the body mass index were found to
include male sex, higher preoperative body mass index, total
gastrectomy, and advanced TNM stage (13), while the independent risk factors for
severe lean body mass loss were surgical complications, total
gastrectomy, and male sex (10). In
the present study, total gastrectomy was the only independent risk
factor for SMM loss at 6 months after gastrectomy.
After total gastrectomy, the body weight, lean body
mass, and SMM all decreased. Reduced oral intake is commonly noted
in patients who undergo total gastrectomy. Two reasons for this
reduction in oral intake can be considered, namely loss of
retention ability and/or decreases in ghrelin, an appetizing
hormone that is released from the stomach. Ghrelin has various
physiologic functions, including secretion of growth hormone,
promotion of the appetite signal in the hypothalamus, and
stimulation of gastrointestinal activity. It has been reported that
the serum ghrelin level after total gastrectomy for gastric cancer
decreases to 10% of its preoperative level (14).
The present study also revealed that SMM loss of ≥5%
was an independent risk factor for OS, but not RFS. Similarly, in a
previous study, preoperative sarcopenia was demonstrated to have
associate with poor prognosis in gastric cancer after curative
surgery (9). In other words, primary
sarcopenia was a predictive factor of poor prognosis. On the other
hand, although it is known that the patients' diet, body weight,
SMM, and exercise capacity change after gastrectomy, no previous
report has demonstrated the relationship between the change in SMM
after gastrectomy and prognosis. To our knowledge, the present
study is the first to analyze the postoperative SMM loss and
determine its relationship with the survival of gastric cancer
patients undergoing gastrectomy. As a predictor of OS, the rate of
SMM loss after surgery was found to be more useful than the
preoperative SMM.
There are numerous possible reasons for why the loss
of SMM is a poor prognostic factor. Several studies have reported
that sarcopenia is a poor prognostic factor of various malignant
tumors (15). Further, Richards et
al reported a strong association between sarcopenia and the
presence of a systemic inflammatory response, including with the
C-reactive protein and albumin levels, in patients with colorectal
cancer (16), and Maggio et al
showed that inflammatory cytokines may be involved in sarcopenia by
interfering with insulin-like growth factor-1 signaling in skeletal
muscle (17). A few studies have
moreover reported that postoperative body weight loss is a poor
prognostic factor after gastrectomy (18,19). In
these previous reports, the reasons for this finding were
considered to be related to the immune response and decreases in
dietary intake.
Aoyama et al reported that body weight loss
or lean body mass loss was a risk factor associated with the
continuation of adjuvant chemotherapy (20). In the present study, the relationship
between loss of SMM and adjuvant chemotherapy was unclear. However,
Aoyama et al reported that lean body mass and fat decreased
after gastrectomy in the early postoperative period (12), and it is possible that the same change
may occur in SMM. Accordingly, one of the reasons for the poor
prognosis may be the fact that adjuvant chemotherapy cannot be
continued in these patients.
The development of sarcopenia is associated with
increased risks of falling, need for nursing care, and mortality.
At the same time, the physical ability of patients with sarcopenia
is reduced, and their living function is weakened, thus negatively
affecting their quality of life. Clarifying the mechanisms of
sarcopenia in patients with cancer may help reveal the mechanism of
cachexia, the root of cancer death. Previous studies have shown
that various methods of nutritional support are effective for
improving dietary intake and outcomes in patients who lose weight
after surgery (21,22). Hatao et al found that the use
of oral nutritional supplements after total gastrectomy
significantly diminished postoperative weight loss (21). If oral nutritional supplements can be
demonstrated to also be efficacious for preventing postoperative
muscle mass reduction, such oral nutritional supplements could
consequently be administered to patients with sarcopenia, which is
an especially common finding after total gastrectomy. Further, a
phase II trial was recently performed to examine the effect of
ghrelin in patients who had undergone total gastrectomy, as a means
of preventing body weight loss (23).
In another study, the administration of synthetic ghrelin
successfully lessened the postoperative body weight loss and
improved appetite and food intake after total gastrectomy (24). Thus, the administration of oral
nutritional supplements or ghrelin after gastrectomy may help to
prevent postoperative sarcopenia. Consistent with this suggestion,
a prior study found that increased ghrelin signaling improved
skeletal muscle atrophy in an aged mouse model (25).
The current study has some limitations. First, this
was a retrospective single-center study with a limited number of
patients. The body weight index is one of the most common
parameters used to represent nutritional status. However, herein,
we did not evaluate the changes in body weight of the patients.
Moreover, we did not measure the SMM in the early postoperative
period. Thereby, we could not clarify exactly when the SMM
decreased postoperatively. If the SMM decreases early in the
postoperative period, the cause of muscle mass loss may be the
invasiveness of the surgery itself or postoperative complications.
If the SMM decreases after discharge, it may be due to a decrease
in dietary quantity or the use of adjuvant chemotherapy. Further
research is necessary to clarify these issues.
In conclusion, the present study revealed that total
gastrectomy was a significant and independent risk factor for SMM
losses of ≤5% at 6 months after gastrectomy. Moreover, we found
that the postoperative SMM is a novel predictive marker of poor
prognosis.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rosenberg I: Summary comments:
Epidemiological and methodological problems in determining
nutritional status of older persons. Am J Clin Nutr. 50:1231–1233.
1989. View Article : Google Scholar
|
|
2
|
Harimoto N, Yoshizumi T, Shimokawa M,
Sakata K, Kimura K, Itoh S, Ikegami T, Ikeda T, Shirabe K and
Maehara Y: Sarcopenia is a poor prognostic factor following hepatic
resection in patients aged 70 years and older with hepatocellular
carcinoma. Hepatol Res. 46:1247–1255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki Y, Okamoto T, Fujishita T, Katsura
M, Akamine T, Takamori S, Morodomi Y, Tagawa T, Shoji F and Maehara
Y: Clinical implications of sarcopenia in patients undergoing
complete resection for early non-small cell lung cancer. Lung
Cancer. 101:92–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirasawa Y, Nakashima J, Yunaiyama D,
Sugihara T, Gondo T, Nakagami Y, Horiguchi Y, Ohno Y, Namiki K,
Ohori M, et al: Sarcopenia as a novel preoperative prognostic
predictor for survival in patients with bladder cancer undergoing
radical cystectomy. Ann Surg Oncol. 23(Suppl 5): S1048–S1054. 2016.
View Article : Google Scholar
|
|
5
|
Tan BH, Birdsell LA, Martin L, Baracos VE
and Fearon KC: Sarcopenia in an overweight or obese patient is an
adverse prognostic factor in pancreatic cancer. Clin Cancer Res.
15:6973–6979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sabel MS, Lee J, Cai S, Englesbe MJ,
Holcombe S and Wang S: Sarcopenia as a prognostic factor among
patients with stage III melanoma. Ann Surg Oncol. 18:3579–3585.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M,
Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Yoshida
M, et al: Sarcopenia is a negative prognostic factor after curative
resection of colorectal cancer. Ann Surg Oncol. 22:2663–2668. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M,
Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Watanabe
M and Baba H: Negative impact of skeletal muscle loss after
systemic chemotherapy in patients with unresectable colorectal
cancer. PLoS One. 10:e01297422015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhuang CL, Huang DD, Pang WY, Zhou CJ,
Wang SL, Lou N, Ma LL, Yu Z and Shen X: Sarcopenia is an
independent predictor of severe postoperative complications and
long-term survival after radical gastrectomy for gastric cancer:
Analysis from a large-scale cohort. Medicine (Baltimore).
95:e31642016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aoyama T, Sato T, Segami K, Maezawa Y,
Kano K, Kawabe T, Fujikawa H, Hayashi T, Yamada T, Tsuchida K, et
al: Risk factors for the loss of lean body mass after gastrectomy
for gastric cancer. Ann Surg Oncol. 23:1963–1970. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamaoka Y, Fujitani K, Tsujinaka T,
Yamamoto K, Hirao M and Sekimoto M: Skeletal muscle loss after
total gastrectomy, exacerbated by adjuvant chemotherapy. Gastric
Cancer. 18:382–389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aoyama T, Kawabe T, Fujikawa H, Hayashi T,
Yamada T, Tsuchida K, Yukawa N, Oshima T, Rino Y, Masuda M, et al:
Loss of lean body mass as an independent risk factor for
continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann
Surg Oncol. 22:2560–2566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aoyama T, Kawabe T, Hirohito F, Hayashi T,
Yamada T, Tsuchida K, Sato T, Oshima T, Rino Y, Masuda M, et al:
Body composition analysis within 1 month after gastrectomy for
gastric cancer. Gastric Cancer. 19:645–650. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takachi K, Doki Y, Ishikawa O, Miyashiro
I, Sasaki Y, Ohigashi H, Murata K, Nakajima H, Hosoda H, Kangawa K,
et al: Postoperative ghrelin levels and delayed recovery from body
weight loss after distal or total gastrectomy. J Surg Res. 130:1–7.
2016. View Article : Google Scholar
|
|
15
|
Joglekar S, Nau PN and Mezhir JJ: The
impact of sarcopenia on survival and complications in surgical
oncology: A review of the current literature. J Surg Oncol.
112:503–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Richards CH, Roxburgh CS, MacMillan MT,
Isswiasi S, Robertson EG, Guthrie GK, Horgan PG and McMillan DC:
The relationships between body composition and the systemic
inflammatory response in patients with primary operable colorectal
cancer. PLoS One. 7:e418832012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maggio M, de Vita F, Lauretani F, Buttò V,
Bondi G, Cattabiani C, Nouvenne A, Meschi T, Dall'Aglio E and Ceda
GP: IGF-1, the cross road of the nutritional, inflammatory and
hormonal pathways to frailty. Nutrients. 5:4184–4205. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee HH, Park JM, Song KY, Choi MG and Park
CH: Survival impact of postoperative body mass index in gastric
cancer patients undergoing gastrectomy. Eur J Cancer. 52:129–137.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kubo H, Komatsu S, Ichikawa D, Kawaguchi
T, Kosuga T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H and
Otsuji E: Impact of body weight loss on recurrence after curative
gastrectomy for gastric cancer. Anticancer Res. 36:807–813.
2016.PubMed/NCBI
|
|
20
|
Aoyama T, Yoshikawa T, Shirai J, Hayashi
T, Yamada T, Tsuchida K, Hasegawa S, Cho H, Yukawa N, Oshima T, et
al: Body weight loss after surgery is an independent risk factor
for continuation of S-1 adjuvant chemotherapy for gastric cancer.
Ann Surg Oncol. 20:2000–2006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hatao F, Chen KY, Wu JM, Wang MY, Aikou S,
Onoyama H, Shimizu N, Fukatsu K, Seto Y and Lin MT: Randomized
controlled clinical trial assessing the effects of oral nutritional
supplements in postoperative gastric cancer patients. Langenbecks
Arch Surg. 402:203–211. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imamura H, Nishikawa K, Kishi K, Inoue K,
Matsuyama J, Akamaru Y, Kimura Y, Tamura S, Kawabata R, Kawada J,
et al: Effects of an oral elemental nutritional supplement on
post-gastrectomy body weight loss in gastric cancer patients: A
randomized controlled clinical trial. Ann Surg Oncol. 23:2928–2935.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adachi S, Takiguchi S, Okada K, Yamamoto
K, Yamasaki M, Miyata H, Nakajima K, Fujiwara Y, Hosoda H, Kangawa
K, et al: Effects of ghrelin administration after total
gastrectomy: A prospective, randomized, placebo-controlled phase II
study. Gastroenterology. 138:1312–1320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takiguchi S, Hiura Y, Takahashi T,
Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Mori M, Hosoda H,
Kangawa K and Doki Y: Effect of rikkunshito, a Japanese herbal
medicine, on gastrointestinal symptoms and ghrelin levels in
gastric cancer patients after gastrectomy. Gastric Cancer.
16:167–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fujitsuka N, Asakawa A, Morinaga A,
Amitani MS, Amitani H, Katsuura G, Sawada Y, Sudo Y, Uezono Y,
Mochiki E, et al: Increased ghrelin signaling prolongs survival in
mouse models of human aging through activation of sirtuin1. Mol
Psychiatry. 21:1613–1623. 2016. View Article : Google Scholar : PubMed/NCBI
|