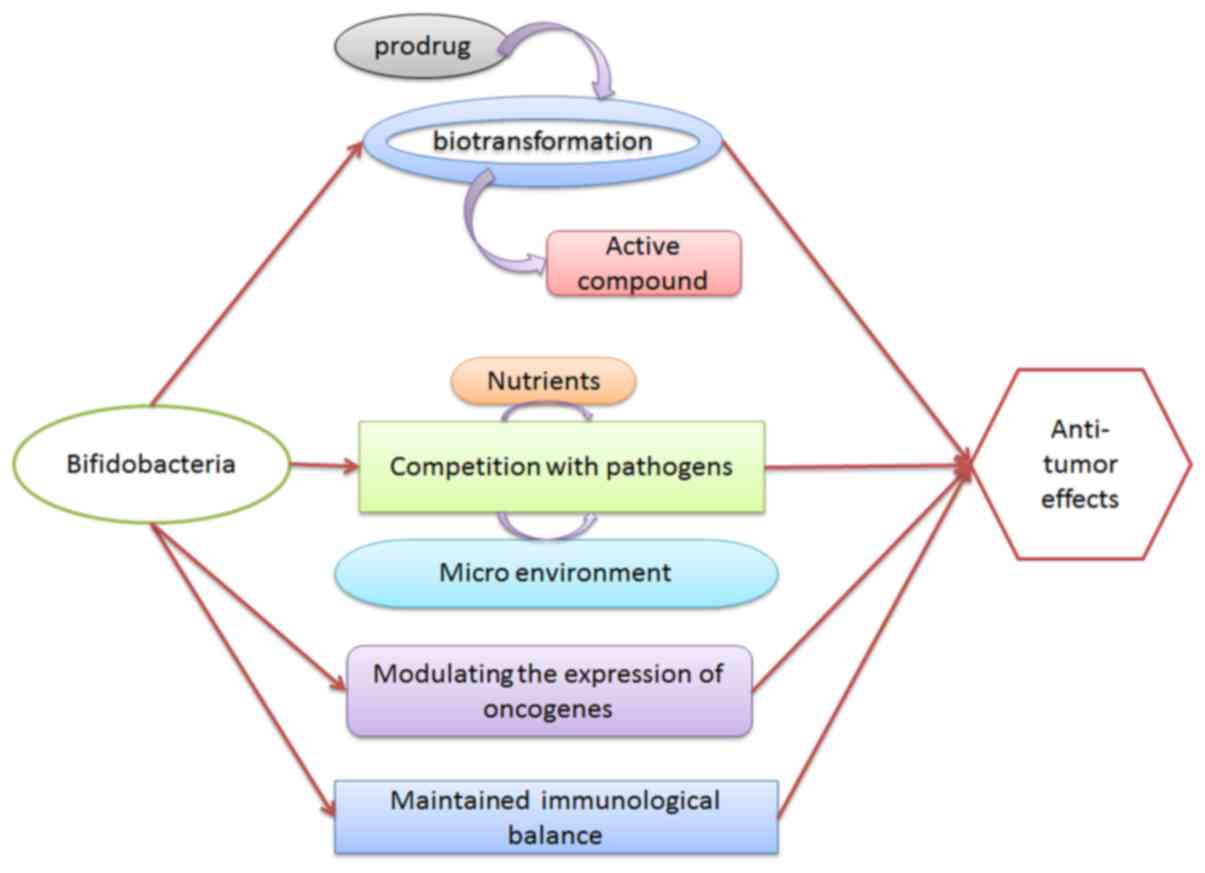
Bifidobacterial species are part of the normal human
microflora, and exert probiotic effects in humans (1). Previously, several studies reported that
bifidobacteria exhibit certain antitumor effects on the development
of cancer (2–4). It may work through the mechanisms of
fermentation (5), biotransformation
(6) and strengthening the intestinal
barrier (7), and may potentially
function as a treatment method (Fig.
1). For example, Bifidobacterium breve, Bifidobacterium
bifidum and Bifidobacterium longum strains isolated from
breastfed infants were associated with the fermentation of caprine
milk oligosaccharides (8). Owing to
the expression of β-galactosidases (9), they were able to utilize 3′- and
6′-sialyl-lactose as growth substrates when they were included as
the only carbon source (8).
Apart from their fermentation function,
bifidobacteria may also serve an important function in
bioconversion, as they may convert ginsenoside into a
deglycosylated form under controlled conditions (10). Furthermore, they affect the
composition of gut microbiota; for example, B. breve UCC2003
exhibited growth properties in a mucin-based medium only in the
presence of B. bifidum PRL2010, which was demonstrated to
metabolize mucin (11). Additionally,
the production of specific molecules secreted by B. bifidum
prevented adhesion attachment and invasion by food-borne pathogens
(12). Lactic acid bacteria,
including bifidobacteria, were revealed to exert chemopreventative
effects on colon, bladder, liver, breast and gastric cancer types
(13). The present review discusses
the mechanisms involved in the antitumor effect of
bifidobacteria.
Biotransformation is among one of the mechanisms by
which bifidobacteria exhibit antitumor effects. Essentially,
biotransformation function is fulfilled by the conversion of a
compound into a usable energy source via a biological process. High
numbers of Bifidobacterium may be involved in enterolactone
production, which has antitumor effects (14). Bifidobacterium spp. may ferment
polyunsaturated fatty acid (linoleic acid) into pectic
oligosaccharides (POS), which may delay the development of leukemia
and associated cachexia in mice, and, consequently, POS may
increase the amount of Bacteroides spp. (15).
Bifidobacteria may also metabolize certain drugs
into therapeutically active compounds against a tumor in
vitro (6), including lapachol and
5-fluorocytosine. Oliveira Silva et al (16) performed experiments using two
probiotic strains from the human gut: Bifidobacterium spp.
and Lactobacillus acidophilus. Each of them was incubated
with lapachol, an anticancer drug, for 12 h in an anaerobic
atmosphere at 37°C (16). The culture
broths were extracted twice using ethyl acetate, prior to analysis
of the chemical profiles of all crude extracts by an injection of
20 µl of all crude extracts (tests and controls) from the culture
broths at 1 mg·ml−1 using reversed-phase
high-performance liquid chromatography. A total of 106
colony-forming units (CFU)/ml of bifidobacteria was able to convert
lapachol into an active compound against breast cancer cell at 37°C
in an anaerobic atmosphere (16).
Additionally, they may convert nontoxic prodrugs
into therapeutically active compounds based on the expression of
certain enzymes, including cytosine deaminase (17). This enzyme has the ability to convert
the nontoxic prodrug 5-fluorocytosine into 5-fluorouracil, which
may inhibit the proliferation of carcinoma cells (18). An antitumor drug named ginsenoside Rb1
may be metabolized into the bioactive compound K when incubated
with Bifidobacterium spp. (19). Furthermore, the gut microbiota was
additionally analyzed in people with differing levels of
ginsenoside Rb1 degradation into compound K (6). A total of 5 samples with fecal activity
potently metabolizing ginsenoside Rb1 to compound K (FPG) and 5
samples with fecal activity not metabolizing ginsenoside Rb1 to
compound K (FNG) were selected from a pool of 100 patients, and the
fecal microbiota were analyzed using 16S ribosomal RNA gene
pyrosequencing. It was revealed that the population levels of
Bifidobacterium were substantially increased in the FPG
group (6). Additionally, lactic
fermentation with Bifidobacterium longum subsp.
infantis may enhance the antitumor cell proliferation effect
of soymilk against HT-29 and Caco-2 colorectal cancer (CRC) cell
lines (5).
Secondly, the competitive exclusion of pathogenic
microbiota by probiotics includes the competition for nutrients and
adhesion at the intestinal mucosa. It has been reported that
probiotics compete for limited nutrients available at the distal
colon and grow at the expense of other bacteria (34–36).
Non-pathogens may compete with pathogens by altering pH and
inducing metabolic changes (37,38).
Vaginal lactobacilli may produce biosurfactants that are composed
of a mixture of proteins, lipids and carbohydrates that help to
displace dense mixed cultures of uropathogenic Escherichia
coli and Enterococcus faecalis (39,40). The
mechanism involved in anti-mutagenic activities is the ability of
bifidobacteria to bind to the mutagens of microbial cells (41).
Finally, bifidobacteria have also been demonstrated
to exhibit anti-mutagenic activities against heterocyclic amines,
N-nitroso compounds and aflatoxins (45,46).
Bifidobacteria also exhibit antitumor effects via
altering the expression of cancer-associated genes and cytokines.
B. longum may suppress azoxymethane-induced colonic tumor
types, and this effect was associated with a decrease in colonic
mucosal proliferation and ornithine decarboxylase and ras-p21
activity (25,47,48).
Animal studies have demonstrated that probiotic preparations
consisting of bifidobacteria are able to decrease the activity of
procarcinogenic enzymes, including B. bifidum, which may
decrease the activity of β-glucosidase (49), and B. longum, which was
revealed to lower β-glucosidase and β-glucuronidase activity
(50). In addition,
microbiota-associated inflammatory processes may contribute to
carcinogenesis in the affected organs. For example, chronic
Helicobacter pylori infection results in gastric cancer, and
hepatitis B virus results in hepatic cancer (51,52).
However, the question of how they promote colon
tumor formation remains unanswered. Patients with CRC exhibit
bacteria adhering to tumor tissue (53) and have indirect evidence of bacterial
invasion (54,55). Human gut commensal bifidobacteria
strains have the ability to adhere to human epithelial cells
(56). Exposure of B. longum
subsp. infantis to human milk oligosaccharides resulted in
an increased ability to adhere to intestinal cells and increase the
expression of the anti-inflammatory cytokine interleukin (IL)-10
(57–59). In an animal model of post-infectious
irritable bowel syndrome, which was established by infection with
Trichinella spiralis for 8 weeks, B. longum
intervention may increase the expression level of NLR family pyrin
domain-containing 3 inflammasome and the downstream cytokines IL-8
and IL-1β (60). For immune cells,
B. longum BB536 serves an important function in the
development and maturation of the immune system. Interferon γ
(IFN-γ) secreting cells and the proportion of IFN-γ/IL-4 secreting
T helper (Th) cells (Th1/Th2) were increased in newborn infants
supplemented with B. longum BB536 (61). Wu et al (62) explored the immunological mechanism of
colonic carcinogenesis using enterotoxigenic Bacteroides
fragilis (ETBF) and revealed a signal transducer and activator
of transcription 3- and Th17-dependent pathway used in
inflammation-induced cancer by ETBF. The beneficial effects of
probiotics are thought to maintain the balance between types of T
cell responses including T regulatory (Treg) and Th17 responses.
The beneficial effects of probiotics are thought to keep balance
between types of T cell response such as T regulatory (Treg) and
Th17 responses (63,64). Th17 cells serve a key function in
inducing tissue inflammation in autoimmune disease (65), and Treg cells function as master
regulators of the immune response (66). However, B. animalis subsp.
lactis was demonstrated to increase forkhead box P3+ mRNA,
which defines the Treg lineage and expression in peribronchial
lymph nodes, suggesting the induction of Treg cells by this strain
(67). Bifidobacterium-treated
mice revealed significantly limited tumor growth in comparison with
non-Bifidobacterium-treated counterparts, which was
accompanied by the induction of tumor-specific T cells in the
periphery and an increased accumulation of antigen-specific cluster
of differentiation 8 (CD8)+ T cells within the tumor
(68).
As bifidobacteria are non-pathogenic and anaerobic,
and may selectively localize and proliferate in a hypoxic
environment in a number of solid tumor types (75), transfected or constructed
bifidobacteria as a novel delivery system for specific genes is a
promising therapeutic method for treating a tumor. For example,
vascular angiogenesis is required for the growth and metastasis of
a tumor (76). B. longum
subsp. infantis transfected with fms-like tyrosine kinase
receptor, a receptor for vascular endothelial growth factor, was
verified to exert a potential effect on Lewis lung carcinoma in
mice (77). Additionally, tumstatin
(Tum) is an endogenous angiogenesis inhibitor (78). Wei et al (79) developed a delivery system for Tum
using engineered B. longum (BL-Tum), used them to treat
tumor-bearing mice, and revealed that the tumor in the BL-Tum group
grew slowly with no metastasis observed. In another study, Nakamura
et al (18) transfected B.
longum with the plasmid Pbles100-S-eCD, which included the gene
encoding cytosine deaminase. This transfected B. longum,
which produced active cytosine deaminase that converted
5-fluorocytosine into 5-fluorouracil in hypoxic solid tumor types,
resulted in 5-fluorouracil concentrations increasing proportionally
with the number of the bacilli (with a baseline of 103
CFU/ml). A similar study observed similar results in B.
longum subsp. infantis (80).
In the present review, the role of bifidobacteria in
the development of cancer was summarized, as transfected
Bifidobacterium have been used to treat tumors in animal
models (81). In the future, this
novel technology may contribute to the discovery of treatment
methods with fewer adverse effects for patients with cancer.
However, further investigation of the use of bifidobacteria in the
prevention and treatment of cancer is warranted.
Not applicable.
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81300270 and
81470801).
All data generated or analyzed during this study are
included in this published article.
LC and HW collected the data and wrote the
manuscript. GL, JY and FLi collected the data. YZ and FLu analyzed
the data. YY conceived the idea for the study and revised the
manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bottacini F, van Sinderen D and Ventura M:
Omics of bifidobacteria: Research and insights into their
health-promoting activities. Biochem J. 474:4137–4152. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biarc J, Nguyen IS, Pini A, Gossé F,
Richert S, Thiersé D, Van Dorsselaer A, Leize-Wagner E, Raul F,
Klein JP and Schöller-Guinard M: Carcinogenic properties of
proteins with pro-inflammatory activity from Streptococcus
infantarius (formerly S. bovis). Carcinogenesis. 25:1477–1484.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei C, Xun AY, Wei XX, Yao J, Wang JY, Shi
RY, Yang GH, Li YX, Xu ZL, Lai MG, et al: Bifidobacteria expressing
tumstatin protein for antitumor therapy in tumor-bearing mice.
Technol Cancer Res Treat. 15:498–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liboredo JC, Anastácio LR, Pelúzio Mdo C,
Valente FX, Penido LC, Nicoli JR and Correia MI: Effect of
probiotics on the development of dimethylhydrazine-induced
preneoplastic lesions in the mice colon. Acta Cir Bras. 28:367–372.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lai LR, Hsieh SC, Huang HY and Chou CC:
Effect of lactic fermentation on the total phenolic, saponin and
phytic acid contents as well as anti-colon cancer cell
proliferation activity of soymilk. J Biosci Bioeng. 115:552–556.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KA, Jung IH, Park SH, Ahn YT, Huh CS
and Kim DH: Comparative analysis of the gut microbiota in people
with different levels of ginsenoside Rb1 degradation to compound K.
PloS One. 8:e624092013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schroeder BO, Birchenough GMH, Stahlman M,
Arike L, Johansson MEV, Hansson GC and Bäckhed F: Bifidobacteria or
fiber protects against diet-induced microbiota-mediated colonic
mucus deterioration. Cell Host Microbe. 23:27–40.e7. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thum C, Roy NC, McNabb WC, Otter DE and
Cookson AL: In vitro fermentation of caprine milk oligosaccharides
by bifidobacteria isolated from breast-fed infants. Gut Microbes.
6:352–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Asakuma S, Hatakeyama E, Urashima T,
Yoshida E, Katayama T, Yamamoto K, Kumagai H, Ashida H, Hirose J
and Kitaoka M: Physiology of consumption of human milk
oligosaccharides by infant gut-associated bifidobacteria. J Biol
Chem. 286:34583–34592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ku S, You HJ, Park MS and Ji GE: Effects
of ascorbic acid on α-l-arabinofuranosidase and
α-l-arabinopyranosidase activities from RD47 and its application to
whole cell bioconversion of ginsenoside. J Korean Soc Appl Biol
Chem. 58:857–865. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Egan M, Motherway MO, Kilcoyne M, Kane M,
Joshi L, Ventura M and van Sinderen D: Cross-feeding by
Bifidobacterium breve UCC2003 during co-cultivation with
Bifidobacterium bifidum PRL2010 in a mucin-based medium. BMC
Microbiol. 14:2822014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bayoumi MA and Griffiths MW: In vitro
inhibition of expression of virulence genes responsible for
colonization and systemic spread of enteric pathogens using
Bifidobacterium bifidum secreted molecules. Int J Food Microbiol.
156:255–263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JE, Kim JY, Lee KW and Lee HJ: Cancer
chemopreventive effects of lactic acid bacteria. J Microbiol
Biotechnol. 17:1227–1235. 2007.PubMed/NCBI
|
|
14
|
Oikarinen S, Heinonen S, Karppinen S,
Mättö J, Adlercreutz H, Poutanen K and Mutanen M: Plasma
enterolactone or intestinal Bifidobacterium levels do not explain
adenoma formation in multiple intestinal neoplasia (Min) mice fed
with two different types of rye-bran fractions. Br J Nutr.
90:119–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bindels LB, Neyrinck AM, Salazar N,
Taminiau B, Druart C, Muccioli GG, Francois E, Blecker C, Richel A,
Daube G, et al: Non digestible oligosaccharides modulate the gut
microbiota to control the development of leukemia and associated
cachexia in mice. PloS One. 10:e01310092015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Silva Oliveira E, de Carvalho Cruz T,
Parshikov IA, dos Santos Alves R, Emery Silva F and Furtado
Jacometti Cardoso NA: Cytotoxicity of lapachol metabolites produced
by probiotics. Lett Appl Microbiol. 59:108–114. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hidaka A, Hamaji Y, Sasaki T, Taniguchi S
and Fujimori M: Exogenous cytosine deaminase gene expression in
Bifidobacterium breve I-53-8w for tumor-targeting enzyme/ prodrug
therapy. Biosci Biotechnol Biochem. 71:2921–2926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura T, Sasaki T, Fujimori M, Yazawa
K, Kano Y, Amano J and Taniguchi S: Cloned cytosine deaminase gene
expression of Bifidobacterium longum and application to
enzyme/pro-drug therapy of hypoxic solid tumors. Biosci Biotechnol
Biochem. 66:2362–2366. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bae EA, Park SY and Kim DH: Constitutive
beta-glucosidases hydrolyzing ginsenoside Rb1 and Rb2 from human
intestinal bacteria. Biol Pharm Bull. 23:1481–1485. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Apás AL, Dupraz J, Ross R, González SN and
Arena ME: Probiotic administration effect on fecal mutagenicity and
microflora in the goat's gut. J Biosci Bioeng. 110:537–540. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lo PR, Yu RC, Chou CC and Huang EC:
Determinations of the antimutagenic activities of several probiotic
bifidobacteria under acidic and bile conditions against
benzo[a]pyrene by a modified Ames test. Int J Food Microbiol.
93:249–257. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Osswald A, Westermann C, Sun Z and Riedel
CU: A phytase-based reporter system for identification of
functional secretion signals in bifidobacteria. PloS One.
10:e01288022015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang L, Xiao X, Ren J, Tang Y, Weng H,
Yang Q, Wu M and Tang W: Proteomic analysis of bladder cancer
indicates Prx-I as a key molecule in BI-TK/GCV treatment system.
PloS One. 9:e987642014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xiao X, Jin R, Li J, Bei Y and Wei T: The
antitumor effect of suicide gene therapy using Bifidobacterium
infantis-mediated herpes simplex virus thymidine kinase/ganciclovir
in a nude mice model of renal cell carcinoma. Urology. 84(982):
e15–e20. 2014.PubMed/NCBI
|
|
25
|
Reddy BS: Possible mechanisms by which
pro- and prebiotics influence colon carcinogenesis and tumor
growth. J Nutr. 129 7 Suppl:S1478–S1482. 1999. View Article : Google Scholar
|
|
26
|
Rodrigues MA, Silva LA, Salvadori DM, De
Camargo JL and Montenegro MR: Aberrant crypt foci and colon cancer:
Comparison between a short- and medium-term bioassay for colon
carcinogenesis using dimethylhydrazine in Wistar rats. Braz J Med
Biol Res. 35:351–355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kulkarni N and Reddy BS: Inhibitory effect
of Bifidobacterium longum cultures on the azoxymethane-induced
aberrant crypt foci formation and fecal bacterial
beta-glucuronidase. Proc Soc Exp Biol Med. 207:278–283. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Femia AP, Luceri C, Dolara P, Giannini A,
Biggeri A, Salvadori M, Clune Y, Collins KJ, Paglierani M and
Caderni G: Antitumorigenic activity of the prebiotic inulin
enriched with oligofructose in combination with the probiotics
Lactobacillus rhamnosus and Bifidobacterium lactis on
azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis.
23:1953–1960. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin Y, Wang RR, Wang Y, Wang JJ and Xu GX:
Preparation of selenium-enriched Bifidobacterium longum and its
effect on tumor growth and immune function of tumor-bearing mice.
Asian Pac J Cancer Prev. 15:3681–3686. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sekine K, Toida T, Saito M, Kuboyama M,
Kawashima T and Hashimoto Y: A new morphologically characterized
cell wall preparation (whole peptidoglycan) from Bifidobacterium
infantis with a higher efficacy on the regression of an established
tumor in mice. Cancer Res. 45:1300–1307. 1985.PubMed/NCBI
|
|
31
|
Rowland IR, Rumney CJ, Coutts JT and
Lievense LC: Effect of Bifidobacterium longum and inulin on gut
bacterial metabolism and carcinogen-induced aberrant crypt foci in
rats. Carcinogenesis. 19:281–285. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simone M, Gozzoli C, Quartieri A, Mazzola
G, Di Gioia D, Amaretti A, Raimondi S and Rossi M: The probiotic
Bifidobacterium breve B632 inhibited the growth of
Enterobacteriaceae within colicky infant microbiota cultures.
Biomed Res Int. 2014:3010532014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mogna L, Del Piano M and Mogna G:
Capability of the two microorganisms Bifidobacterium breve B632 and
Bifidobacterium breve BR03 to colonize the intestinal microbiota of
children. J Clin Gastroenterol. 48 Suppl 1:S37–S39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fooks LJ and Gibson GR: Probiotics as
modulators of the gut flora. Br J Nutr. 88 Suppl 1:S39–S49. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dhanani AS and Bagchi T: Lactobacillus
plantarum CS24.2 prevents E. coli adhesion to HT-29 cells and also
down-regulates enteropathogen induced TNF-α and IL-8 expression.
Microbiol Immunol. 57:309–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tareb R, Bernardeau M, Gueguen M and
Vernoux JP: In vitro characterization of aggregation and adhesion
properties of viable and heat-killed forms of two probiotic
Lactobacillus strains and interaction with foodborne zoonotic
bacteria, especially Campylobacter jejuni. J Med Microbiol.
62:637–649. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kolenbrander PE, Palmer RJ Jr, Periasamy S
and Jakubovics NS: Oral multispecies biofilm development and the
key role of cell-cell distance. Nat Rev Microbiol. 8:471–480. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cadieux PA, Burton J, Devillard E and Reid
G: Lactobacillus by-products inhibit the growth and virulence of
uropathogenic Escherichia coli. J Physiol Pharmacol. 60 Suppl
6:S13–S18. 2009.
|
|
39
|
McMillan A, Dell M, Zellar MP, Cribby S,
Martz S, Hong E, Fu J, Abbas A, Dang T, Miller W and Reid G:
Disruption of urogenital biofilms by lactobacilli. Colloids Surf B
Biointerfaces. 86:58–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Velraeds MM, van de Belt-Gritter B, van
der Mei HC, Reid G and Busscher HJ: Interference in initial
adhesion of uropathogenic bacteria and yeasts to silicone rubber by
a Lactobacillus acidophilus biosurfactant. J Med Microbiol.
47:1081–1085. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Apás AL, González SN and Arena ME:
Potential of goat probiotic to bind mutagens. Anaerobe. 28:8–12.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Asaduzzaman SM and Sonomoto K:
Lantibiotics: Diverse activities and unique modes of action. J
Biosci Bioeng. 107:475–487. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xu HY, Tian WH, Wan CX, Jia LJ, Wang LY,
Yuan J, Liu CM, Zeng M and Wei H: Antagonistic potential against
pathogenic microorganisms and hydrogen peroxide production of
indigenous lactobacilli isolated from vagina of Chinese pregnant
women. Biomed Environ Sci. 21:365–371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Martín R and Suárez JE: Biosynthesis and
degradation of H2O2 by vaginal lactobacilli. Appl Environ
Microbiol. 76:400–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lankaputhra WE and Shah NP: Antimutagenic
properties of probiotic bacteria and of organic acids. Mutat Res.
397:169–182. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sreekumar O and Hosono A: The
antimutagenic properties of a polysaccharide produced by
Bifidobacterium longum and its cultured milk against some
heterocyclic amines. Can J Microbiol. 44:1029–1036. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Reddy BS: Prevention of colon cancer by
pre- and probiotics: Evidence from laboratory studies. Br J Nutr.
80:S219–S223. 1998.PubMed/NCBI
|
|
48
|
Singh J, Rivenson A, Tomita M, Shimamura
S, Ishibashi N and Reddy BS: Bifidobacterium longum, a lactic
acid-producing intestinal bacterium inhibits colon cancer and
modulates the intermediate biomarkers of colon carcinogenesis.
Carcinogenesis. 18:833–841. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Verma A and Shukla G: Probiotics
Lactobacillus rhamnosus GG, Lactobacillus acidophilus suppresses
DMH-induced procarcinogenic fecal enzymes and preneoplastic
aberrant crypt foci in early colon carcinogenesis in Sprague Dawley
rats. Nutr Cancer. 65:84–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lee do K, Jang S, Baek EH, Kim MJ, Lee KS,
Shin HS, Chung MJ, Kim JE, Lee KO and Ha NJ: Lactic acid bacteria
affect serum cholesterol levels, harmful fecal enzyme activity, and
fecal water content. Lipids Health Dis. 8:212009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Suerbaum S and Michetti P: Helicobacter
pylori infection. N Engl J Med. 347:1175–1186. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
El-Serag HB and Rudolph KL: Hepatocellular
carcinoma: Epidemiology and molecular carcinogenesis.
Gastroenterology. 132:2557–2576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Swidsinski A, Khilkin M, Kerjaschki D,
Schreiber S, Ortner M, Weber J and Lochs H: Association between
intraepithelial Escherichia coli and colorectal cancer.
Gastroenterology. 115:281–286. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abdulamir AS, Hafidh RR, Mahdi LK,
Al-jeboori T and Abubaker F: Investigation into the controversial
association of Streptococcus gallolyticus with colorectal cancer
and adenoma. BMC Cancer. 9:4032009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Gupta A, Madani R and Mukhtar H:
Streptococcus bovis endocarditis, a silent sign for colonic tumour.
Colorectal Dis. 12:164–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bernet MF, Brassart D, Neeser JR and
Servin AL: Adhesion of human bifidobacterial strains to cultured
human intestinal epithelial cells and inhibition of
enteropathogen-cell interactions. Appl Environ Microbiol.
59:4121–4128. 1993.PubMed/NCBI
|
|
57
|
Kavanaugh DW, O'Callaghan J, Buttó LF,
Slattery H, Lane J, Clyne M, Kane M, Joshi L and Hickey RM:
Exposure of Bifidobacterium longum subsp. infantis to milk
oligosaccharides increases adhesion to epithelial cells and induces
a substantial transcriptional response. PloS One. 8:e672242013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wickramasinghe S, Pacheco AR, Lemay DG and
Mills DA: Bifidobacteria grown on human milk oligosaccharides
downregulate the expression of inflammation-related genes in Caco-2
cells. BMC Microbiol. 15:1722015. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chichlowski M, De Lartigue G, German JB,
Raybould HE and Mills DA: Bifidobacteria isolated from infants and
cultured on human milk oligosaccharides affect intestinal
epithelial function. J Pediatr Gastroenterol Nutr. 55:321–327.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Gu QY, Zhang J and Feng YC: Role of NLRP3
inflammasome in Bifidobacterium longum-regulated visceral
hypersensitivity of postinfectious irritable bowel syndrome. Artif
Cells Nanomed Biotechnol. 44:1933–1937. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu BB, Yang Y, Xu X and Wang WP: Effects
of Bifidobacterium supplementation on intestinal microbiota
composition and the immune response in healthy infants. World J
Pediatr. 12:177–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wu S, Rhee KJ, Albesiano E, Rabizadeh S,
Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al: A
human colonic commensal promotes colon tumorigenesis via activation
of T helper type 17 T cell responses. Nat Med. 15:1016–1022. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mortaz E, Adcock IM, Folkerts G, Barnes
PJ, Vos Paul A and Garssen J: Probiotics in the management of lung
diseases. Mediators Inflamm. 2013:7510682013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Helwig U, Lammers KM, Rizzello F, Brigidi
P, Rohleder V, Caramelli E, Gionchetti P, Schrezenmeir J, Foelsch
UR, Schreiber S and Campieri M: Lactobacilli, bifidobacteria and E.
coli nissle induce pro- and anti-inflammatory cytokines in
peripheral blood mononuclear cells. World J Gastroenterol.
12:5978–5986. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Korn T, Bettelli E, Oukka M and Kuchroo
VK: IL-17 and Th17 cells. Ann Rev Immunol. 27:485–517. 2009.
View Article : Google Scholar
|
|
66
|
Lin W, Truong N, Grossman WJ, Haribhai D,
Williams CB, Wang J, Martin MG and Chatila TA: Allergic
dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J
Allergy Clin Immunol. 116:1106–1115. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Feleszko W, Jaworska J, Rha RD,
Steinhausen S, Avagyan A, Jaudszus A, Ahrens B, Groneberg DA, Wahn
U and Hamelmann E: Probiotic-induced suppression of allergic
sensitization and airway inflammation is associated with an
increase of T regulatory-dependent mechanisms in a murine model of
asthma. Clin Exp Allergy. 37:498–505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal Bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Yin Y, Kou L, Wang JJ and Xu GX:
Therapeutic efficacy of Bifidobacterium longum-mediated human
interleukin-2 with endostatin or TRAIL in transplanted tumors in
mice. Exp Ther Med. 3:481–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Bordonaro M, Lazarova DL and Sartorelli
AC: Butyrate and Wnt signaling: A possible solution to the puzzle
of dietary fiber and colon cancer risk? Cell Cycle. 7:1178–1183.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Mäkivuokko H, Tiihonen K, Tynkkynen S,
Paulin L and Rautonen N: The effect of age and non-steroidal
anti-inflammatory drugs on human intestinal microbiota composition.
Br J Nutr. 103:227–234. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bergmann KR, Liu SX, Tian R, Kushnir A,
Turner JR, Li HL, Chou PM, Weber CR and De Plaen IG: Bifidobacteria
stabilize claudins at tight junctions and prevent intestinal
barrier dysfunction in mouse necrotizing enterocolitis. Am J
Pathol. 182:1595–1606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ewaschuk JB, Diaz H, Meddings L,
Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M and Madsen
KL: Secreted bioactive factors from Bifidobacterium infantis
enhance epithelial cell barrier function. Am J Physiol Gastrointest
Liver Physiol. 295:G1025–G1034. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Becker S, Oelschlaeger TA, Wullaert A,
Vlantis K, Pasparakis M, Wehkamp J, Stange EF and Gersemann M:
Bacteria regulate intestinal epithelial cell differentiation
factors both in vitro and in vivo. PloS One. 8:e556202013.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
de Vrese M and Schrezenmeir J: Probiotics,
prebiotics, and synbiotics. Adv Biochem Eng Biotechnol. 111:1–66.
2008.PubMed/NCBI
|
|
76
|
Ricciuti B, Foglietta J, Bianconi V,
Sahebkar A and Pirro M: Enzymes involved in tumor-driven
angiogenesis: A valuable target for anticancer therapy. Semin
Cancer Biol: S1044-579X(17)30043-3. 2017. View Article : Google Scholar
|
|
77
|
Zhu H, Li Z, Mao S, Ma B, Zhou S, Deng L,
Liu T, Cui D, Zhao Y, He J, et al: Antitumor effect of sFlt-1 gene
therapy system mediated by Bifidobacterium infantis on Lewis lung
cancer in mice. Cancer Gene Ther. 18:884–896. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sudhakar A and Boosani CS: Inhibition of
tumor angiogenesis by tumstatin: Insights into signaling mechanisms
and implications in cancer regression. Pharm Res. 25:2731–2739.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wei C, Xun AY, Wei XX, Yao J, Wang JY, Shi
RY, Yang GH, Li YX, Xu ZL, Lai MG, et al: Bifidobacteria expressing
tumstatin protein for antitumor therapy in tumor-bearing mice.
Technol Cancer Res Treat. 15:498–508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yi C, Huang Y, Guo ZY and Wang SR:
Antitumor effect of cytosine deaminase/5-fluorocytosine suicide
gene therapy system mediated by Bifidobacterium infantis on
melanoma. Acta Pharmacol Sin. 26:629–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sasaki T, Fujimori M, Hamaji Y, Hama Y,
Ito K, Amano J and Taniguchi S: Genetically engineered
Bifidobacterium longum for tumor-targeting enzyme-prodrug therapy
of autochthonous mammary tumors in rats. Cancer Sci. 97:649–657.
2006. View Article : Google Scholar : PubMed/NCBI
|