Introduction
Inflammation serves a pivotal role in carcinogenesis
and the promotion, malignant conversion and migration of malignant
tumours (1). It has been established
that inflammatory conditions in certain organs increase the risk of
cancer. Tumour-associated inflammation also enables cancer cells to
escape from the surveillance of adaptive immunity and diminishes
the cellular response to chemotherapeutic drugs (2). Therefore, inflammation is considered a
hallmark of cancer (2,3). Studies have revealed the molecular and
cellular pathways that are vital for linking inflammation and
cancer (1). The effect of immune
cells on tumour cells partly depends on the production of
cytokines, chemokines, growth factors and reactive oxygen species.
Pro- and anti-tumorigenic effects are exerted by tumour-associated
cytokines; interleukin (IL)-1, IL-6 and IL-8 have been revealed to
promote carcinogenesis and tumour growth and invasion (4–6). By
contrast, IL-10 and IL-27 induce apoptosis in cancer cells and
prevent the development of cancer (7,8). The
pro-inflammatory cytokine IL-32 has also been implicated in the
development of various types of cancer, including hepatocellular
carcinoma, pancreatic cancer, oesophageal cancer, lung cancer,
gastric cancer, colon cancer, breast cancer and cutaneous T-cell
lymphoma (9–16).
IL-32 was first identified as natural killer cell
transcript 4 (NK4) as it was detected in activated natural killer
cells and T cells (17). The
biological function of IL-32 remained unknown until 2005 when Kim
et al (18) demonstrated for
the first time that recombinant NK4 could induce several
pro-inflammatory cytokines, including tumour necrosis factor
(TNF)-α and IL-8 (18). IL-32 is
located at human chromosome 16p13.3 and contains eight small exons.
According to the GenBank Database, IL-32 has more than nine
isoforms, which are transformed into four major splice variants:
IL-32α, IL-32β, IL-32γ and IL-32δ (19). Among the variants, IL-32β appears the
most abundant and IL32γ is the most broadly studied (20,21).
Furthermore, IL-32γ can be spliced into IL-32β in vitro and
in vivo. The overexpression of splice-resistant IL-32γ in
THP1 cells or rheumatoid arthritis (RA) synovial fibroblasts
revealed a greater induction of IL-6 and C-X-C motif chemokine
ligand 8 than in the same models overexpressing IL-32β (22). In addition to inducing cytokine
production, the transient overexpression of endogenous IL-32β or
IL-32γ resulted in cell death, whereas IL-32α overexpression failed
to induce cell death (23). Notably,
restoring the IL-8 survival-signalling pathway by co-overexpressing
C-X-C motif chemokine receptor 1 with IL-32β or IL-32γ in 293 cells
prevented IL-32β but not IL-32γ from inducing cell death (23). These studies indicated functional
differences between these diverse splice variants. The biological
properties of each isoform have been described in several reports
(24–26). IL-32α increases IL-6 expression in
THP-1 promonocytic cells (27). This
result was also supported by a report that revealed that IL-32α
induced IL-6 production by inhibiting B-cell lymphoma 6 (28). However, IL-32θ-expressing THP-1 cells
revealed a reduced expression of C-C motif chemokine ligand 5
(29). These studies suggested that
IL-32 functions as an intracellular mediator of inflammation.
Additionally, numerous studies have provided evidence that IL-32
has diverse functions due to the intracellular interactions of its
isoforms (26,30).
IL-32γ, originally known as NK4, was anticipated to
be a secreted protein because it contains a signal peptide sequence
and lacks a transmembrane region (18). Kim et al (18) demonstrated that induced the
overexpression of either IL-32α or IL-32β resulted in the secretion
of their proteins and stimulated peripheral blood mononuclear cells
(PBMCs), NK cells and A549 cells to release IL-32. Another study
revealed that IL-32 was secreted from T cells undergoing apoptosis
(20). By contrast, Heinhuis et
al (22) reported that the
overexpression of spliced or splice-resistant IL-32γ led to the
secretion of IL-32 but did not result in the release of
intracellular lactate dehydrogenase (22). Taken together, these results suggested
that endogenous IL-32 can be secreted via non-classical and
classical secretory pathways (31). A
definitive receptor has not been discovered for IL-32, but
proteinase 3 has been recently recognized as a specific IL-32
binding protein (32). Furthermore,
IL-32 can bind the extracellular domain of integrins, which might
act as receptors through the focal adhesion kinase 1 (FAK-1)
signalling pathway (33). As an
extracellular protein, IL-32 has been revealed to serve a crucial
role in the process of monocyte differentiation into
macrophage-like cells with phagocytic capacities (34). More recently, IL-32 was reported to
promote monocyte differentiation into CD1c+ dendritic cells and
CD163+CD68+ macrophages (35).
Therefore, IL-32 not only induces the production of
pro-inflammatory cytokines but also directly affects the
development and maturation of specific immune cells. IL-32 is also
involved in numerous inflammatory and infectious diseases,
including rheumatoid arthritis, chronic obstructive pulmonary
disease, mycobacterium tuberculosis infections and inflammatory
bowel disease (36–39).
IL-32 expression in cancer
A number of studies on a diverse range of tumour
types have investigated the clinical significance of IL-32
expression as a prognostic factor. A higher expression of IL-32 in
cancerous tissues of the human liver, pancreas, oesophagus, lung
and stomach has been revealed in comparison with the expression in
normal tissue or serum (Table I)
(9–11,13,40). The
expression of IL-32α was upregulated in tissues and serum from
patients with hepatocellular carcinoma (HCC) (9). Additionally, surgical tissues from
patients with pancreatic cancer revealed significantly higher
expression levels of IL-32 compared with that of healthy pancreatic
or normal tissues (10).
Immunohistochemical studies of oesophageal cancer tissues have
revealed that oesophageal cancer cells stain positively for IL-32,
whereas normal oesophageal cells display minimal staining (11). In human lung cancer, Sorrentino and
Sorrentino et al (40)
reported that high IL-32 expression was present in adenocarcinoma
(AC), large-cell carcinoma and small cell lung cancer but not in
squamous-cell carcinoma (SCC). Notably, in the present study, IL-32
expression was associated with a poor clinical outcome. These
results are in line with those of a study that revealed that IL-32
is associated with tumour cell invasion and metastasis in primary
lung adenocarcinoma (12).
Furthermore, the positive correlation between IL-32 and cancer
invasion that was demonstrated in lung cancer was also reported in
gastric and breast cancer (13,15). Tsai
et al (13) demonstrated that
the overexpression of IL-32 in gastric cancer appeared to correlate
with the aggressiveness of cancer and a poor prognosis. In a study
that involved breast cancer, a positive link was demonstrated
between IL-32 expression and tumour size, the number of lymph node
metastases and tumour stage (15).
However, there remains controversy regarding the expression and
action of IL-32 in tumours. A recent report noted that, although
cervical cancer tissues expressed a higher level of IL-32, the
expression of IL-32 was not correlated with patient mortality
(41). In chronic myelomonocytic
leukaemia, IL-32 expression was lower than in healthy donors.
Furthermore, IL-32 expression in stromal cells from the bone marrow
of patients with leukaemia served an important role in inducing
apoptosis in leukaemia cells (42).
 | Table I.Expression of IL-32 in human tumour
tissues. |
Table I.
Expression of IL-32 in human tumour
tissues.
| Tumour | Methods | IL-32 expression
and prognosis | (Refs.) |
|---|
| Hepatocellular
carcinoma | DNA microarray
chips, RT-PCR, IHC, WB | Higher in tumour
tissues than in normal tissues |
(9) |
| Pancreatic
cancer | RT-PCR, IHC | Higher in tumour
tissues than in pancreatic or normal tissues | (10) |
| Oesophageal
cancer | RT-PCR, IHC WB,
ELISA | Higher in tumour
tissues and patients' serum than in normal subjects | (11) |
| Lung cancer | RT-PCR, IHC
LCM | High in tumour
tissues with a histology-specific association; correlation with
worse prognosis revealed | (40) |
| Gastric cancer | RT-PCR, IHC | Higher in gastric
carcinomas than in corresponding normal mucosa; correlates with
tumour progression and poor prognosis | (13) |
| Breast cancer | RT-PCR, IHC | Higher in tumour
tissues than in normal tissues; correlates with tumour size, number
of lymph node metastases and tumour stage | (15) |
| Cutaneous T-cell
lymphoma | RT-PCR, IHC | Higher in tumour
tissues than in normal tissues; correlated with CCL17 and CCL18
expression | (16) |
|
| ELISA | Correlated with
disease activity |
|
In these studies, IL-32 served either an oncogenic
or a tumour suppressive role, likely due to disparities in the
predominant IL-32 isoform expressed in the tumour tissue. Genetic
variations among different ethnic groups and the different clinical
stages of tumours may also contribute to these contradictory
results. The expression of IL-32 may be a valuable independent
prognostic tumour marker for overall survival rates and the degree
of metastasis in patients with various forms of cancer.
The role of IL-32 in cancer
The mitogenic properties of IL-32
IL-32 has been demonstrated to be involved in cancer
development. A recent report noted that IL-32 can act as an
important growth factor for human cutaneous T-cell lymphoma (CTCL)
cells (16). The study revealed that
IL-32 accelerated the proliferation of CTCL cell lines in a
dose-dependent manner. Furthermore, the exposure of certain CTCL
cell lines to anti-IL-32 antibodies in culture inhibited cell
proliferation. These results indicated that IL-32 may be involved
in the pathogenesis of CTCL as an autocrine growth factor.
Similarly, extracellular IL-32 has also been proposed to act as a
mitogenic factor in breast cancer (43). Our group recently revealed that IL-32
promoted multiple myeloma cell growth through inducing the
production of IL-6 in bone marrow stromal cells (44). Intracellular IL-32 also affects tumour
growth. In hepatocellular carcinoma cells transfected with IL-32
small interfering RNA (siRNA), intrinsic apoptosis was increased
and cell growth was decreased compared with the level of these
activities in the control siRNA-transfected cells (9). These results are consistent with those
of one previous study demonstrating that IL-32 suppression abated
anti-apoptotic protein expression in pancreatic cancer cells
(10).
Contrary to the oncogenic role of IL-32 in cancer
cell proliferation and death, a number of reports have demonstrated
that IL-32 inhibits the proliferation of several types of cancer,
including colon cancer, prostate cancer and melanoma (45,46).
Induction of IL-32 expression inhibited the growth of colon cancer
cells, while suppression of endogenous IL-32 was reported to
reverse this inhibitory effect. This observation was also
reproduced in prostate cancer and melanoma cells (45,46).
In addition to its role in tumour proliferation,
IL-32 has also been linked to antitumour activities that are
dependent on the influence of other cytokines and immune cells.
Chronic myeloid leukaemia cell lines that overexpressed IL-32,
upregulated the expression of Fas and UL16-binding protein 2
(47). In accordance with the results
of the present study, Park et al (48) indicated that enforced overexpression
of IL-32 in colon cancer cells potentiated the inhibitory effect of
TNF-α and promoted the apoptosis of colon cancer cells. Other
studies have demonstrated that the inhibitory growth effects of
IL-32 were partly dependent on lymphocytes, dendritic cells and
other cytokines (46,49).
These results indicated contradictory functions of
IL-32 on tumour growth. It is possible that alternative splicing of
IL-32 leads to discrepancies in functional studies. Heinhuis et
al (23) revealed that 293 cells
overexpressing IL-32β or IL-32γ underwent cell death, while human
mammalian cell lines expressing high levels of IL-32α did not.
These opposing functions have also been reported in the alternative
splice variants of IL-6 and IL-24 (50,51). One
function of IL-6 is to promote cell proliferation, but an
alternatively spliced IL-6 variant can counteract this effect.
Similarly, IL-24-induced cell death can be blocked by an IL-24
splice variant.
The motogenic properties of IL-32
IL-32 has also been demonstrated to influence tumour
cell motility, a critical factor in tumour cell invasion and
metastasis. In gastric cancer, Tsai et al (13) established stable IL-32-expressing cell
sublines and revealed that all IL-32-overexpressing cells had
protrusions at their leading edges with elongated, spindle-like
morphology compared with that of the control cells. In addition to
these morphological characteristics, IL-32-overexpressing cells
displayed significantly higher invasive, metastatic and wound
healing capacities compared with those of control cells. Similar
observations were also repeatedly obtained in doxycycline-induced
IL-32-overexpressing cells. This study also revealed that the
underlying mechanism of IL-32-triggered cell invasion was an
increase in the expression levels of IL-8, vascular endothelial
growth factor (VEGF), matrix metalloproteinase (MMP)2 and MMP9, and
activation of protein kinase B (Akt), β-catenin and hypoxia
inducible factor 1α (HIF-1α) (13).
These findings are in line with those of previous studies, which
revealed that the IL-32-acquired invasive and migration phenotype
of lung cancer was mediated through the co-expression of IL-8 and
VEGF (40).
The involvement of IL-32 in tumour migration and
invasion has also been demonstrated in breast cancer (15). Previous study has reported that
overexpression of IL-32β increased tumour cell migration and
invasion in human breast cancer cells. By contrast, knockdown of
IL-32 negated these effects. Additionally, the motogenic effect of
IL-32 was stimulated by IL-32β-induced VEGF (15). In addition, Guenin et al
(52) demonstrated that IL-32 induced
the migration of head and neck squamous cell carcinoma cells by
regulating the expression of Snail. In HCC, IL-32α-expressing
tumour cells invaded blood vessels, which may suggest an
association between IL-32α expression and tumour metastasis
(9). More recently, studies have
revealed a motogenic effect of IL-32 in osteosarcoma and melanoma
(53,54).
Taken together, these results suggested that IL-32
expression increases the migration and invasion capacities of
tumour cells. By contrast, a recent study revealed that
overexpression of a different IL-32 isoform (IL-32θ) in colon cells
displayed a decrease in their invasiveness and in their oncogenic
capabilities (55). Therefore, the
detrimental role of IL-32 in multiple types of cancer may be
clinically relevant.
The angiogenic properties of
IL-32
In human pulmonary arterial hypertension and
glioblastoma multiforme, hyper-proliferative endothelial cells
(ECs) revealed a significant increase in IL-32 expression (56). Furthermore, suppression of IL-32
negatively affected the proliferation of ECs and downregulated
expression levels of nitric oxide, IL-8 and MMP-9 in adult and
neonatal ECs (56). Functional
studies that used co-culture-based angiogenesis assays revealed an
IL-32 increased tube formation in a dose-dependent manner. By
contrast, an αVβ3 inhibitor attenuated this response and reduced
the expression of IL-32γ-induced IL-8. These studies demonstrated
that IL-32 promotes angiogenesis partially through the integrin
αVβ3. The direct association between IL-32 and tumour angiogenesis
remains undetermined, and associated studies are limited. In
gastric cancer, IL-32 can induce the expression of IL-8, VEGF, MMP2
and MMP9 (13). Notably, IL-8 is a
particularly well-established potent promoter of angiogenesis.
IL-32 may indirectly promote tumour angiogenesis via inducing other
cytokines and growth factors.
It may be beneficial to evaluate the angiogenic
activity of IL-32 in human cancer, as angiogenesis serves a crucial
role in the pathogenesis and progression of cancer. In summary,
IL-32 is involved in multiple aspects of cancer development,
including growth, migration and angiogenesis (Fig. 1).
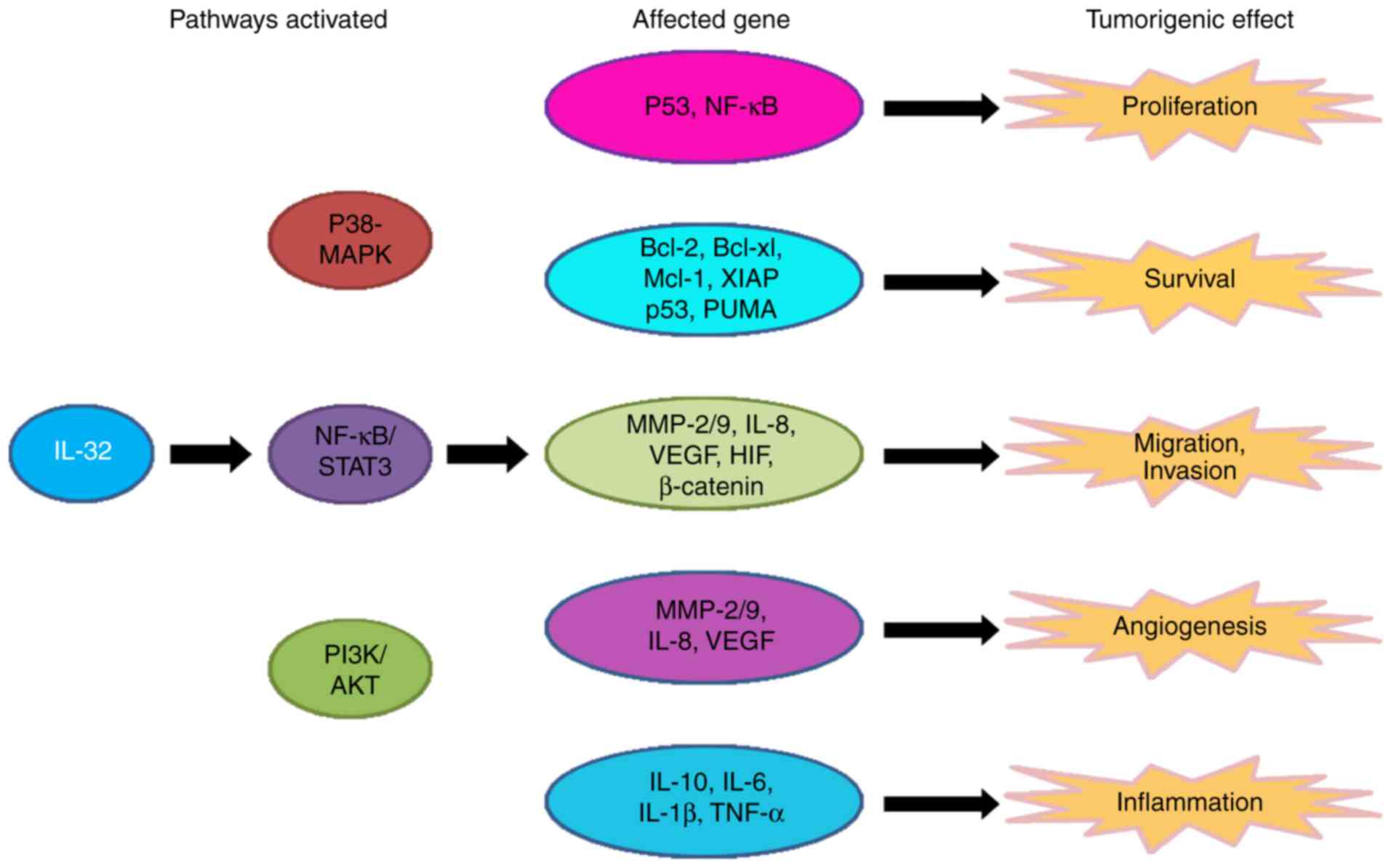 | Figure 1.The role and molecular pathway of
IL-32 in cancer. IL-32 activates 3 pathways: The P38-MAPK,
NF-κB/STAT-3 and PI3K/Akt pathways. The activation of these
pathways modifies the expression of several genes that affect cell
proliferation, survival, migration and invasion, and carcinogenic
angiogenesis and inflammation, causing tumorigenic effects. IL,
interleukin; MAPK, mitogen-activated protein kinase; NF-κB, nuclear
factor-κB; Bcl, B-cell lymphoma; Mcl-1, induced myeloid leukemia
cell differentiation protein 1; XIAP, x-linked inhibitor of
apoptosis; PUMA, p53 upregulated modulator of apoptosis; MMP,
matrix metalloproteinase; VEGF, vascular endothelial growth factor;
STAT, signal transducer and activator of transcription; PI3K,
phosphoinositide 3-kinase; Akt, protein kinase B; HIF,
hypoxia-inducible factor; TNF, tumour necrosis factor. |
The mechanisms of IL-32 in cancer
To directly investigate the mechanisms of
IL-32-mediated tumour growth and metastasis, various human tumour
cell lines have been transfected with the IL-32 gene. The effect of
IL-32-transfection on tumour inhibition and promotion depends on
the tumour cell type. For example, IL-32β-transfected breast cancer
cells demonstrated stronger migration and invasion abilities than
those of control tumour cells, and this process was mainly
dependent on the IL-32β-VEGF-STAT3 pathway (15). In addition, Tsai et al
(13) revealed that ectopic
expression of IL-32 in gastric cancer cell lines increased
metastatic potential via increased activity of Akt, β-catenin and
HIF-1α. Tumour cells were also used to monitor cell survival and
tumour development following transfection with IL-32-specific small
interfering RNA. In pancreatic cancer, IL-32 suppression markedly
stimulated apoptosis by downregulating anti-apoptotic proteins.
This finding was confirmed in human HCC (9).
However, in chronic myeloid leukaemia,
IL-32-transfected cells were more susceptible to NK cell-mediated
killing than control tumour cells (47). In another study, IL-32 overexpression
inhibited cancer development in cervical cancer cells compared with
mock-control cells (41).
Additionally, overexpression of IL-32 in colon and prostate cancer
cell lines inhibited tumour growth. It was hypothesised that IL-32
may promote TNF-α-induced cell growth inhibition or induce death
through the TNFR1 signalling pathway (14,48).
Notably, in vivo tumour growth was significantly slower in
mice with IL-32γ-transfected HCT116 colon cancer cells compared
with control cells (45,46). Additionally, the anticancer effect of
IL-32 was associated with the increased infiltration of CD8+ T
cells, lymphocytes and NK cells.
These results illustrated the discrepant effects of
IL-32 in cancer. The inconsistent observations among these results
might originate from malignancy-specific variations in downstream
pathways of IL-32. IL-32 is associated with various pathways,
including the nuclear factor-κB and p38 mitogen-activated protein
kinase pathways (18), extracellular
signal-regulated kinase-1/2 and phosphoinositide 3-kinase/Akt
pathways (57), a caspase-1-mediated
pathway that can be synchronized with nucleotide-binding
oligomerization domain-containing protein 1 and 2 ligands (58), and a caspase-3-dependent pathway
(59), depending on the tumour type
and tumour microenvironment (Fig. 1).
Determining the modulatory effects of IL-32 on pathways is
challenging, particularly since it may be affected by variations in
the tumour microenvironment.
The regulation of IL-32 in cancer
Aberrant expression levels of IL-32 serve an
important role in cancer, and therefore represent a potential
therapeutic target. The mechanisms of IL-32 expression regulation
in cancer have been studied by several groups. In epithelial
cell-derived thyroid carcinoma (TC) (60), Plantinga et al (60) revealed that patients with TC have an
overrepresentation of the ancient T allele, and
lipopolysaccharide-induced expression of IL-32 was higher in cells
homozygous for the ancient T allele than in cells without this
allele. These results clearly reveal that genetic variations of
IL-32 can lead to an increase in IL-32 gene expression. In
addition, the transcription factor Oct4 also increased IL-32
expression at the mRNA and protein levels in colorectal cancer
cells (61). It has been reported
that microRNA-205 induces an increase in IL-32 mRNA and protein
expression levels (62). These
results are consistent with those of another study that revealed
that the inhibition of miR-23b-3p induces a reduction in IL-32
expression in renal cancer (63). In
summary, IL-32 expression levels appear to correlate with genetic
factors, including genetic variations, transcription factor and
microRNAs.
Using immunostaining and reverse
transcription-polymerase quantitative chain reaction, Suga et
al (16) revealed that IL-32 was
more predominantly expressed in skin lesions of patients with
cutaneous T-cell lymphoma than in normal skin, suggesting that
hypoxia may contribute to the overexpression of IL-32. It has been
confirmed that hypoxia induces IL-32 in human breast cancer cells
(15,64); hypoxic challenges led to a
time-dependent increase in the expression of IL-32 in breast cancer
cells. A recent study reported that IL-32 was induced by hypoxia
and secreted from multiple myeloma cells in extracellular vesicles
(65). Furthermore, IL-32β expression
levels were upregulated by a hypoxia mimetic chemical,
CoCl2. These results demonstrated that hypoxia enhances
the expression of IL-32, which may contribute to the progression
and metastasis of human cancer. Additionally, acidic conditions
also contribute to increases in IL-32 expression (66). Identification of the mechanisms
underlying IL-32 modulation are required prior to this novel
cytokine being used as a target in human cancer treatments.
Conclusion and future directions
Inflammatory microenvironments serve a critical role
in tumorigenesis, imposing significant challenges in the design of
effective cancer therapy. Among growth factors and cytokines, IL-32
may be an additional mediator of human cancer development and
therefore may provide a novel therapeutic strategy. Understanding
the biological activity of IL-32 in cancer progression requires a
comprehensive analysis of the results obtained from clinical
studies, cell cultures and animal models. Nevertheless, further
investigations are required to elucidate the paradoxical role of
IL-32 in cancer, which appears to be due in part to IL-32 isoforms
and variations in the tumour microenvironment. It is apparent that
various mechanisms are involved in IL-32 actions, including direct
effects on tumour cell proliferation, angiogenesis and metastasis,
and indirect effects via inflammatory cells, including neutrophils
and macrophages. The mechanisms of the antitumour effect of IL-32
must be identified, and tumour-stimulating actions must be
neutralised. Identifying the optimal IL-32 isoform to function in a
particular disease may indicate new directions for the development
of antitumor strategies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 91429302, 81201868
and 81560030).
Availability of data and materials
Not applicable.
Authors' contributions
ZC and HY designed and conceived the study. DH, XH,
EZ, QC, RX, XL, FZ and ZC provided advice and assistance. HY wrote
the manuscript. All authors have contributed to and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: Links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hanahan D and Weinberg R: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Liu Z, Balivada S, Shrestha T,
Bossmann S, Pyle M, Pappan L, Shi J and Troyer D: Interleukin-1β
and transforming growth factor-β cooperate to induce neurosphere
formation and increase tumorigenicity of adherent LN-229 glioma
cells. Stem Cell Res Ther. 3:52012. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin L, Liu A, Peng Z, Lin HJ, Li PK, Li C
and Lin J: STAT3 is necessary for proliferation and survival in
colon cancer-initiating cells. Cancer Res. 71:7226–7237. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srivastava SK, Bhardwaj A, Arora S, Tyagi
N, Singh AP, Carter JE, Scammell JG, Fodstad Ø and Singh S:
Interleukin-8 is a key mediator of FKBP51-induced melanoma growth,
angiogenesis and metastasis. Br J Cancer. 112:1772–1781. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tao H, Lu L, Xia Y, Dai F, Wang Y, Bao Y,
Lundy SK, Ito F, Pan Q, Zhang X, et al: Antitumor effector B cells
directly kill tumor cells via the Fas/FasL pathway and are
regulated by IL-10. Eur J Immunol. 45:999–1009. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yoshimoto T, Chiba Y, Furusawa J, Xu M,
Tsunoda R, Higuchi K and Mizoguchi I: Potential clinical
application of interleukin-27 as an antitumor agent. Cancer Sci.
106:1103–1110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang YH, Park MY, Yoon DY, Han SR, Lee CI,
Ji NY, Myung PK, Lee HG, Kim JW, Yeom YI, et al: Dysregulation of
overexpressed IL-32α in hepatocellular carcinoma suppresses cell
growth and induces apoptosis through inactivation of NF-κB and
Bcl-2. Cancer Lett. 318:226–233. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishida A, Andoh A, Inatomi O and Fujiyama
Y: Interleukin-32 expression in the pancreas. J Biol Chem.
284:17868–17876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yousif NG, Al-Amran FG, Hadi N, Lee J and
Adrienne J: Expression of IL-32 modulates NF-κB and p38 MAP kinase
pathways in human esophageal cancer. Cytokine. 61:223–227. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zeng Q, Li S, Zhou Y, Ou W, Cai X, Zhang
L, Huang W, Huang L and Wang Q: Interleukin-32 contributes to
invasion and metastasis of primary lung adenocarcinoma via
NF-kappaB induced matrix metalloproteinases 2 and 9 expression.
Cytokine. 65:24–32. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsai CY, Wang CS, Tsai MM, Chi HC, Cheng
WL, Tseng YH, Chen CY, Lin CD, Wu JI, Wang LH and Lin KH:
Interleukin-32 increases human gastric cancer cell invasion
associated with tumor progression and metastasis. Clin Cancer Res.
20:2276–2288. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yun HM, Park KR, Kim EC, Han SB, Yoon DY
and Hong JT: IL-32α suppresses colorectal cancer development via
TNFR1-mediated death signaling. Oncotarget. 6:9061–9072. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park JS, Choi SY, Lee JH, Lee M, Nam ES,
Jeong AL, Lee S, Han S, Lee MS, Lim JS, et al: Interleukin-32β
stimulates migration of MDA-MB-231 and MCF-7cells via the
VEGF-STAT3 signaling pathway. Cell Oncol (Dordr). 36:493–503. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suga H, Sugaya M, Miyagaki T, Kawaguchi M,
Fujita H, Asano Y, Tada Y, Kadono T and Sato S: The role of IL-32
in cutaneous T-cell lymphoma. J Invest Dermatol. 134:1428–1435.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dahl CA, Schall RP, He HL and Cairns JS:
Identification of a novel gene expressed in activated natural
killer cells and T cells. J Immunol. 148:597–603. 1992.PubMed/NCBI
|
|
18
|
Kim SH, Han SY, Azam T, Yoon DY and
Dinarello CA: Interleukin-32: A cytokine and inducer of TNFalpha.
Immunity. 22:131–142. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kang JW, Park YS, Lee DH, Kim MS, Bak Y,
Ham SY, Park SH, Kim H, Ahn JH, Hong JT and Yoon DY: Interaction
network mapping among IL-32 isoforms. Biochimie. 101:248–251. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goda C, Kanaji T, Kanaji S, Tanaka G,
Arima K, Ohno S and Izuhara K: Involvement of IL-32 in
activation-induced cell death in T cells. Int Immunol. 18:233–240.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi JD, Bae SY, Hong JW, Azam T,
Dinarello CA, Her E, Choi WS, Kim BK, Lee CK, Yoon DY, et al:
Identification of the most active interleukin-32 isoform.
Immunology. 126:535–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heinhuis B, Koenders MI, van de Loo FA,
Netea MG, van den Berg WB and Joosten LA: Inflammation-dependent
secretion and splicing of IL-32{gamma} in rheumatoid arthritis.
Proc Natl Acad Sci USA. 108:4962–4967. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heinhuis B, Plantinga TS, Semango G,
Küsters B, Netea MG, Dinarello CA, Smit JWA, Netea-Maier RT and
Joosten LAB: Alternatively spliced isoforms of IL-32 differentially
influence cell death pathways in cancer cell lines. Carcinogenesis.
37:197–205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jung MY, Son MH, Kim SH, Cho D and Kim TS:
IL-32gamma induces the maturation of dendritic cells with Th1- and
Th17-polarizing ability through enhanced IL-12 and IL-6 production.
J Immunol. 186:6848–6859. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yun HM, Kim JA, Hwang CJ, Jin P, Baek MK,
Lee JM, Hong JE, Lee SM, Han SB, Oh KW, et al: Neuroinflammatory
and amyloidogenic activities of IL-32β in Alzheimer's disease. Mol
Neurobiol. 52:341–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong JT, Son DJ, Lee CK, Yoon DY, Lee DH
and Park MH: Interleukin 32, Inflammation and Cancer. Pharmacol
Ther. 174:127–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kang JW, Park YS, Lee DH, Kim JH, Kim MS,
Bak Y, Hong J and Yoon DY: Intracellular interaction of interleukin
(IL)-32α with protein kinase Cε (PKCε) and STAT3 protein augments
IL-6 production in THP-1 promonocytic cells. J Biol Chem.
287:35556–35564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park YS, Kang JW, Lee DH, Kim MS, Bak Y,
Yang Y, Lee HG, Hong J and Yoon DY: Interleukin-32α downregulates
the activity of the B-cell CLL/lymphoma 6 protein by inhibiting
protein kinase Cε-dependent SUMO-2 modification. Oncotarget.
5:8765–8777. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bak Y, Kang JW, Kim MS, Park YS, Kwon T,
Kim S, Hong J and Yoon DY: IL-32θ downregulates CCL5 expression
through its interaction with PKCδ and STAT3. Cell Signal.
26:3007–3015. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kang JW, Park YS, Lee DH, Kim MS, Bak Y,
Park SH, Ham SY, Yang Y, Hong JT and Yoon DY: Interleukin-32δ
interacts with IL-32β and inhibits IL-32β-mediated IL-10
production. FEBS Lett. Oct 25–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
31
|
Hasegawa H, Thomas HJ, Schooley K and Born
TL: Native IL-32 is released from intestinal epithelial cells via a
non-classical secretory pathway as a membrane-associated protein.
Cytokine. 53:74–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Novick D, Rubinstein M, Azam T, Rabinkov
A, Dinarello CA and Kim SH: Proteinase 3 is an IL-32 binding
protein. Proc Natl Acad Sci USA. 103:3316–3321. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heinhuis B, Koenders MI, van den Berg WB,
Netea MG, Dinarello CA and Joosten LA: Interleukin 32 (IL-32)
contains a typical α-helix bundle structure that resembles focal
adhesion targeting region of focal adhesion kinase-1. J Biol Chem.
287:5733–5743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Netea MG, Lewis EC, Azam T, Joosten LA,
Jaekal J, Bae SY, Dinarello CA and Kim SH: Interleukin-32 induces
the differentiation of monocytes into macrophage-like cells. Proc
Natl Acad Sci USA. 105:3515–3520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohmatsu H, Humme D, Gonzalez J, Gulati N,
Möbs M, Sterry W and Krueger JG: IL-32 induces indoleamine
2,3-dioxygenase+CD1c+ dendritic cells and
indoleamine 2,3-dioxygenase+CD163+
macrophages: Relevance to mycosis fungoides progression.
OncoImmunology. 6:e11812372016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Joosten LA, Netea MG, Kim SH, Yoon DY,
Oppers-Walgreen B, Radstake TR, Barrera P, van de Loo FA, Dinarello
CA and van den Berg WB: IL-32, a proinflammatory cytokine in
rheumatoid arthritis. Proc Natl Acad Sci USA. 103:3298–3303. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Calabrese F, Baraldo S, Bazzan E, Lunardi
F, Rea F, Maestrelli P, Turato G, Lokar-Oliani K, Papi A, Zuin R,
et al: IL-32, a novel proinflammatory cytokine in chronic
obstructive pulmonary disease. Am J Respir Crit Care Med.
178:894–901. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi J, Bae S, Hong J, Ryoo S, Jhun H,
Hong K, Yoon D, Lee S, Her E, Choi W, et al: Paradoxical effects of
constitutive human IL-32{gamma} in transgenic mice during
experimental colitis. Proc Natl Acad Sci USA. 107:21082–21086.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai X, Kim SH, Azam T, McGibney MT, Huang
H, Dinarello CA and Chan ED: IL-32 is a host protective cytokine
against Mycobacterium tuberculosis in differentiated THP-1 human
macrophages. J Immunol. 184:3830–3840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sorrentino C and Di Carlo E: Expression of
IL-32 in human lung cancer is related to the histotype and
metastatic phenotype. Am J Respir Crit Care Med. 180:769–779. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee S, Kim JH, Kim H, Kang JW, Kim SH,
Yang Y, Kim J, Park J, Park S, Hong J and Yoon DY: Activation of
the interleukin-32 pro-inflammatory pathway in response to human
papillomavirus infection and over-expressionof interleukin-32
controls the expression of the humanpapillomavirus oncogene.
Immunology. 132:410–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Marcondes AM, Mhyre AJ, Stirewalt DL, Kim
SH, Dinarello CA and Deeg HJ: Dysregulation of IL-32 in
myelodysplastic syndrome and chronic myelomonocytic leukemia
modulates apoptosis and impairs NK function. Proc Natl Acad Sci
USA. 105:2865–2870. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang S, Chen F and Tang L: IL-32 promotes
breast cancer cell growth and invasiveness. Oncol Lett. 9:305–307.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lin X, Yang L, Wang G, Zi F, Yan H, Guo X,
Chen J, Chen Q, Huang X, Li Y, et al: Interleukin-32α promotes the
proliferation of multiple myeloma cells by inducing production of
IL-6 in bone marrow stromal cells. Oncotarget. 8:92841–92854. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oh JH, Cho MC, Kim JH, Lee SY, Kim HJ,
Park ES, Ban JO, Kang JW, Lee DH, Shim JH, et al: IL-32γ inhibits
cancer cell growth through inactivation of NF-κB and STAT3 signals.
Oncogene. 30:3345–3359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yun HM, Oh JH, Shim JH, Ban JO, Park KR,
Kim JH, Lee DH, Kang JW, Park YH, Yu D, et al: Antitumor activity
of IL-32β through the activation of lymphocytes, and the
inactivation of NF-κB and STAT3 signals. Cell Death Dis.
4:e6402013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheon S, Lee JH, Park S, Bang SI, Lee WJ,
Yoon DY, Yoon SS, Kim T, Min H, Cho BJ, et al: Overexpression of
IL-32alpha increases natural killer cell-mediated killing through
up-regulation of Fas and UL16-binding protein 2 (ULBP2) expression
in human chronic myeloid leukemia cells. J Biol Chem.
286:12049–12055. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Park ES, Yoo JM, Yoo HS, Yoon DY, Yun YP
and Hong J: IL-32γ enhances TNF-α-induced cell death in colon
cancer. Mol Carcinog. 53 Suppl 1:E23–E35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qu Y, Taylor JL, Bose A and Storkus WJ:
Therapeutic effectiveness of intratumorally delivered dendritic
cells engineered to express the pro-inflammatory cytokine,
interleukin (IL)-32. Cancer Gene Ther. 18:663–673. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alberti L, Bachelot T, Duc A, Biota C and
Blay JY: A spliced isoform of interleukin 6 mRNA produced by renal
cell carcinoma encodes for an interleukin 6 inhibitor. Cancer Res.
65:2–5. 2005.PubMed/NCBI
|
|
51
|
Sahoo A, Jung YM, Kwon HK, Yi HJ, Lee S,
Chang S, Park ZY, Hwang KC and Im SH: A novel splicing variant of
mouse interleukin (IL)-24 antagonizes IL-24-induced apoptosis. J
Biol Chem. 283:28860–28872. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guenin S, Mouallif M, Hubert P, Jacobs N,
Krusy N, Duray A, Ennaji MM, Saussez S and Delvenne P:
Interleukin-32 expression is associated with a poorer prognosis in
head and neck squamous cell carcinoma. Mol Carcinog. 53:667–673.
2014.PubMed/NCBI
|
|
53
|
Lee J, Kim KE, Cheon S, Song JH, Houh Y,
Kim TS, Gil M, Lee KJ, Kim S, Kim D, et al: Interleukin-32α induces
migration of human melanoma cells through downregulation of
E-cadherin. Oncotarget. 7:65825–65836. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou Y, Hu Z, Li N and Jiang R:
Interleukin-32 stimulates osteosarcoma cell invasion and motility
via AKT pathway-mediated MMP-13 expression. Int J Mol Med.
35:1729–1733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Bak Y, Kwon T, Bak IS, Hong J, Yu DY and
Yoon DY: IL-32θ inhibits stemness and epithelial-mesenchymal
transition of cancer stem cells via the STAT3 pathway in colon
cancer. Oncotarget. 7:7307–7317. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nold-Petry CA, Rudloff I, Baumer Y, Ruvo
M, Marasco D, Botti P, Farkas L, Cho SX, Zepp JA, Azam T, et al:
IL-32 promotes angiogenesis. J Immunol. 192:589–602. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mabilleau G and Sabokbar A: Interleukin-32
promotes osteoclast differentiation but not osteoclast activation.
PLoS One. 4:e41732009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Netea MG, Azam T, Ferwerda G, Girardin SE,
Walsh M, Park JS, Abraham E, Kim JM, Yoon DY, Dinarello CA and Kim
SH: IL-32 synergizes with nucleotide oligomerization domain (NOD) 1
and NOD2 ligands for IL-1beta and IL-6 production through a caspase
1-dependent mechanism. Proc Natl Acad Sci USA. 102:16309–16314.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Joosten LA, Heinhuis B, Netea MG and
Dinarello CA: Novel insights into the biology of interleukin-32.
Cell Mol Life Sci. 70:3883–3892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Plantinga TS, Costantini I, Heinhuis B,
Huijbers A, Semango G, Kusters B, Netea MG, Hermus AR, Smit JW,
Dinarello CA, et al: A promoter polymorphism in human
interleukin-32 modulates its expression and influences the risk and
the outcome of epithelial cell-derived thyroid carcinoma.
Carcinogenesis. 34:1529–1535. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chang CJ, Chien Y, Lu KH, Chang SC, Chou
YC, Huang CS, Chang CH, Chen KH, Chang YL, Tseng LM, et al:
Oct4-related cytokine effects regulate tumorigenic properties of
colorectal cancer cells. Biochem Biophys Res Commun. 415:245–251.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Majid S, Dar AA, Saini S, Yamamura S,
Hirata H, Tanaka Y, Deng G and Dahiya R: MicroRNA-205-directed
transcriptional activation of tumor suppressor genes in prostate
cancer. Cancer. 116:5637–5649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zaman MS, Thamminana S, Shahryari V,
Chiyomaru T, Deng G, Saini S, Majid S, Fukuhara S, Chang I, Arora
S, et al: Inhibition of PTEN gene expression by oncogenic
miR-23b-3p in renal cancer. PLoS One. 7:e502032012. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Park JS, Lee S, Jeong AL, Han S, Ka HI,
Lim JS, Lee MS, Yoon DY, Lee JH and Yang Y: Hypoxia-induced IL-32β
increases glycolysis in breast cancer cells. Cancer Lett.
356:800–808. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zahoor M, Westhrin M, Aass KR, Moen SH,
Misund K, Psonka-Antonczyk KM, Giliberto M, Buene G, Sundan A,
Waage A, et al: Hypoxia promotes IL-32 expression in myeloma cells,
and high expression is associated with poor survival and bone loss.
Blood Adv. 1:2656–2666. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fukamachi T, Ikeda S, Wang X, Saito H,
Tagawa M and Kobayashi H: Gene expressions for signal transduction
under acidic conditions. Genes (Basel). 4:65–85. 2013. View Article : Google Scholar : PubMed/NCBI
|















