Introduction
Esophageal carcinoma (EC) remains one of the leading
causes of cancer-associated mortality (1), with a 5-year survival rate of <20%
(2). EC usually occurs as either
adenocarcinoma or squamous cell carcinoma (ESCC), the latter of
which is more dominant in East Asia and accounts for 95% of all
Chinese EC cases (3). Given this,
there is an urgent requirement for research to prevent and treat
this disease.
Fentanyl, a strongly anesthetic analgesic drug, is
an agonist for the µ-opioid receptor and is widely used in surgery,
including tumor radical resection (4). Furthermore, it is considered to be an
effective analgesic for breakthrough cancer pain in patients with
terminal cancer (5). Recently, an
increasing number of studies reported that fentanyl is able to
inhibit cancer progression, including proliferation, cell cycle,
apoptosis, invasion and chemotherapy sensitivity (6–10). In
brief, fentanyl may serve a potential therapeutic role in cancer
treatment. However, the effect of fentanyl on ESCC and the
mechanism underpinning this remain unknown.
MicroRNAs (miRNAs) represent a class of small
non-coding RNAs that regulate gene expression at the
post-transcriptional level. One study demonstrated that miRNAs were
aberrantly regulated in different oncogenic pathways and/or various
types of cancer, indicating that certain miRNAs may function as
oncogenes or tumor suppressor genes (11). Previous studies revealed the
expression profiles of different miRNAs and identified certain
specific miRNAs with biological functions and significance in ESCC
(12–14). miR-302b, which was downregulated in
ESCC, inhibited cell proliferation, induced apoptosis and reversed
invasion in ESCC (13). In addition,
it was believed that miR-302b inhibited the malignant behaviors of
ESCC by directly targeting ErbB4, a molecular therapeutic target
for ESCC (13).
Since fentanyl is able to change miRNA expression
profiles in human cancer cells (8),
we hypothesized that fentanyl may inhibit the proliferation and
invasion of ESCC cells through the miR-302b/ErbB4 pathway.
Therefore, in the present study, the effects of fentanyl on the
proliferation, apoptosis and invasion of ESCC Eca109 and TE1 cells
were investigated. Furthermore, the regulatory effect of fentanyl
on the expression of miR-302b and its target, ErbB4, was also
examined in order to elucidate the exact mechanism of the antitumor
effect of fentanyl in ESCC.
Materials and methods
Cell culture and reagents
The ESCC Eca109 and TE1 cell lines were obtained
from the Shanghai Institute for Biological Sciences (http://www.cellbank.org.cn/), Chinese Academy of
Sciences (Beijing, China). Cells were cultured in RPMI-1640 medium
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), supplemented with
10% fetal bovine serum and 100 U/ml penicillin and streptomycin, at
37°C in a humidified atmosphere with 5% CO2. Fentanyl
was purchased from Sigma-Aldrich; Merck KGaA, was dissolved in
dimethyl sulfoxide (DMSO) and was added into the culture medium at
various concentrations (0, 0.5, 5, 50 and 500 ng/ml) for in
vitro assays.
Cell proliferation assay
Cells were seeded at a density of 5×103
cells/well in 96-well plates at a final volume of 180 µl in
incubation, at 37°C in a 5% CO2 atmosphere. Following
various incubation times (24, 48 and 72 h), 20 µl of 5 mg/ml
solution of MTT (Sigma-Aldrich; Merck KGaA) in 1xphosphate-buffered
saline (PBS) was added to each well. The plates were subsequently
incubated for 4 h at 37°C, prior to the reaction being solubilized
in 100% DMSO (20 µl/well) and agitated at 37°C for 15 min. The
absorbance of each well was measured on a multi-detection
microplate reader (BMG Labtech GmbH, Ortenburg, Germany) at a
wavelength of 570 nm.
Apoptosis analysis
The cells were washed twice with cold 10 mM 1xPBS
and were resuspended in 1xbinding buffer (BD Biosciences, San Jose,
CA, USA). Cells were then washed twice with PBS and 400 µl 1×
binding buffer was added followed by 5 µl Annexin V-fluorescein
isothiocyanate (FITC) conjugate from the FITC Annexin V Apoptosis
Detection kit (cat. no. 556547; BD Biosciences). The cells were
then incubated in the dark for 15 min at 2–8°C, then 5 µl PI was
added and incubation was continued for 5 min. The samples were
analyzed using a flow cytometer (FACSCalibur; BD Biosciences) and
analyzed by the Cell Quest software (version 3.3; BD
Biosciences).
Cell invasion assay
For the invasion assay, the membrane invasion
culture system Transwell membranes with a diameter of 6.5 mm
diameter and a pore size of 8 µm; Costar (Corning Incorporated,
Corning, NY, USA) was used according to the manufacturer's
protocol. Briefly, harvested cells (1×105), resuspended
in 100 µl of serum-free RPMI-1640 medium, were added into the upper
chamber. A total of 1,000 µl conditioned RPMI-1640 medium with 20%
(v/v) fetal bovine serum was used as a chemoattractant and was
placed into the lower chamber. After 48 h, the un-invaded cells on
the upper surface of the membrane were removed with a cotton swab.
The transformed cells that had invaded through the Matrigel matrix
and stuck to the lower surface of the membrane were fixed with 4%
paraformaldehyde for 1 h at room temperature and stained with 1%
crystal purple for 15 min at room temperature. The invasive cells
were then counted (in 5 high-power fields/chamber) using an
inverted microscope (Olympus Corporation, Tokyo, Japan;
magnification, ×200). Each experiment was repeated in
triplicate.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from Eca109 and TE1 cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol.
RT-qPCR was performed using a Bio-Rad iQ5 Real-Time PCR Detection
system to confirm the mRNA expression levels. A reverse
transcription kit and SYBR-Green both from Takara Biotechnology
Co., Ltd. (Dalian, China) were used. In brief, reverse
transcription (RT) was performed in a 20 µl volume with 1 µg total
RNA, by incubation at 16°C for 30 min, 42°C for 42 min and 85°C for
5 min. A total of 1 µl of the RT product was used in each PCR. The
PCR cycling began with template denaturing at 95°C for 5 min,
followed by 40 cycles of 95°C for 10 sec, 60°C for 20 sec, 72°C for
20 sec and 78°C for 20 sec. Final PCR products were resolved by
agarose gel electrophoresis and a single band of expected size
indicated the specificity of the reaction. Relative quantification
was performed using the 2−ΔΔCq method normalized to
GAPDH (15). Each PCR amplification
was performed in triplicate to verify the results. The primers were
as previously described (14).
Western blot analysis
Total proteins were extracted from cells using lysis
buffer containing phenylmethyl sulfonylfluoride (both from Beyotime
Institute of Biotechnology, Haimen, China) at 25°C. The protein
concentration was determined using BCA Protein Assay kit (Beyotime
Institute of Biotechnology). For western blot analyses, 20 µg total
protein was electrophoresed on a 10% SDS gel, transferred onto
polyvinylidene difluoride membranes, blocked with 5% (w/v) non-fat
dry milk in Tris-buffered saline with 0.1% Tween-20 for 1 h at room
temperature, and incubated with anti-ErbB4 (cat no. sc-283; 1:500;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and anti-β-actin
(cat no. sc-7210; 1:200; Santa Cruz Biotechnology, Inc.) primary
antibodies at 4°C for 12 h. A corresponding bovine anti-rabbit IgG
horseradish peroxidase-conjugated secondary antibody (cat no.
sc-2370; 1:1,000; Santa Cruz Biotechnology, Inc.) was subsequently
applied at room temperature for 2 h. Following chemiluminescence
reactions with enhanced chemiluminescence detection reagents kits
(GE Healthcare, Chicago, IL, USA), according to the manufacturer's
protocol, the membranes were visualized by exposure to X-ray film
in the dark. Densitometric analysis was performed using Scion Image
software (Scion Corporation, Frederick, MD, USA).
Anti-miR design and transfection
miR-302b inhibitor (A) and miR Inhibit or Negative
Control (NC) were purchased from AngRang Inc. (Xi'an, China). The
sequence for miR-302b inhibitor was 5′-CTACTAAAACATGGAAGCACTTA-3′.
Cells were seeded on to a 24-well plate at a concentration of
1×105 cells/well. RNA oligonucleotides transfection (50
nM) was performed with Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Fresh growth medium (RPMI-1640) was changed 6 h after transfection,
and the cells were harvested for analysis 48 h after
transfection.
Statistical analysis
Data are expressed as the mean ± standard error of
the mean from ≥3 separate experiments performed in triplicate.
Differences among groups were assessed by a one-way analysis of
variance (followed by Student-Newman-Keuls) using SPSS 13.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of fentanyl on cell
proliferation, apoptosis and invasion
The present study initially investigated the effects
of fentanyl on cell proliferation, apoptosis and invasion. The
Eca109 and TE1 cell lines were cultured in the presence of various
concentrations (0.5, 5, 50 and 500 ng/ml) of fentanyl and the cell
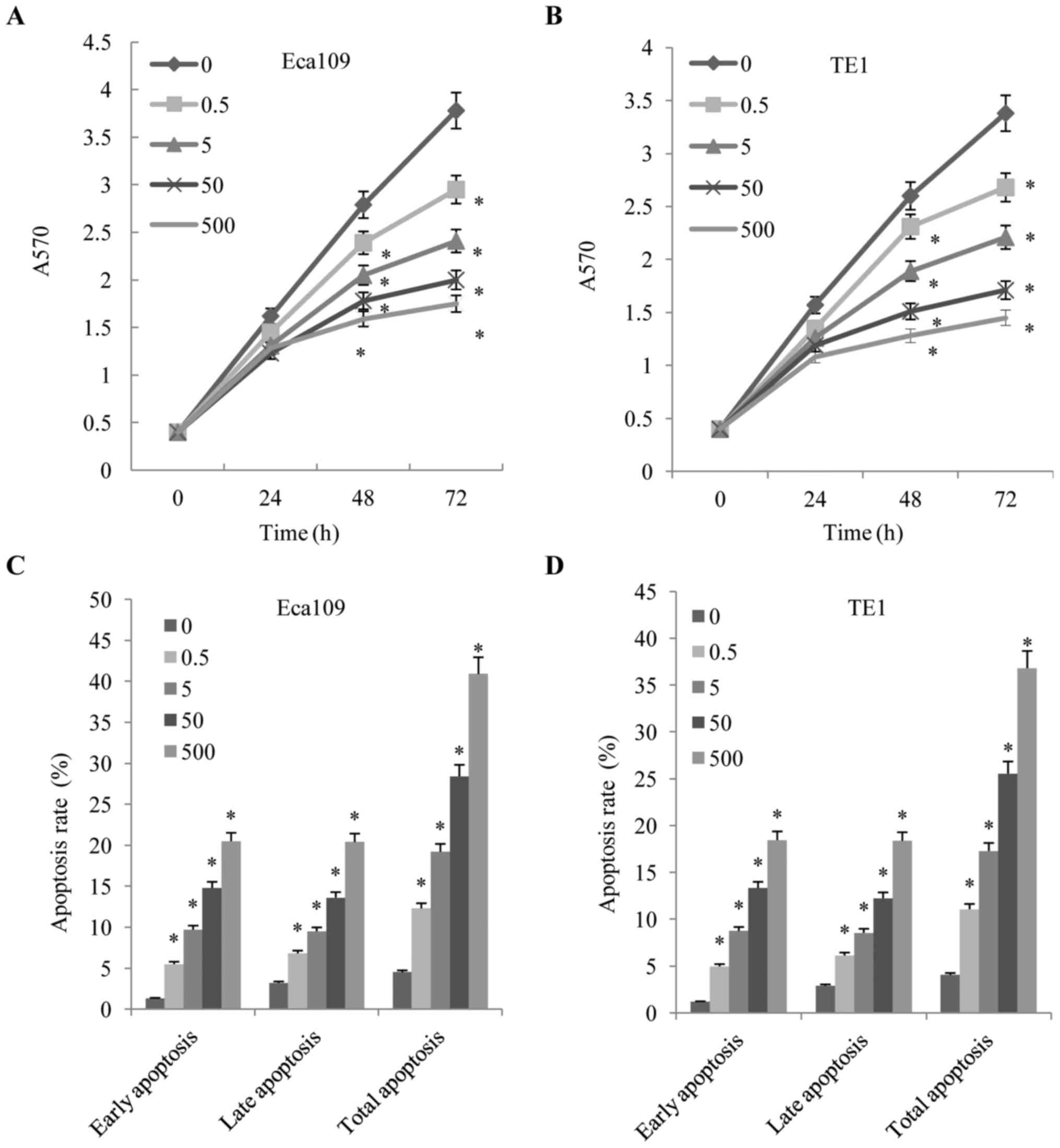
proliferation were measured using MTT assays. As demonstrated in
Fig. 1A and B, the proliferation of
the Eca109 and TE1 cells was inhibited by fentanyl in a dose- and
time-dependent manner. Fentanyl significantly inhibited cell
proliferation at 48 and 72 h. In order to further quantify cell
death, Annexin V/PI analysis was performed. Following exposure to
fentanyl for 48 h, Eca109 cells exhibited a decreasing rate of
apoptosis (Fig. 1C and D). The cell
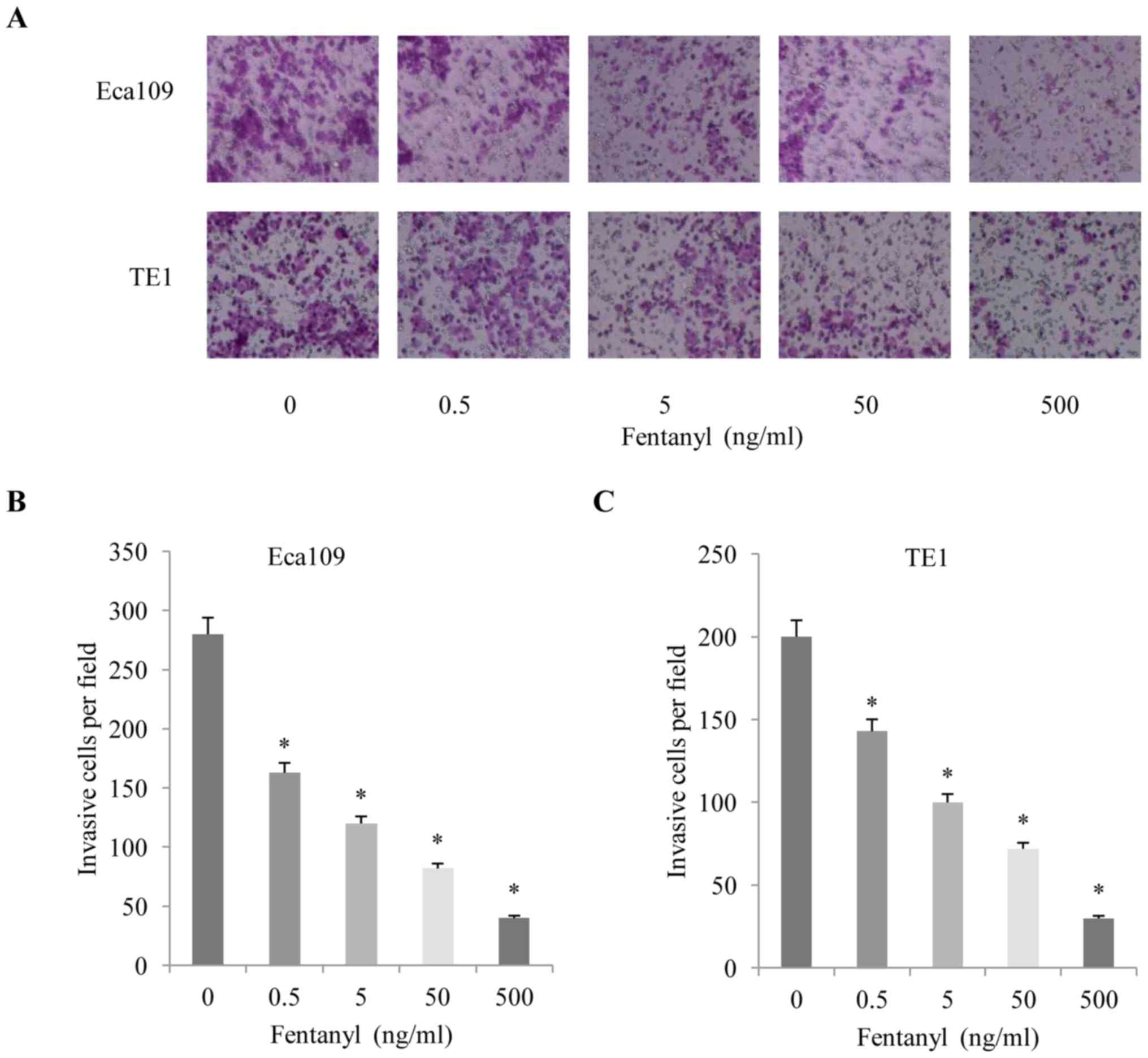
invasion assay also revealed that fentanyl significantly stimulated
invasion in a concentration-dependent manner (Fig. 2A-C). Concentrations of fentanyl >5
ng/ml exhibited a significant inhibitory effect on cell
proliferation and metastasis. Therefore, a concentration of 5 ng/ml
was selected for the subsequent experiments.
miR-302b is involved in the effect of
fentanyl on ESCC behaviors
It was previously revealed that miR-302b suppressed
proliferation by inducing apoptosis and repressed the invasion of
ESCC cells through targeting ErbB4 (13). The present study further investigated
whether or not miR-302b is also involved in the effect of fentanyl
on the biological behaviors of ESCC. An miR-302b inhibitor
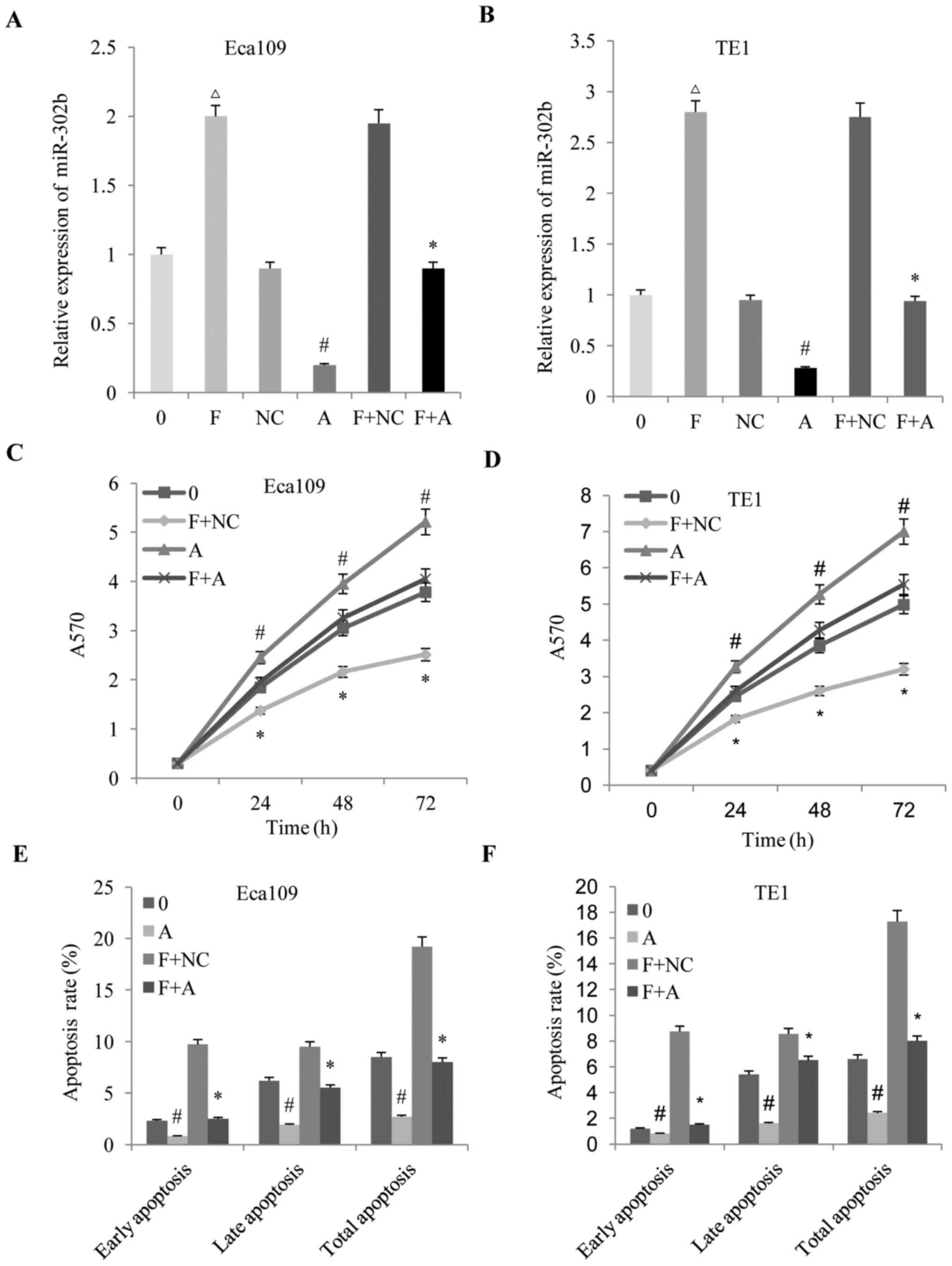
(anti-miR-302b) was used to block miR-302b expression in ESCC
cells, with the results demonstrating that fentanyl increased the
expression of miR-302b, and that anti-miR-302b reversed the
upregulation of miR-302b (Fig. 3A and
B). Subsequently, the effects of altered miR-302b expression on
the anti-proliferation, pro-apoptosis and anti-invasion effects
induced by fentanyl in ESCC were detected. It was revealed that
downregulation of miR-302b reversed the anti-proliferation
(Fig. 3C and D), pro-apoptosis
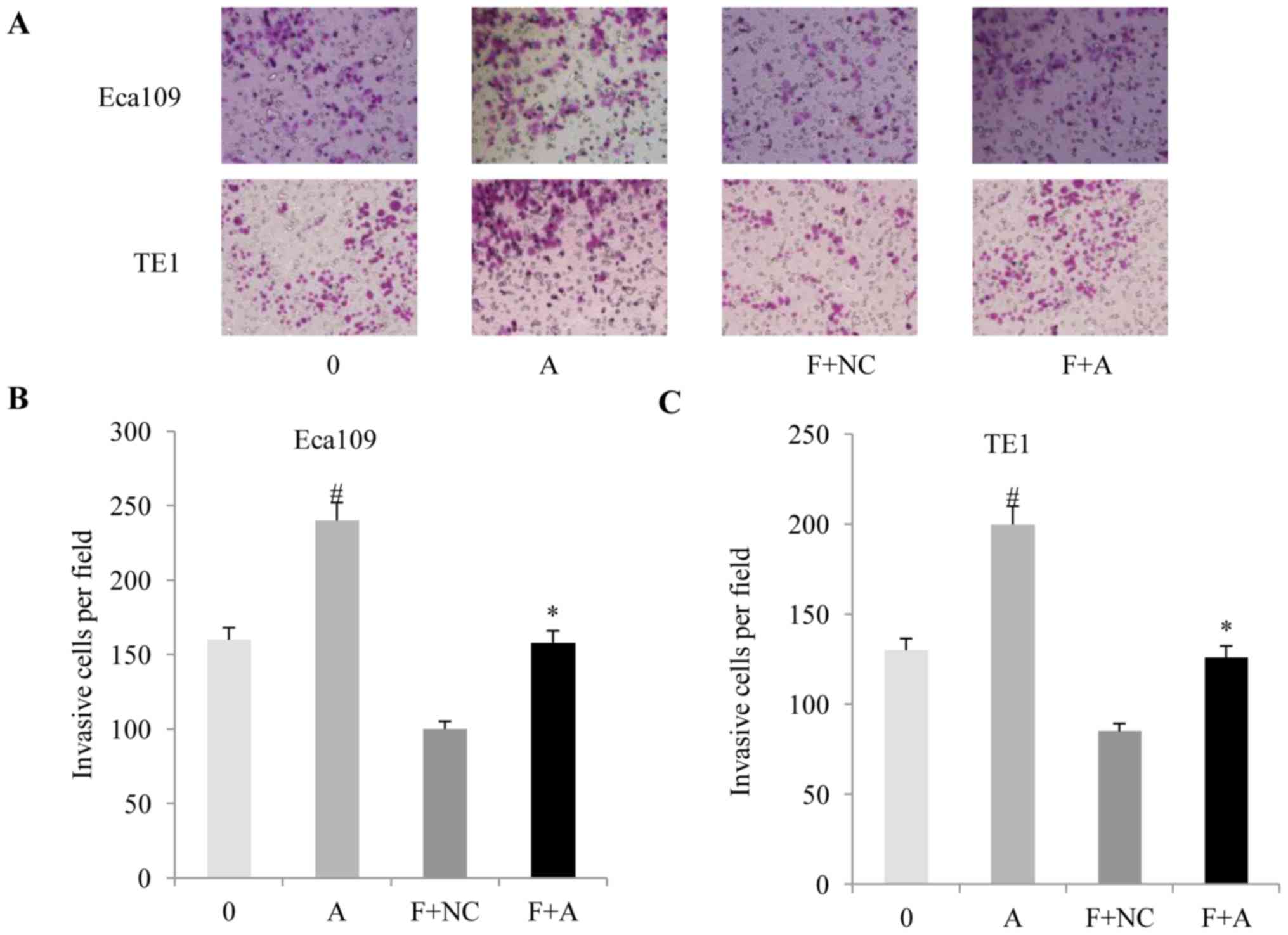
(Fig. 3E and F) and anti-invasion
(Fig. 4A-C) effects of fentanyl in
the two ESSC cell lines.
Fentanyl upregulated the expression of
miR-302b in ESSC cells
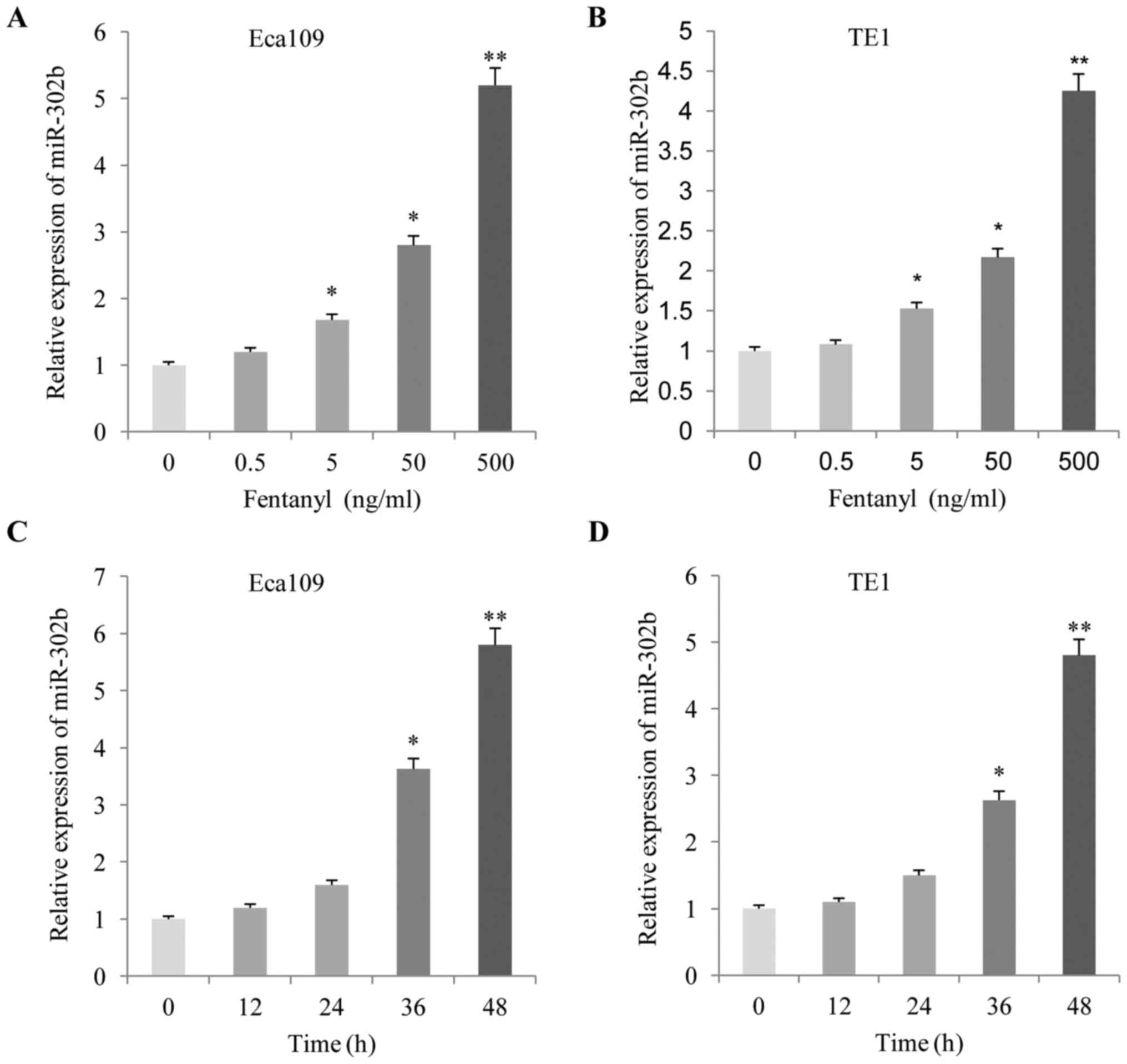
The present study analyzed the effects of fentanyl
on the expression levels of miR-302b. As demonstrated in Fig. 5, following treatment with fentanyl for
48 h, the expression level of miR-302b increased significantly in
the two ESCC cells in a dose-(Fig. 5A and
B) and time-(Fig. 5C and D)
dependent manner, according to the RT-qPCR results.
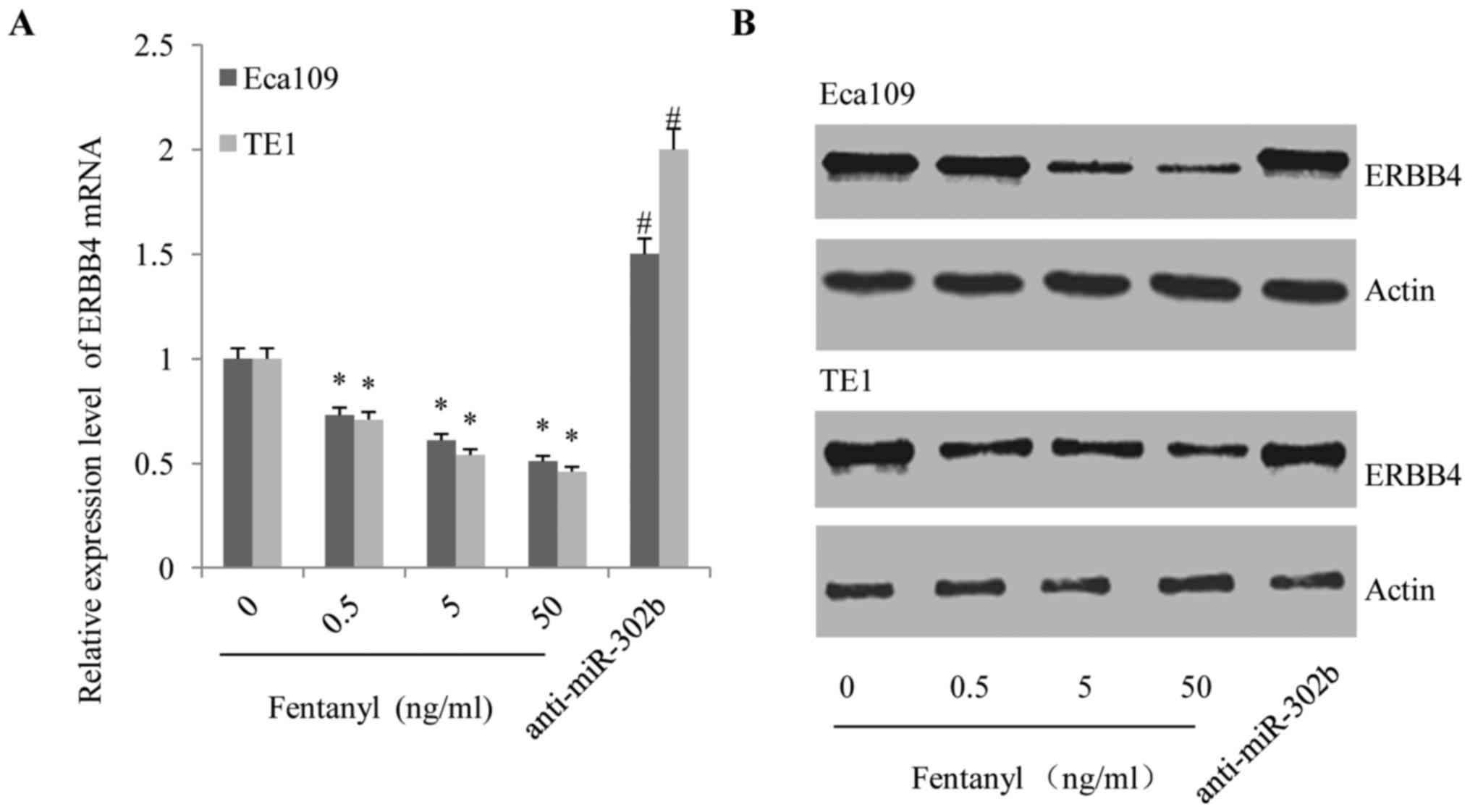
Fentanyl downregulated the expression
of ErbB4, but this effect was reversed by anti-miR-302b
transfection in ESSC cells
ERBB4 is one of the down-stream targets of miR-302b
(14); therefore, we hypothesized
that fentanyl modified the behaviors of ESCC cells through
suppressing ErbB4. As demonstrated in Fig. 6, fentanyl decreased the expression of
ErbB4 at the transcriptional (Fig.
6A) and translational levels (Fig.
6B) in a dose-dependent manner. However, the suppressive effect
of fentanyl on ErbB4 expression was subsequently reversed by
anti-miR-302b transfection (Fig. 6A and
B), demonstrating the effects of miR-302b on the ability of
fentanyl to inhibit the activation of ErbB4.
Discussion
Cancer is a major public health issue in the
majority of countries, including China. Cancer is often treated by
chemotherapy, immunotherapy, radiation and surgery. Anesthesia
serves an important role in surgery, ensuring the safety and
comfort of patients during procedures (16). However, numerous anesthetic agents are
used without knowledge of their effects on cancer. Recently, it has
been suggested that certain anesthetic drugs may modify malignant
biological behaviors, including proliferation, angiogenesis and
apoptosis in certain cancer cells (17). Nevertheless, the possible role of
anesthetic drugs in cancer development and progression remains
unclear.
Fentanyl is widely used in clinic as an anesthetic,
particularly in the treatment of different types of cancer,
including ESCC (18). Previous
studies have reported the potential antitumor effects of fentanyl,
but there are limited reports regarding its role in ESCC (6–10). The
results of the present study suggested that the mechanisms of the
anti-proliferation and anti-invasion effects of fentanyl were
associated with miR-302b expression in ESCC Eca109 and TE1 cell
lines. Accompanied by the malignant biological behaviors changes,
the expression of miR-302b was elevated by fentanyl treatment.
Furthermore, ErbB4 was targeted and inhibited by increased miR-302b
expression. Notably, the application of anti-miR-302b impaired the
anti-proliferation and anti-invasion effects of fentanyl. These
results supported our hypothesis that fentanyl inhibited the
proliferation and invasion of ESCC cells by stimulating the
expression of miR-302b which, in turn, downregulated the expression
of ErbB4.
miR-302b is a member of the miR-302 cluster, which
regulates the regulatory circuitry controlling ES cell ‘stemness’
(19). Previously, it was revealed
that miR-302b acted as a tumor suppressor by post-transcriptionally
regulating different types of oncogenes. miR-302b was able to
inhibit proliferation (20,21), induce apoptosis (22,23) and
enhance chemotherapy sensitivity (24,25). A
previous study revealed that miR-302b was a potential molecular
marker of ESCC and that it acts as a tumor suppressor by targeting
ErbB4 (13).
ErbB4, one of the potential targets in ESCC, is one
of members of the ErbB/HER subfamily, which regulates cellular
proliferation, differentiation and programmed cell death (26). Xu et al (27) revealed that extra-nuclear ErbB4 had
negative effects on the progression of ESCC, while the nuclear
translocation of ErbB4 exhibited a tumor-promoting property. Zhao
et al (28) reported that
ErbB4 served as a potential molecular target in the treatment of
ESCC. Therefore, activation of ErbB4 may promote proliferation and
invasion in ESCC. The results of the present study demonstrated
that, accompanied by elevation of miR-302b in ESCC cells, fentanyl
treatment also downregulated the expression of ErbB4, thereby
inhibiting proliferation and invasion. Furthermore, fentanyl failed
to downregulate the expression of ErbB4 in cells transfected with
anti-miR-302b, which also impaired the inhibitory effects of
fentanyl against the proliferation and invasion of these cells
through miR-302b.
In summary, to the best of our knowledge, the
present study is the first to identify the involvement of miR-302b
in the anti-proliferation and anti-invasion effects of fentanyl in
ESCC. Based on the results in the present study, it was concluded
that fentanyl inhibited the proliferation and invasion of ESCC
cells by elevating the expression of miR-302b and, in turn,
suppressing the activation of ErbB4. However, the manner in which
fentanyl treatment regulatesmiR-302b expression in ESCC cells
remains unknown and requires further study.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NW and ZZ conceived the study. NW and JL wrote and
edited the main manuscript. JL and ZZ designed the experiments. NW,
ZZ and JL performed the experiments and analyzed the data.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nuckols TK, Anderson L, Popescu I, Diamant
AL, Doyle B, Di Capua P and Chou R: Opioid prescribing: A
systematic review and critical appraisal of guidelines for chronic
pain. Ann Intern Med. 160:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mercadante S: Fentanyl buccal tablet for
the treatment of cancer-related breakthrough pain. Expert Rev Clin
Pharmacol. 8:9–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin Y, Li L, Chen J, Tang X, Liao C, Xie Y
and Xiao Q: Fentanyl inhibits progression of human gastric cancer
MGC-803 cells by NF-kappaB downregulation and PTEN upregulation in
vitro. Oncol Res. 20:61–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nomura Y, Kawaraguchi Y, Sugimoto H,
Furuya H and Kawaguchi M: Effects of morphine and fentanyl on
5-fluorouracil sensitivity in human colon cancer HCT116 cells. J
Anesth. 28:298–301. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang XL, Chen ML and Zhou SL: Fentanyl
inhibits proliferation and invasion of colorectal cancer via
β-catenin. Int J Clin Exp Pathol. 8:227–235. 2015.PubMed/NCBI
|
|
9
|
Li AX, Xin WQ and Ma CG: Fentanyl inhibits
the invasion and migration of colorectal cancer cells via
inhibiting the negative regulation of Ets-1 on BANCR. Biochem
Biophys Res Commun. 465:594–600. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miao J, Wang L, Chen L, Yang T, Jin L and
Lin L: Fentanyl inhibits cell viability in human pancreatic cancer
cell line and tumor growth in pancreatic cancer cell-transplanted
mice. Int J Clin Exp Med. 8:17684–17693. 2015.PubMed/NCBI
|
|
11
|
Ohtsuka M, Ling H, Doki Y, Mori M and
Calin GA: MicroRNA processing and human cancer. J Clin Med.
4:1651–1667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang M, Zhou S, Zhang L, Zhang J, Cai H,
Zhu J, Huang C and Wang J: miR-518b is down-regulated, and involved
in cell proliferation and invasion by targeting Rap1b in esophageal
squamous cell carcinoma. FEBS Lett. 586:3508–3521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Yang Q, Zhang L, Zhou S, Ye W,
Yao Q, Li Z, Huang C, Wen Q and Wang J: miR-302b is a potential
molecular marker of esophageal squamous cell carcinoma and
functions as a tumor suppressor by targeting ErbB4. J Exp Clin
Cancer Res. 33:102014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou S, Ye W, Ren J, Shao Q, Qi Y, Liang J
and Zhang M: MicroRNA-381 increases radiosensitivity in esophageal
squamous cell carcinoma. Am J Cancer Res. 5:267–277.
2014.PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ciechanowicz SJ and Ma D: Anaesthesia for
oncological surgery-can it really influence cancer recurrence?
Anaesthesia. 71:127–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santamaria LB, Schifilliti D, La Torre D
and Fodale V: Drugs of anaesthesia and cancer. Surg Oncol.
19:63–81. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tai YH, Wu HL, Chang WK, Tsou MY, Chen HH
and Chang KY: Intraoperative fentanyl consumption does not impact
cancer recurrence or overall survival after curative colorectal
cancer resection. Sci Rep. 7:108162017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin SL, Chang DC, Chang-Lin S, Lin CH, Wu
DT, Chen DT and Ying SY: Mir-302 reprograms human skin cancer cells
into a pluripotent ES-cell-like state. RNA. 14:2115–2124. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Yao J, Shi X, Hu L, Li Z, Song T
and Huang C: MicroRNA-302b suppresses cell proliferation by
targeting EGFR in human hepatocellular carcinoma SMMC-7721 cells.
BMC Cancer. 13:4482013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L, Yao J, Zhang X, Guo B, Le X,
Cubberly M, Li Z, Nan K, Song T and Huang C: miRNA-302b suppresses
human hepatocellular carcinoma by targeting AKT2. Mol Cancer Res.
12:190–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Y, Hu H, Song L, Cai L, Wei R and
Jin W: Epirubicin-mediated expression of miR-302b is involved in
osteosarcoma apoptosis and cell cycle regulation. Toxicol Lett.
222:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen PH, Shih CM, Chang WC, Cheng CH, Lin
CW, Ho KH, Su PC and Chen KC: MicroRNA-302b-inhibited E2F3
transcription factor is related to all trans retinoic acid-induced
glioma cell apoptosis. J Neurochem. 131:731–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai D, He K, Chang S, Tong D and Huang C:
MicroRNA-302b Enhances the Sensitivity of Hepatocellular Carcinoma
Cell Lines to 5-FU via Targeting Mcl-1 and DPYD. Int J Mol Sci.
16:23668–23682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cataldo A, Cheung DG, Balsari A, Tagliabue
E, Coppola V, Iorio MV, Palmieri D and Croce CM: miR-302b enhances
breast cancer cell sensitivity to cisplatin by regulating E2F1 and
the cellular DNA damage response. Oncotarget. 7:786–797. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Ma J, Han Y, Liu J, Zhou W, Hong
L and Fan D: Targeted therapy in esophageal cancer. Expert Rev
Gastroenterol Hepatol. 10:595–604. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu S, Kitayama J, Yamashita H, Souma D and
Nagawa H: Nuclear translocation of HER-4/c-erbB-4 is significantly
correlated with prognosis of esophageal squamous cell carcinoma. J
Surg Oncol. 97:44–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao K, Chen BJ and Chen ZG: ErbB4 as a
potential molecular target in the treatment of esophageal squamous
cell cancers. ScientificWorldJournal. 2014:1241052014. View Article : Google Scholar : PubMed/NCBI
|